Functionalized Carbon Nanotubes (CNTs) for Water and Wastewater Treatment: Preparation to Application
Abstract
:1. Introduction
1.1. Historical Background
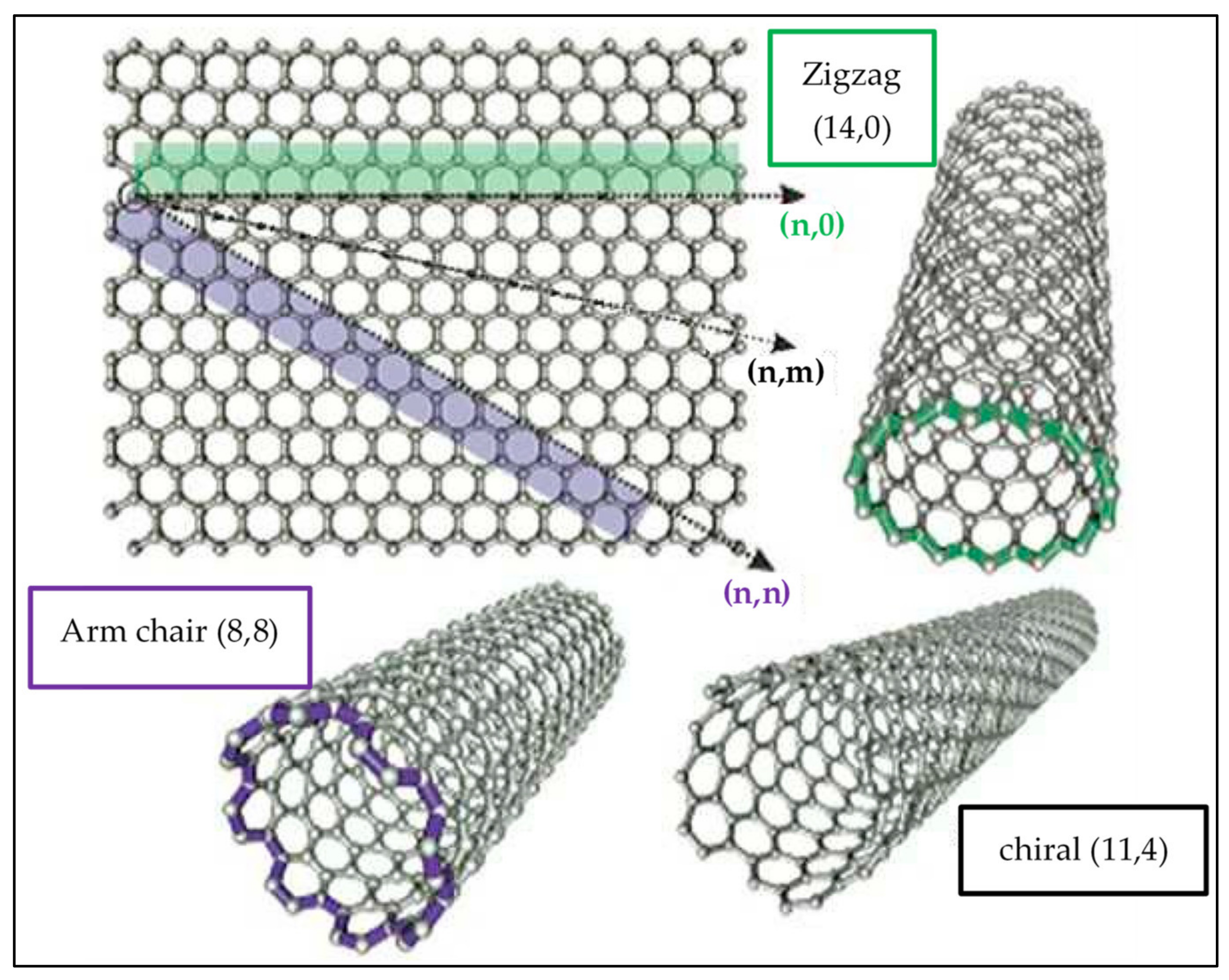
1.2. Types of CNTs and Structure
- 1.
- Single-walled carbon nanotubes (SWCNTs)
- 2.
- Multi-walled carbon nanotubes (MWCNTs).
2. Synthesis of CNTs
- (a)
- Arc discharge
- (b)
- Laser ablation
- (c)
- Chemical vapor deposition (CVD).
| Parameters | Arc Discharge Method | Chemical Vapor Deposition | Laser Ablation (Vaporization) | Ref. | |
|---|---|---|---|---|---|
| Method | Source of energy | Direct current | Temperature (ignition) | High intensity laser beam | [90,91] |
| Source of carbon | Carbon or graphite electrodes | Hydrocarbon gases or carbon monoxide (CH4, CO, or acetylene) | Graphite object | ||
| Temperature (°C) | 3000 to 4000 | 500 to 1100 | About 3000 | [84,85] | |
| Cost per unit synthesis | Costly | Economic | Costly | [83,92] | |
| CNTs selectivity | Less | High | Less | [93] | |
| Availability of carbon source | Complex | Easy | Difficult | [94] | |
| Purification level | More | Less | More | [95] | |
| Nature of synthesis process | Batch | Continuous | Batch | [64,96] | |
| Control on synthesis parameters | Difficult | Easy | Difficult | [97,98] | |
| Energy requirement | High | Low | High | [99] | |
| Design of reactor | Hard | Simple and easy | Hard | [100] | |
| Nanotube graphitization | High | Moderate | High | [101,102,103] | |
| Typical yield | 30 to 90% | 20 to 100% | Up to 70% | [84,91,95,104,105,106] | |
| Typical Diameter | SWCNTs | 0.6 to 1.4 nm | 0.6 to 4 nm | 1 to 2 nm | [87,91,107,108] |
| MWCNTs | Inner: 1 to 3 nm Outer: ~10 nm | 0.1 to several nanometers | 10 to 20 nm | [79,83,91,104,109] | |
| Advantages | 1. Synthesis of both SWCNTs and MWCNTs is easy 2. MWCNTs can be produced without any catalyst 3. Costly process but less than laser ablation method 4. Synthesis of CNTs is possible in open air 5. High degree of structural perfection | 1. Bulk production is easy 2. More extensive length CNTs than other methods 3. Simple and easy process 4. Quite pure 5. Alignment of produced CNTs is good 6. Diameter and number of layers can be controlled | 1. Primarily for SWCNTs 2. Diameter of CNTs can be controlled 3. Lower numbers of defects 4. High degree of structural perfection 5. Tubes’ length can vary from 5 to 20 mm | [98,110,111,112,113] | |
| Disadvantages | 1. Received with some structural defects 2. Short and randomly distributed in length and direction 3. Lot of structural purification is needed 4. Contains carbon impurities | 1. Only used to produce MWCNTs 2. Higher structural defect density | 1. Costly technique due to expensive lasers beams 3. Power needs are high 4. Low yield | [65,86,98,109] | |
| Figures |  |  |  | [114] | |
3. CNT Purifications
- Oxidation
- Acid treatment
- Surfactant based sonication.
| Technologies | Methods | Characteristics | ||
|---|---|---|---|---|
| Advantages | Limitations | |||
| Physical method | Filtration | 1. Non-destructive 2. Retains the inherency and intrinsic structure necessary to elucidate the properties of CNTs 3. More suitable as an auxiliary step in combination with chemical purification 4. Improve crystallinity 5. High selectivity to metal 6. CNTs can be separated on the bases of difference in length and conductivity | 1. Not very effective 2. CNT samples need to be extremely dispersed 3. Purification of samples can be done in a limited quantity at a time | |
| Centrifugation | ||||
| Solubilization with functional groups | ||||
| High temperature annealing | ||||
| Chromatography, electrophoresis | ||||
| Chemical method | Gas phase | Air, Cl2, H2O, HCl, H2O, Ar, O2, C2H2F4, SF6 | 1. Opens the lid of the CNTs without affecting sidewalls or associated functional groups 2. Eliminates polyhedral and amorphous carbon and metallic impurities at the cost of substantial amounts of CNTs or damage to the CNT structure 3. Leads to functional groups 4. Does not disrupt or affect the alignments of CNTs | 1. Low yield 2. Produces more defects on sidewalls, breaks into different shorter length, and also the alignment and structure are affected greatly, thus limiting the final applications of CNTs |
| Liquid phase | HNO3, H2O2, HCl, Mixture of acid or KMnO4, Microwave in inorganic acid | |||
| Electrochemical | Alkali or acid solution | |||
| Multi step method | Oxidation, sonication, centrifugation, filtration, wet grinding, and HIDE | 1. High-purity with respect to metal 2. Metal free, improving crystallinity 3. Effectively removes carbonaceous and metallic impurities 4. Better purification yield due to the early removal of metallic impurities that can oxidize CNTs | 1. Low yield | |
| Filtration/magnetic filtration, oxidation, annealing | ||||
| Filtration, sonication in HNO3, HF, H2O2, or SDS | ||||
| Annealing at high temperature, extraction | ||||
| Technique | Residual Material | Assessment Techniques | Advantages | Limitations |
|---|---|---|---|---|
| Thermo-gravimetric analysis (TGA) | Carbonaceous impurities Metal impurities | After oxidation of material, the residual metallic impurities are calculated by weighing ash and the carbonaceous impurities by area ratio of DTG | Accurate measurement of impurities | Completely oxidize/destroy the CNTs |
| Raman spectrometry | Carbonaceous impurities Structure defects Conductivity characteristics | The pure CNTs are associated with G-band by RBM as well as no D-band | Conductivity features and quality of CNTs can be measure along with their diameter | Difficult or even unacceptable for MWCNTs and metallic contents |
| Electron microscopy (SEM, TEM) | Defects in CNTs Amorphous carbon | Directly observes and qualitatively evaluates the adhesion defects on the CNT wall, the amount of amorphous carbon, fullerene | Absolute scrutiny can be undertaken | Can analyze the sample in a very small amount |
| UV–vis-NIR | Carbonaceous impurities conductivity characteristics | Absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region | Conductivity features and contents of CNTs can be analyzed exactly | A standard sample is needed with 100% purity |
| X-ray photoelectron spectroscopy (XPS) | Support material/functional groups (fine alumina, magnesium oxide, silica, zeolite, etc.) | Quantitatively characterizes the type and contents of functional groups or support materials | Analysis of functional groups on CNTs can be undertaken exactly | Unacceptable for purity |
| Energy-dispersive X-ray spectroscopy (EDS) | Metal impurities | Analytical technique used for chemical and/or elemental analysis of a sample | Contents and traces of different elements can be analyzed | Evaluation of the contents of CNTs is invalid |
4. Functionalization of CNTs
- With π conjugated network of CNTs through covalent bonds;
- Attachment of different chemical groups via non-covalent bonds by using hydrophobicity of CNTs such as hydrogen bonds, π–π interactions, or ionic bonds;
- Inline filling (endohedral) of hollow tubes of CNTs. The two methods are more common for CNTs functionalization and variously used by the researchers.
