Abstract
Appropriately disposing of and reusing waste is a major step in promoting environmentally sustainable development. Following the rise in environmental awareness, agricultural waste has been applied as a reusable organic resource and as a cost-efficient material for preparing hydrogel adsorbents. The present study combined rice bran with sodium alginate and chitosan to prepare two new types of hydrogel beads. The beads were then modified using simple methods, and their material characteristics were analyzed using a Fourier transform infrared spectroscope, a scanning electron microscope, and thermogravimetric analysis. Specifically, the effects of pH, adsorbent dosage, adsorption time, and adsorption temperature on the performance of the hydrogel beads in the adsorption of dyes with various properties were examined. The results revealed that the optimal conditions for the rice bran/alginate hydrogel beads to adsorb crystal violet were pH 5, a dosage of 30 mg, at 30 °C, for 6 h of adsorption; furthermore, the kinetic and isothermal adsorption data were found to be consistent with the pseudo-second-order model and the Freundlich isotherm model, respectively. The optimal conditions for the rice bran/chitosan hydrogel beads to adsorb reactive blue 4 were pH 3, a dosage of 40 mg, at 50 °C, for 7 h of adsorption, and the kinetic and isothermal adsorption data were consistent with the pseudo-first-order model and the Langmuir isotherm model, respectively. This study applied natural polymers and agricultural waste to prepare cost-efficient and environmentally friendly adsorbents, which satisfy today’s environmental protection trends and economic values because of their low environmental impact and favorable adsorptive and regenerative properties. They can be prepared without high-temperature and high-pressure processing, and can be recycled through the separation of water bodies using simple filter methods, thus substantially reducing energy and monetary costs.
1. Introduction
Human survival is strongly associated with water, which plays a critical role in biochemical and metabolic reactions in the body. Although industrial development has facilitated economic prosperity, it has also brought water pollution [1]. In the face of water pollution and increasingly severe water shortages, the appropriate disposal and reuse of waste is pivotal for environmental protection and sustainable development [2,3]. Organic dyes are widely used to color final products in various industries, including cosmetics, textiles, plastics, food, leather, rubber, and paper [4,5]. Approximately 10,000 types of dyes are manufactured globally each year, which has led to the aggravation of water pollution and damage to the balance of the ecosystem [6,7]. Currently, dye wastewater is treated using various physical and chemical methods such as adsorption, chemical oxidation, photocatalytic degradation, electrochemistry, membrane filtration, biodegradation, and ion exchange [8]. In particular, adsorption has been prevalently used as a wastewater treatment approach because of its high efficiency, low selectivity, ease of use, simple operation, and cost efficiency [9,10,11].
In view of the issues mentioned above, studies have employed numerous materials to develop highly efficient adsorbents. Although activated carbon exhibits the highest adsorption effectiveness, it is costly to produce and requires a complex procedure to regenerate [12,13]. Therefore, studies have focused on developing cost-efficient adsorbents [14,15,16,17]. Selecting the base materials for preparing adsorbents requires one to consider whether there are sufficient sources for the materials and whether said materials are cost-efficient and environmentally friendly [18,19,20,21,22]. Additionally, to ensure satisfactory adsorption effectiveness, these materials must contain large amounts of carbon and oxygen as well as abundant functional groups with varied characteristics [17,23]. Currently, biosorbents have the highest developmental potential; these adsorbents adsorb target substances through the use of materials composed of biological extracts, substrates, or tissues. Materials used to create biosorbents include chitosan, alginate, fungi, plants, and agricultural wastes [24,25,26,27]. Because of their biodegradability, biocompatibility, nontoxicity, environmental friendliness, cost efficiency, and reproducibility, biosorbents are suitable for use in treating wastewater [23,28,29].
Sodium alginate is a type of natural polysaccharide composed of d-mannuronic and l-guluronic acids and can be extracted from brown seaweed [24,30]. Compared with other natural polymers, sodium alginate is cost-efficient and nontoxic to cells, and it exhibits satisfactory biocompatibility and abundant oxygen-containing functional groups (e.g., carboxyl and hydroxyl). Therefore, sodium alginate has been broadly applied in drug release, tissue engineering, cellular solidification, and food engineering [31,32]. Studies have reported that sodium alginate adsorbs contaminants effectively, such as metal ions, antibiotics, and organic dyes; it can also be applied to water purification [33,34,35]. Chitosan is a type of polysaccharide extracted from chitin through alkaline hydrolysis. Chitin is one of the richest natural amino polysaccharides in nature, second only to lignocellulose biomass, and is extracted from the exoskeletons of crustaceans and insects [36,37]. Chitosan, which features abundant amino groups (–NH2) and hydroxyls –OH), is cost-efficient and exhibits favorable biocompatibility, nontoxicity, and antibacterial properties as well as biodegradability, and it has been broadly applied in biomedicine and environmental technology [38,39,40]. However, as an adsorbent, sodium alginate is relatively poor in its adsorption capacity and selectivity; chitosan washes out easily with water, is low in mechanical strength, and is poor in acid resistance and stability. These shortcomings have limited the applicability of the two polysaccharides [24,39]. In recent years, many studies have pointed out that modified hydrogel beads may improve the shortcomings of the adsorbent. In addition, after acetic acid or ammonia modification, oxygenic functional groups of hydrogel beads can be increased and the surface of hydrogel beads can be positively charged [15,26].
Rice bran, a prevalent type of agricultural waste in Asia, is a type of yellow hull acquired from rice milling. A large amount of rice bran is produced in Taiwan. Rice bran has been usually used as feed or discarded directly. If rice bran in nature can be used to prepare adsorbents for wastewater treatment, it is facilitating waste recycling benefits and added value [10,41]. Additionally, the adsorption process is low cost, highly efficient, has easy operational procedures, and insensitivity to harmful substances. Studies have examined the effectiveness of rice bran/Fe3O4 and rice bran/SnO2/Fe3O4 adsorbents at adsorbing dyes, revealing that these adsorbents must be recycled through magnetic approaches [9,15]. Recent studies have modified chitosan and sodium alginate to prepare environmentally friendly adsorbents [24,40]. However, few have explored the possibility of combining agricultural waste with these two saccharides to improve the adsorption performance. In this study, rice bran was combined with sodium alginate and chitosan to prepare hydrogel adsorbents, which can be recycled easily without the use of magnetic forces, and can exhibit improved mechanical strength. To the best of our knowledge, this work is the first attempt to prepare rice bran-based hydrogel beads to remove dye from aqueous solutions; it exhibits applicational potentiality for removal of dye from wastewater. Furthermore, all of the materials employed are highly biocompatible and biodegradable; they can be acquired from a wide range of sources, are cost-efficient, and are green and safe.
In this study, rice bran was combined with sodium alginate and chitosan to create two types of new hydrogel adsorbents for removing the dyes crystal violet (CV) and reactive blue 4 (RB4) from aqueous solutions, respectively. To enhance their dye removal rates, the effects of various controlling factors of adsorption effectiveness were investigated, such as pH, adsorbent dosage, adsorption temperature, and adsorption time. Subsequently, kinetic and isothermal adsorption analyses were conducted, and mathematical models were employed to analyze the CV and RB4 adsorption behaviors of the adsorbents. Finally, the feasibility of recycling and reusing the adsorbent materials was evaluated. The newly developed hydrogel adsorbents effectively processed industrial waste and enabled them to be reused as resources; thus, they possess considerable potential for environmentally friendly material development.
2. Materials and Methods
2.1. Rice Bran Hydrogel Beads
Rice bran from the rice variety Tainan No. 11 was purchased from Taichung, Taiwan, and a 100 mesh sifting screen was used to select bran powder less than 100 meshes in diameter. The powder was placed in a sealed glass bottle and stored in a refrigerator at 4 °C for preprocessing. Subsequently, 400 mg of rice bran was added to 40 mL of deionized water and was stirred with a magnet for 30 min to dissolve; 10 mL of the rice bran solution was added to 200 mg of sodium alginate powder, which was then stirred with a magnet for 1 h in order for the sodium alginate to completely dissolve. An infusion pump with a syringe (aperture: 2 mm) was used to drop the solution to the 5 wt.% calcium chloride solution at 75 mL/h in order to create rice bran/alginate hydrogel beads (RAHBs). The RAHBs were immersed in the solution for 1 h, separated through the filter, and rinsed multiple times using deionized water. Approximately 4.5 g of the RAHBs were immersed in 200 mL of a 4.4 M acetic acid solution, placed in a constant-temperature water tank set to 30 °C, and oscillated at 100 rpm for 5 h. The RAHBs were separated through the filter, rinsed multiple times using deionized water, and dried in an oven at 50 °C for several hours, thus completing the modified RAHBs (MRAHBs).
Another 500 mg of rice bran was added to 49.5 mL of deionized water and stirred with a magnet for 10 min for dissolution; 1.2 g of chitosan powder was then added to the solution. Subsequently, the solution was added to 500 µL of a 1% acetic acid solution and was stirred at 40 °C for 2 h for thorough dissolution. An infusion pump was employed to drop the solution to the 0.5 M sodium hydroxide solution at 75 mL/h to create rice bran/chitosan hydrogel beads (RCHBs). The RCHBs were immersed in the solution for 22 h, separated through the filter, and rinsed multiple times using deionized water. Approximately 4.5 g of the RCHBs was immersed in 200 mL of 4.4 M ammonia solution, placed in the constant-temperature water tank set to 30 °C, and oscillated for 5 h. The RCHBs were separated through the filter, rinsed multiple times using deionized water, and dried in an oven at 50 °C for several hours, thus completing the modified RCHBs (MRCHBs).
2.2. Hydrogel Beads Adsorption Experiments
First, 20 mg extracts of the MRAHBs and MRCHBs were placed in 20 mL of 200 mg/L CV and RB4 solutions, respectively. The effects of control variables, such as the pH (2–10) of the dye solution, temperature (30°C–60 °C), adsorbent dose (0.5–3.0 g/L), and adsorption time (0–600 min), were studied. In all of the experiments, the clear liquid in the upper layers was extracted and diluted, and ultraviolet–visible spectroscopy (UV–VIS, Thermo Scientific, Dreieich, Germany) was performed to measure the absorbance. A calibration curve was subsequently employed to calculate the adsorption capacity, thus clarifying the effect of the control variables on the dye adsorption effectiveness.
The removal rate was calculated according to the following equation [15]:
where Co and Ce are the dye concentration (mg/L) at the start and equilibrium, respectively.
2.3. Adsorbent Regeneration
The solvent employed for MRAHB regeneration was comprised of 5 wt.% calcium chloride and 4.4 M acetic acid solutions, and that applied for MRCHB regeneration consisted of 0.5 M sodium hydroxide and 4.4 M ammonia solutions. The adsorbents were immersed in the solvents for regeneration after dye adsorption. The solutions were placed in the constant-temperature water tank, set to 30 °C, for oscillation. The regenerated adsorbents were rinsed multiple times using deionized water and dried in the oven at 50 °C for reuse. The desorption effectiveness of the regeneration solvents was compared, and those with an optimal desorption effectiveness were selected for five additional adsorption–desorption cycle tests, thus clarifying the feasibility of reusing the adsorbents.
3. Results and Discussion
3.1. Hydrogel Beads Material Surface Morphology Analysis
The surface morphology of hydrogel bead material was observed by scanning electron microscopy (SEM, S-3000H, Hitachi Science and Technology, Tokyo, Japan). Figure 1a–c illustrate the results of the SEM analysis of the RAHBs before and after modification and after dye adsorption, and Figure 2a–c depict those of the RCHBs. Both adsorbents featured smooth surfaces before modification; the surfaces became rough and wrinkled after modification, exhibiting additional adsorption surface areas. Additional wrinkles emerged after dye adsorption because of the dyes that had been adsorbed.

Figure 1.
SEM images of the rice bran/alginate hydrogel beads (RAHBs): (a) before modification, (b) after modification, and (c) after dye adsorption.

Figure 2.
SEM images of the rice bran/chitosan hydrogel beads (RCHBs): (a) before modification, (b) after modification, and (c) after dye adsorption.
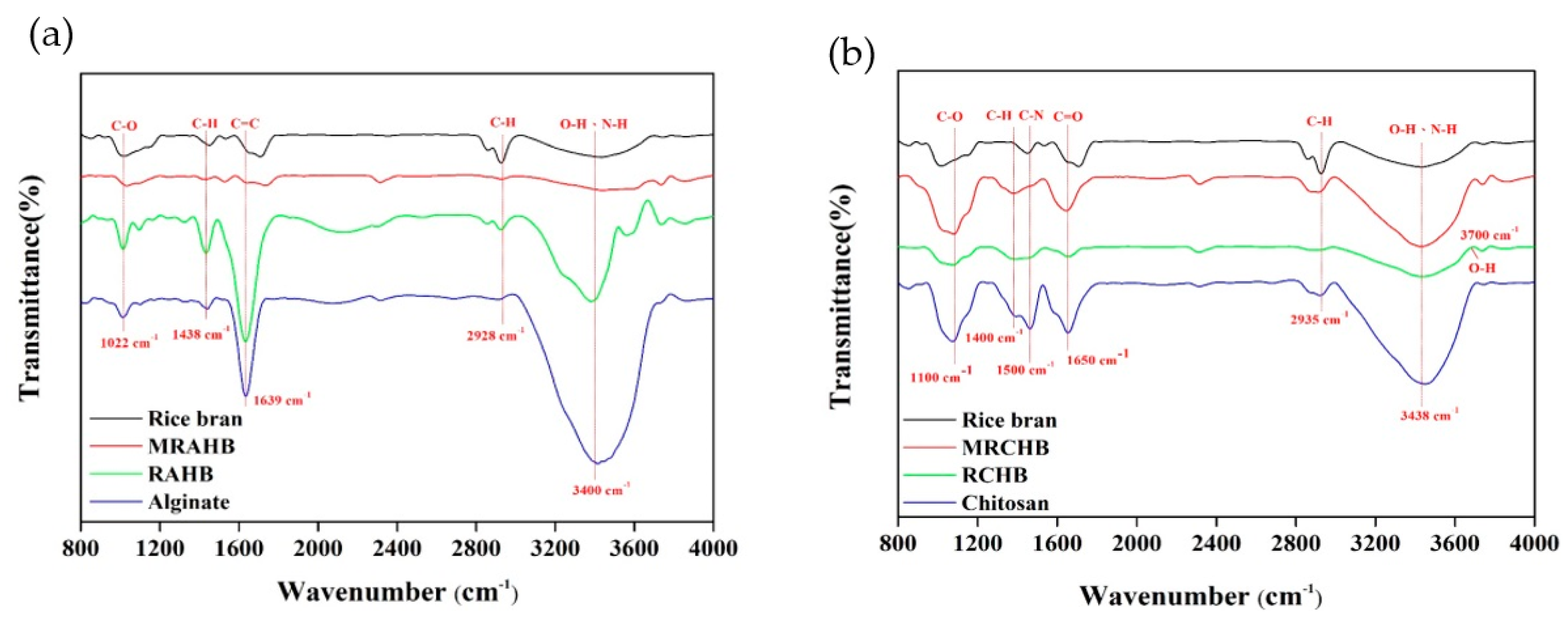
Fourier transform infrared spectroscopy (FTIR, 6600 Series, Jasco, Tokyo, Japan) was employed to analyze the differences between and the changes in the functional groups of the RAHBs and RCHBs (Figure 3). Figure 3a presents FTIR maps of the rice bran, sodium alginate, RAHBs, and MRAHBs. At 3400–3500 cm−1, the rice bran exhibited active sites in the O–H and N–H groups; at 2800–3000 cm−1, C–H stretching vibrations were observed; at 1600–1800 cm−1, C=C stretching vibrations were identified; at 1300–1500 cm−1, C–H bending vibrations were noted; and at 1000–1200 cm−1, C–O stretching vibrations were observed [15,42]. At 3000–3600 cm−1, the sodium alginate exhibited a strong and wide O–H group absorption peak; at 1650 cm−1, C=O bonding was observed inside the carboxylate; at 1400–1500 cm−1, asymmetric COOH groups were noted; and at 1000 cm−1, symmetric COOH groups were identified [43]. At 3000–3600 cm−1, the RAHBs were observed to provide N–H groups to the rice bran as well as O–H groups to both the rice bran and sodium alginate; at 2800–3200 cm−1, a stretching effect was identified in the C–H bonds in the rice bran; at 1600–1800 cm−1, C=C stretching vibrations were observed in the celluloses of the rice bran; at 1300–1400 cm−1, C–H bending vibrations were identified; and at 1000–1200 cm−1, symmetric COOH groups and internal C–O bonding were observed. At 1600–1800 cm−1, the MRAHBs exhibited two absorption peaks, namely C=C stretching vibrations and carboxylic acid C=O bonding; the absorption peaks increased substantially after acetic acid modification at 1000 cm−1 (–COOH), indicating more incorporation of –COO− groups. After the rice bran was loaded into the surface of the alginate hydrogel beads, the transmittance intensity was significantly attenuated. This also indicated that the rice bran was indeed attached to the alginate hydrogel beads. Figure 3b presents FTIR maps of the rice bran, chitosan, RCHBs, and MRCHBs. At 3000–3600 cm−1, N–H and O–H groups in the chitosan were noted [44]; at 2800–3200 cm−1, a stretching effect was identified in the internal C–H bond; at 1650 cm−1, C=O stretching vibrations were observed; and at 1500, 1400, and 1100 cm−1, C-N bending vibrations, C–H bending vibrations, and C–O and C–N vibrations were noted, respectively. At 3700 cm−1, free O–H tensile vibrations were noted in the RCHBs, and at 2900 cm−1, C–H stretching vibrations were caused by the celluloses and hemicelluloses in the chitosan and rice bran. At 3200–3600 cm−1, an absorption peak generated by the N–H group was noted in the MRCHBs; due to the vibration intensity increasing after the surface concentration increased through ammonia modification, the O–H group absorption peak at 1100 cm−1 increased substantially in intensity after ammonia modification [24].
