Genetic Technologies for Sustainable Management of Insect Pests and Disease Vectors
Abstract
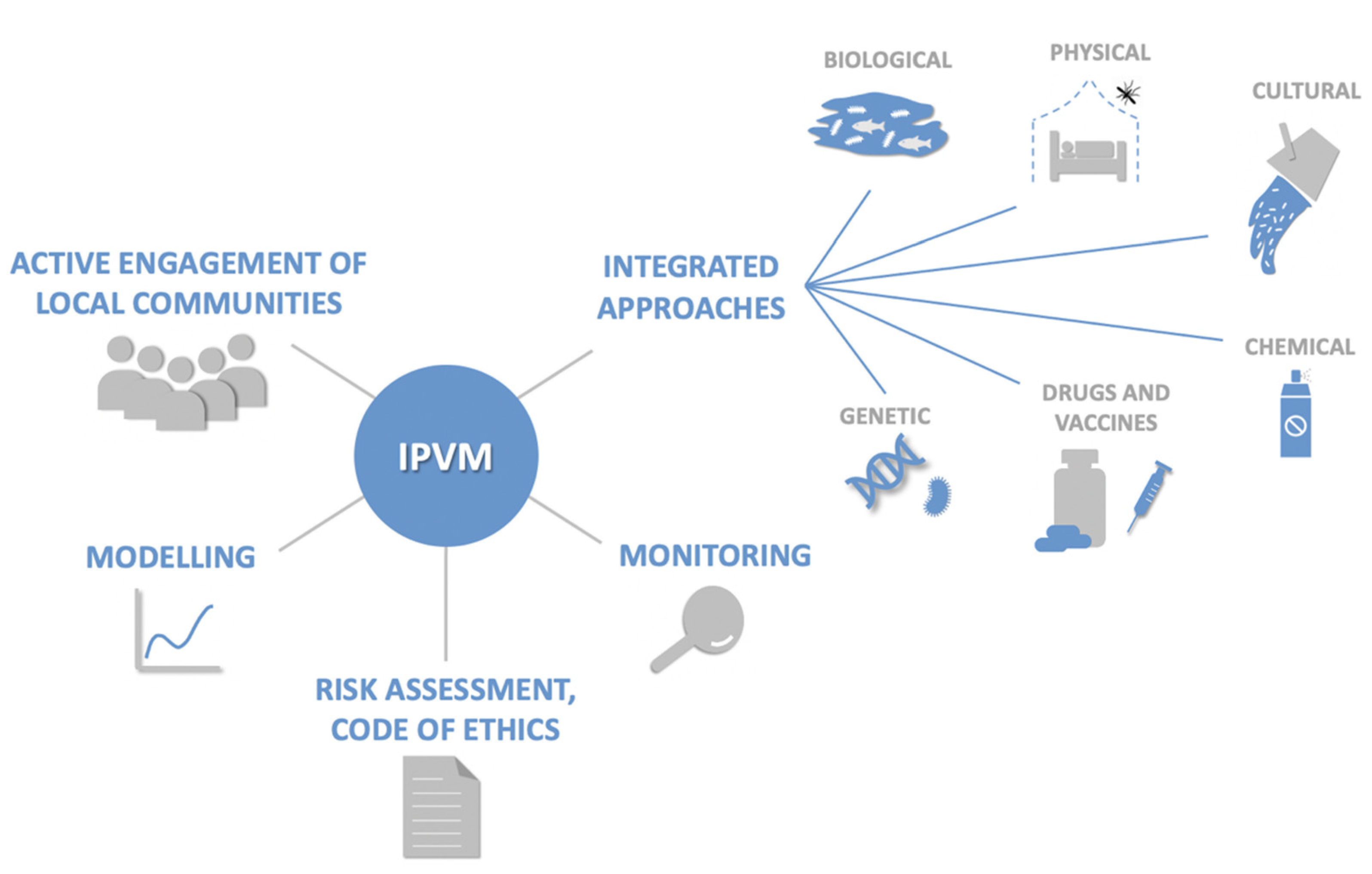
:1. Introduction
2. Genetic Control
Paratransgenesis
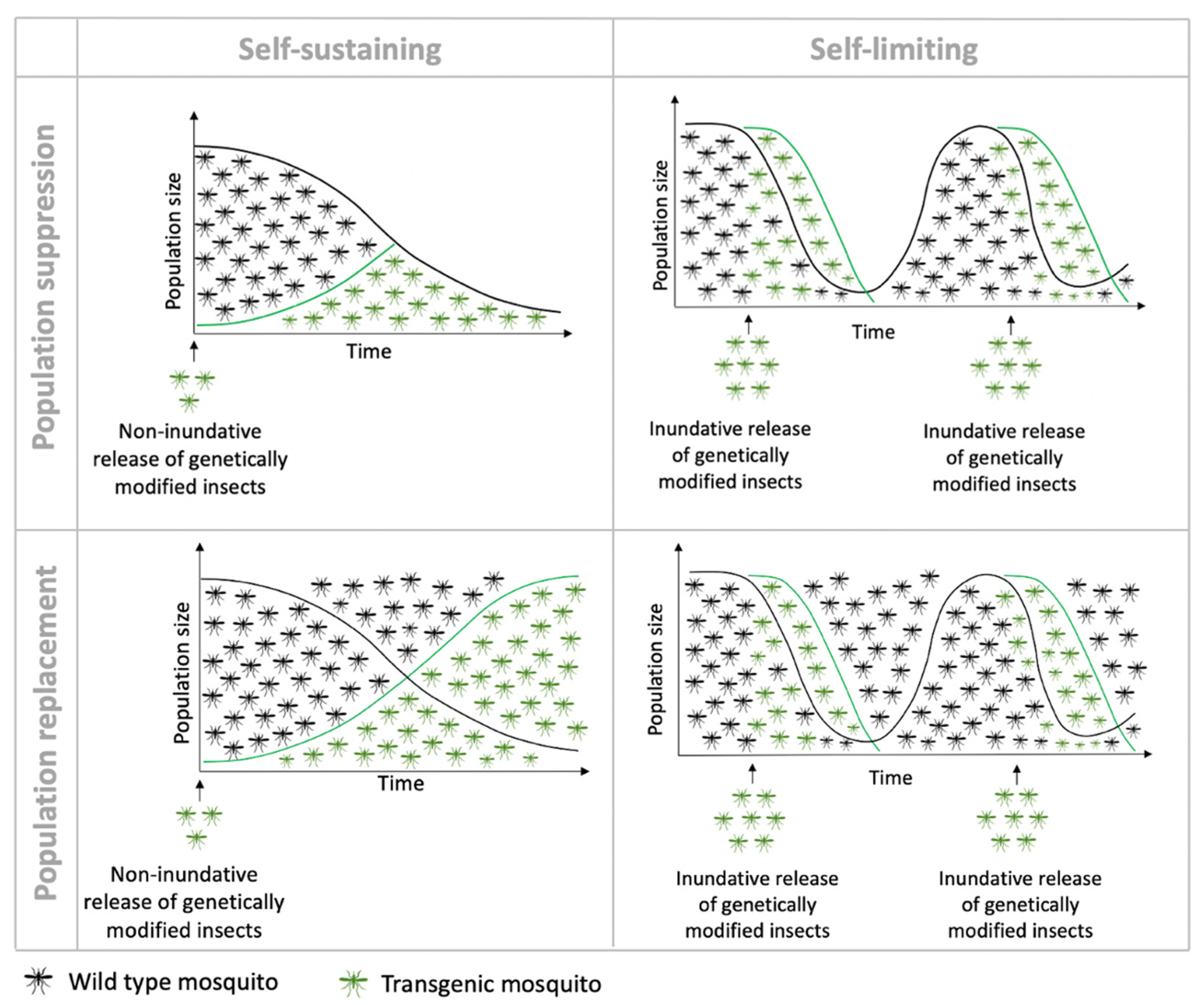
3. Self-Limiting Strategies
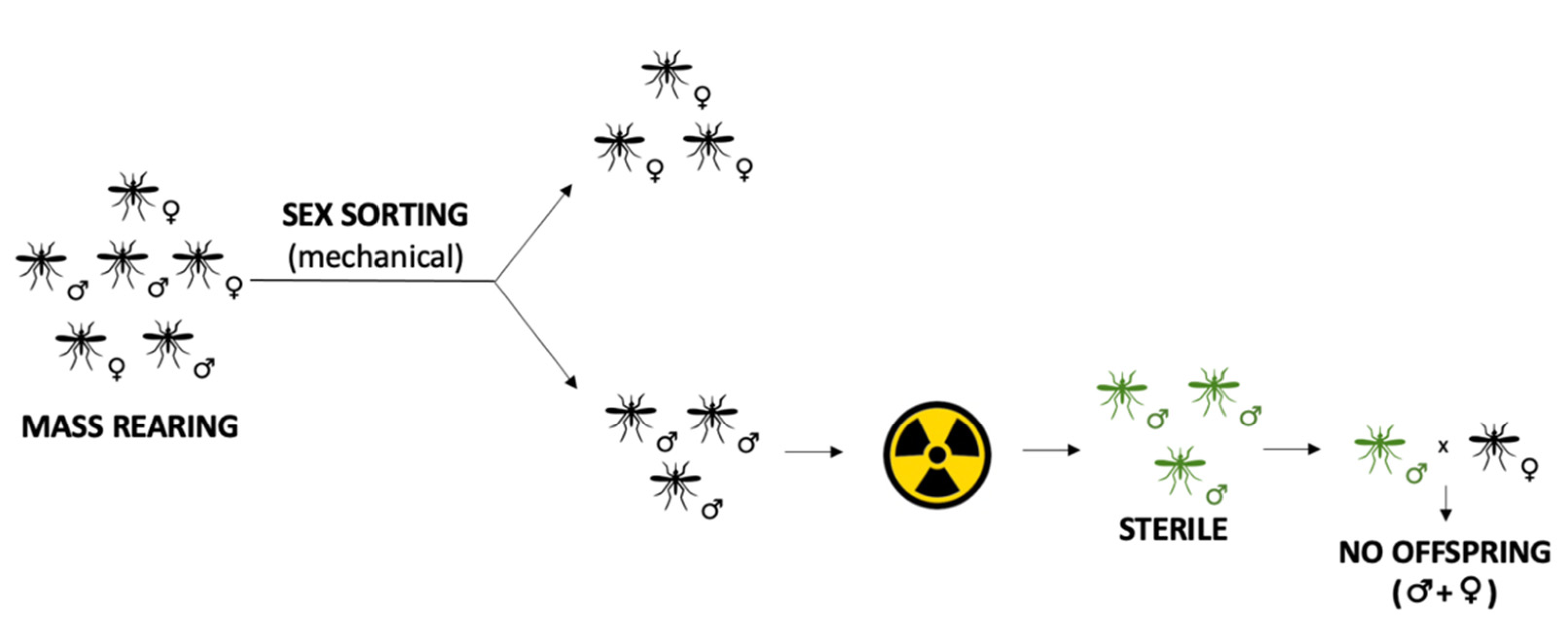
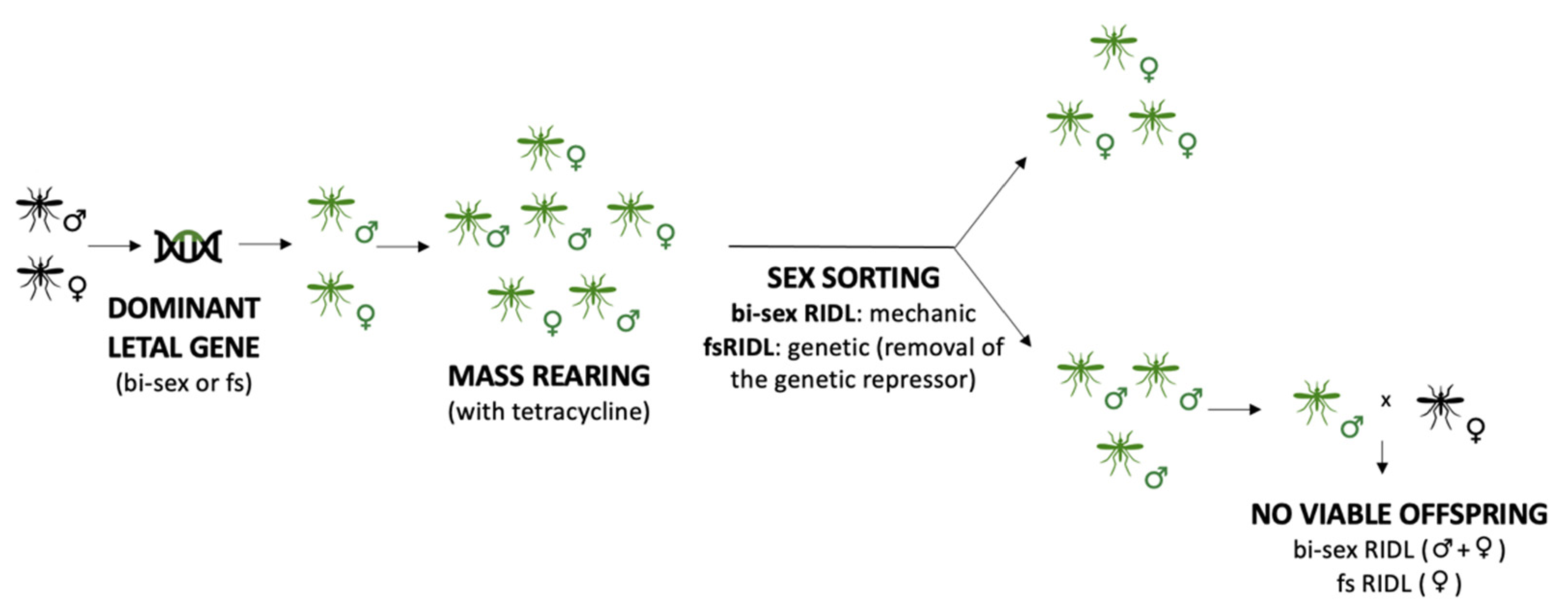
3.1. Sterile Insects
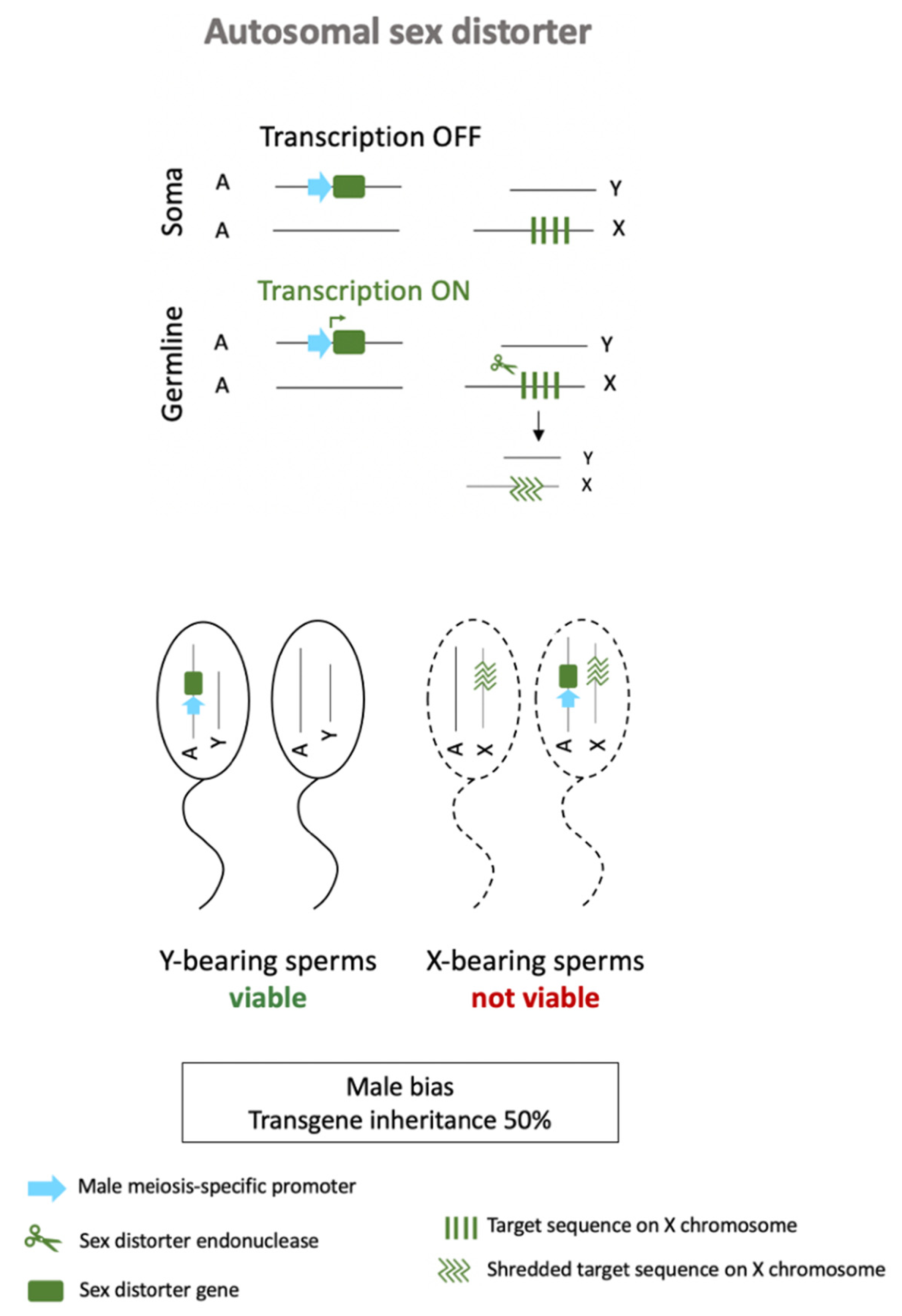
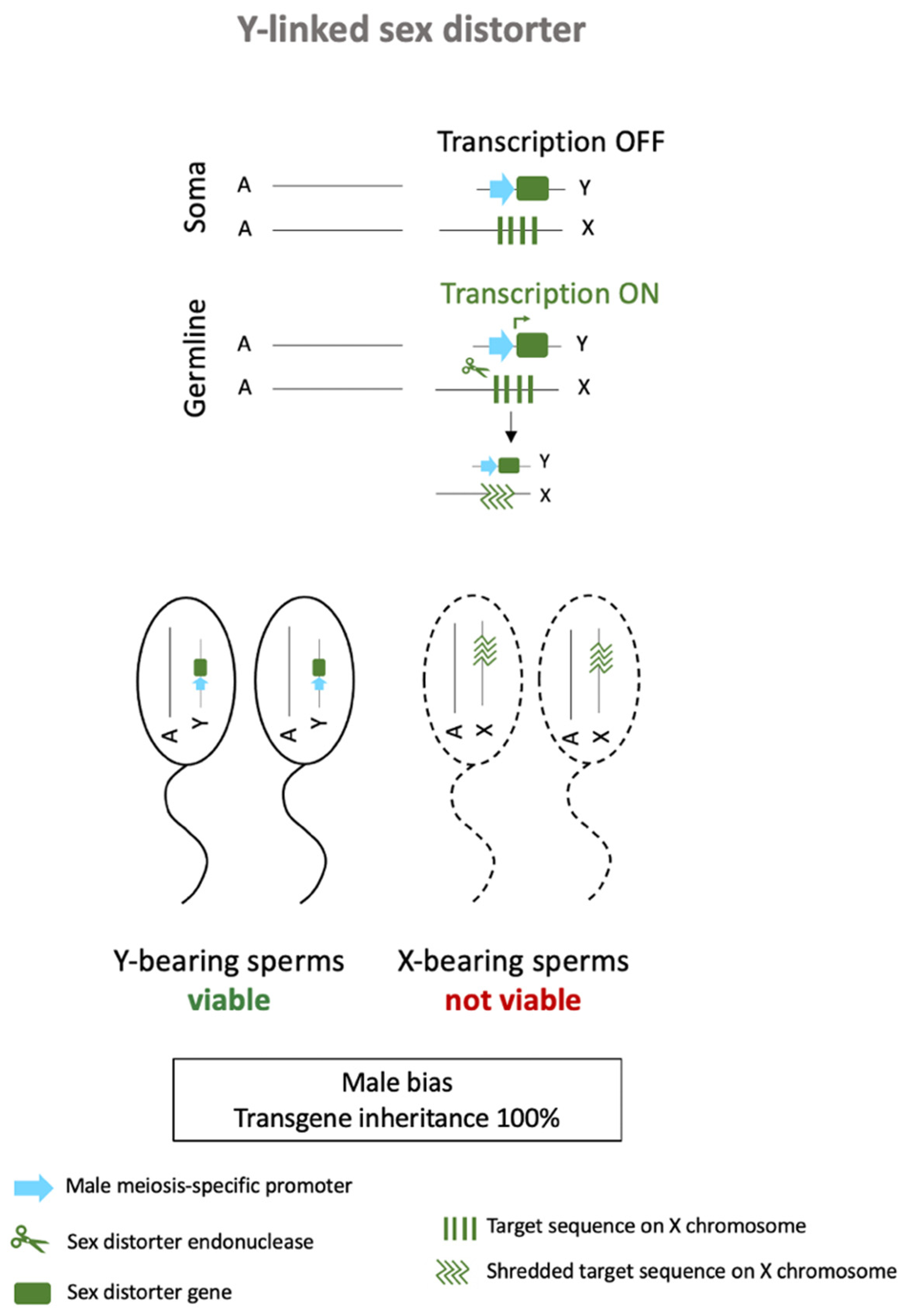
3.2. Autosomal Sex-Ratio Distorters
4. Self-Sustaining Strategies
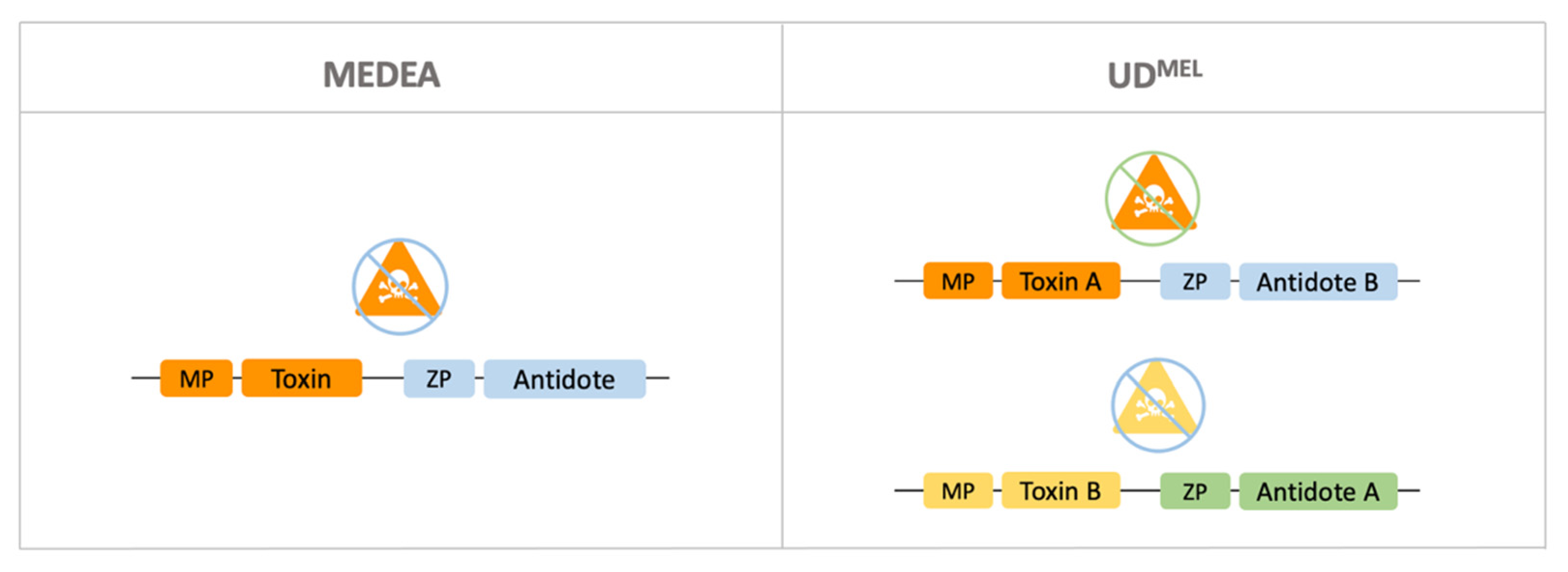
4.1. Toxin-Antidote-Based Gene Drives
4.2. Meiotic Drives
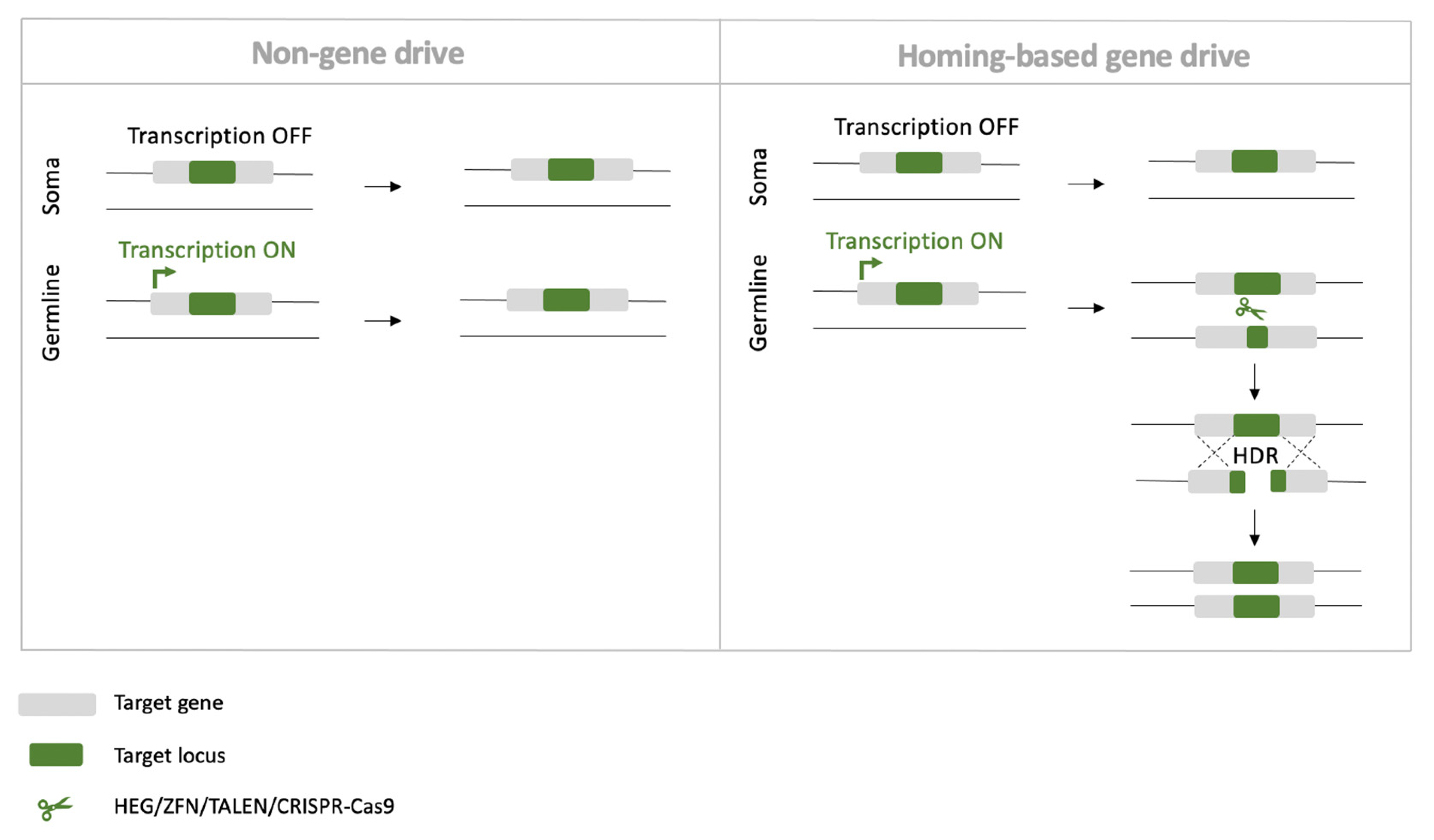
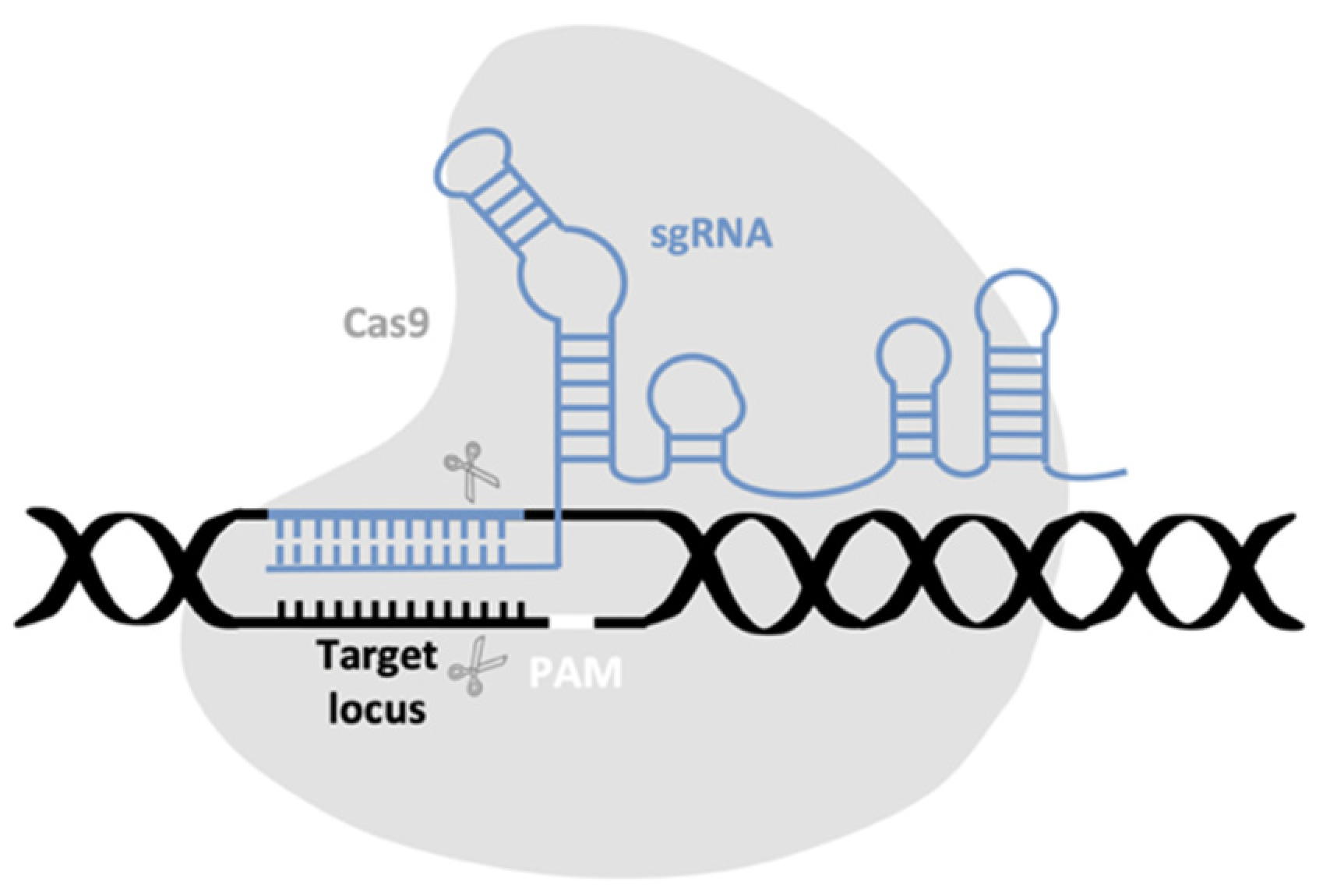
4.3. Homing-Based Gene Drives
5. Split Drives
6. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization Vector-Borne Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 11 December 2020).
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.M.; Katak, R.D.M.; Campos de Oliveira, J.; Araujo, M.D.S.; Carlos, B.C.; Galizi, R.; Tripet, F.; Marinotti, O.; Souza-Neto, J.A. Vector-Focused Approaches to Curb Malaria Transmission in the Brazilian Amazon: An Overview of Current and Future Challenges and Strategies. Trop. Med. Infect. Dis. 2020, 5, 161. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; Reuss, F.; Kendrovski, V.; Montag, D. Vector-Borne diseases. In Biodiversity and Health in the Face of Climate Change; Marselle, M., Stadler, J., Korn, H., Irvine, K., Bonn, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 67–90. [Google Scholar]
- Succo, T.; Leparc-Goffart, I.; Ferré, J.-B.; Roiz, D.; Broche, B.; Maquart, M.; Noel, H.; Catelinois, O.; Entezam, F.; Caire, D.; et al. Autochthonous Dengue Outbreak in Nîmes, South of France, July to September 2015. Euro Surveill 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Rezza, G. Chikungunya Is Back in Italy: 2007–2017. J. Travel Med. 2018, 25. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. World Malaria Report 2020: 20 Years of Global Progress & Challenges. Available online: https://www.who.int/publications-detail-redirect/9789240015791 (accessed on 15 April 2021).
- World Health Organization Integrated Vector Management (IVM). Available online: http://www.who.int/neglected_diseases/vector_ecology/ivm_concept/en/ (accessed on 19 February 2021).
- Beier, J.C.; Keating, J.; Githure, J.I.; Macdonald, M.B.; Impoinvil, D.E.; Novak, R.J. Integrated vector management for malaria control. Malar. J. 2008, 7, S4. [Google Scholar] [CrossRef] [Green Version]
- van Emden, H.; Service, M. Environmental/cultural control. In Pest and Vector Control; Cambridge University Press: Cambridge, UK, 2004; pp. 123–146. [Google Scholar]
- World Health Organization. Mosquitoes. In Pesticides and their Application for the Control of Vectors and Pests of Public Health Importance; World Health Organization: Geneva, Switzerland, 2006; pp. 22–37. [Google Scholar]
- Himeidan, Y.E.; Temu, E.A.; Kweka, E.J. Insecticides for Vector-Borne Diseases: Current Use, Benefits, Hazard and Resistance. In Insecticides—Advances in Integrated Pest Management; Perveen, F., Ed.; InTech: Rijeka, Croatia, 2012; pp. 683–708. [Google Scholar]
- Sampaio, M.V.; Bueno, V.H.P.; Silveira, L.C.P.; Auad, A.M. Biological control of insect pests in the tropics. In Tropical biology and Conservation Management; Del Claro, K., Oliveira, P.S., Rico-Gray, V., Eds.; UNESCO Publishing-Eolss: Oxford, UK, 2010; Volume 3, pp. 28–70. [Google Scholar]
- Van den Berg, H.; Zaim, M.; Yadav, R.S.; Soares, A.; Ameneshewa, B.; Mnzava, A.; Hii, J.; Dash, A.P.; Ejov, M. Global Trends in the Use of Insecticides to Control Vector-Borne Diseases. Environ. Health Perspect. 2012, 120, 577–582. [Google Scholar] [CrossRef]
- Vanaerschot, M.; Huijben, S.; Van den Broeck, F.; Dujardin, J.-C. Drug Resistance in Vectorborne Parasites: Multiple Actors and Scenarios for an Evolutionary Arms Race. FEMS Microbiol. Rev. 2014, 38, 41–55. [Google Scholar] [CrossRef] [Green Version]