5. Characterization of CNTs
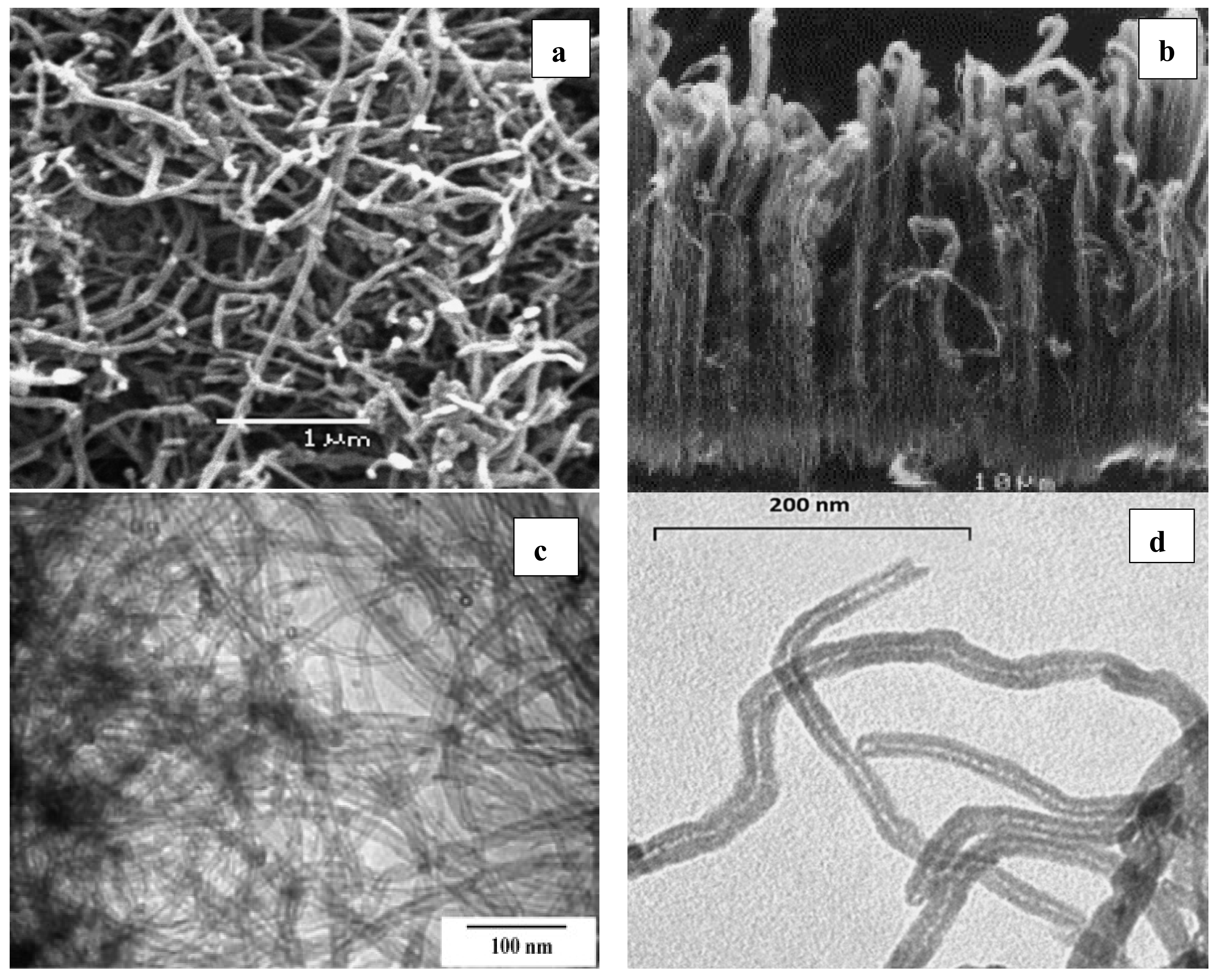
6. Applications of CNTs
6.1. Removal of Heavy Metals
| Adsorbate | Adsorbent | Surface Area (m2/g) | Diameter (nm) | Qe/RE | Experimental Conditions | Removal Mechanism | Model | Comments | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | IC | AL | |||||||||
| As(III) | MWCNTs | 9.1 | 10–40 | 91% | 6 | 40 µg/L | 2.0 g/L | Liquid film diffusion, ion exchange | Tempkin, Dubinin-Radushkevic, Langmuir, Freundlich | In column operation, the removal As(III) was up to 13.5 µg/L | [217] |
| Zero-valent iron-doped MWCNT | - | - | 200 mg/g | 4 | 10 mg/L | 0.2–4.0 g/L | Ion exchange, surface complexation | Langmuir | Maximum As(III) removal efficiency was 98.5% | [218] | |
| Floating catalyst CNTs (FCNT) | 74 | 18.6 | 1.22 mg/g | 6.5 | 0.1–10 mg/L | 1 g/L | Electrostatic attraction, surface complexation | Langmuir | Potential adsorbent for removal to total arsenic | [219] | |
| Oxidized-FCNT | 129 | 10.7 | 1.90 mg/g | ||||||||
| Heat-treated oxidized CNTs (FCNT-HOX) | 168 | 7 | 5.99 mg/g | ||||||||
| Zero-valent iron immobilized on MWCNTs | 78.78 | 111.1 mg/g | 7 | 0.1–1 mg/L | 0.05 g | Surface complexation | Langmuir | Reusability of adsorbent was up to 5 cycles | [219] | ||
| MWCNT-ZrO2 | 152 | 20–40 | 2 mg/g | 6 | 100 μg/L | 100 mg/10 mL | Chemisorption/ physisorption | Langmuir | The adsorption capacity of AS (III) is not associated with pH value | [203] | |
| Iron-oxide-coated multi-walled carbon nanotubes | 153 | 20–40 | 1.723 mg/g | 4 | 100 μg/L | 10 mg/10 mL | Electrostatic interaction, surface complexation | Langmuir | Suggesting that modifying MWCNTs with other groups can develop potential adsorbents for water treatment | [203] | |
| As(V) | Iron-oxide-coated SWCNTs | - | - | 49.65 mg/g | 4 | 5–50 mg/L | - | Surface complexation | Freundlich | Adsorption was very fast for low concentration of As(V) | [220] |
| MWCNTs | 9.1 | 10–40 | 92% | 6 | 40 µg/L | 2.0 g/L | Liquid film diffusion, ion exchange | Tempkin, Dubinin-Radushkevic, Langmuir, Freundlich | In column operation, the removal As(III) was up to 14.0 µg/L | [217] | |
| Zero valent iron doped MWCNTs | - | - | 250 mg/g | 4 | 10 mg/L | 0.2–4.0 g/L | Ion exchange, surface complexation | Langmuir | Maximum As(V) removal efficiency was 98.5% | [218] | |
| Floating catalyst CNTs (FCNT) | 74 | 18.6 | 0.88 mg/g | 6.5 | 0.1–10 mg/L | 1 g/L | Electrostatic attraction, surface complexation | Langmuir | Potential adsorbent for removal to total Arsenic | [219] | |
| Oxidized-FCNT | 129 | 10.7 | 2.51 mg/g | ||||||||
| Heat-treated oxidized CNTs (FCNT-HOX) | 168 | 7 | 6.37 mg/g | ||||||||
| 3-(2-aminoethylamino) propyltrimethoxysilane modified MWCNTs | - | - | 8.01 mg/g | 2.2 | 1.0 mg/L | 40 mg | - | - | Cr(IV) was selectively adsorbed in the micro-column packed with adsorbent | [221] | |
| Zero-valent iron immobilized on MWCNTs | 78.78 | 167 mg/g | 7 | 0.1–1 mg/L | 0.05 g | Surface complexation | Langmuir | Successfully applied to ground water with high pH | [219] | ||
| Iron(III)-oxide-coated ethylenediamine functionalized MWCNTs | 198.5 | 5–10 | 23.5 mg/g | 4 | 100 μg/L | 50 mg/10 mL | Ion exchange | Langmuir | Greater efficiency to remove As(V) due to enormous adsorbing sites | [222] | |
| MWCNT–zirconia nanohybrid | 152 | 20–40 | 5.0 mg/g | 6 | 100 μg/L | 100 mg/10 mL | Chemisorption/ physisorption | Langmuir | The adsorption capacity of As(V) is not associated with pH value | [223] | |
| Iron-oxide-coated MWCNTs | 153 | 20–40 | 0.189 mg/g | 4 | 100 μg/L | 10 mg/10 mL | Electrostatic interaction, surface complexation | Langmuir | Modifying MWCNTs with other groups can develop potential adsorbents for water treatment | [203] | |
| Cr(III) | Iron oxide/carbon nanotubes/chitosan magnetic composite film | 64.4 | 66.25 mg/g | 2–10 | 100 mg/L | 0.3 mg/g | Electrostatic | Langmuir | Decrease in efficiency was 12% after reusing the adsorbent for ten cycles | [224] | |
| Nitrogen-doped magnetic carbon nanoparticles | - | - | 83.7 mmol/g | 8 | 200 mg/L | 10 mg/500 mL | Chemical adsorption | Langmuir | 10-fold greater removals than activated carbon due to large SSA | [225] | |
| Acid modified MWCNTs | - | 23 | 0.5 mg/g | 7 | 1 mg/L | 120 mg/500 mL | Electrostatic interaction | Pseudo-second order | Increasing removal of Cr with increasing the dose of CNTs | [215] | |
| Cr(IV) | Iron oxide/carbon nanotubes/chitosan magnetic composite film | 64.4 | - | 449.3 mg/g | 10-Feb | 100 mg/L | 0.3 mg/g | Electrostatic | Langmuir | Decrease in efficiency was 6% after reusing the adsorbent for ten cycles | [224] |
| Nitrogen-doped magnetic CNTs | 116.4 | - | 970.9 mg/g | 1 | 40–1000 mg/L | 0.5–3.5 g/L | Surface complexation | Langmuir | Recycled adsorbent was successfully used for excellent electrochemical reduction of CO2 | [163] | |
| Chitosan-modified MWCNTs | - | 30–50 | 164.0 mg/g | 2 | 50 mg/L | 50 mg | Electrostatic | Langmuir | Adsorbent can be recycled up to 4 times | [226] | |
| Magnetic iron oxide MWCNTs | - | ~50 | 42.0 mg/g | 2 | 5 mg/L | 0.4–1.0 g/L | Electrostatic | Langmuir | Absorbent highly showed durability, selectivity, easy regeneration ability | [227] | |
| Chitin magnetite MWCNTs | 69.1 | - | 100% | 2 | 50 mg/L | - | Physical | - | Removal of Cr(IV) was enhanced after mixing MWCNTs with chitin | [228] | |
| Magnetic MWCNTs | 200 | 20–40 | 16.23 mg/g | 3 | 25 mg/L | - | - | Langmuir | The adsorption capacity of adsorbent increases with initial concentration of Cr(VI) and contact time, but decreases with the increase of adsorbent dosage | [229] | |
| 3-(2-aminoethylamino) propyltrimethoxysilane-modified MWCNTs | - | - | 9.79 mg/g | 2.2 | 1.0 mg/L | 40 mg | - | - | Cr(IV) was selectively adsorbed in the micro-column packed with adsorbent | [221] | |
| Activated-carbon-coated CNTs | - | 10–20 | 9.0 mg/g | 2 | 0.2–0.5 mg/L | 2 mg/50 mL | - | Langmuir | The f-CNT can be used largely for the removal of Cr ions | [215] | |
| Ceria-supported CNTs nanoparticles | - | 20–80 | 31.55 mg/g | 7 | 35.3 mg/L | 100 mg/100 mL | Ion exchange | Langmuir | Suggesting that CeO2/ACNTs has high potential for heavy metal removals | [230] | |
| Pb(II) | Thiol-functionalizedMWCNTs/Fe3O4 | 97.367 | - | 65.4 mg/g | 6.5 | 50 mg/L | 100 mg/100 mL | Lewis acid–base interactions | Langmuir | The adsorbent removed heavy metal ions effectively at various pH values | [231] |
| Magnetic MWCNTs | 295.4 | - | N/A | 6 | 100 mg/L | 1000 mg | - | Experimental | High removal efficiency due to intrinsic properties, large SSA, and porous structure | [232] | |
| MWCNTs/Fe3O4 | 108.37 | 10–20 | 22.04 mg/g | 5.3 | 30 mg/L | 500 mg/1000 mL | Electrostatic, hydrophobic, and π–π interactions | Langmuir | Easily regenerate the adsorbent by external magnetic field after several cycles | [233] | |
| MWCNTs/Fe3O4 modified with 3-aminopropyltriethoxysilane | 90.68 | 10–20 | 75.02 mg/g | 5.3 | 30 mg/L | 500 mg/1000 mL | Electrostatic, hydrophobic, and π–π interactions | Langmuir | Easily regenerate the adsorbent by external magnetic field after several cycles | [233] | |
| MWCNTs grafted/PAAM membrane | - | - | 98% | - | 10 mg/L | 1000 mg/1000 mL | Electrostatic interaction | - | The f-CNT membrane potentially enhances the water flux and removal of heavy metals | [234] | |
| Oxidized CNT sheets | - | - | 117.65 mg/g | 7 | 1200 mg/L | 50 mg/25 mL | Chemical interaction | Langmuir | Considering the oxidize CNT sheets promising nanomaterial for adsorption | [235] | |
| MWCNTs grafted with 2-Vinylpyridine | - | - | 37.0 mg/g | 6 | 10 mg/L | 640 mg/1000 mL | Ion exchange, electrostatic interaction | Langmuir | Showed high suitability for preconcentration and immobilization of heavy metal ions from water | [236] | |
| Oxidized MWCNTs | 142.29 | 10–30 | 0.021 mmol/g | 4.1 | 0.83 mmol/L | 0.75 g/L | Chemical, electrostatic, hydrophobic, and π–π interactions | Langmuir | High removal efficiency toward heavy metal ions in wastewater | [214] | |
| Alumina-coated MWCNTs | - | - | 99% | Different | - | 10 mg/25 mL | N/A | - | The composite can be used largely to remove lead from industrial wastewater. Adsorption efficiency increased with the pH (3 to 7) | [236] | |
| Nitrogen-doped magnetic carbon nanoparticles | - | 6.74 mmol/g | 8 | 200 mg/L | 10 mg/500 mL | Chemical adsorption | Experimental | High removal efficiency toward Pb compared to Cr | [225] | ||
| Titanium Dioxide /MWCNT composites | - | - | 137.0 mg/g | 6 | 10 mg/L | 20 mg/10 mL | - | Langmuir | Important adsorption ability to remove large amount of Pb(II) in short period | [204] | |
| Pb(II) | Oxidize MWCNTs | - | 20–30 | - | - | 10 mg/L | 3000 mg/1000 mL | - | - | The sorption of Pb largely depends on foreign ions and ionic strength | [237] |
| Manganese oxide-coated CNTs | 275 | 2.60 | 78.74 mg/g | 5 | 30 mg/L | 50 mg/100 mL | Electrostatic interaction, surface complexation | Langmuir | 300% greater adsorption capacity than raw CNTs | [238] | |
| Acidified MWCNTs | 237.3 | 29.0 | 85 mg/g | 5 | 50 mg/L | 25 mg/50 mL | Physical adsorption | Langmuir | The regeneration of Pb increasing with decreasing pH and can be used for several cycles | [239] | |
| Cd(II) | Alumina-decorated MWCNTs | 109.8 | 10–20 | 27.21 mg/g | 7 | 1 mg/L | 50 mg/L | Electrostatic interaction, physical adsorption, surface precipitation | Langmuir | Capable of removing both metallic and organic Contaminants | [240] |
| Oxidized MWCNTs | 78.5 | 16.09 | 24.15 mg/g | - | 5 mg/L | 1 mg/10 mL | Chemisorption | Langmuir | The sorption capacity is strongly dependent on pH due to surface charge and showed best performance in the pH ranging from 6 to 10 | [209] | |
| Ethylenediamine-functionalized MWCNTs | 101.2 | 21.25 | 25.7 mg/g | - | 5 mg/L | 1 mg/10 mL | Chemisorption | Langmuir | The sorption capacity is strongly dependent on pH due to surface charge and showed best performance in the pH ranging from 6 to 10 | [209] | |
| Oxidized CNT sheets | - | - | 92.59 mg/g | 7 | 1200 mg/L | 50 mg/25 mL | Chemical interaction | Langmuir | Excellent removal of heavy metal ions | [235] | |
| Acid-modified CNTs | 170 | 10–20 | 4.35 mg/g | 7 | - | 50 mg | Electrostatic interaction | Langmuir | Potential material for water purification | [241] | |
| MWCNTs modified with Chitosan | - | 60–100 | - | - | - | 2000 mg | Electrostatic interaction | - | The removal efficiency increases with increase of mass of both MWCNTs and chitosan | [242] | |
| Hg(II) | MnO2-coated CNTs | 110.4 | 30–50 | 58.82 mg/g | 5–7 | 10 mg/L | 200 mg/20 mL | Electrostatic interaction | Langmuir | Higher adsorption affinity to other heavy metals rather than Hg | [149] |
| Thiol-derivatized SWCNTs | - | - | 131.58 mg/g | 5 | 40 mg/L | 0.25 mg/mL | Electrostatic interaction | Langmuir | Easily desorb/regenerate Hg after treatment of water | [243] | |
| Amino and thiolated functionalized-MWCNTs | - | 5–10 | 84.66 mg/g | 6 | 100 mg/L | 60 mg | Physisorption | Langmuir | Highly efficient removal from real wastewater and further research is necessary to commercialize | [244] | |
| Iodide-incorporated MWCNT (CNT-I) | 153 | 10–20 | 123.45 mg/g | 6 | 100–500 mg/L | 2500 mg/1000 mL | Ion exchange | Langmuir | Successfully used for the adsorption and desorption of Hg(II) | [205] | |
| Sulphur-containing CNTs | - | - | 72.8 μg/g | 12.15 | 0.1mg/L | 100 mg/20 mL | Chemisorption | Freundlich | Greater treatment ability for industrial wastewater containing Hg and other anions and cations | [245] | |
| Hg(II) | Thiol-functionalized-MWCNTs/Fe3O4 | 97.2 | - | 65.52 mg/g | 6.5 | 50 mg/L | 1000 mg/100 mL | Lewis acid–base interactions | Langmuir | Better removal of heavy metals in different pH concentration | [231] |
| Oxidized MWCNTs | - | - | 3.83 mg/g | 7 | 10–100 μg/L | 25 mg/50 mL | Electrostatic interaction | Langmuir | Small diameter of CNTs removing greater amount of Hg(II) from aqueous solution | [246] | |
| Zn(II) | Functionalized MWCNTs | 250 | 10–25 | 2.42 mg/g | 10 | 1.1 mg/L | 0.09 g | Electrostatic interaction | Langmuir | Excellent potential for the removal of heavy metal ions | [247] |
| Oxidized CNTs | - | - | 74.63 mg/g | 7 | 1200 mg/L | 50 mg/25 mL | Chemical interaction | Langmuir | Economically feasible material with excellent heavy metal ion removal efficiency without any CNTs leakage | [235] | |
| Chitosan-MWCNTs | - | 60–100 | N/A | 7 | - | 200 mg | Electrostatic interaction | N/A | The removal efficiency increases with increase of mass of both MWCNTs and Chitosan | [242] | |
| Nitrogen-doped magnetic carbon nanoparticles | - | - | 9.31 mmol/g | 8 | 12.82 mg/L | 10 mg/500 mL | Chemical adsorption | Langmuir | Higher specific surface area and nitrogen make the nanomaterial an excellent adsorbent | [225] | |
| Zn(II) | Oxidized MWCNTS | - | 14 | 0.27 mmol/g | 6.5–6.8 | 15 mg/L | 5 mg/5 mL | Electrostatic interaction | Langmuir | Further research is necessary to understand the full mechanism | [199] |
| Sodium-hypochlorite-treated MWCNTs | - | <10 | 34.36 mg/g | - | 60 mg/L | 50 mg/100 mL | Electrostatic interaction | Langmuir | Zinc ion could be easily regenerated, and the adsorbent can be used for many cycles | [211] | |
| Cu(II) | Sulfonated MWCNTs | 28.7 | - | 43.16 mg/g | 5 | 20 mg/L | 25 mg/50 mL | Electrostatic interaction, surface complexation | D–R model | Enabling CNTs for wastewater treatment and composite formation or physical blending | [248] |
| Magnetic MWCNTs | - | 10–20 | 38.91 mg/g | 30 mg/L | 200 mg/1000 mL | Electrostatic interaction, physical interaction | - | Easily regenerate the Cu after removal from polluted water | [232] | ||
| Oxidized CNT sheets | - | - | 64.93 mg/g | 7 | 200 mg/L | 50 mg/25 mL | Chemical interaction | Langmuir | Considering the oxidize CNT sheets promising nanomaterial for heavy metal adsorption | [235] | |
| Chitosan/ poly(vinyl) functionalized MWCNTs | - | 5–20 | 11.1 mg/g | 5.5 | 30 mg/L | 0.5–2 wt% | Ion exchange | Langmuir | No loss in the adsorption capacity after four regeneration cycles | [249] | |
| MWCNTs modified with Chitosan | - | 60–100 | >95% | - | - | 2000 mg | Electrostatic interaction | - | The removal efficiency increases with increase of mass of both MWCNTs and chitosan | [242] | |
| Chitosan-grafted MWCNTs | - | - | 24.0 mg/g | - | 10mg/L | 1000 mg/1000 mL | N/A | - | Effective preconcentration and solidification of heavy metals in aqueous samples | [250] | |
| Co(II) | Poly(acrylic acid)-grafted MWCNTs | - | - | 1.66 × 10−4 mol/g | 6.8 | 1.69 × 10−4 mol/L | 1.0 g/L | Surface complexation | Langmuir | Promising ability to use in water purification | [251] |
| MWCNTS/ iron oxide | - | - | 0.18 mmol/g | 6.4 | 4.2 mg/L | 0.5 g/L | Ion exchange, surface complexation | Langmuir | Highlights the interaction between heavy metals and organic substances in wastewater | [252] | |
| Oxidized CNT sheets | - | - | 85.74 mg/g | 7 | 1200 mg/L | 50 mg/25 mL | Chemical interaction | Langmuir | Considering the oxidized CNT sheets promising material for the removal of heavy metal ions | [235] | |
| Ni(II) | HNO3-treated MWCNTs | 102 | 10–20 | 17.86 mg/g | 6.5 | 20 mg/L | 0.8 g/L | Ion exchange | Langmuir | Better removal efficiency toward heavy metal ions | [253] |
| MWCNTs modified with Chitosan | - | 60–100 | 90% | - | - | 2000 mg | Electrostatic interaction | - | The removal efficiency increases with an increase of mass of both MWCNTs and Chitosan | [242] | |
| Nitrogen-doped magnetic carbon nanoparticles | - | - | 8.06 mmol/g | 8 | 12.82 mg/L | 10 mg/500 mL | Chemical adsorption | Langmuir | The removal efficiency was not very good for Ni compared to Cr | [225] | |
| Poly(acrylic acid) (PAA)-oxidized MWCNTs | 197 | - | 6.615 × 10−6 mol/g | 5.4 | 5 mg/L | 0.8 g/L | Electrostatic interaction, π–π interaction | Langmuir | Effective preconcentration and solidification of Ni(II) in liquid samples | [60] | |
| NaClO-modified SWCNTs | - | 380 | 47.86 mg/g | 7 | 10–80 mg/L | 50 mg/100 mL | Electrostatic interaction | Langmuir | High removal affinity to heavy metals and can be used for water treatment | [216] | |
| MWCNTs/ Iron oxide | - | - | 9.18 mg/g | - | 6 mg/L | 0.75 g/L | Ion exchange | Langmuir | Promising candidate for the solidification and preconcentration of heavy metal ions as well as for radionuclides from water | [201] | |
| Oxidized MWCNTs | - | 5.5–14 | 49.26 mg/g | - | 10–200 mg/L | 20 mg/50 mL | Electrostatic interaction | - | Greater adsorption ability than raw MWCNTs in water | [254] | |
| Oxidized MWCNTs | 197 | 10–30 | >80% | 8 | 6–20 mg/L | 50 mg/200 mL | Electrostatic interaction | - | Excellent material for the adsorption of metal ions. | [255] | |
| Ni(II) | MWCNTs | 40–600 | 40–60 | 6.09 mg/g | 7 | 25 mg/L | 5 g/L | Ion exchange, surface complexation, chemical interaction | - | Excellent sorption of Ni+2 ions with smaller equilibrium time | [256] |
| U(II) | Diglycolamide-functionalized MWCNT (DGA-MWCNTs) | 300–600 | - | 133.74 mg/g | 7 | - | 1–10 mg | - | Langmuir | Adsorption efficiency increased with the increasing dose of adsorbent and temperature | [257] |
| Sr(II) | Oxidized-MWCNTs | - | - | 36% | 2-11 | - | 3 g/L | - | Diffuse layer model | Adsorption efficiency increased with increasing pH but decreased with the ionic strength | [258] |
| Eu (III) | Oxidized-MWCNTs | - | - | 96% | 2-11 | - | 3 g/L | - | Diffuse layer model | Higher adsorption efficiency for Eu(III) than Sr(II) | [258] |
6.2. Removal of Organics
| Adsorbent | Dye Pollutants | Surface area (m2/g) | Q (mg/g) | Removal percentage (%) | Optimum conditions | Remarks | Ref. |
| Oxidized SWCNT | Basic red 46 (BR 46) | 400 | 49.45 | - | pH 9, IC = 150 mg/L, AL = 0.05 g, Contact time = 100 min, 298 K, | Exothermic process favored at lower temperature range Orderly adsorption of dye due to negative entropy | [282] |
| HNO3-oxidized MWCNTs | Bromothymol blue (BTB) | 96.8 | 55 | 97 | pH 1, IC = 30 mg/L, AL = 0.02 g, T = 293.15 K, | Endothermic process of adsorption significantly affected by pH, initial concentration, sorbent dosage, and contact time | [262] |
| Functionalized CNT/Mg(Al)O | Congo red | 148 | 1250 | 94 | pH 7, AL = 30 mg, contact time = 75 min | Strong electrostatic interactions between dye particles and functional groups associated with the surface of nanomaterial | [283] |
| Magnetic MWCNTs-Fe3C nanocomposite | Direct red 23 | 38.7 | 85.5 | - | pH 3.7, IC = 54 mg/L, AL = 0.04 g, T = 333 K, | Spontaneous endothermic adsorption process due to positive enthalpy | [284] |
| SWCNT–COOH | Malachite Green | 400 | 22.33 | - | pH 7, IC = 10 mg/L, 300 K, | Adsorption significantly affected by ionic strength, initial concentration, sorbent mass, contact time, and temperature More active functional groups on SWCNT-NH2 adsorbed more dye than SWCNT-COOH | [285] |
| SWCNT-NH2 | Malachite Green | 400 | 29.36 | - | pH 7, IC = 10 mg/L, T = 300 K, | ||
| SWCNT–COOH | Methyl orange | 400 | 25 | - | pH 7, IC = 10 mg/L, T = 300 K, | ||
| SWCNT-NH2 | Methyl orange | 400 | 27.15 | - | pH 7, IC = 10 mg/L, T = 300 K, | ||
| Oxidized MWCNTs | Methyl orange | 165 | 10 | - | AL = 20 mg/L, T = 313 K, stirring speed = 500 rpm | Initially, rapid adsorption was observed, but it slowed down with the time As the mixture temperature, agitation speed, and initial concentration increased, the adsorption efficiency also increased | [286] |
| Functionalized-CNTs loaded TiO2 | Methyl orange | - | 42.85 | 100 | pH 6.5,IC = 5 mg/L, contact time = 30 min, T = 298 K | Highly active hydroxyl and amine functional groups made TiO2-CNT composite an effective adsorbent | [287] |
| Thiol-functionalized MWCNT (MWCNT-SH) | Methylene blue | 400 | 166.67 | - | pH 6,IC = 10 mg/L, AL = 20 mg, T = 298 K, Contact time = 60 min, | As the temperature and initial concentration increased, the adsorption efficiency also increased | [288] |
| Adsorbent | Phenol and its derivatives pollutants | Q (mg/g) | Surface area (m2/g) | Removal percentage (%) | Optimum conditions | Remarks | Ref. |
| KOH-modified MWCNTs | Bisphenol-A | 0.20 mmol/g | 494.48 | - | pH 6, IC = 40 mg/L, contact time = 5 min, T = 298 K, | As the pH increased, the adsorption capacity decreased because of deprotonating; both the negatively charged function groups and adsorbates repel each other | [289] |
| HNO3-modified MWCNTs | Bisphenol-A | 0.59 mmol/g | 153.79 | - | pH 6, IC = 40 mg/L, contact time = 30 min, T = 298 K, | ||
| SOCl2/NH4OH-modified CNT | Bisphenol A | 69.93 | 94.8 | - | pH 6.5, IC = 10 mg/L, AL = 0.125 g/L, T = 280 K, | Adsorption efficiency increased with the initial concentrations | [290] |
| NH3-treated MWCNTs | Chlorophenols (CP) | 110.3 | 195 | - | pH 3.8, T = 298 K, | The adsorption capacity increased due to higher pores size, π–π interactions, and hydrophobicity of nanocomposite Effective nanomaterial with smaller equilibrium time | [291] |
| HNO3 and KMnO4-Functionalized MWCNTs | Phenol | 76.92 | - | 88 | IC = 500 mg/L, Agitation speed = 200 rpm, T = 298 K | Adsorption capacity can be greatly affected by pH and adsorbent mass | [292] |
| Oxidized SWNTs | p-Nitrophenol (PNP) | 206 | - | 97.9 | IC = 0.01 mg/mL, agitation time = 30 min T = 293 K | Open ends of nanotubes, higher surface area, and the functional groups (hydroxyl, carbonyl, and carboxyl) were responsible for higher adsorption | [293] |
| Nitrogen-doped carbon nanotubes (CNx) | Phenol | 0.16 mmol/g | 102 | - | pH 7, AL = 0.6mmol/L, 298 K, | π–π interaction occurred between the functional groups and phenol; more oxidized CNTs adsorbed less phenol | [294] |
| MWCNT-COOH | Phenol | 0.15 mmol/g | - | - | IC = 0.417 mg/L, AL = 10 mg, T = 293 K | Higher adsorption of CP than phenol resulted because of the different solubility of these contaminants | [295] |
| 3-Chlorophenol (CP) | 0.37 mmol/g | - | 95 | IC = 1.25 mg/L, AL = 10 mg, T = 293 K | |||
| Acid-functionalized MWCNT (MWCNT-COOH) | 2-Nitrophenol | 256.41 | 197.83 | - | pH 5.5, IC = 45 mg/L, T = 298 K, | Excellent adsorbent due to strong interactions between 2–Nitrophenol and surface functional groups | [296] |
6.3. Removal of Microorganisms
| Contaminants | Adsorbents | RE | Removal Mechanism | Comments | Ref | ||
|---|---|---|---|---|---|---|---|
| Types | AL | IC | |||||
| Escherichia coli (E. coli) | Silver-doped CNT membrane | - | 1 × 106 CFU/mL | 100% | - | All the bacteria were inactivated by membrane with 10% silver loadings in 60 min only | [312] |
| Silver-nanoparticle-loaded CNTs | 2.5 µg/mL | 106 CFU/mL | 89% | - | Effectively inactivate the pathogen from wastewater effluents, resistance toward bacterial adhesion | [313] | |
| Chitosan/CNT nanocomposites | 2 wt% | 1.5 × 108 to 5.0 × 108 CFU/mL | 2.89 log reduction | Physical interaction and surface complexation | Higher antimicrobial activity at the low contact time (10 min) and low concentration (1%) | [314] | |
| Acidic-conditioned MWCNTs | 200 µg/mL | 106 to 109 CFU/mL | - | Steric obstruction | Inactivation of pathogens was due to both MWCNT functionalization and nutrition level | [315] | |
| 1-octadecanol-functionalized MWCNTS | 0.2 g/100 mL | 3.5 × 107 CFU/mL | 100% | Polarization | The interaction of microwaves with f-CNTs is an innovative approach that has the potential to be employed for water disinfection | [316] | |
| Staphylococcusaureus | CNT–Ag nanohybrid | 2.5 µg/mL | 106 CFU/mL | 100% | - | Effectively inactivate the pathogen from wastewater effluents, resistance toward bacterial adhesion | [313] |
| Chitosan/CNTs nanocomposites | 2 wt% | 1.5 × 108 to 5.0 × 108 CFU/mL | 4.9 log reduction | Physical interaction and surface complexation | Higher antimicrobial activity at the low contact time (10 min) and low concentration (1%) | [314] | |
| Aspergillus flavus | Chitosan/CNTs nanocomposites | 2 wt% | 1.5 × 108 to 5.0 × 108 CFU/mL | 5.5 log reduction | Physical interaction and surface complexation | Higher antimicrobial activity at the low contact time (10 min) and low concentration (1%) | [314] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, I.; Li, J.; Peng, C.; Qasim, M.; Khan, Z.M.; Naz, I.; Sultan, M.; Rauf, M.; Iqbal, W.; Sharif, H.M.A. 3-Dimensional membrane capsules: Synthesis modulations for the remediation of environmental pollutants—A critical review. Crit. Rev. Environ. Sci. Technol. 2020, 1–62. [Google Scholar] [CrossRef]
- Usman, M.; Waseem, M.; Mani, N.; Andiego, N. Optimization of soil aquifer treatment by chemical oxidation with hydrogen peroxide addition. Pollution 2018, 4, 369–379. [Google Scholar] [CrossRef]
- Usman, M. New Applications of Fine-Grained Iron Oxyhydroxides as Cost-Effective Arsenic Adsorbents in Water Treatment. Ph.D. Thesis, Technische Universität Hamburg, Hamburg, Germany, 2020. [Google Scholar] [CrossRef]
- Islam, T.; Peng, C.; Ali, I.; Li, J.; Khan, Z.M.; Sultan, M.; Naz, I. Synthesis of rice husk-derived magnetic biochar through Liquefaction to adsorb anionic and cationic dyes from aqueous solutions. Arab. J. Sci. Eng. 2021, 46, 233–246. [Google Scholar] [CrossRef]
- Khan, S.U.; Farooqi, I.H.; Usman, M.; Basheer, F. Energy efficient rapid removal of arsenic in an electrocoagulation reactor with hybrid Fe/Al electrodes: Process optimization using CCD and kinetic modeling. Water 2020, 12, 2876. [Google Scholar] [CrossRef]
- Usman, M.; Katsoyiannis, I.; Rodrigues, J.H.; Ernst, M. Arsenate removal from drinking water using by-products from conventional iron oxyhydroxides production as adsorbents coupled with submerged microfiltration unit. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amjed, M.A.; Peng, C.; Dai, M.; Chang, Q.; Ali, I.; Sultan, M.; Naz, I.; Farooq, M.Z.; Kashif, M. Recent updates on the solar-assisted biochar production and potential usage for water treatment. Fresenius Environ. Bull. 2020, 29, 5616–5632. [Google Scholar]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.M.A.; Khan, Z.M.; Sultan, M.; Niaz, Y.; Mahmood, M.H.; Shoaib, M.; Shakoor, A.; Ahmad, M. Performance evaluation of trickling filter-based wastewater treatment system utilizing cotton sticks as filter media. Polish J. Environ. Stud. 2017, 26, 1955–1962. [Google Scholar] [CrossRef]
- Khan, Z.M.; Kanwar, R.M.A.; Farid, H.U.; Sultan, M.; Arsalan, M.; Ahmad, M.; Shakoor, A.; Aslam, M.M.A. Wastewater evaluation for multan, pakistan: Characterization and agricultural reuse. Polish J. Environ. Stud. 2019, 28, 2159–2174. [Google Scholar] [CrossRef]
- Aslam, M.M.A.; Den, W.; Kuo, H.W. Encapsulated chitosan-modified magnetic carbon nanotubes for aqueous-phase CrVI uptake. J. Water Process Eng. 2021, 40, 101793. [Google Scholar] [CrossRef]
- Den, W.; Wang, C.J. Removal of silica from brackish water by electrocoagulation pretreatment to prevent fouling of reverse osmosis membranes. Sep. Purif. Technol. 2008, 59, 318–325. [Google Scholar] [CrossRef]
- Su, Y.N.; Lin, W.S.; Hou, C.H.; Den, W. Performance of integrated membrane filtration and electrodialysis processes for copper recovery from wafer polishing wastewater. J. Water Process Eng. 2014, 4, 149–158. [Google Scholar] [CrossRef]
- Ali, I.; Peng, C.; Khan, Z.M.; Naz, I.; Sultan, M.; Ali, M.; Abbasi, I.A.; Islam, T.; Ye, T. Overview of microbes based fabricated biogenic nanoparticles for water and wastewater treatment. J. Environ. Manag. 2019, 230, 128–150. [Google Scholar] [CrossRef]
- Ali, I.; Peng, C.; Khan, Z.M.; Naz, I.; Sultan, M. An overview of heavy metal removal from wastewater using magnetotactic bacteria. J. Chem. Technol. Biotechnol. 2018, 93, 2817–2832. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Saleh, T.A. Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res. 2011, 45, 2207–2212. [Google Scholar] [CrossRef]
- Usman, M.; Belkasmi, A.I.; Katsoyiannis, I.A.; Ernst, M. Pre-deposited dynamic membrane adsorber formed of microscale conventional iron oxide-based adsorbents to remove arsenic from water: Application study and mathematical modeling. J. Chem. Technol. Biotechnol. 2021. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Usman, M.; Zarebanadkouki, M.; Waseem, M.; Katsoyiannis, I.A.; Ernst, M. Mathematical modeling of arsenic(V) adsorption onto iron oxyhydroxides in an adsorption-submerged membrane hybrid system. J. Hazard. Mater. 2020, 400, 123221. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Matis, K.A. Nanoadsorbents for pollutants removal: A review. J. Mol. Liq. 2015, 203, 159–168. [Google Scholar] [CrossRef]
- Trujillo-Reyes, J.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Supported and unsupported nanomaterials for water and soil remediation: Are they a useful solution for worldwide pollution? J. Hazard. Mater. 2014, 280, 487–503. [Google Scholar] [CrossRef]
- Sharma, V.K.; McDonald, T.J.; Kim, H.; Garg, V.K. Magnetic graphene–carbon nanotube iron nanocomposites as adsorbents and antibacterial agents for water purification. Adv. Colloid Interface Sci. 2015, 225, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Alvarez, P.J.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Katsoyiannis, I.; Mitrakas, M.; Zouboulis, A.; Ernst, M. Performance evaluation of small sized powdered ferric hydroxide as arsenic adsorbent. Water 2018, 10, 957. [Google Scholar] [CrossRef] [Green Version]
- Goh, K.; Karahan, H.E.; Wei, L.; Bae, T.-H.; Fane, A.G.; Wang, R.; Chen, Y. Carbon nanomaterials for advancing separation membranes: A strategic perspective. Carbon N. Y. 2016, 109, 694–710. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Hilal, N. Nano-enabled membranes technology: Sustainable and revolutionary solutions for membrane desalination? Desalination 2016, 380, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Goh, P.S.; Matsuura, T.; Ismail, A.F.; Hilal, N. Recent trends in membranes and membrane processes for desalination. Desalination 2016, 391, 43–60. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Saleh, T.A. Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J. Hazard. Mater. 2011, 185, 17–23. [Google Scholar] [CrossRef]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef]
- Pendergast, M.M.; Hoek, E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946. [Google Scholar] [CrossRef] [Green Version]
- Bethi, B.; Sonawane, S.H.; Bhanvase, B.A.; Gumfekar, S.P. Nanomaterials-based advanced oxidation processes for wastewater treatment: A review. Chem. Eng. Process. Process Intensif. 2016, 109, 178–189. [Google Scholar] [CrossRef]
- Holmes, A.B.; Gu, F.X. Emerging nanomaterials for the application of selenium removal for wastewater treatment. Environ. Sci. Nano 2016, 3, 982–996. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, S.; Liu, Z. Progress and challenges of carbon nanotube membrane in water treatment. Crit. Rev. Environ. Sci. Technol. 2016, 46, 999–1046. [Google Scholar] [CrossRef]
- Olivera, S.; Muralidhara, H.B.; Venkatesh, K.; Guna, V.K.; Gopalakrishna, K.; Kumar, K.Y. Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: A review. Carbohydr. Polym. 2016, 153, 600–618. [Google Scholar] [CrossRef]
- Ong, C.S.; Goh, P.S.; Lau, W.J.; Misdan, N.; Ismail, A.F. Nanomaterials for biofouling and scaling mitigation of thin film composite membrane: A review. Desalination 2016, 393, 2–15. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P.; Ok, Y.S. Review on nano zerovalent iron (nZVI): From synthesis to environmental applications. Chem. Eng. J. 2016, 287, 618–632. [Google Scholar] [CrossRef]
- Monthioux, M.; Kuznetsov, V.L. Who should be given the credit for the discovery of carbon nanotubes? Carbon N. Y. 2006, 44, 1621–1623. [Google Scholar] [CrossRef]
- Radushkevich, L.V.; Lukyanovich, V.M. About the structure of carbon formed by thermal decomposition of carbon monoxide on iron substrate. J. Phys. Chem. 1952, 26, 88–95. [Google Scholar]
- Oberlin, A.; Endo, M.; Koyama, T. Filamentous growth of carbon through benzene decomposition. J. Cryst. Growth 1976, 32, 335–349. [Google Scholar] [CrossRef]
- Abrahamson, J.; Wiles, P.G.; Rhoades, B.L. Structure of carbon fibres found on carbon arc anodes. Carbon N. Y. 1999, 37, 1873–1874. [Google Scholar] [CrossRef]
- Hirlekar, R.; Yamagar, M.; Garse, H.; Vij, M.; Kadam, V.; Vidyapeeth, B. Carbon nanotubes and its applications: A review. Asian J. Pharm. Clin. Res. 2009, 2, 17–27. [Google Scholar]
- Tennent, H.G.; Barber, J.J.; Hoch, R. Carbon Fibrils, Method for Producing Same and Compositions Containing Same. U.S. Patent 4,663,230, 5 May 1987. [Google Scholar]
- Bethune, D.S.; Kiang, C.H.; de Vries, M.S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Nakayama-Ratchford, N.; Dai, H. Supramolecular Chemistry on Water-Soluble Carbon Nanotubes for Drug Loading and Delivery. ACS Nano 2007, 1, 50–56. [Google Scholar] [CrossRef]
- Singh, B.G.P.; Baburao, C.; Pispati, V.; Pathipati, H.; Muthy, N.; Prassana, S.R.V.; Rathode, B.G. Carbon nanotubes. A novel drug delivery system. Int. J. Res. Pharm. Chem. 2012, 2, 523–532. [Google Scholar]
- Lam, C.; James, J.T.; McCluskey, R.; Arepalli, S.; Hunter, R.L. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit. Rev. Toxicol. 2006, 36, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Bekyarova, E.; Ni, Y.; Malarkey, E.B.; Montana, V.; McWilliams, J.L.; Haddon, R.C.; Parpura, V. Applications of Carbon Nanotubes in Biotechnology and Biomedicine. J. Biomed. Nanotechnol. 2005, 1, 3–17. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Pham-Huy, L.A.; Dramou, P.; Xiao, D.; Zuo, P.; Pham-Huy, C. Carbon Nanotubes: Applications in Pharmacy and Medicine. Biomed. Res. Int. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Reilly, R.M. Carbon Nanotubes: Potential Benefits and Risks of Nanotechnology in Nuclear Medicine. J. Nucl. Med. 2007, 48, 1039–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Mai, Y.; Zhou, X. Dispersion and alignment of carbon nanotubes in polymer matrix: A review. Mater. Sci. Eng. R Rep. 2005, 49, 89–112. [Google Scholar] [CrossRef]
- Ihsanullah, A.A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Chemically Functionalized Carbon Nanotubes. Small 2005, 1, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Monea, B.F.; Ionete, E.I.; Spiridon, S.I.; Ion-Ebrasu, D.; Petre, E. Carbon Nanotubes and Carbon Nanotube Structures Used for Temperature Measurement. Sensors 2019, 19, 2464. [Google Scholar] [CrossRef] [Green Version]
- Digge, M.; Moon, R.; Gattani, S. Application of Carbon Nanotubes in Drug Delivery: A Review. Int. J. PharmTech Res. 2011, 4, 839–847. [Google Scholar]
- Kateb, B.; Yamamoto, V.; Alizadeh, D.; Zhang, L.; Manohara, H.M.; Bronikowski, M.J.; Badie, B. Multi-walled Carbon Nanotube (MWCNT) Synthesis, Preparation, Labeling, and Functionalization. In Immunotherapy of Cancer; Humana Press: Totowa, NJ, USA, 2010; pp. 307–317. [Google Scholar]
- Liao, H.; Paratala, B.; Sitharaman, B.; Wang, Y. Applications of Carbon Nanotubes in Biomedical Studies. In Biomedical Nanotechnology; Humana Press: Totowa, NJ, USA, 2011; pp. 223–241. [Google Scholar]
- Usui, Y.; Haniu, H.; Tsuruoka, S.; Saito, N. Carbon nanotubes innovate on medical technology. Med. Chem 2012, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Yang, F.; Hu, J.; Long, J.; Wang, C.; Fu, D.; Ni, Q. Hydrophilic multi-walled carbon nanotubes decorated with magnetite nanoparticles as lymphatic targeted drug delivery vehicles. Chem. Commun. 2009, 4447. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, Y.; Yan, B. Functionalized carbon nanotubes for potential medicinal applications. Drug Discov. Today 2010, 15, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Cassell, A.M.; Raymakers, J.A.; Kong, J.; Dai, H. Large Scale CVD Synthesis of Single-Walled Carbon Nanotubes. J. Phys. Chem. B 1999, 103, 6484–6492. [Google Scholar] [CrossRef]
- Sinnott, S.B.; Andrews, R.; Qian, D.; Rao, A.M.; Mao, Z.; Dickey, E.C.; Derbyshire, F. Model of carbon nanotube growth through chemical vapor deposition. Chem. Phys. Lett. 1999, 315, 25–30. [Google Scholar] [CrossRef]
- Vinciguerra, V.; Buonocore, F.; Panzera, G.; Occhipinti, L. Growth mechanisms in chemical vapour deposited carbon nanotubes. Nanotechnology 2003, 14, 655. [Google Scholar] [CrossRef]
- Helveg, S.; López-Cartes, C.; Sehested, J.; Hansen, P.L.; Clausen, B.S.; Rostrup-Nielsen, J.R.; Abild-Pedersen, F.; Nørskov, J.K. Atomic-scale imaging of carbon nanofibre growth. Nature 2004, 427, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Maser, W.K.; Benito, A.M.; Martınez, M.T. Production of carbon nanotubes: The light approach. Carbon N. Y. 2002, 40, 1685–1695. [Google Scholar] [CrossRef]
- Kingston, C.T.; Simard, B. Fabrication of Carbon Nanotubes. Anal. Lett. 2003, 36, 3119–3145. [Google Scholar] [CrossRef]
- Hutchison, J.L.; Kiselev, N.A.; Krinichnaya, E.P.; Krestinin, A.V.; Loutfy, R.O.; Morawsky, A.P.; Muradyan, V.E.; Obraztsova, E.D.; Sloan, J.; Terekhov, S.V.; et al. Double-walled carbon nanotubes fabricated by a hydrogen arc discharge method. Carbon N. Y. 2001, 39, 761–770. [Google Scholar] [CrossRef]
- Shi, Z.; Lian, Y.; Zhou, X.; Gu, Z.; Zhang, Y.; Iijima, S.; Zhou, L.; Yue, K.T.; Zhang, S. Mass-production of single-wall carbon nanotubes by arc discharge method11This work was supported by the National Natural Science Foundation of China, No. 29671030. Carbon N. Y. 1999, 37, 1449–1453. [Google Scholar] [CrossRef]
- Sano, N.; Wang, H.; Chhowalla, M.; Alexandrou, I.; Amaratunga, G.A.J. Synthesis of carbon “onions” in water. Nature 2001, 414, 506–507. [Google Scholar] [CrossRef]
- Li, H.; Guan, L.; Shi, Z.; Gu, Z. Direct Synthesis of High Purity Single-Walled Carbon Nanotube Fibers by Arc Discharge. J. Phys. Chem. B 2004, 108, 4573–4575. [Google Scholar] [CrossRef]
- Imasaka, K.; Kanatake, Y.; Ohshiro, Y.; Suehiro, J.; Hara, M. Production of carbon nanoonions and nanotubes using an intermittent arc discharge in water. Thin Solid Films 2006, 506–507, 250–254. [Google Scholar] [CrossRef]
- Sagara, T.; Kurumi, S.; Suzuki, K. Growth of linear Ni-filled carbon nanotubes by local arc discharge in liquid ethanol. Appl. Surf. Sci. 2014, 292, 39–43. [Google Scholar] [CrossRef]
- Ben Belgacem, A.; Hinkov, I.; Yahia, S.B.; Brinza, O.; Farhat, S. Arc discharge boron nitrogen doping of carbon nanotubes. Mater. Today Commun. 2016, 8, 183–195. [Google Scholar] [CrossRef]
- Berkmans, J.; Jagannatham, M.; Reddy, R.; Haridoss, P. Synthesis of thin bundled single walled carbon nanotubes and nanohorn hybrids by arc discharge technique in open air atmosphere. Diam. Relat. Mater. 2015, 55, 12–15. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Y. Carbon nanomaterials synthesized by arc discharge hot plasma. Carbon N. Y. 2015, 83, 90–99. [Google Scholar] [CrossRef]
- Guo, T.; Nikolaev, P.; Thess, A.; Colbert, D.T.; Smalley, R.E. Catalytic growth of single-walled manotubes by laser vaporization. Chem. Phys. Lett. 1995, 243, 49–54. [Google Scholar] [CrossRef]
- Guo, T.; Nikolaev, P.; Rinzler, A.G.; Tomanek, D.; Colbert, D.T.; Smalley, R.E. Self-assembly of tubular fullerenes. J. Phys. Chem. 1995, 99, 10694–10697. [Google Scholar] [CrossRef]
- Nagy, J.B.; Bister, G.; Fonseca, A.; Méhn, D.; Kónya, Z.; Kiricsi, I.; Horváth, Z.E.; Biró, L.P. On the Growth Mechanism of Single-Walled Carbon Nanotubes by Catalytic Carbon Vapor Deposition on Supported Metal Catalysts. J. Nanosci. Nanotechnol. 2004, 4, 326–345. [Google Scholar] [CrossRef]
- Bandaru, P.R. Electrical Properties and Applications of Carbon Nanotube Structures. J. Nanosci. Nanotechnol. 2007, 7, 1239–1267. [Google Scholar] [CrossRef]
- Thess, A.; Lee, R.; Nikolaev, P.; Dai, H.; Petit, P.; Robert, J.; Xu, C.; Lee, Y.H.; Kim, S.G.; Rinzler, A.G.; et al. Crystalline Ropes of Metallic Carbon Nanotubes. Science 1996, 273, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Journet, C.; Bernier, P. Production of carbon nanotubes. Appl. Phys. A Mater. Sci. Process. 1998, 67, 1–9. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Hiura, H.; Fujita, J.; Ochiai, Y.; Matsui, S.; Tanigaki, K. Patterns in the bulk growth of carbon nanotubes. Chem. Phys. Lett. 1993, 209, 83–90. [Google Scholar] [CrossRef]
- José-Yacamán, M.; Miki-Yoshida, M.; Rendón, L.; Santiesteban, J.G. Catalytic growth of carbon microtubules with fullerene structure. Appl. Phys. Lett. 1993, 62, 202–204. [Google Scholar] [CrossRef]
- Ren, Z.F. Synthesis of Large Arrays of Well-Aligned Carbon Nanotubes on Glass. Science 1998, 282, 1105–1107. [Google Scholar] [CrossRef] [Green Version]
- Oliver, J. Global Markets and Technologies for Carbon Nanotubes: NAN024F BCC Research; BCC Publishing: Wellesley, MA, USA, 2015. [Google Scholar]
- Kumar, S.; Rani, R.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Carbon nanotubes: A novel material for multifaceted applications in human healthcare. Chem. Soc. Rev. 2017, 46, 158–196. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Franceschi, W.; Menezes, B.R.C.; Biagioni, A.F.; Coutinho, A.R.; Cividanes, L.S. Synthesis, Characterization, and Applications of Carbon Nanotubes. In Carbon-Based Nanofillers and Their Rubber Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–45. [Google Scholar]
- Donaldson, K.; Aitken, R.; Tran, L.; Stone, V.; Duffin, R.; Forrest, G.; Alexander, A. Carbon Nanotubes: A Review of Their Properties in Relation to Pulmonary Toxicology and Workplace Safety. Toxicol. Sci. 2006, 92, 5–22. [Google Scholar] [CrossRef] [Green Version]
- Ando, Y.; Zhao, X.; Sugai, T.; Kumar, M. Growing carbon nanotubes. Mater. Today 2004, 7, 22–29. [Google Scholar] [CrossRef]
- Muataz, A.A.; Fakhrul-Razi, A.; Dayang, B.A.R.; El-Sadig, M.; Chuah, G.T.; Maan, F.A.; Sunny, I.; Faizah, Y.; Abdul Hamid, M.; Halim, M. Production of vapor growth carbon fiber (vgcf) by using cvd. In Proceedings of the 17th Symposium of Malaysian Chemical Engineers (SOMChE 2003) “Role of Chemical Engineers for Sustainability of Small Medium Industries (SMI)”, Penang, Malaysia, 29–30 December 2003; pp. 596–602. [Google Scholar]
- Cao, Z.; Sun, Z.; Guo, P.; Chen, Y. Effect of acetylene flow rate on morphology and structure of carbon nanotube thick films grown by thermal chemical vapor deposition. Front. Mater. Sci. China 2007, 1, 92–96. [Google Scholar] [CrossRef]
- Fonseca, A.; Hernadi, K.; Piedigrosso, P.; Colomer, J.-F.; Mukhopadhyay, K.; Doome, R.; Lazarescu, S.; Biro, L.P.; Lambin, P.; Thiry, P.A.; et al. Synthesis of single- and multi-wall carbon nanotubes over supported catalysts. Appl. Phys. A Mater. Sci. Process. 1998, 67, 11–22. [Google Scholar] [CrossRef]
- Zheng, L.X.; O’Connell, M.J.; Doorn, S.K.; Liao, X.Z.; Zhao, Y.H.; Akhadov, E.A.; Hoffbauer, M.A.; Roop, B.J.; Jia, Q.X.; Dye, R.C.; et al. Ultralong single-wall carbon nanotubes. Nat. Mater. 2004, 3, 673–676. [Google Scholar] [CrossRef]
- Kim, N.S.; Lee, Y.T.; Park, J.; Han, J.B.; Choi, Y.S.; Choi, S.Y.; Choo, J.; Lee, G.H. Vertically Aligned Carbon Nanotubes Grown by Pyrolysis of Iron, Cobalt, and Nickel Phthalocyanines. J. Phys. Chem. B 2003, 107, 9249–9255. [Google Scholar] [CrossRef]
- Bustero, I.; Ainara, G.; Isabel, O.; Roberto, M.; Inés, R.; Amaya, A. Control of the Properties of Carbon Nanotubes Synthesized by CVD for Application in Electrochemical Biosensors. Microchim. Acta 2006, 152, 239–247. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Ghanem, M.A.; Khairy, M.; Naguib, E.; Alotaibi, N.H. Zinc oxide incorporated carbon nanotubes or graphene oxide nanohybrids for enhanced sonophotocatalytic degradation of methylene blue dye. Appl. Surf. Sci. 2019, 487, 539–549. [Google Scholar] [CrossRef]
- Kong, J.; Cassell, A.M.; Dai, H. Chemical vapor deposition of methane for single-walled carbon nanotubes. Chem. Phys. Lett. 1998, 292, 567–574. [Google Scholar] [CrossRef]
- Pérez-Cabero, M.; Monzón, A.; Rodrıguez-Ramos, I.; Guerrero-Ruız, A. Syntheses of CNTs over several iron-supported catalysts: Influence of the metallic precursors. Catal. Today 2004, 93–95, 681–687. [Google Scholar] [CrossRef]
- Zhu, S.; Su, C.-H.; Lehoczky, S.L.; Muntele, I.; Ila, D. Carbon nanotube growth on carbon fibers. Diam. Relat. Mater. 2003, 12, 1825–1828. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Miser, D.E.; Chan, W.G.; Hajaligol, M.R. Characterization of multiwalled carbon nanotubes prepared by carbon arc cathode deposit. Mater. Chem. Phys. 2003, 82, 638–647. [Google Scholar] [CrossRef]
- Collins, P.G.; Avouris, P. Nanotubes for electronics. Sci. Am. 2000, 283, 62–69. [Google Scholar] [CrossRef]
- Colomer, J.-F.; Stephan, C.; Lefrant, S.; Van Tendeloo, G.; Willems, I.; Kónya, Z.; Fonseca, A.; Laurent, C.; Nagy, J. Large-scale synthesis of single-wall carbon nanotubes by catalytic chemical vapor deposition (CCVD) method. Chem. Phys. Lett. 2000, 317, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Ebbesen, T.W.; Ajayan, P.M. Large-scale synthesis of carbon nanotubes. Nature 1992, 358, 220–222. [Google Scholar] [CrossRef]
- Ren, Z.F.; Huang, Z.P.; Wang, D.Z.; Wen, J.G.; Xu, J.W.; Wang, J.H.; Calvet, L.E.; Chen, J.; Klemic, J.F.; Reed, M.A. Growth of a single freestanding multiwall carbon nanotube on each nanonickel dot. Appl. Phys. Lett. 1999, 75, 1086–1088. [Google Scholar] [CrossRef] [Green Version]
- Yudasaka, M.; Kikuchi, R.; Matsui, T.; Ohki, Y.; Yoshimura, S.; Ota, E. Specific conditions for Ni catalyzed carbon nanotube growth by chemical vapor deposition. Appl. Phys. Lett. 1995, 67, 2477–2479. [Google Scholar] [CrossRef]
- Yudasaka, M.; Kikuchi, R.; Ohki, Y.; Ota, E.; Yoshimura, S. Behavior of Ni in carbon nanotube nucleation. Appl. Phys. Lett. 1997, 70, 1817–1818. [Google Scholar] [CrossRef]
- Eklund, P.C.; Pradhan, B.K.; Kim, U.J.; Xiong, Q.; Fischer, J.E.; Friedman, A.D.; Holloway, B.C.; Jordan, K.; Smith, M.W. Large-Scale Production of Single-Walled Carbon Nanotubes Using Ultrafast Pulses from a Free Electron Laser. Nano Lett. 2002, 2, 561–566. [Google Scholar] [CrossRef]
- Maser, W.K.; Muñoz, E.; Benito, A.M.; Martınez, M.T.; de la Fuente, G.F.; Maniette, Y.; Anglaret, E.; Sauvajol, J.-L. Production of high-density single-walled nanotube material by a simple laser-ablation method. Chem. Phys. Lett. 1998, 292, 587–593. [Google Scholar] [CrossRef]
- Bolshakov, A.P.; Uglov, S.A.; Saveliev, A.V.; Konov, V.I.; Gorbunov, A.A.; Pompe, W.; Graff, A. A novel CW laser–powder method of carbon single-wall nanotubes production. Diam. Relat. Mater. 2002, 11, 927–930. [Google Scholar] [CrossRef]
- Scott, C.D.; Arepalli, S.; Nikolaev, P.; Smalley, R.E. Growth mechanisms for single-wall carbon nanotubes in a laser-ablation process. Appl. Phys. A Mater. Sci. Process. 2001, 72, 573–580. [Google Scholar] [CrossRef]
- Aqel, A.; El-Nour, K.M.M.A.; Ammar, R.A.A.; Al-Warthan, A. Carbon nanotubes, science and technology part (I) structure, synthesis and characterisation. Arab. J. Chem. 2012, 5, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Gong, K.; Ci, L. Process for purification of carbon nanotubes. Carbon 2008, 46, 2003–2025. [Google Scholar]
- Hou, P.; Bai, S.; Yang, Q.; Liu, C.; Cheng, H. Multi-step purification of carbon nanotubes. Carbon N. Y. 2002, 40, 81–85. [Google Scholar] [CrossRef]
- Hajime, G.; Terumi, F.; Yoshiya, F.; Toshiyuki, O. Method of Purifying Single Wall Carbon Nanotubes from Metal Catalyst Impurities; JP Patent Filed & Issued; Honda Giken Kogyo Kabushiki Kaisha: Tokyo, Japan, 2002. [Google Scholar]
- Borowiak-Palen, E.; Pichler, T.; Liu, X.; Knupfer, M.; Graff, A.; Jost, O.; Pompe, W.; Kalenczuk, R.; Fink, J. Reduced diameter distribution of single-wall carbon nanotubes by selective oxidation. Chem. Phys. Lett. 2002, 363, 567–572. [Google Scholar] [CrossRef]
- Huang, S.; Dai, L. Plasma Etching for Purification and Controlled Opening of Aligned Carbon Nanotubes. J. Phys. Chem. B 2002, 106, 3543–3545. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Chen, C.-H.; Chiang, Y.-C.; Chen, S.-L. Circulating inclined fluidized beds with application for desiccant dehumidification systems. Appl. Energy 2016, 175, 199–211. [Google Scholar] [CrossRef]
- Harutyunyan, A.R.; Pradhan, B.K.; Chang, J.; Chen, G.; Eklund, P.C. Purification of Single-Wall Carbon Nanotubes by Selective Microwave Heating of Catalyst Particles. J. Phys. Chem. B 2002, 106, 8671–8675. [Google Scholar] [CrossRef]
- Farkas, E.; Elizabeth Anderson, M.; Chen, Z.; Rinzler, A.G. Length sorting cut single wall carbon nanotubes by high performance liquid chromatography. Chem. Phys. Lett. 2002, 363, 111–116. [Google Scholar] [CrossRef]
- Chiang, I.W.; Brinson, B.E.; Huang, A.Y.; Willis, P.A.; Bronikowski, M.J.; Margrave, J.L.; Smalley, R.E.; Hauge, R.H. Purification and Characterization of Single-Wall Carbon Nanotubes (SWNTs) Obtained from the Gas-Phase Decomposition of CO (HiPco Process). J. Phys. Chem. B 2001, 105, 8297–8301. [Google Scholar] [CrossRef] [Green Version]
- Chiang, I.W.; Brinson, B.E.; Smalley, R.E.; Margrave, J.L.; Hauge, R.H. Purification and Characterization of Single-Wall Carbon Nanotubes. J. Phys. Chem. B 2001, 105, 1157–1161. [Google Scholar] [CrossRef]
- Kajiura, H.; Tsutsui, S.; Huang, H.; Murakami, Y. High-quality single-walled carbon nanotubes from arc-produced soot. Chem. Phys. Lett. 2002, 364, 586–592. [Google Scholar] [CrossRef]
- Moon, J.-M.; An, K.H.; Lee, Y.H.; Park, Y.S.; Bae, D.J.; Park, G.-S. High-Yield Purification Process of Singlewalled Carbon Nanotubes. J. Phys. Chem. B 2001, 105, 5677–5681. [Google Scholar] [CrossRef]
- Doi, M.; Ikuga, Y.; Kimura, T.; Mitsuzuka, H. Laminated Sheet and Diaper and Article for Sanitary Use. JP Patent JPH10713A, Application JP8116796A, 10 May 1996. [Google Scholar]
- Bandow, S.; Rao, A.M.; Williams, K.A.; Thess, A.; Smalley, R.E.; Eklund, P.C. Purification of Single-Wall Carbon Nanotubes by Microfiltration. J. Phys. Chem. B 1997, 101, 8839–8842. [Google Scholar] [CrossRef]
- Sajid, M.I.; Jamshaid, U.; Jamshaid, T.; Zafar, N.; Fessi, H.; Elaissari, A. Carbon nanotubes from synthesis to in vivo biomedical applications. Int. J. Pharm. 2016, 501, 278–299. [Google Scholar] [CrossRef]
- Hou, P.-X.; Liu, C.; Cheng, H.-M. Purification of carbon nanotubes. Carbon N. Y. 2008, 46, 2003–2025. [Google Scholar] [CrossRef]
- Sato, Y.; Ogawa, T.; Motomiya, K.; Shinoda, K.; Jeyadevan, B.; Tohji, K.; Kasuya, A.; Nishina, Y. Purification of MWNTs Combining Wet Grinding, Hydrothermal Treatment, and Oxidation. J. Phys. Chem. B 2001, 105, 3387–3392. [Google Scholar] [CrossRef]
- Das, R.; Abd Hamid, S.B.; Ali, M.E.; Ismail, A.F.; Annuar, M.S.M.; Ramakrishna, S. Multifunctional carbon nanotubes in water treatment: The present, past and future. Desalination 2014, 354, 160–179. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Carbon Nanotubes as Superior Sorbent for Dioxin Removal. J. Am. Chem. Soc. 2001, 123, 2058–2059. [Google Scholar] [CrossRef]
- Jun, L.Y.; Mubarak, N.M.; Yee, M.J.; Yon, L.S.; Bing, C.H.; Khalid, M.; Abdullah, E.C. An overview of functionalised carbon nanomaterial for organic pollutant removal. J. Ind. Eng. Chem. 2018, 67, 175–186. [Google Scholar] [CrossRef]
- Ali, S.; Rehman, S.A.U.; Luan, H.-Y.; Farid, M.U.; Huang, H. Challenges and opportunities in functional carbon nanotubes for membrane-based water treatment and desalination. Sci. Total Environ. 2019, 646, 1126–1139. [Google Scholar] [CrossRef]
- Ihsanullah; Al Amer, A.M.; Laoui, T.; Abbas, A.; Al-Aqeeli, N.; Patel, F.; Khraisheh, M.; Atieh, M.A.; Hilal, N. Fabrication and antifouling behaviour of a carbon nanotube membrane. Mater. Des. 2016, 89, 549–558. [Google Scholar] [CrossRef] [Green Version]
- Ihsanullah; Al-Khaldi, F.A.; Abu-Sharkh, B.; Abulkibash, A.M.; Qureshi, M.I.; Laoui, T.; Atieh, M.A. Effect of acid modification on adsorption of hexavalent chromium (Cr(VI)) from aqueous solution by activated carbon and carbon nanotubes. Desalin. Water Treat. 2016, 57, 7232–7244. [Google Scholar] [CrossRef]
- Garzia Trulli, M.; Sardella, E.; Palumbo, F.; Palazzo, G.; Giannossa, L.C.; Mangone, A.; Comparelli, R.; Musso, S.; Favia, P. Towards highly stable aqueous dispersions of multi-walled carbon nanotubes: The effect of oxygen plasma functionalization. J. Colloid Interface Sci. 2017, 491, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Bourlinos, A.; Gournis, D.; Tsoufis, T.; Trapalis, C.; Mateo-Alonso, A.; Prato, M. Multipurpose Organically Modified Carbon Nanotubes: From Functionalization to Nanotube Composites. J. Am. Chem. Soc. 2008, 130, 8733–8740. [Google Scholar] [CrossRef] [PubMed]
- Bounos, G.; Andrikopoulos, K.S.; Moschopoulou, H.; Lainioti, G.C.; Roilo, D.; Checchetto, R.; Ioannides, T.; Kallitsis, J.K.; Voyiatzis, G.A. Enhancing water vapor permeability in mixed matrix polypropylene membranes through carbon nanotubes dispersion. J. Memb. Sci. 2017, 524, 576–584. [Google Scholar] [CrossRef]
- Oyetade, O.A.; Skelton, A.A.; Nyamori, V.O.; Jonnalagadda, S.B.; Martincigh, B.S. Experimental and DFT studies on the selective adsorption of Pb2+ and Zn2+ from aqueous solution by nitrogen-functionalized multiwalled carbon nanotubes. Sep. Purif. Technol. 2017, 188, 174–187. [Google Scholar] [CrossRef]
- Ali, I. New Generation Adsorbents for Water Treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef]
- Bahgat, M.; Farghali, A.A.; El Rouby, W.M.A.; Khedr, M.H. Synthesis and modification of multi-walled carbon nano-tubes (MWCNTs) for water treatment applications. J. Anal. Appl. Pyrolysis 2011, 92, 307–313. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.; Xu, H.; Liu, H.; Wang, M.; He, Y.; Pan, B. Nanomaterials-enabled water and wastewater treatment. Nanoimpact 2016, 3–4, 22–39. [Google Scholar] [CrossRef]
- Upadhyayula, V.K.K.; Deng, S.; Mitchell, M.C.; Smith, G.B. Application of carbon nanotube technology for removal of contaminants in drinking water: A review. Sci. Total Environ. 2009, 408, 1–13. [Google Scholar] [CrossRef]
- Rao, G.; Lu, C.; Su, F. Sorption of divalent metal ions from aqueous solution by carbon nanotubes: A review. Sep. Purif. Technol. 2007, 58, 224–231. [Google Scholar] [CrossRef]
- Ren, X.; Chen, C.; Nagatsu, M.; Wang, X. Carbon nanotubes as adsorbents in environmental pollution management: A review. Chem. Eng. J. 2011, 170, 395–410. [Google Scholar] [CrossRef]
- Liang, J.; Li, L.; Chen, D.; Hajagos, T.; Ren, Z.; Chou, S.-Y.; Hu, W.; Pei, Q. Intrinsically stretchable and transparent thin-film transistors based on printable silver nanowires, carbon nanotubes and an elastomeric dielectric. Nat. Commun. 2015, 6, 7647. [Google Scholar] [CrossRef] [Green Version]
- Moghaddam, H.K.; Pakizeh, M. Experimental study on mercury ions removal from aqueous solution by MnO2/CNTs nanocomposite adsorbent. J. Ind. Eng. Chem. 2015, 21, 221–229. [Google Scholar] [CrossRef]
- Tang, W.-W.; Zeng, G.-M.; Gong, J.-L.; Liu, Y.; Wang, X.-Y.; Liu, Y.-Y.; Liu, Z.-F.; Chen, L.; Zhang, X.-R.; Tu, D.-Z. Simultaneous adsorption of atrazine and Cu (II) from wastewater by magnetic multi-walled carbon nanotube. Chem. Eng. J. 2012, 211–212, 470–478. [Google Scholar] [CrossRef]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon N. Y. 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- Hassellöv, M.; Readman, J.W.; Ranville, J.F.; Tiede, K. Nanoparticle analysis and characterization methodologies in environmental risk assessment of engineered nanoparticles. Ecotoxicology 2008, 17, 344–361. [Google Scholar] [CrossRef]
- Belin, T.; Epron, F. Characterization methods of carbon nanotubes: A review. Mater. Sci. Eng. B 2005, 119, 105–118. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Ren, Z.; Chou, T.-W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef] [Green Version]
- Herrero-Latorre, C.; Álvarez-Méndez, J.; Barciela-García, J.; García-Martín, S.; Peña-Crecente, R.M. Characterization of carbon nanotubes and analytical methods for their determination in environmental and biological samples: A review. Anal. Chim. Acta 2015, 853, 77–94. [Google Scholar] [CrossRef]
- Täschner, C.; Pácal, F.; Leonhardt, A.; Spatenka, P.; Bartsch, K.; Graff, A.; Kaltofen, R. Synthesis of aligned carbon nanotubes by DC plasma-enhanced hot filament CVD. Surf. Coat. Technol. 2003, 174–175, 81–87. [Google Scholar] [CrossRef]
- Xiao, L.; Ha, J.W.; Wei, L.; Wang, G.; Fang, N. Determining the Full Three-Dimensional Orientation of Single Anisotropic Nanoparticles by Differential Interference Contrast Microscopy. Angew. Chem. 2012, 124, 7854–7858. [Google Scholar] [CrossRef]
- Korneva, G.; