Sustainable Food Production: The Contribution of Genome Editing in Livestock
Abstract
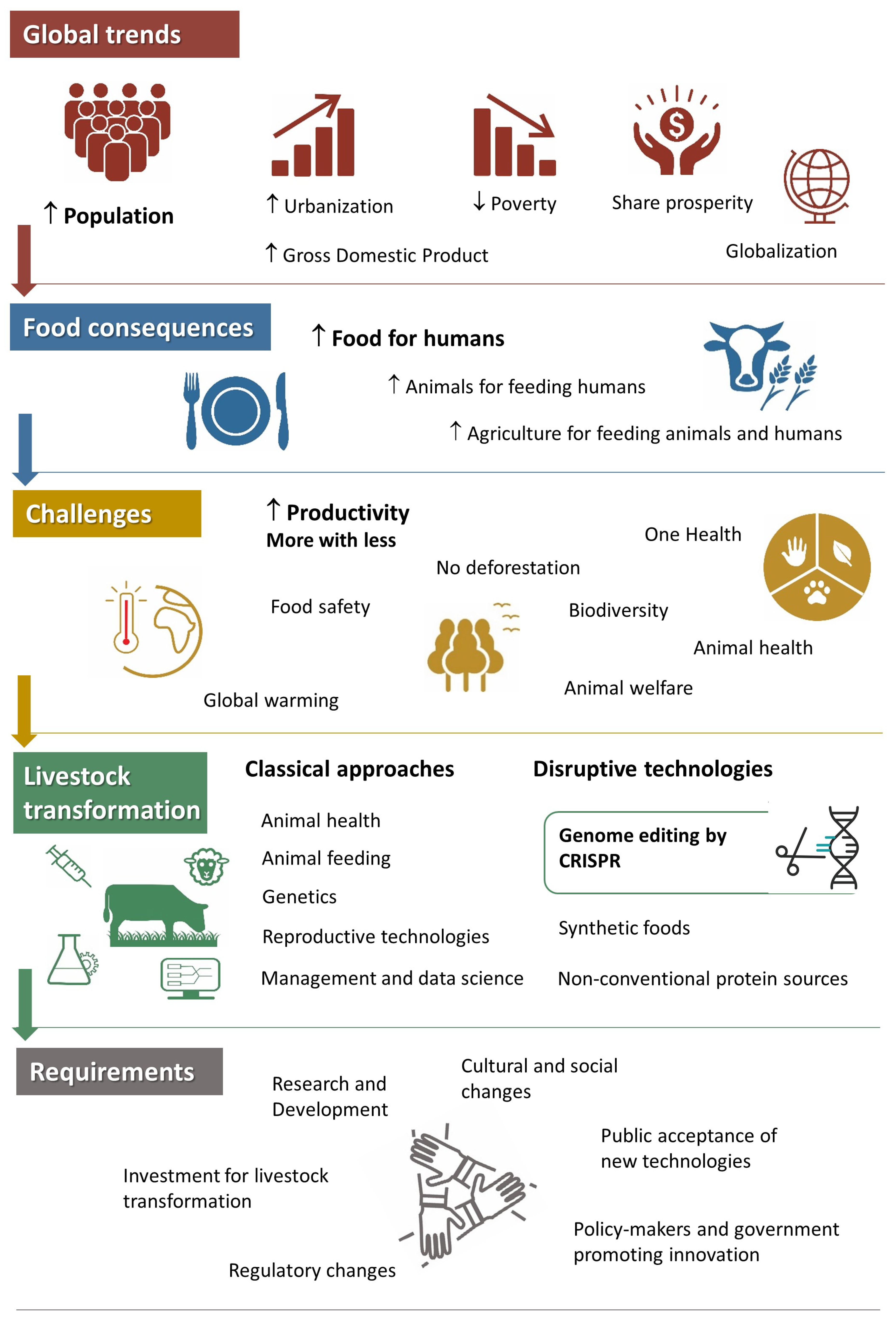
:1. Introduction
2. Livestock and Sustainability
3. The Arrival of Genome Editing
4. How Genome Editing Improves Livestock
4.1. Yield and Quality Related Traits
4.2. Animal Welfare
4.3. Animal Health
4.4. Pest and Disease Vector Control
4.5. Invasive Species
5. Limitations Beyond Science
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. World Population Prospects: The 2017 Revision; United Nations: New York, NY, USA, 2017. [Google Scholar]
- World Bank. Poverty and Shared Prosperity 2020: Reversals of Fortune; World Bank: Washington, DC, USA, 2020. [Google Scholar]
- FAO. Shaping the Future of Livestock Sustainably, Responsibly, Efficiently; FAO: Berlin, Germany, 2018. [Google Scholar]
- ICSU; ISSC. Review of the Sustainable Development Goals: The Science Perspective; International Council for Science (ICSU): Paris, France, 2015. [Google Scholar]
- Ritchie, H.; Roser, M. Land Use-Our World in Data. Available online: https://ourworldindata.org/land-use (accessed on 15 February 2021).
- Capper, J.L.; Cady, R.A.; Bauman, D.E. The Environmental Impact of Dairy Production: 1944 Compared with 2007. J. Anim. Sci. 2009, 87, 2160–2167. [Google Scholar] [CrossRef]
- White, R.R.; Brady, M.; Capper, J.L.; McNamara, J.P.; Johnson, K.A. Cow-Calf Reproductive, Genetic, and Nutritional Management to Improve the Sustainability of Whole Beef Production Systems. J. Anim. Sci. 2015, 93, 3197–3211. [Google Scholar] [CrossRef]
- Menchaca, A.; dos Santos-Neto, P.C.; Mulet, A.P.; Crispo, M. CRISPR in Livestock: From Editing to Printing. Theriogenology 2020, 150, 247–254. [Google Scholar] [CrossRef]
- Hennig, S.L.; Owen, J.R.; Lin, J.C.; Young, A.E.; Ross, P.J.; Van Eenennaam, A.L.; Murray, J.D. Evaluation of Mutation Rates, Mosaicism and off Target Mutations When Injecting Cas9 MRNA or Protein for Genome Editing of Bovine Embryos. Sci. Rep. 2020, 10, 22309. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, H.; Shivalila, C.S.; Dawlaty, M.M.; Cheng, A.W.; Zhang, F.; Jaenisch, R. One-Step Generation of Mice Carrying Mutations in Multiple Genes by CRISPR/Cas-Mediated Genome Engineering. Cell 2013, 153, 910–918. [Google Scholar] [CrossRef] [Green Version]
- Hai, T.; Teng, F.; Guo, R.; Li, W.; Zhou, Q. One-Step Generation of Knockout Pigs by Zygote Injection of CRISPR/Cas System. Cell Res. 2014, 24, 372–375. [Google Scholar] [CrossRef] [Green Version]
- Whitworth, K.M.; Lee, K.; Benne, J.A.; Beaton, B.P.; Spate, L.D.; Murphy, S.L.; Samuel, M.S.; Mao, J.; O’Gorman, C.; Walters, E.M.; et al. Use of the CRISPR/Cas9 System to Produce Genetically Engineered Pigs from In Vitro-Derived Oocytes and Embryos1. Biol. Reprod. 2014, 91, 78–79. [Google Scholar] [CrossRef] [Green Version]
- Ni, W.; Qiao, J.; Hu, S.; Zhao, X.; Regouski, M.; Yang, M.; Polejaeva, I.A.; Chen, C. Efficient Gene Knockout in Goats Using CRISPR/Cas9 System. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crispo, M.; Mulet, A.P.; Tesson, L.; Barrera, N.; Cuadro, F.; Dos Santos-Neto, P.C.; Nguyen, T.H.; Crénéguy, A.; Brusselle, L.; Anegón, I.; et al. Efficient Generation of Myostatin Knock-out Sheep Using CRISPR/Cas9 Technology and Microinjection into Zygotes. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Wu, H.; Wang, Y.; Liu, X.; Chen, L.; Li, Q.; Cui, C.; Liu, X.; Zhang, J.; Zhang, Y. Single Cas9 Nickase Induced Generation of NRAMP1 Knockin Cattle with Reduced Off-Target Effects. Genome Biol. 2017, 18, 13. [Google Scholar] [CrossRef] [Green Version]
- Hammer, R.E.; Pursel, V.G.; Rexroad, C.E.; Wall, R.J.; Bolt, D.J.; Ebert, K.M.; Palmiter, R.D.; Brinster, R.L. Production of Transgenic Rabbits, Sheep and Pigs by Microinjection. Nature 1985, 315, 680–683. [Google Scholar] [CrossRef] [Green Version]
- Menchaca, A.; Anegon, I.; Whitelaw, C.B.A.; Baldassarre, H.; Crispo, M. New Insights and Current Tools for Genetically Engineered (GE) Sheep and Goats. Theriogenology 2016, 86, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Capecchi, M. Altering the Genome by Homologous Recombination. Science 1989, 244, 1288–1292. [Google Scholar] [CrossRef] [Green Version]
- Tan, W.; Proudfoot, C.; Lillico, S.G.; Whitelaw, C.B.A. Gene Targeting, Genome Editing: From Dolly to Editors. Transgenic Res. 2016, 25, 273–287. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yu, H.; Lei, A.; Zhou, J.; Zeng, W.; Zhu, H.; Dong, Z.; Niu, Y.; Shi, B.; Cai, B.; et al. Generation of Gene-Modified Goats Targeting MSTN and FGF5 via Zygote Injection of CRISPR/Cas9 System. Sci. Rep. 2015, 5, 13878. [Google Scholar] [CrossRef] [Green Version]
- Kalds, P.; Crispo, M.; Li, C.; Tesson, L.; Anegon, I.; Chen, Y.; Wang, X.; Menchaca, A. Generation of Double-Muscled Sheep and Goats by CRISPR/Cas9-Mediated Knockout of the Myostatin Gene. Methods Mol. Biol. 2021, in press. [Google Scholar]
- Zhou, W.; Wan, Y.; Guo, R.; Deng, M.; Deng, K.; Wang, Z.; Zhang, Y.; Wang, F. Generation of Beta-Lactoglobulin Knock-out Goats Using CRISPR/Cas9. PLoS ONE 2017, 12, e0186056. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Wagner, S.; Maclean, P.; Brophy, B.; Cole, S.; Smolenski, G.; Carlson, D.F.; Fahrenkrug, S.C.; Wells, D.N.; Laible, G. Cattle with a Precise, Zygote-Mediated Deletion Safely Eliminate the Major Milk Allergen Beta-Lactoglobulin. Sci. Rep. 2018, 8, 7661. [Google Scholar] [CrossRef] [Green Version]
- Owen, J.R.; Hennig, S.L.; McNabb, B.R.; Mansour, T.A.; Smith, J.M.; Lin, J.C.; Young, A.E.; Trott, J.F.; Murray, J.D.; Delany, M.E.; et al. One-Step Generation of a Targeted Knock-in Calf Using the CRISPR-Cas9 System in Bovine Zygotes. BMC Genom. 2021, 22. [Google Scholar] [CrossRef]
- Kurtz, S.; Petersen, B. Pre-Determination of Sex in Pigs by Application of CRISPR/Cas System for Genome Editing. Theriogenology 2019, 137, 67–74. [Google Scholar] [CrossRef]
- Ideta, A.; Yamashita, S.; Seki-Soma, M.; Yamaguchi, R.; Chiba, S.; Komaki, H.; Ito, T.; Konishi, M.; Aoyagi, Y.; Sendai, Y. Generation of Exogenous Germ Cells in the Ovaries of Sterile NANOS3-Null Beef Cattle. Sci. Rep. 2016, 6, 24983. [Google Scholar] [CrossRef]
- Park, K.E.; Kaucher, A.V.; Powell, A.; Waqas, M.S.; Sandmaier, S.E.S.; Oatley, M.J.; Park, C.H.; Tibary, A.; Donovan, D.M.; Blomberg, L.A.; et al. Generation of Germline Ablated Male Pigs by CRISPR/Cas9 Editing of the NANOS2 Gene. Sci. Rep. 2017, 7, 40176. [Google Scholar] [CrossRef]
- Ciccarelli, M.; Giassetti, M.I.; Miao, D.; Oatley, M.J.; Robbins, C.; Lopez-Biladeau, B.; Waqas, M.S.; Tibary, A.; Whitelaw, B.; Lillico, S.; et al. Donor-Derived Spermatogenesis Following Stem Cell Transplantation in Sterile NANOS2 Knockout Males. Proc. Natl. Acad. Sci. USA 2020, 117, 24195–24204. [Google Scholar] [CrossRef]