- Hammond, A.; Galizi, R.; Kyrou, K.; Simoni, A.; Siniscalchi, C.; Katsanos, D.; Gribble, M.; Baker, D.; Marois, E.; Russel, S.; et al. A CRISPR-Cas9 Gene Drive System Targeting Female Reproduction in the Malaria Mosquito Vector Anopheles Gambiae. Nat. Biotechnol. 2016, 34, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Kyrou, K.; Hammond, A.; Galizi, R.; Kranjc, N.; Burt, A.; Beaghton, A.K.; Nolan, T.; Crisanti, A. A CRISPR–Cas9 Gene Drive Targeting Doublesex Causes Complete Population Suppression in Caged Anopheles Gambiae Mosquitoes. Nat. Biotechnol. 2018, 36, 1062–1066. [Google Scholar] [CrossRef] [Green Version]
- Galizi, R.; Doyle, L.A.; Menichelli, M.; Bernardini, F.; Deredec, A.; Burt, A.; Stoddard, B.L.; Windbichler, N.; Crisanti, A. A synthetic sex ratio distortion system for the control of the human malaria mosquito. Nat. Commun. 2014, 5, 3977. [Google Scholar] [CrossRef] [Green Version]
- Galizi, R.; Hammond, A.; Kyrou, K.; Taxiarchi, C.; Bernardini, F.; O’Loughlin, S.M.; Papathanos, P.-A.; Nolan, T.; Windibichler, N.; Crisanti, A. A CRISPR-Cas9 Sex-Ratio Distortion System for Genetic Control. Sci. Rep. 2016, 6, 31139. [Google Scholar] [CrossRef] [Green Version]
- Simoni, A.; Hammond, A.M.; Beaghton, A.K.; Galizi, R.; Taxiarchi, C.; Kyrou, K.; Meacci, D.; Morselli, G.; Burt, A.; Nolan, T.; et al. A Male-Biased Sex-Distorter Gene Drive for the Human Malaria Vector Anopheles Gambiae. Nat. Biotechnol. 2020, 38, 1054–1060. [Google Scholar] [CrossRef]
- Burt, A. Heritable Strategies for Controlling Insect Vectors of Disease. Philos. Trans. R Soc. Lond. B Biol. Sci. 2014, 369, 20130432. [Google Scholar] [CrossRef]
- Aksoy, S.; Weiss, B.; Attardo, G. Paratransgenesis Applied for Control of Tsetse Transmitted Sleeping Sickness. Adv. Exp. Med. Biol. 2008, 627, 35–48. [Google Scholar] [CrossRef]
- Durvasula, R.V.; Gumbs, A.; Panackal, A.; Kruglov, O.; Aksoy, S.; Merrifield, R.B.; Richards, F.F.; Beard, C.B. Prevention of Insect-Borne Disease: An Approach Using Transgenic Symbiotic Bacteria. Proc. Natl. Acad. Sci. USA 1997, 94, 3274–3278. [Google Scholar] [CrossRef] [Green Version]
- Riehle, M.A.; Moreira, C.K.; Lampe, D.; Lauzon, C.; Jacobs- Lorena, M. Using bacteria to express and display anti-Plasmodium molecules in the mosquito midgut. Int. J. Parasitol. 2007, 37, 595–603. [Google Scholar] [CrossRef]
- Bisi, D.C.; Lampe, D.J. Secretion of Anti-Plasmodium Effector Proteins from a Natural Pantoea Agglomerans Isolate by Using PelB and HlyA Secretion Signals. Appl. Env. Microbiol. 2011, 77, 4669–4675. [Google Scholar] [CrossRef] [Green Version]
- Favia, G.; Ricci, I.; Damiani, C.; Raddadi, N.; Crotti, E.; Marzorati, M.; Rizzi, A.; Urso, R.; Brusetti, L.; Borin, S.; et al. Bacteria of the Genus Asaia Stably Associate with Anopheles Stephensi, an Asian Malarial Mosquito Vector. Proc. Natl. Acad. Sci. USA 2007, 104, 9047–9051. [Google Scholar] [CrossRef] [Green Version]
- Bongio, N.J.; Lampe, D.J. Inhibition of Plasmodium berghei Development in Mosquitoes by Effector Proteins Secreted from Asaia sp. Bacteria Using a Novel Native Secretion Signal. PLoS ONE 2015, 10, 0143541. [Google Scholar] [CrossRef] [Green Version]
- Damiani, C.; Ricci, I.; Crotti, E.; Rossi, P.; Rizzi, A.; Scuppa, P.; Capone, A.; Ulissi, U.; Epis, S.; Genchi, M.; et al. Mosquito-bacteria symbiosis: The case of Anopheles gambiae and Asaia. Microb. Ecol. 2010, 60, 644–654. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, S.; Ioka, D.; Matsuoka, H.; Endo, H.; Ishii, A. Bacteria Expressing Single-Chain Immunotoxin Inhibit Malaria Parasite Development in Mosquitoes. Mol. Biochem. Parasitol. 2001, 113, 89–96. [Google Scholar] [CrossRef]
- Wang, S.; Ghosh, A.K.; Bongio, N.; Stebbings, K.A.; Lampe, D.J.; Jacobs-Lorena, M. Fighting Malaria with Engineered Symbiotic Bacteria from Vector Mosquitoes. Proc. Natl. Acad. Sci. USA 2012, 109, 12734–12739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasgon, J.L. Using Infections to Fight Infections: Paratransgenic Fungi Can Block Malaria Transmission in Mosquitoes. Future Microbiol. 2011, 6, 851–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci, I.; Mosca, M.; Valzano, M.; Damiani, C.; Scuppa, P.; Rossi, P.; Crotti, E.; Cappelli, A.; Ulissi, U.; Capone, A.; et al. Different Mosquito Species Host Wickerhamomyces Anomalus (Pichia Anomala): Perspectives on Vector-Borne Diseases Symbiotic Control. Antonie Van Leeuwenhoek 2011, 99, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappelli, A.; Ulissi, U.; Valzano, M.; Damiani, C.; Epis, S.; Gabrielli, M.G.; Conti, S.; Polonelli, L.; Bandi, C.; Favia, G.; et al. A Wickerhamomyces Anomalus Killer Strain in the Malaria Vector Anopheles Stephensi. PLoS ONE 2014, 9, e95988. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Vega-Rodríguez, J.; Ghosh, A.K.; Jacobs-Lorena, M.; Kang, A.; St Leger, R.J. Development of Transgenic Fungi That Kill Human Malaria Parasites in Mosquitoes. Science 2011, 331, 1074–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; Hoiczyk, E.; Rasgon, J.L. Viral Paratransgenesis in the Malaria Vector Anopheles Gambiae. PLoS Pathog. 2008, 4, e1000135. [Google Scholar] [CrossRef]
- Wilke, A.B.B.; Marrelli, M.T. Paratransgenesis: A Promising New Strategy for Mosquito Vector Control. Parasit. Vectors 2015, 8. [Google Scholar] [CrossRef] [Green Version]
- Demirbas-Uzel, G.; De Vooght, L.; Parker, A.G.; Vreysen, M.J.B.; Mach, R.L.; Van Den Abbeele, J.; Abd-Alla, A.M.M. Combining Paratransgenesis with SIT: Impact of Ionizing Radiation on the DNA Copy Number of Sodalis Glossinidius in Tsetse Flies. BMC Microbiol. 2018, 18, 160. [Google Scholar] [CrossRef] [Green Version]
- Shane, J.L.; Grogan, C.L.; Cwalina, C.; Lampe, D.J. Blood Meal-Induced Inhibition of Vector-Borne Disease by Transgenic Microbiota. Nat. Commun. 2018, 9, 4127. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Wang, S.; Jacobs-Lorena, M. Self-Limiting Paratransgenesis. PLoS Negl. Trop. Dis. 2020, 14, e0008542. [Google Scholar] [CrossRef]
- Wang, S.; Dos-Santos, A.L.A.; Huang, W.; Liu, K.C.; Oshaghi, M.A.; Wei, G.; Agre, P.; Jacobs-Lorena, M. Driving Mosquito Refractoriness to Plasmodium Falciparum with Engineered Symbiotic Bacteria. Science 2017, 357, 1399–1402. [Google Scholar] [CrossRef] [Green Version]
- Mancini, M.V.; Spaccapelo, R.; Damiani, C.; Accoti, A.; Tallarita, M.; Petraglia, E.; Rossi, P.; Cappelli, A.; Capone, A.; Peruzzi, G.; et al. Paratransgenesis to Control Malaria Vectors: A Semi-Field Pilot Study. Parasites & Vectors 2016, 9, 140. [Google Scholar] [CrossRef] [Green Version]
- Ricci, I.; Valzano, M.; Ulissi, U.; Epis, S.; Cappelli, A.; Favia, G. Symbiotic Control of Mosquito Borne Disease. Pathog. Glob. 2012, 106, 380–385. [Google Scholar] [CrossRef] [Green Version]
- Damiani, C.; Ricci, I.; Crotti, E.; Rossi, P.; Rizzi, A.; Scuppa, P.; Esposito, F.; Bandi, C.; Daffonchio, D.; Favia, G. Paternal Transmission of Symbiotic Bacteria in Malaria Vectors. Curr. Biol. 2008, 18, R1087–R1088. [Google Scholar] [CrossRef] [Green Version]
- Arora, A.K.; Forshaw, A.; Miller, T.A.; Durvasula, R. A Delivery System for Field Application of Paratransgenic Control. BMC Biotechnol. 2015, 15, 59. [Google Scholar] [CrossRef] [Green Version]
- Hendrichs, J.; Vreysen, M.J.B.; Enkerlin, W.R.; Cayol, J.P. Strategic options in using sterile insects for area-wide integrated pest management. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 563–600. [Google Scholar]
- Klassen, W. Area-wide integrated pest management and the sterile insect technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 39–68. [Google Scholar]
- Klassen, W.; Curtis, C.F. History of the sterile insect technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 3–36. [Google Scholar]
- Bourtzis, K.; Lees, R.S.; Hendrichs, J.; Vreysen, M.J.B. More than One Rabbit out of the Hat: Radiation, Transgenic and Symbiont-Based Approaches for Sustainable Management of Mosquito and Tsetse Fly Populations. Acta Trop. 2016, 157, 115–130. [Google Scholar] [CrossRef]
- Enkerlin, W.R. Impact of fruit fly control programmes using the sterile insect technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 651–676. [Google Scholar]
- Rendón, P.; McInnis, D.; Lance, D.; Stewart, J. Medfly (Diptera: Tephritidae) Genetic Sexing: Large-Scale Field Comparison of Males-Only and Bisexual Sterile Fly Releases in Guatemala. J. Econ. Entomol. 2004, 97, 1547–1553. [Google Scholar] [CrossRef]
- Slade, G.; Morrison, N. Developing GM Insects for Sustainable Pest Control in Agriculture and Human Health. BMC Proc. 2014, 8, O43. [Google Scholar] [CrossRef] [Green Version]
- Pagendam, D.E.; Trewin, B.J.; Snoad, N.; Ritchie, S.A.; Hoffmann, A.A.; Staunton, K.M.; Paton, C.; Beebe, N. Modelling the Wolbachia Incompatible Insect Technique: Strategies for Effective Mosquito Population Elimination. BMC Biol. 2020, 18, 161. [Google Scholar] [CrossRef]
- Zhang, D.; Zheng, X.; Xi, Z.; Bourtzis, K.; Gilles, J.R.L. Combining the Sterile Insect Technique with the Incompatible Insect Technique: I-Impact of Wolbachia Infection on the Fitness of Triple- and Double-Infected Strains of Aedes Albopictus. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Zhang, D.; Li, Y.; Yang, C.; Wu, Y.; Liang, X.; Liang, Y.; Pan, X.; Hu, L.; Sun, Q.; et al. Incompatible and Sterile Insect Techniques Combined Eliminate Mosquitoes. Nature 2019, 572, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; Donnelly, C.A.; Wood, R.J.; Alphey, L.S. Insect population control using a dominant, repressible, lethal genetic system. Science 2000, 287, 2474–2476. [Google Scholar] [CrossRef] [PubMed]
- Schetelig, M.F.; Caceres, C.; Zacharopoulou, A.; Franz, G.; Wimmer, E.A. Conditional Embryonic Lethality to Improve the Sterile Insect Technique in Ceratitis Capitata (Diptera: Tephritidae). BMC Biol. 2009, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Ogaugwu, C.E.; Schetelig, M.F.; Wimmer, E.A. Transgenic Sexing System for Ceratitis Capitata (Diptera: Tephritidae) Based on Female-Specific Embryonic Lethality. Insect. Biochem. Mol. Biol. 2013, 43, 1–8. [Google Scholar] [CrossRef]
- Concha, C.; Yan, Y.; Arp, A.; Quilarque, E.; Sagel, A.; de León, A.P.; McMillan, W.O.; Skoda, S.; Scott, M.J. An Early Female Lethal System of the New World Screwworm, Cochliomyia Hominivorax, for Biotechnology-Enhanced SIT. BMC Genet. 2020, 21, 143. [Google Scholar] [CrossRef]
- Schetelig, M.F.; Handler, A.M. A Transgenic Embryonic Sexing System for Anastrepha Suspensa (Diptera: Tephritidae). Insect Biochem. Mol. Biol. 2012, 42, 790–795. [Google Scholar] [CrossRef]
- Yan, Y.; Williamson, M.E.; Davis, R.J.; Andere, A.A.; Picard, C.J.; Scott, M.J. Improved Transgenic Sexing Strains for Genetic Control of the Australian Sheep Blow Fly Lucilia Cuprina Using Embryo-Specific Gene Promoters. Mol. Genet. Genom. 2020, 295, 287–298. [Google Scholar] [CrossRef]
- Kandul, N.P.; Liu, J.; Sanchez C., H.M.; Wu, S.L.; Marshall, J.M.; Akbari, O.S. Transforming Insect Population Control with Precision Guided Sterile Males with Demonstration in Flies. Nat. Commun. 2019, 10, 84. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Yang, T.; Bui, M.; Gamez, S.; Wise, T.; Kandul, N.P.; Liu, J.; Alcantara, L.; Lee, H.; Edula, J.R.; et al. Eliminating Mosquitoes with Precision Guided Sterile Males. bioRxiv 2021. [Google Scholar] [CrossRef]
- Haghighat-Khah, R.E.; Sharma, A.; Wunderlich, M.R.; Morselli, G.; Marston, L.A.; Bamikole, C.; Hall, A.; Kranjc, N.; Taxiarchi, C.; Sharakhov, I.; et al. Cellular Mechanisms Regulating Synthetic Sex Ratio Distortion in the Anopheles Gambiae Germline. Pathog. Glob. 2020, 114, 370–378. [Google Scholar] [CrossRef]
- Fasulo, B.; Meccariello, A.; Morgan, M.; Borufka, C.; Papathanos, P.A.; Windbichler, N. A Fly Model Establishes Distinct Mechanisms for Synthetic CRISPR/Cas9 Sex Distorters. PLoS Genet. 2020, 16, e1008647. [Google Scholar] [CrossRef] [Green Version]
- Meccariello, A.; Krsticevic, F.; Colonna, R.; Del Corsano, G.; Fasulo, B.; Papathanos, P.A.; Windbichler, N. Engineered Sex Ratio Distortion by X-Shredding in the Global Agricultural Pest Ceratitis Capitata. BMC Biol. 2021, 19, 78. [Google Scholar] [CrossRef]
- Pollegioni, P.; North, A.R.; Persampieri, T.; Bucci, A.; Minuz, R.L.; Groneberg, D.A.; Nolan, T.; Papathanos, P.A.; Crisanti, A.; Müller, R. Detecting the Population Dynamics of an Autosomal Sex Ratio Distorter Transgene in Malaria Vector Mosquitoes. J. Appl. Ecol. 2020, 57, 2086–2096. [Google Scholar] [CrossRef]
- Burt, A.; Deredec, A. Self-Limiting Population Genetic Control with Sex-Linked Genome Editors. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180776. [Google Scholar] [CrossRef] [Green Version]
- Burt, A.; Crisanti, A. Gene Drive: Evolved and Synthetic. ACS Chem. Biol. 2018, 13, 343–346. [Google Scholar] [CrossRef] [Green Version]
- Beeman, R.; Friesen, K.; Denell, R. Maternal-Effect Selfish Genes in Flour Beetles. Science 1992, 256, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Huang, H.; Ward, C.M.; Su, J.T.; Schaeffer, L.V.; Guo, M.; Hay, B.A. A Synthetic Maternal-Effect Selfish Genetic Element Drives Population Replacement in Drosophila. Science 2007, 316, 597–600. [Google Scholar] [CrossRef]
- Buchman, A.; Marshall, J.M.; Ostrovski, D.; Yang, T.; Akbari, O.S. Synthetically Engineered Medea Gene Drive System in the Worldwide Crop Pest Drosophila Suzukii. Proc. Natl. Acad. Sci. USA 2018, 115, 4725–4730. [Google Scholar] [CrossRef] [Green Version]
- Akbari, O.S.; Chen, C.H.; Marshall, J.M.; Huang, H.; Antoshechkin, I.; Hay, B.A. Novel Synthetic Medea Selfish Genetic Elements Drive Population Replacement in Drosophila; A Theoretical Exploration of Medea-Dependent Population Suppression. ACS Synth. Biol. 2014, 3, 915–928. [Google Scholar] [CrossRef] [Green Version]
- Davis, S.A.; Bax, N.; Grewe, P. Engineered Underdominance Allows Efficient and Economical Introgression of Traits into Pest Populations. J. Biol. 2001, 212, 83–98. [Google Scholar] [CrossRef]
- Akbari, O.S.; Matzen, K.D.; Marshall, J.M.; Huang, H.; Ward, C.M.; Hay, B.A. A Synthetic Gene Drive System for Local, Reversible Modification and Suppression of Insect Populations. Curr. Biol. 2013, 23, 671–677. [Google Scholar] [CrossRef] [Green Version]
- Reeves, R.G.; Bryk, J.; Altrock, P.M.; Denton, J.A.; Reed, F.A. First Steps towards Underdominant Genetic Transformation of Insect Populations. PLoS ONE 2014, 9, e97557. [Google Scholar] [CrossRef] [Green Version]
- Curtis, C.F. Possible Use of Translocations to Fix Desirable Genes in Insect Pest Populations. Nature 1968, 218, 368–369. [Google Scholar] [CrossRef]
- Buchman, A.B.; Ivy, T.; Marshall, J.M.; Akbari, O.S.; Hay, B.A. Engineered Reciprocal Chromosome Translocations Drive High Threshold, Reversible Population Replacement in Drosophila. ACS Synth. Biol. 2018, 7, 1359–1370. [Google Scholar] [CrossRef] [Green Version]
- Alphey, L.S.; Crisanti, A.; Randazzo, F.; Akbari, O.S. Standardizing the Definition of Gene Drive. Proc. Natl. Acad. Sci. USA 2020, 117, 30864–30867. [Google Scholar] [CrossRef]
- Gokhale, C.S.; Reeves, R.G.; Reed, F.A. Dynamics of a Combined Medea-Underdominant Population Transformation System. BMC Evol. Biol. 2014, 14, 98. [Google Scholar] [CrossRef] [Green Version]
- Burt, A.; Trivers, R. Selfish Genetic Elements. In Genes in Conflict: The Biology of Selfish Genetic Elements; Harvard University Press: Cambridge, MA, USA, 2009; pp. 1–18. [Google Scholar]
- Hickey, W.A.; Craig, G.B., Jr. Genetic Distortion of Sex Ratio in a Mosquito, Aedes Aegypti. Genetics 1966, 53, 1177–1196. [Google Scholar] [CrossRef]
- Sweeny, T.L.; Barr, A.R. Sex Ratio Distortion Caused by Meiotic Drive in a Mosquito, Culex Pipiens, L. Genetics 1978, 88, 427–446. [Google Scholar] [CrossRef]
- Alcalay, Y.; Fuchs, S.; Galizi, R.; Bernardini, F.; Haghighat-Khah, R.E.; Rusch, D.B.; Adrion, J.R.; Hahn, M.W.; Tortosa, P.; Papathanos, P.A. The Potential for a Released Autosomal X-Shredder Becoming a Driving-Y Chromosome and Invasively Suppressing Wild Populations of Malaria Mosquitoes. bioRxiv 2019, 860551. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.M. Meiotic Sex Chromosome Inactivation. Development 2007, 134, 1823–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taxiarchi, C.; Kranjc, N.; Kriezis, A.; Kyrou, K.; Bernardini, F.; Russel, S.; Nolan, T.; Crisanti, A.; Galizi, R. High-Resolution Transcriptional Profiling of Anopheles Gambiae Spermatogenesis Reveals Mechanisms of Sex Chromosome Regulation. Sci. Rep. 2019, 9, 14841. [Google Scholar] [CrossRef] [PubMed]
- North, A.R.; Burt, A.; Godfray, H.C.J. Modelling the Potential of Genetic Control of Malaria Mosquitoes at National Scale. BMC Biol. 2019, 17, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burt, A. Site-Specific Selfish Genes as Tools for the Control and Genetic Engineering of Natural Populations. Proc. R. Soc. Lond. B 2003, 270, 921–928. [Google Scholar] [CrossRef] [Green Version]
- Windbichler, N.; Papathanos, P.A.; Catteruccia, F.; Ranson, H.; Burt, A.; Crisanti, A. Homing endonuclease mediated gene targeting in Anopheles gambiae cells and embryos. Nucleic Acids Res. 2007, 35, 5922–5933. [Google Scholar] [CrossRef] [Green Version]
- Windbichler, N.; Menichelli, M.; Papathanos, P.A.; Thyme, S.B.; Li, H.; Ulge, U.Y.; Hovde, B.T.; Baker, D.; Monnat, R.J.; Burt, A.; et al. A Synthetic Homing Endonuclease-Based Gene Drive System in the Human Malaria Mosquito. Nature 2011, 473, 212–215. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.-S.; Naujoks, D.; Huen, D.; Russell, S. Insect Population Control by Homing Endonuclease-Based Gene Drive: An Evaluation in Drosophila Melanogaster. Genetics 2011, 188, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.-S.; Huen, D.S.; Glauert, R.; Whiteway, E.; Russell, S. Optimising Homing Endonuclease Gene Drive Performance in a Semi-Refractory Species: The Drosophila Melanogaster Experience. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F. ZFN, TALEN and CRISPR/Cas-Based Methods for Genome Engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef] [Green Version]
- Simoni, A.; Siniscalchi, C.; Chan, Y.-S.; Huen, D.S.; Russel, S.; Windbichler, W.; Crisanti, A. Development of Synthetic Selfish Elements Based on Modular Nucleases in Drosophila Melanogaster. Nucleic Acids Res. 2014, 42, 7461–7472. [Google Scholar] [CrossRef]
- Makarova, K.S.; Grishin, N.V.; Shabalina, S.A.; Wolf, Y.I.; Koonin, E.V. A Putative RNA-Interference-Based Immune System in Prokaryotes: Computational Analysis of the Predicted Enzymatic Machinery, Functional Analogies with Eukaryotic RNAi, and Hypothetical Mechanisms of Action. Biol. Direct. 2006, 1. [Google Scholar] [CrossRef] [Green Version]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E.A. Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Doudna, J.A.; Charpentier, E. The New Frontier of Genome Engineering with CRISPR-Cas9. Science 2014, 346. [Google Scholar] [CrossRef]
- Hammond, A.; Karlsson, X.; Morianou, I.; Kyrou, K.; Beaghton, A.; Gribble, M.; Kranjc, N.; Galizi, R.; Burt, A.; Crisanti, A.; et al. Regulating the Expression of Gene Drives Is Key to Increasing Their Invasive Potential and the Mitigation of Resistance. PLoS Genet. 2021, 17. [Google Scholar] [CrossRef]
- Gantz, V.M.; Jasinskiene, N.; Tatarenkova, O.; Fazekas, A.; Macias, V.M.; Bier, E.; James, A.A. Highly Efficient Cas9-Mediated Gene Drive for Population Modification of the Malaria Vector Mosquito Anopheles Stephensi. Proc. Natl. Acad. Sci. USA 2015, 112, 6736–6743. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Yang, T.; Kandul, N.P.; Bui, M.; Gamez, S.; Raban, R.; Bennett, J.; Sánchez C., H.M.; Lanzaro, G.C.; Schmidt, H.; et al. Development of a Confinable Gene Drive System in the Human Disease Vector Aedes Aegypti. eLife 2020, 9, e51701. [Google Scholar] [CrossRef]
- Carballar-Lejarazú, R.; James, A.A. Population Modification of Anopheline Species to Control Malaria Transmission. Pathog. Glob. Health 2017, 111, 424–435. [Google Scholar] [CrossRef]
- Carballar-Lejarazú, R.; Ogaugwu, C.; Tushar, T.; Kelsey, A.; Pham, T.B.; Murphy, J.; Schmidt, H.; Lee, Y.; Lanzaro, G.C.; James, A.A. Next-Generation Gene Drive for Population Modification of the Malaria Vector Mosquito, Anopheles Gambiae. Proc. Natl. Acad. Sci. USA 2020, 117, 22805–22814. [Google Scholar] [CrossRef]
- Deredec, A.; Godfray, H.C.J.; Burt, A. Requirements for Effective Malaria Control with Homing Endonuclease Genes. Proc. Natl. Acad. Sci. USA 2011, 108, E874–E880. [Google Scholar] [CrossRef] [Green Version]
- Hammond, A.M.; Kyrou, K.; Bruttini, M.; North, A.; Galizi, R.; Karlsson, X.; Kranic, N.; Carpi, F.M.; D’Aurizio, R.; Crisanti, A.; et al. The Creation and Selection of Mutations Resistant to a Gene Drive over Multiple Generations in the Malaria Mosquito. PLoS Genet. 2017, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noble, C.; Olejarz, J.; Esvelt, K.M.; Church, G.M.; Nowak, M.A. Evolutionary Dynamics of CRISPR Gene Drives. Sci. Adv. 2017, 3, e1601964. [Google Scholar] [CrossRef] [Green Version]
- Beaghton, A.K.; Hammond, A.M.; Nolan, T.; Crisanti, A.; Burt, A. Gene Drive for Population Genetic Control: Non-Functional Resistance and Parental Effects. Proc. R. Soc. B 2019, 286, 20191586. [Google Scholar] [CrossRef]
- Deredec, A.; Burt, A.; Godfray, H.C. The Population Genetics of Using Homing Endonuclease Genes in Vector and Pest Management. Genetics 2008, 179, 2013–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaghton, A.; Hammond, A.; Nolan, T.; Crisanti, A.; Godfray, H.C.J.; Burt, A. Requirements for Driving Anti-Pathogen Effector Genes into Populations of Disease Vectors by Homing. Genetics 2017, 205, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Beaghton, A.; Beaghton, P.J.; Burt, A. Gene Drive through a Landscape: Reaction-Diffusion Models of Population Suppression and Elimination by a Sex Ratio Distorter. Theor. Popul. Biol. 2016, 108, 51–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Champer, J.; Reeves, R.; Oh, S.Y.; Liu, C.; Liu, J.; Clark, A.G.; Messer, P.W. Novel CRISPR/Cas9 Gene Drive Constructs Reveal Insights into Mechanisms of Resistance Allele Formation and Drive Efficiency in Genetically Diverse Populations. PLoS Genet. 2017, 13. [Google Scholar] [CrossRef] [Green Version]
- Kranjc, N.; Crisanti, A.; Nolan, T.; Bernardini, F. Anopheles Gambiae Genome Conservation as a Resource for Rational Gene Drive Target Site Selection. Insects 2021, 12, 97. [Google Scholar] [CrossRef]
- Marshall, J.M.; Buchman, A.; Sánchez, C.H.M.; Akbari, O.S. Overcoming Evolved Resistance to Population Suppressing Homing-Based Gene Drives. Sci. Rep. 2017, 7, 3776. [Google Scholar] [CrossRef] [Green Version]
- Champer, J.; Buchman, A.; Akbari, O.S. Cheating Evolution: Engineering Gene Drives to Manipulate the Fate of Wild Populations. Nat. Rev. Genet. 2016, 17, 146–159. [Google Scholar] [CrossRef] [Green Version]
- Champer, S.E.; Oh, S.Y.; Liu, C.; Wen, Z.; Clark, A.G.; Messer, P.W.; Champer, J. Computational and Experimental Performance of CRISPR Homing Gene Drive Strategies with Multiplexed GRNAs. Sci. Adv. 2020, 6, eaaz0525. [Google Scholar] [CrossRef] [Green Version]
- Nash, A.; Urdaneta, G.M.; Beaghton, A.K.; Hoermann, A.; Papathanos, P.A.; Christophides, G.K.; Windbichler, N. Integral Gene Drives for Population Replacement. Biol. Open 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Oberhofer, G.; Ivy, T.; Hay, B.A. Cleave and Rescue, a Novel Selfish Genetic Element and General Strategy for Gene Drive. Proc. Natl. Acad. Sci. USA 2019, 116, 6250–6259. [Google Scholar] [CrossRef] [Green Version]
- Converting Endogenous Genes of the Malaria Mosquito into Simple Non-Autonomous Gene Drives for Population Replacement | ELife. Available online: https://elifesciences.org/articles/58791 (accessed on 17 May 2021).
- Champer, J.; Yang, E.; Lee, E.; Liu, J.; Clark, A.G.; Messer, P.W. A CRISPR Homing Gene Drive Targeting a Haplolethal Gene Removes Resistance Alleles and Successfully Spreads through a Cage Population. Proc. Natl. Acad. Sci. USA 2020, 117, 24377–24383. [Google Scholar] [CrossRef]
- Champer, J.; Lee, E.; Yang, E.; Liu, C.; Clark, A.G.; Messer, P.W. A Toxin-Antidote CRISPR Gene Drive System for Regional Population Modification. Nat. Commun. 2020, 11, 1082. [Google Scholar] [CrossRef]
- Adolfi, A.; Gantz, V.M.; Jasinskiene, N.; Lee, H.-F.; Hwang, K.; Terradas, G.; Bulger, E.A.; Ramaiah, A.; Bennett, J.B.; Emerson, J.J.; et al. Efficient Population Modification Gene-Drive Rescue System in the Malaria Mosquito Anopheles Stephensi. Nat. Commun. 2020, 11, 5553. [Google Scholar] [CrossRef]
- Akbari, O.S.; Bellen, H.J.; Bier, E.; Bullock, S.L.; Burt, A.; Church, G.M.; Cook, K.R.; Duchek, P.; Edwards, O.R.; Esvelt, K.M.; et al. Safeguarding Gene Drive Experiments in the Laboratory. Science 2015, 349, 927–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Champer, J.; Chung, J.; Lee, Y.L.; Liu, C.; Yang, E.; Wen, Z.; Clark, A.G.; Messer, P.W. Molecular Safeguarding of CRISPR Gene Drive Experiments. eLife 2019, 8, e41439. [Google Scholar] [CrossRef]
- Edgington, M.P.; Harvey-Samuel, T.; Alphey, L. Split Drive Killer-Rescue Provides a Novel Threshold-Dependent Gene Drive. Sci. Rep. 2020, 10, 20520. [Google Scholar] [CrossRef] [PubMed]
- Noble, C.; Min, J.; Olejarz, J.; Buchthal, J.; Chavez, A.; Smidler, A.L.; DeBenedictis, E.A.; Church, G.M.; Nowak, M.A.; Esvelt, K.M. Daisy-Chain Gene Drives for the Alteration of Local Populations. Proc. Natl. Acad. Sci. USA 2019, 116, 8275–8282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backus, G.A.; Delborne, J.A. Threshold-Dependent Gene Drives in the Wild: Spread, Controllability, and Ecological Uncertainty. BioScience 2019, 69, 900–907. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, S.L. The Use of Wolbachia by the World Mosquito Program to Interrupt Transmission of Aedes aegypti Transmitted Viruses. In Dengue and Zika: Control and Antiviral Treatment Strategies; Hilgenfeld, R., Vasudevan, S.G., Eds.; Advances in Experimental Medicine and Biology; Springer Singapore: Singapore, 2018; Volume 1062, pp. 355–360. ISBN 978-981-10-8726-4. [Google Scholar]
- Nazni, W.A.; Hoffmann, A.A.; NoorAfizah, A.; Cheong, Y.L.; Mancini, M.V.; Golding, N.; Kamarul, G.M.R.; Arif, M.A.K.; Thohir, H.; NurSyamimi, H.; et al. Establishment of Wolbachia Strain WAlbB in Malaysian Populations of Aedes Aegypti for Dengue Control. Curr. Biol. 2019, 29, 4241–4248.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Target Malaria. Target Malaria Proceeded with a Small-Scale Release of Genetically Modified Sterile Male Mosquitoes in Bana, a Village in Burkina Faso. 2019. Available online: https://targetmalaria.org/target-malaria-proceeded-with-a-small-scale-release-of-genetically-modified-sterile-male-mosquitoes-in-bana-a-village-in-burkina-faso/ (accessed on 11 May 2021).
- Target Malaria. Results from Months of Monitoring Following the First Release of Non Gene Drive Genetically Modified Mosquitoes in Africa. 2021. Available online: https://targetmalaria.org/results-from-months-of-monitoring-following-the-first-release-of-non-gene-drive-genetically-modified-mosquitoes-in-africa/ (accessed on 9 May 2021).
- Shelton, A.M.; Long, S.J.; Walker, A.S.; Bolton, M.; Collins, H.L.; Revuelta, L.; Johnson, L.M.; Morrison, N.I. First Field Release of a Genetically Engineered, Self-Limiting Agricultural Pest Insect: Evaluating Its Potential for Future Crop Protection. Front. Bioeng. Biotechnol. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Simmons, G.S.; McKemey, A.R.; Morrison, N.I.; O’Connell, S.; Tabashnik, B.E.; Claus, J.; Fu, G.; Tang, G.; Sledge, M.; Walker, A.S.; et al. Field Performance of a Genetically Engineered Strain of Pink Bollworm. PLoS ONE 2011, 6, e24110. [Google Scholar] [CrossRef]
- Harris, A.F.; Nimmo, D.; McKemey, A.R.; Kelly, N.; Scaife, S.; Donnelly, C.A.; Beech, C.; Petrie, W.D.; Alphey, L. Field Performance of Engineered Male Mosquitoes. Nat. Biotechnol. 2011, 29, 1034–1037. [Google Scholar] [CrossRef]
- Harris, A.F.; McKemey, A.R.; Nimmo, D.; Curtis, Z.; Black, I.; Morgan, S.A.; Oviedo, M.N.; Lacroix, R.; Naish, N.; Morrison, N.I.; et al. Successful Suppression of a Field Mosquito Population by Sustained Release of Engineered Male Mosquitoes. Nat. Biotechnol. 2012, 30, 828–830. [Google Scholar] [CrossRef]
- Carvalho, D.O.; McKemey, A.R.; Garziera, L.; Lacroix, R.; Donnelly, C.A.; Alphey, L.; Malavasi, A.; Capurro, M.L. Suppression of a Field Population of Aedes Aegypti in Brazil by Sustained Release of Transgenic Male Mosquitoes. PLoS Negl. Trop. Dis. 2015, 9, e0003864. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, R.; McKemey, A.R.; Raduan, N.; Wee, L.K.; Ming, W.H.; Ney, T.G.; Siti, R.A.A.; Salman, S.; Subramaniam, S.; Nordin, O.; et al. Open Field Release of Genetically Engineered Sterile Male Aedes Aegypti in Malaysia. PLoS ONE 2012, 7, e42771. [Google Scholar] [CrossRef]
- Waltz, E. First Genetically Modified Mosquitoes Released in the United States. Nature 2021. Available online: https://www.nature.com/articles/d41586-021-01186-6 (accessed on 9 May 2021).
- Hammond, A.; Pollegioni, P.; Persampieri, T.; North, A.; Minuz, R.; Trusso, A.; Bucci, A.; Kyrou, A.; Morianou, I.; Simoni, A.; et al. Population Suppression of the Malaria Vector Anopheles Gambiae by Gene Drive Technology: A Large-Cage Indoor Study Bridging the Gap between Laboratory and Field Testing. Res. Square 2021. [Google Scholar] [CrossRef]
- Long, K.C.; Alphey, L.; Annas, G.J.; Bloss, C.S.; Campbell, K.J.; Champer, J.; Chen, C.-H.; Choudhary, A.; Church, G.M.; Collins, J.P.; et al. Core Commitments for Field Trials of Gene Drive Organisms. Science 2020, 370, 1417–1419. [Google Scholar] [CrossRef]
- Annas, G.J.; Beisel, C.L.; Clement, K.; Crisanti, A.; Francis, S.; Galardini, M.; Galizi, R.; Grünewald, J.; Immobile, G.; Khalil, A.S.; et al. A Code of Ethics for Gene Drive Research. Cris. J. 2021, 4, 19–24. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grilli, S.; Galizi, R.; Taxiarchi, C. Genetic Technologies for Sustainable Management of Insect Pests and Disease Vectors. Sustainability 2021, 13, 5653. https://doi.org/10.3390/su13105653
Grilli S, Galizi R, Taxiarchi C. Genetic Technologies for Sustainable Management of Insect Pests and Disease Vectors. Sustainability. 2021; 13(10):5653. https://doi.org/10.3390/su13105653
Chicago/Turabian StyleGrilli, Silvia, Roberto Galizi, and Chrysanthi Taxiarchi. 2021. "Genetic Technologies for Sustainable Management of Insect Pests and Disease Vectors" Sustainability 13, no. 10: 5653. https://doi.org/10.3390/su13105653
APA StyleGrilli, S., Galizi, R., & Taxiarchi, C. (2021). Genetic Technologies for Sustainable Management of Insect Pests and Disease Vectors. Sustainability, 13(10), 5653. https://doi.org/10.3390/su13105653