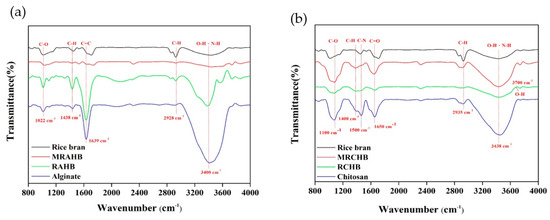
Figure 3.
FTIR analysis results on the adsorbents: (a) RAHB and (b) RCHB.
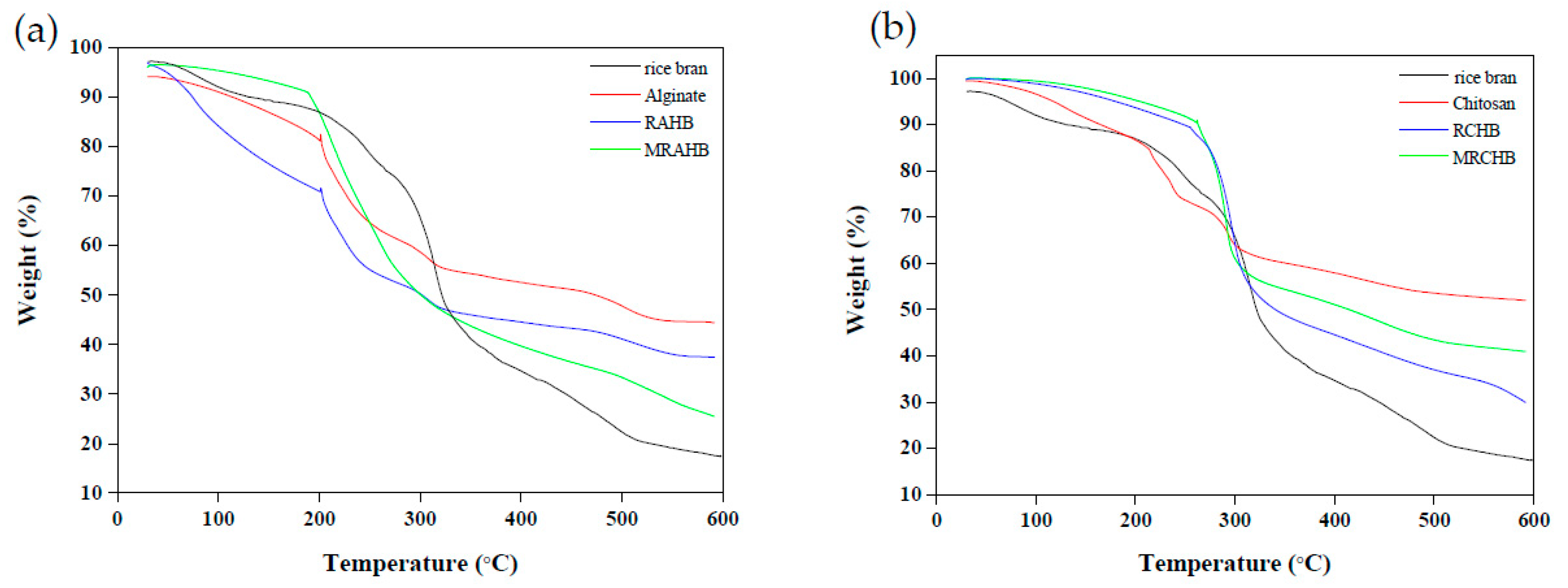
Figure 4 presents the results of the thermogravimetric analysis (TGA; STA7300, Hitachi Science and Technology, Tokyo, Japan) performed on the rice bran, sodium alginate, chitosan, RAHBs, RCHBs, MRAHBs, and MRCHBs. The 5% weight loss temperature of the MRAHBs was 109.43 °C, higher than that of the rice bran, sodium alginate, and RAHBs (76 °C, 34.53 °C, and 48.61 °C, respectively; Figure 4a). The 5% weight loss temperature of the MRCHBs, rice bran, chitosan, and RCHBs was 204.27 °C, 71.61 °C, 117.53 °C, and 179.87 °C, respectively (Figure 4b). The thermogravimetric analysis of the MRCHBs compared with the rice bran, chitosan, and RCHBs, were found to be higher thermal stability. According to Figure 4a, weight loss at temperatures below 200 °C was attributed to water evaporation in the material, which, at temperatures between 220 °C and 320 °C, was attributed to breaking of the main chain in the sodium alginate and, at temperatures higher than 320 °C, was attributed to the decomposition of the carboxyl groups [45]. According to Figure 4b, the 50% weight loss that occurred at temperatures between 200 °C and 500 °C was caused by the decomposition of the crosslinking structure in the chitosan [46].
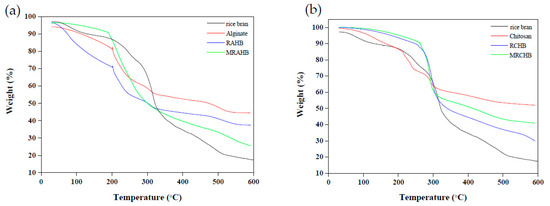
Figure 4.
TGA results for (a) RAHBs and (b) RCHBs.
The swell rate (S %) was determined by the following equation [24,47]:
where Ww and Wd are the weights (g) of the wet and dried hydrogel beads, respectively.
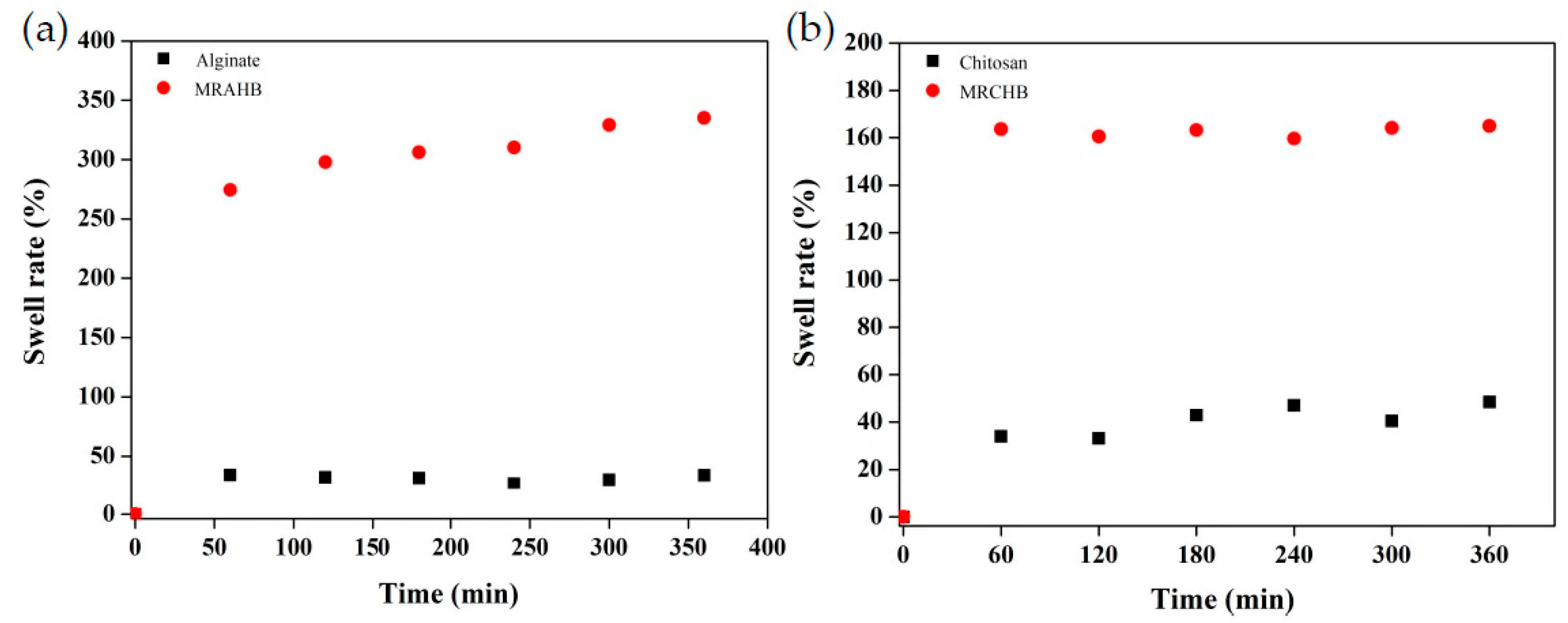
The swell rate reveals the mechanical and hydrophilic properties of hydrogel beads (Figure 5). The swell rate of the MRAHBs and MRCHBs were higher than those of the sodium alginate and chitosan, indicating that the modified hydrogel beads exhibited higher hydrophilic properties than the sodium alginate and chitosan. This increased the adsorbents’ effectiveness at adsorbing the dyes [48].

Figure 5.
Expansion ratios of the hydrogel beads: (a) modified RAHBs (MRAHBs) and (b) modified MRCHBs (MRCHBs).
3.2. The Effects of Control Variables
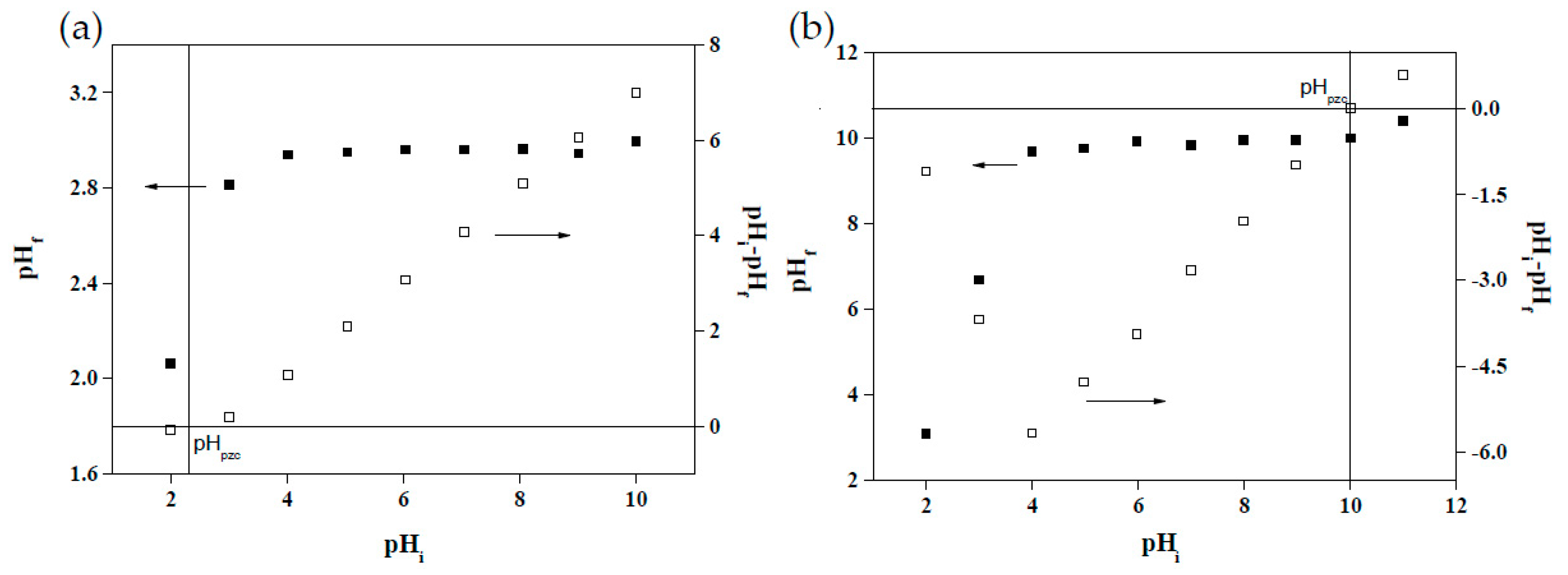
Changes in the pH of solutions affect the surface charge between adsorbents and adsorbates as well as alter the structures and forms of functional groups. The surface charge of adsorbents critically influences their dye adsorption effectiveness [15]. As depicted in Figure 6a, when the pH rose from 2 to 4, the CV adsorption capacity of the MRAHBs increased substantially, but the capacity did not increase considerably at pH values higher than 4. Figure 7a illustrates the changes in the surface charge of the MRAHBs, the pHpzc of which was 2.40. When pH < pHpzc, the adsorption capacity decreased because the adsorbent surface carried a positive charge and could not attract CV, which also carried a positive charge [43]. Conversely, when pH > pHpzc, the adsorbent surface carried a negative charge, enabling it to attract CV. Because the adsorption equilibrium was reached when pH = 4–10 and the unadjusted pH value of CV was 5, the optimal pH for CV adsorption was set as 5. As demonstrated in Figure 6b, the RB4 adsorption capacity of the MRCHBs decreased as the pH rose; according to the surface charge of the MRCHBs (depicted in Figure 7b), the pHpzc of the MRCHBs was 10. When pH > pHpzc, the surface charge of the MRCHBs was negative, preventing the adsorbent from adsorbing RB4, which also carried a negative charge [49]. Conversely, when pH < pHpzc, the RB4 adsorption capacity increased following the rise in the positive surface charge of the MRCHBs. Accordingly, the optimal pH for the MRCHBs in adsorbing RB4 was set as 3.

Figure 6.
Effect of pH on adsorption effectiveness: (a) MRAHBs and (b) MRCHBs.
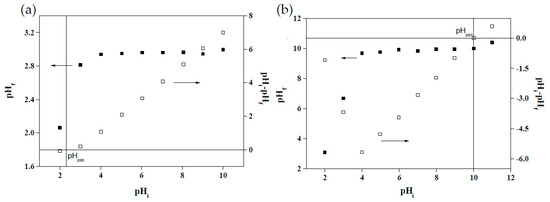
Figure 7.
Points of zero charge: (a) MRAHBs and (b) MRCHBs.
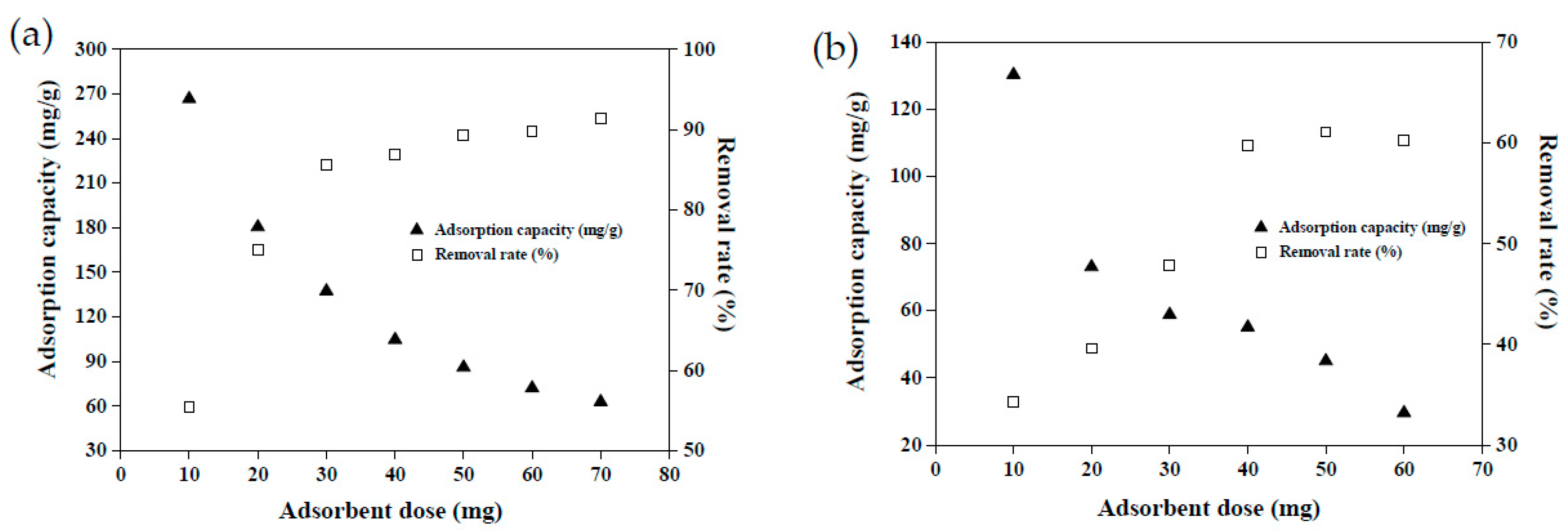
Adsorbent dosage is a key factor in the dye adsorption system and determines the effectiveness of dye removal [24]. Examining the dosage required to remove each unit of dye solution is pivotal for maximizing the cost efficiency and benefits of adsorbents. Figure 8 illustrates the effects of adsorbent dosage on the dye adsorption capacity and removal rate. When 50 mg of MRAHBs was applied, the CV adsorption capacity was 86 mg/g, constituting a removal rate of 89%. When the MRAHB dosage increased continually beyond 50 mg, the adsorption capacity decreased gradually, but the dye removal rate increased gradually, eventually reaching an equilibrium (Figure 8a). This is because the amount of dye in the solution was fixed. Excessive adsorbent dosage caused the active sites of the adsorbent to compete, leading to a decrease in the amount of dye adsorbed per unit surface area; the excessive adsorbent concentration also caused these active sites to overlap [11]. Accordingly, the optimal MRAHB dosage for CV removal was set as 50 mg. Figure 8b demonstrates the effect of MRCHB dosage on RB4 removal. When 40 mg of MRCHBs was implemented, the RB4 adsorption capacity was 57 mg/g, constituting a removal rate of 60%. When the MRCHB dosage increased beyond 40 mg, the RB4 removal rate did not change substantially but the adsorption capacity decreased gradually. According to the material surface morphology analysis and the effects of control variables discussion, the surface of MRCHBs is positively charged under acidic conditions and CV adsorption onto MRAHBs may be due to the cationic dye easily reacting with oxygenic functional groups. Therefore, the adsorption mechanism might contain the hydrogen bond and electrostatic attraction.
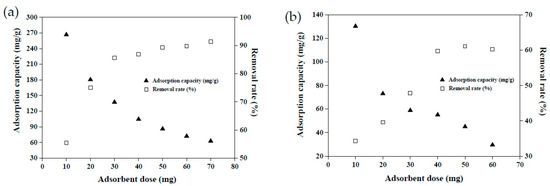
Figure 8.
Effect of the adsorbent dosage on dye adsorption: (a) MRAHBs and (b) MRCHBs.
Temperature critically affects dye adsorption effectiveness [15,50]. Figure 9 illustrates the relationship between temperature and the effectiveness of dye adsorption, which involved endothermic and exothermic reactions. The CV adsorption capacity of the MRAHBs decreased following a rise in temperature, demonstrating an exothermic reaction. Therefore, the optimal CV adsorption temperature was determined to be 30 °C. By contrast, the RB4 adsorption by the MRCHBs involved an endothermic reaction. Because the instability in the MRCHB structure at 60 °C led to its dissolution, the optimal RB4 adsorption temperature for the MRCHBs was set as 50 °C.

Figure 9.
Effect of temperature on dye adsorption: (a) MRAHBs and (b) MRCHBs.
3.3. Kinetics and Isotherm Adsorption
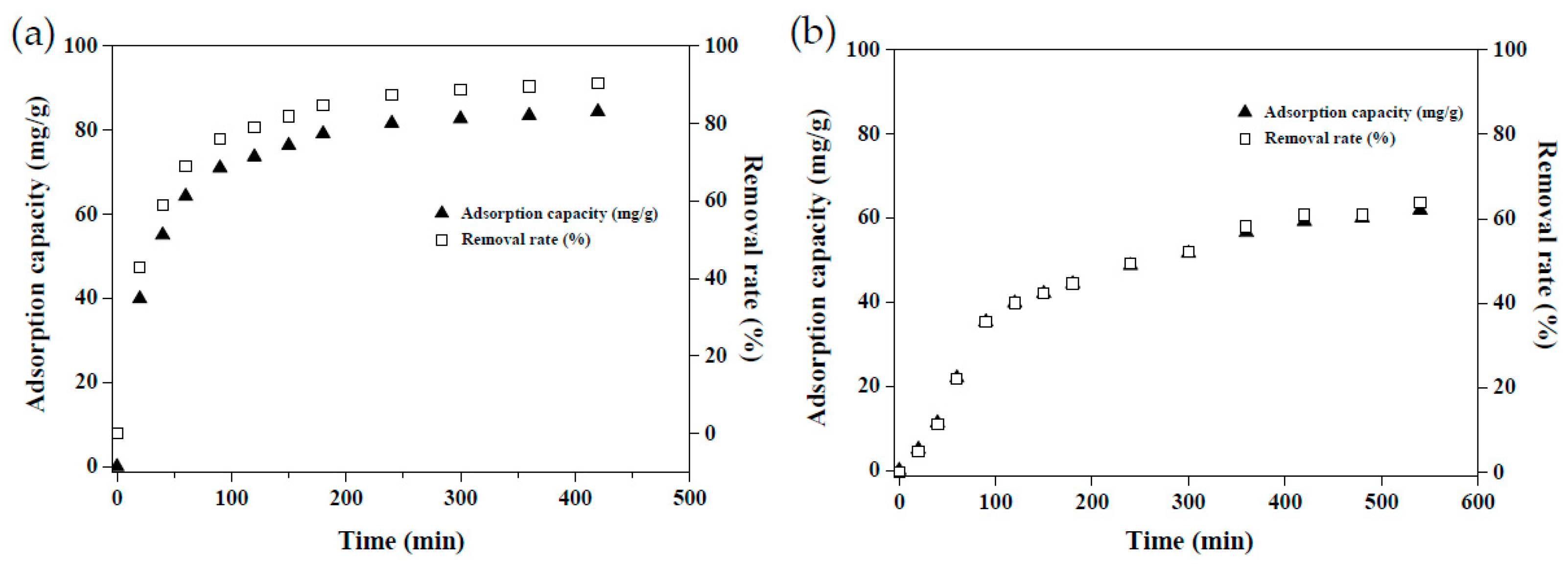
The kinetic dye adsorption test was conducted using single-factor optimal conditions (pH 5, at 30 °C, and 50 mg of MRAHBs for CV adsorption; pH 3, at 50 °C, and 40 mg of MRACBs for RB4 adsorption), yielding the results presented in Figure 10. The dye adsorption capacities of the MRAHBs and MRCHBs increased over time, and the equilibria for the MRAHBs and MRCHBs were reached in the 6th and 7th hours, respectively. Theoretical calculations were performed on the kinetic adsorption data using the pseudo-first-order model (PFOM), pseudo-second-order model (PSOM), and intraparticle diffusion model (IPDM). The following equations were employed [9,15,24]:
where k1 (min−1) and k2 (g/mg·min) are the adsorption rate constants of PFOM and PSOM, respectively. The qt (mg/g) is the adsorption capacity at time t (min). kid (mg/g·min0.5) is the intraparticle diffusion rate constant. C is the boundary layer thickness.

Figure 10.
Kinetic dye adsorption: (a) MRAHBs and (b) MRCHBs.
Table 1 lists the kinetic adsorption model calculation results. For both CV and RB4 adsorption by the MRAHBs and MRCHBs, respectively, the qe values calculated using the PSOM were closer to the measured values than those calculated using the PFOM, and their R2 values were also closer to 1. Therefore, the PSOM was more suitable for describing the kinetic dye adsorption behaviors of the adsorbents and dyes. Due to the dye adsorption behaviors of the adsorbents being complex and potentially involving numerous types of reaction mechanisms, IPDM was employed to describe the diffusion of the dye molecules from the solvents to the adsorbent bodies. According to the kinetic adsorption data, the process from the start of adsorption to the final equilibrium consisted of several different linear stages. The first stage involved rapid adsorption; due to many active sites being on the adsorbent surfaces, the dyes in the solutions rapidly occupied the sites, leading to a high adsorption rate. The second stage involved pore diffusion; specifically, the adsorption slowed because of the diffusion of the dyes within the pores of the adsorbents. The third stage involved adsorption equilibrium, in which the adsorption rate was maintained at a fixed value. A higher C indicated a thicker boundary layer. Table 1 reveals that all C values were higher in the second stage than in the first stage and that all of the adsorption rates were lower in the second stage than in the first stage.

Table 1.
Kinetic dye adsorption model calculation results.
The isothermal dye adsorption data were calculated using the Langmuir and Freundlich isotherm models. The following equations were applied [15,24,51]:
where Ce is the dye concentration (mg/L) at equilibrium, and qm and KL are the maximum adsorption capacity (L/mg) and the Langmuir constant, respectively. KF and n are Freundlich isotherm constant (mg/g) and adsorption intensity, respectively.
Table 2 lists the model calculation results. The isothermal CV adsorption behavior of the MRAHBs was more consistent with the Freundlich model than the Langmuir model. The Langmuir model revealed that the maximal CV adsorption capacity of the MRAHBs (qm) was 454.55 mg/g; moreover, the CV adsorption capacity of the MRAHBs decreased following a rise in the temperature. Accordingly, the adsorption process involved an exothermic reaction. The isothermal RB4 adsorption behavior of the MRCHBs was more consistent with the Langmuir model, which revealed the maximal adsorption capacity (qm) to be 212.77 mg/g; the RB4 adsorption capacity of the MRCHB increased following a rise in temperature. Accordingly, the adsorption process involved an endothermic reaction. The MRAHBs and MRCHBs exhibited considerably higher adsorption capacities than the other adsorbents created using agricultural waste [9,15], thereby demonstrating a higher potential for practical application.

Table 2.
Isothermal dye adsorption model calculation results.
3.4. Adsorbent Regeneration
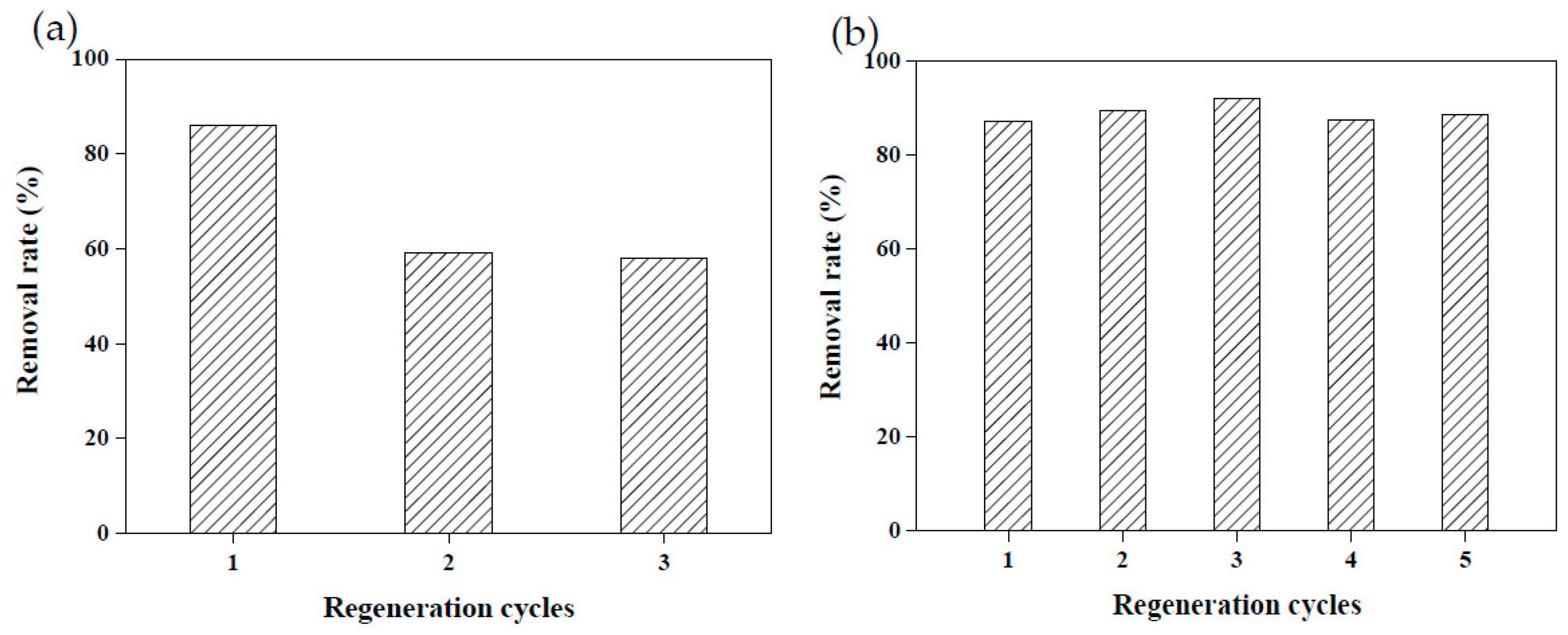
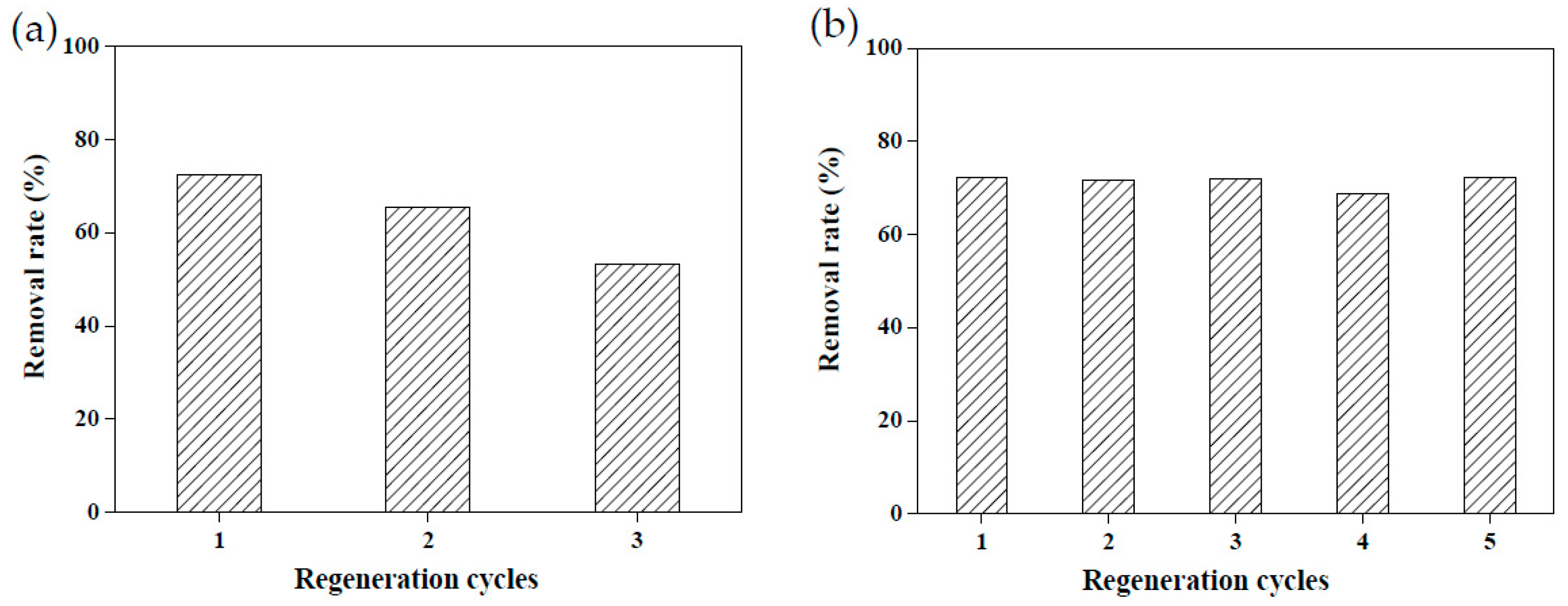
Due to the raw materials used to create adsorbents being agricultural waste, they are relatively unlikely to cause secondary pollution. However, recycling adsorbents prepared using agricultural waste remains a challenge [3,18]. Reusable adsorbents reduce the cost of wastewater treatment considerably. To explore the reusability of the adsorbents created in this study, 5 wt.% calcium chloride and 4.4 M acetic acid were employed to conduct a desorption test on MRAHBs that had completed dye adsorption. Both solutions effectively desorbed the dye from the MRAHBs. In particular, acetic acid provided numerous anionic groups to enhance the adsorption capacity of the MRAHBs in addition to regenerating the adsorbent. After five repeated tests, the desorption process exhibited nearly no effect on the CV removal rate by the MRAHBs (Figure 11). Figure 12a,b illustrate the use of 0.5 M sodium hydroxide and 4.4 M ammonia in the MRCHB desorption test, respectively. The RB4 removal rate of the MRCHBs after being regenerated using sodium hydroxide three times decreased by 20%, but their RB4 removal rate remained satisfactory after they were regenerated using ammonia five times. This is because the ammonia provided numerous cationic functional groups, which enhanced the adsorption capacity of the MRCHBs.
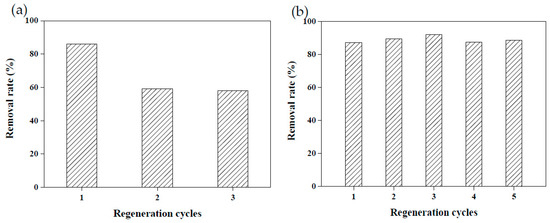
Figure 11.
MRAHB regeneration results: (a) 5 wt.% calcium chloride and (b) 4.4 M acetic acid.
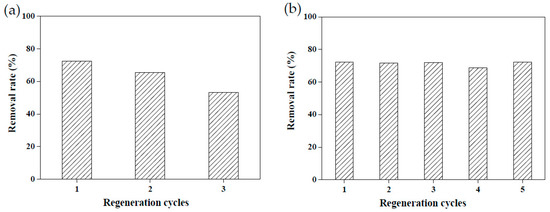
Figure 12.
MRCHB regeneration results: (a) 0.5 M sodium hydroxide and (b) 4.4 M ammonia.
4. Conclusions
The topic of a circular economy has received great attention from governments worldwide. Industrial waste recycling technology has been heavily promoted to mitigate environmental impacts and to enable sustainable development of the environment. This study combined rice bran with sodium alginate and chitosan to create two new types of hydrogel adsorbents. The adsorption tests revealed that the optimal conditions for the MRAHBs to adsorb the cationic 200 mg/L CV were pH 5 at a dosage of 30 mg at 30 °C for 6 h of adsorption; the kinetic and isothermal adsorption data were consistent with the pseudo-second-order model and the Freundlich isotherm model. The optimal conditions for the MRCHBs for adsorbing anionic 200 mg/L RB4 were pH 3 at a dosage of 40 mg at 50 °C for 7 h of adsorption; the kinetic and isothermal adsorption data were consistent with the pseudo-first-order model and the Langmuir isotherm model. The regeneration tests revealed 4.4 M acetic acid as being the most effective solution for desorbing CV from the MRAHBs, whereas 4.4 M ammonia was revealed as the most effective solution for desorbing RB4 from the MRCHBs. Therefore, these adsorbents prepared using rice bran were effective at dye adsorption. Future studies should apply these adsorbents for the removal of heavy metals or emerging pollutants. These new adsorbents will enable an effective reduction in agricultural waste and the creation of agriculture added value, thereby achieving environmental protection and sustainable development. This agricultural waste-reuse technology is expected to open a new door for the circular agricultural economy.
Author Contributions
Conceptualization and methodology, T.-J.Y., G.-B.H. and C.-M.M.; investigation and formal analysis, T.-J.Y.; resources, G.-B.H.; data curation, T.-J.Y.; writing—original draft preparation, T.-J.Y., G.-B.H. and C.-M.M.; writing—review and editing, H.-C.L. and C.-M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Science and Technology, Taiwan (grant number MOST 108-2221-E-027-001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Ministry of Science and Technology, Taiwan, for supporting this research financially.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Dietrich, A.M.; Burlingame, G.A. Critical review and rethinking of USEPA secondary standards for maintaining organoleptic quality of drinking water. Environ. Sci. Technol. 2015, 49, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Hynes, N.R.J.; Kumar, J.K.; Kamyab, H.; Sujana, J.A.J.; Al-Khashman, O.A.; Kuslu, Y.; Ene, A.; Kumar, B.S. Modern enabling techniques and adsorbents based dye removal with sustainability concerns in textile industrial sector—A comprehensive review. J. Clean. Prod. 2020, 272, 122636. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2018, 16, 1193–1226. [Google Scholar] [CrossRef]
- Fernandes, F.H.; Bustos-Obregon, E.; Salvadori, D.M.F. Disperse Red 1 (textile dye) induces cytotoxic and genotoxic effects in mouse germ cells. Reprod. Toxicol. 2015, 53, 75–81. [Google Scholar] [CrossRef]
- Chahinez, H.O.; Abdelkader, O.; Leila, Y.; Tran, H.N. One-stage preparation of palm petiole-derived biochar: Characterization and application for adsorption of crystal violet dye in water. Environ. Technol. Innov. 2020, 19, 100872. [Google Scholar] [CrossRef]
- Hong, G.B.; Wang, Y.K. Synthesis of low-cost adsorbent from rice bran for the removal of reactive dye based on the response surface methodology. Appl. Surf. Sci. 2017, 423, 800–809. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Jabeen, A.; Iqbal, M.; Noreen, S.; Naseem, Z. Adsorptive behavior of rice bran-based composites for malachite green dye: Isotherm, kinetic and thermodynamic studies. J. Mol. Liq. 2017, 237, 322–333. [Google Scholar] [CrossRef]
- Cinar, S.; Kaynar, U.H.; Aydemir, T.; Kaynar, S.C.; Ayvacikli, M. An efficient removal of RB5 from aqueous solution by adsorption onto nano-ZnO/chitosan composite beads. Int. J. Biol. Macromol. 2017, 96, 459–465. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zang, H.; Chen, L.; Meng, Z.; Li, H.; Ci, L. ZnCl2-activated carbon from soybean dregs as a high efficiency adsorbent for cationic dye removal: Isotherm, kinetic, and thermodynamic studies. Environ. Technol. 2018, 30, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.H.; Kadhum, A.M.; Ngoh, Y.S. Applicability of dragon fruit (Hylocereus 77 polyrhizus) peels as low-cost biosorbent for adsorption of methylene blue from aqueous solution: Kinetics, equilibrium and thermodynamics studies. Desalin. Water Treat. 2018, 109, 231–240. [Google Scholar] [CrossRef]
- Karthik, R.; Muthezhilan, R.; Hussain, A.J.; Ramalingam, K.; Rekha, V. Effective removal of methylene blue dye from water using three different low-cost adsorbents. Desalin. Water Treat. 2016, 57, 10626–10631. [Google Scholar] [CrossRef]
- Ma, C.M.; Hong, G.B.; Wang, Y.K. Performance Evaluation and Optimization of Dyes Removal using Rice Bran-Based Magnetic Composite Adsorbent. Materials 2020, 13, 2764. [Google Scholar] [CrossRef]
- Qian, W.C.; Luo, X.P.; Wang, X.; Guo, M.; Li, B. Removal of methylene blue from aqueous solution by modified bamboo hydrochar. Ecotoxicol. Environ. Saf. 2018, 157, 300–306. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M. Green adsorbents for wastewaters: A critical review. Materials 2014, 7, 333–364. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Utilization of punica granatum peel as an eco-friendly biosorbent for the removal of methylene blue dye from aqueous solution. J. Appl. Biotechnol. Bioeng. 2018, 5, 242–249. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Cheng, Z. Removal of organic pollutants from aqueous solution using agricultural wastes: A review. J. Mol. Liq. 2015, 212, 739–762. [Google Scholar] [CrossRef]
- Hamzeh, Y.; Ashori, A.; Azadeh, E.; Abdulkhani, A. Removal of acid orange 7 and remazol black 5 reactive dyes from aqueous solutions using a novel biosorbent. Mater. Sci. Eng. C 2012, 32, 1394–1400. [Google Scholar] [CrossRef]
- Alizadeh, N.; Shariati, S.; Besharati, N. Adsorption of crystal violet and methylene blue on azolla and fig leaves modified with magnetite iron oxide nanoparticles. Int. J. Environ. Res. 2017, 11, 197–206. [Google Scholar] [CrossRef]
- Basu, M.; Guha, A.K.; Ray, L. Adsorption of lead on cucumber peel. J. Clean. Prod. 2017, 151, 603–615. [Google Scholar] [CrossRef]
- Kadhom, M.; Albayati, N.; Alalwan, H.; Al-Furaiji, M. Removal of dyes by agricultural waste. Sustain. Chem. Pharm. 2020, 16, 100259. [Google Scholar] [CrossRef]
- Ma, C.M.; Yang, B.Y.; Hong, G.B. Husk of agarwood fruit-based hydrogel beads for adsorption of cationic and anionic dyes in aqueous solutions. Molecules 2021, 26, 1437. [Google Scholar] [CrossRef] [PubMed]
- Dawood, S.; Sen, T.K.; Phan, C. Performance and dynamic modelling of biochar and kaolin packed bed adsorption column for aqueous phase methylene blue (MB) dye removal. Environ. Technol. 2018, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Quansah, J.O.; Hlaing, T.; Lyonga, F.N.; Kyi, P.P.; Hong, S.H.; Lee, C.G.; Park, S.J. Nascent rice husk as an adsorbent for removing cationic dyes from textile wastewater. Appl. Sci. 2020, 10, 3437. [Google Scholar] [CrossRef]
- Wong, S.; Ghafar, N.A.; Ngadi, N.; Razmi, F.A.; Inuwa, I.M.; Mat, R.; Amin, N.A.S. Effective removal of anionic textile dyes using adsorbent synthesized from coffee waste. Sci. Rep. 2020, 10, 2928. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Sun, Q.; Wang, W.; Lu, L.; Liu, M.; Li, J.; Yang, S.; Sun, Y.; Zhang, K.; Xu, J.; et al. Utilizations of agricultural waste as adsorbent for the removal of contaminants: A review. Chemosphere 2018, 211, 235–253. [Google Scholar] [CrossRef]
- Tan, K.B.; Vakili, M.; Horri, B.A.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Gan, L.; Li, H.; Chen, L.; Xu, L.; Liu, J.; Geng, A.; Mei, C.; Shang, S. Graphene oxide incorporated alginate hydrogel beads for the removal of various organic dyes and bisphenol A in water. Colloid Polym. Sci. 2018, 296, 607–615. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Qin, Y.; Shen, P.; Peng, Q. Structures, properties and application of alginic acid: A review. Int. J. Biol. Macromol. 2020, 162, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Mubarak, N.M.; Jannat, F.T.; Ashfaq, T.; Santulli, C.; Rizwan, M.; Najda, A.; Bin-Jumah, M.; Abdel-Daim, M.M.; Hussain, S.; et al. A critical review on the synthesis of natural sodium alginate based composite materials: An innovative biological polymer for biomedical delivery applications. Processes 2021, 9, 137. [Google Scholar] [CrossRef]
- Hu, Z.H.; Omer, A.M.; Ouyang, X.K.; Yu, D. Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb(II) from aqueous solution. Int. J. Biol. Macromol. 2018, 108, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Butter, B.; Santander, P.; Pizarro, G.D.C.; Oyarzun, D.P.; Tasca, F.; Sanchez, J. Electrochemical reduction of Cr(VI) in the presence of sodium alginate and its application in water purification. J. Environ. Sci. 2021, 101, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.J.; Huang, X.L.; Yang, F.; Zhao, W.F.; Zhou, X.Z.; Zhao, C.S. Engineering sodium alginate-based cross-linked beads with high removal ability of toxic metal ions and cationic dyes. Carbohydr. Polym. 2018, 187, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.W.; Jeon, B.H.; Chon, C.M.; Schwartz, F.W.; Jeong, Y.; Song, H. Magnetic chitosan composite for adsorption of cationic and anionic dyes in aqueous solution. J. Ind. Eng. Chem. 2015, 28, 60–66. [Google Scholar] [CrossRef]
- Perez-Calderon, J.; Santos, M.V.; Zaritzky, N. Reactive RED 195 dye removal using chitosan coacervated particles as bio-sorbent: Analysis of kinetics, equilibrium and adsorption mechanisms. J. Environ. Chem. Eng. 2018, 6, 6749–6760. [Google Scholar] [CrossRef]
- Croisier, F.; Jerome, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef]
- Safie, N.N.; Zahrim, A.Y. Recovery of nutrients from sewage using zeolite-chitosan-biochar adsorbent: Current practices and perspectives. J. Water Process. Eng. 2021, 40, 101845. [Google Scholar] [CrossRef]
- Qi, J.; Li, Y.; Majeed, H.; Goff, H.D.; Rahman, M.R.T.; Fang Zhong, F. Adsorption mechanism modeling using lead (Pb) sorption data on modified rice bran-insoluble fiber as universal approach to assess other metals toxicity. Int. J. Food Prop. 2019, 22, 1397–1410. [Google Scholar] [CrossRef]
- Patel, M.; Naik, N. Gamma-oryzanol from rice bran oil-a review. J. Sci. Ind. Res. 2004, 63, 569–578. [Google Scholar]
- Kong, Y.; Zhuang, Y.; Han, Z.; Yu, J.; Shi, B.; Han, K.; Hao, H. Dye removal by eco-friendly physically cross-linked double network polymer hydrogel beads and their functionalized composites. J. Environ. Sci. 2019, 78, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Lee, M.W.; Woo, S.H. Adsorption of congo red by chitosan hydrogel beads impregnated with carbon nanotubes. Bioresour. Technol. 2010, 101, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Doctorsafaei, A.H.; Zia, K.M. Alginate/calix[4] arenes modified graphene oxide nanocomposite beads: Preparation, characterization, and dye adsorption studies. Int. J. Biol. Macromol. 2018, 120, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.X.; Li, Y.; Wang, L.; Wang, J.; Cao, Y.L. Preparation of supported chitosan adsorbent with high adsorption capacity for Titan Yellow removal. Int. J. Biol. Macromol. 2020, 152, 449–455. [Google Scholar] [CrossRef]
- Elwakeel, K.Z. Removal of reactive black 5 from aqueous solutions using magnetic chitosan resins. J. Hazard. Mater. 2009, 167, 383–392. [Google Scholar] [CrossRef]
- Wu, M.; Chen, W.; Mao, Q.; Bai, Y.; Ma, H. Facile synthesis of chitosan/gelatin filled with graphene bead adsorbent for orange II removal. Chem. Eng. Res. Des. 2019, 144, 35–46. [Google Scholar] [CrossRef]
- Kang, S.; Qin, L.; Zhao, Y.; Wang, W.; Zhang, T.; Yang, L.; Rao, F.; Song, S. Enhanced removal of methyl orange on exfoliated montmorillonite/chitosan gel in presence of methylene blue. Chemosphere 2020, 238, 124693. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saha, P. Sea shell powder as a new adsorbent to remove basic green 4 (malachite green) from aqueous solutions: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2010, 164, 168–177. [Google Scholar] [CrossRef]
- Ong, S.T.; Lee, C.K.; Zainal, Z. Removal of basic and reactive dyes using ethylenediamine modified rice hull. Bioresour. Technol. 2007, 98, 2792–2799. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).