- Jenko, J.; Gorjanc, G.; Cleveland, M.A.; Varshney, R.K.; Whitelaw, C.B.A.; Woolliams, J.A.; Hickey, J.M. Potential of Promotion of Alleles by Genome Editing to Improve Quantitative Traits in Livestock Breeding Programs. Genet. Sel. Evol. 2015, 47, 55. [Google Scholar] [CrossRef] [Green Version]
- Carlson, D.F.; Lancto, C.A.; Zang, B.; Kim, E.S.; Walton, M.; Oldeschulte, D.; Seabury, C.; Sonstegard, T.S.; Fahrenkrug, S.C. Production of Hornless Dairy Cattle from Genome-Edited Cell Lines. Nat. Biotechnol. 2016, 34, 479–481. [Google Scholar] [CrossRef]
- Young, A.E.; Mansour, T.A.; McNabb, B.R.; Owen, J.R.; Trott, J.F.; Brown, C.T.; Van Eenennaam, A.L. Genomic and Phenotypic Analyses of Six Offspring of a Genome-Edited Hornless Bull. Nat. Biotechnol. 2020, 38, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Semex. Recombinetics and Semex Form Alliance to Improve Animal Well-Being. Available online: https://www.semex.com/us/i?lang=en&news=list&id=1527559162 (accessed on 15 March 2021).
- The Alliance to End Surgical Castration of Swine Announces Precision Breeding Successes-Hendrix Genetics Corporate. Available online: https://www.hendrix-genetics.com/en/news/alliance-end-surgical-castration-swine-announces-precision-breeding-successes/ (accessed on 15 March 2021).
- Hansen, P.J. Prospects for Gene Introgression or Gene Editing as a Strategy for Reduction of the Impact of Heat Stress on Production and Reproduction in Cattle. Theriogenology 2020, 154, 190–202. [Google Scholar] [CrossRef]
- This Gene-Edited Calf Could Transform Brazil’s Beef Industry|Moving Upstream-YouTube. Available online: https://www.youtube.com/watch?v=lvDYGSAMiWk (accessed on 15 March 2021).
- WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 15 March 2021).
- Slingenbergh, J.; Gilbert, M.; De Balogh, K.; Wint, W. Ecological Sources of Zoonotic Diseases. OIE Rev. Sci. Tech. 2004, 23, 467–484. [Google Scholar] [CrossRef]
- King, K.C.; Lively, C.M. Does Genetic Diversity Limit Disease Spread in Natural Host Populations. Heredity (Edinb) 2012, 109, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Whitworth, K.M.; Rowland, R.R.R.; Ewen, C.L.; Trible, B.R.; Kerrigan, M.A.; Cino-Ozuna, A.G.; Samuel, M.S.; Lightner, J.E.; McLaren, D.G.; Mileham, A.J.; et al. Gene-Edited Pigs Are Protected from Porcine Reproductive and Respiratory Syndrome Virus. Nat. Biotechnol. 2016, 34, 20–22. [Google Scholar] [CrossRef]
- Burkard, C.; Opriessnig, T.; Mileham, A.J.; Stadejek, T.; Ait-Ali, T.; Lillico, S.G.; Whitelaw, C.B.A.; Archibald, A.L. Pigs Lacking the Scavenger Receptor Cysteine-Rich Domain 5 of CD163 Are Resistant to Porcine Reproductive and Respiratory Syndrome Virus 1 Infection. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [Green Version]
- The Future of Animal Health Is Here-Progressive Dairy. Available online: https://www.progressivedairy.com/topics/a-i-breeding/the-future-of-animal-health-is-here (accessed on 15 March 2021).
- Perspective Opinions and Strategies. Panorama 2020. Available online: https://oiebulletin.com/wp-content/uploads/2020/Panorama2020-1/panorama-2020-1-en.pdf (accessed on 15 March 2021).
- Mason-D’Croz, D.; Bogard, J.R.; Herrero, M.; Robinson, S.; Sulser, T.B.; Wiebe, K.; Willenbockel, D.; Godfray, H.C.J. Modelling the Global Economic Consequences of a Major African Swine Fever Outbreak in China. Nat. Food 2020, 1, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Palgrave, C.J.; Gilmour, L.; Lowden, C.S.; Lillico, S.G.; Mellencamp, M.A.; Whitelaw, C.B.A. Species-Specific Variation in RELA Underlies Differences in NF-B Activity: A Potential Role in African Swine Fever Pathogenesis. J. Virol. 2011, 85, 6008–6014. [Google Scholar] [CrossRef] [Green Version]
- Lillico, S.G.; Proudfoot, C.; Carlson, D.F.; Stverakova, D.; Neil, C.; Blain, C.; King, T.J.; Ritchie, W.A.; Tan, W.; Mileham, A.J.; et al. Live Pigs Produced from Genome Edited Zygotes. Sci. Rep. 2013, 3, 2847. [Google Scholar] [CrossRef] [Green Version]
- Lillico, S.G.; Proudfoot, C.; King, T.J.; Tan, W.; Zhang, L.; Mardjuki, R.; Paschon, D.E.; Rebar, E.J.; Urnov, F.D.; Mileham, A.J.; et al. Mammalian Interspecies Substitution of Immune Modulatory Alleles by Genome Editing. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Tait-Burkard, C.; Doeschl-Wilson, A.; McGrew, M.J.; Archibald, A.L.; Sang, H.M.; Houston, R.D.; Whitelaw, C.B.; Watson, M. Livestock 2.0-Genome Editing for Fitter, Healthier, and More Productive Farmed Animals. Genome Biol. 2018, 19, 204. [Google Scholar] [CrossRef] [Green Version]
- McCleary, S.; Strong, R.; McCarthy, R.R.; Edwards, J.C.; Howes, E.L.; Stevens, L.M.; Sánchez-Cordón, P.J.; Núñez, A.; Watson, S.; Mileham, A.J.; et al. Substitution of Warthog NF-ΚB Motifs into RELA of Domestic Pigs Is Not Sufficient to Confer Resilience to African Swine Fever Virus. Sci. Rep. 2020, 10, 2045–2322. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Pang, D.; Yuan, H.; Jiao, H.; Lu, C.; Wang, K.; Yang, Q.; Li, M.; Chen, X.X.; Yu, T.; et al. Genetically Modified Pigs Are Protected from Classical Swine Fever Virus. PLoS Pathog. 2018, 14, e1007193. [Google Scholar] [CrossRef] [Green Version]
- Whitworth, K.M.; Rowland, R.R.R.; Petrovan, V.; Sheahan, M.; Cino-Ozuna, A.G.; Fang, Y.; Hesse, R.; Mileham, A.; Samuel, M.S.; Wells, K.D.; et al. Resistance to Coronavirus Infection in Amino Peptidase N-Deficient Pigs. Transgenic Res. 2019, 28, 21–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide Pesticide Usage and Its Impacts on Ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef] [Green Version]
- Esvelt, K.M.; Smidler, A.L.; Catteruccia, F. Emerging Technology: Concerning RNA-Guided Gene Drives for the Alteration of Wild Populations|ELife. Elife 2014, 3, e03401. [Google Scholar] [CrossRef]
- Gantz, V.M.; Jasinskiene, N.; Tatarenkova, O.; Fazekas, A.; Macias, V.M.; Bier, E.; James, A.A. Highly Efficient Cas9-Mediated Gene Drive for Population Modification of the Malaria Vector Mosquito Anopheles Stephensi. Proc. Natl. Acad. Sci. USA. 2015, 112, E6736–E6743. [Google Scholar] [CrossRef] [Green Version]
- Dearden, P.K.; Gemmell, N.J.; Mercier, O.R.; Lester, P.J.; Scott, M.J.; Newcomb, R.D.; Buckley, T.R.; Jacobs, J.M.E.; Goldson, S.G.; Penman, D.R. The Potential for the Use of Gene Drives for Pest Control in New Zealand: A Perspective. J. R. Soc. N. Zeal. 2018, 48, 225–244. [Google Scholar] [CrossRef]
- Moro, D.; Byrne, M.; Kennedy, M.; Campbell, S.; Tizard, M. Identifying Knowledge Gaps for Gene Drive Research to Control Invasive Animal Species: The next CRISPR Step. Glob. Ecol. Conserv. 2018, 13, e00363. [Google Scholar] [CrossRef]
- James, S.P. Conclusions of the Malaria Commission of the League of Nations. Br. Med. J. 1927, 2, 340–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. World Malaria Report 2020: 20 Years of Global Progress and Challenges. Available online: https://www.who.int/publications/i/item/9789240015791 (accessed on 15 March 2021).
- Teem, J.L.; Ambali, A.; Glover, B.; Ouedraogo, J.; Makinde, D.; Roberts, A. Problem Formulation for Gene Drive Mosquitoes Designed to Reduce Malaria Transmission in Africa: Results from Four Regional Consultations 2016-2018. Malar. J. 2019, 18, 347. [Google Scholar] [CrossRef] [Green Version]
- Hammond, A.; Galizi, R.; Kyrou, K.; Simoni, A.; Siniscalchi, C.; Katsanos, D.; Gribble, M.; Baker, D.; Marois, E.; Russell, S.; et al. A CRISPR-Cas9 Gene Drive System Targeting Female Reproduction in the Malaria Mosquito Vector Anopheles Gambiae. Nat. Biotechnol. 2016, 34, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Kyrou, K.; Hammond, A.M.; Galizi, R.; Kranjc, N.; Burt, A.; Beaghton, A.K.; Nolan, T.; Crisanti, A. A CRISPR–Cas9 Gene Drive Targeting Doublesex Causes Complete Population Suppression in Caged Anopheles Gambiae Mosquitoes. Nat. Biotechnol. 2018, 36, 1062–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoni, A.; Hammond, A.M.; Beaghton, A.K.; Galizi, R.; Taxiarchi, C.; Kyrou, K.; Meacci, D.; Gribble, M.; Morselli, G.; Burt, A.; et al. A Male-Biased Sex-Distorter Gene Drive for the Human Malaria Vector Anopheles Gambiae. Nat. Biotechnol. 2020, 38, 1054–1060. [Google Scholar] [CrossRef]
- Pham, T.B.; Phong, C.H.; Bennett, J.B.; Hwang, K.; Jasinskiene, N.; Parker, K.; Stillinger, D.; Marshall, J.M.; Carballar-Lejarazú, R.; James, A.A. Experimental Population Modification of the Malaria Vector Mosquito, Anopheles Stephensi. PLoS Genet. 2019, 15, e1008440. [Google Scholar] [CrossRef]
- Carballar-Lejarazú, R.; Ogaugwu, C.; Tushar, T.; Kelsey, A.; Pham, T.B.; Murphy, J.; Schmidt, H.; Lee, Y.; Lanzaro, G.C.; James, A.A. Next-Generation Gene Drive for Population Modification of the Malaria Vector Mosquito, Anopheles Gambiae. Proc. Natl. Acad. Sci. USA 2020, 117, 22805–22814. [Google Scholar] [CrossRef]
- OIE. OIE-Listed Diseases 2021: OIE-World Organisation for Animal Health. Available online: https://www.oie.int/en/animal-health-in-the-world/oie-listed-diseases-2021/ (accessed on 15 March 2021).
- Vargas-Terán, M.; Hofmann, H.C.; Tweddle, N.E. Impact of Screwworm Eradication Programmes Using the Sterile Insect Technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Springer Netherlands: Dordrecht, The Netherlands, 2005; pp. 629–650. [Google Scholar] [CrossRef]
- Grunwald, H.A.; Gantz, V.M.; Poplawski, G.; Xu, X.R.S.; Bier, E.; Cooper, K.L. Super-Mendelian Inheritance Mediated by CRISPR–Cas9 in the Female Mouse Germline. Nature 2019, 566, 105–109. [Google Scholar] [CrossRef]
- GISD. 100 of the World’s Worst Invasive Alien Species. Available online: http://www.iucngisd.org/gisd/100_worst.php (accessed on 15 March 2021).
- McFarlane, G.R.; Whitelaw, C.B.A.; Lillico, S.G. CRISPR-Based Gene Drives for Pest Control. Trends Biotechnol. 2018, 36, 130–133. [Google Scholar] [CrossRef] [Green Version]
- Galizi, R.; Hammond, A.; Kyrou, K.; Taxiarchi, C.; Bernardini, F.; O’Loughlin, S.M.; Papathanos, P.A.; Nolan, T.; Windbichler, N.; Crisanti, A. A CRISPR-Cas9 Sex-Ratio Distortion System for Genetic Control. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Burt, A.; Deredec, A. Self-Limiting Population Genetic Control with Sex-Linked Genome Editors. Proc. R. Soc. B Biol. Sci. 2018, 285. [Google Scholar] [CrossRef] [Green Version]
- Prowse, T.A.A.; Cassey, P.; Ross, J.V.; Pfitzner, C.; Wittmann, T.A.; Thomas, P. Dodging Silver Bullets: Good CRISPR Gene-Drive Design Is Critical for Eradicating Exotic Vertebrates. Proc. R. Soc. B Biol. Sci. 2017, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, T.F.; Van Eenennaam, A.L. Genome Editing Approaches to Augment Livestock Breeding Programs. J. Exp. Biol. 2020, 223 (Suppl. 1). [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EU-SAGE. European Sustainable Agriculture through Genome Editing (EU-SAGE). Available online: https://www.eu-sage.eu/sites/default/files/2020-07/Open Statement EU-SAGE July 2020_EN.pdf (accessed on 15 March 2021).
- Regnault-Roger, C. GMOs and Genome Edited Organisms (GEOs): Regulatory and Geopolitical Challenges; Fondation pour l’innovation Politique (Fondapol): Paris, France, 2021. [Google Scholar]
- Regnault-Roger, C. New Genomic Techniques (NGTs): The European Commission Opens the Door. An Historical Challenge for French EU Presidency. Available online: https://www.europeanscientist.com/en/features/new-genomic-techniques-ngts-the-european-commission-opens-the-door-an-historical-challenge-for-french-eu-presidency/ (accessed on 21 May 2021).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menchaca, A. Sustainable Food Production: The Contribution of Genome Editing in Livestock. Sustainability 2021, 13, 6788. https://doi.org/10.3390/su13126788
Menchaca A. Sustainable Food Production: The Contribution of Genome Editing in Livestock. Sustainability. 2021; 13(12):6788. https://doi.org/10.3390/su13126788
Chicago/Turabian StyleMenchaca, Alejo. 2021. "Sustainable Food Production: The Contribution of Genome Editing in Livestock" Sustainability 13, no. 12: 6788. https://doi.org/10.3390/su13126788
APA StyleMenchaca, A. (2021). Sustainable Food Production: The Contribution of Genome Editing in Livestock. Sustainability, 13(12), 6788. https://doi.org/10.3390/su13126788





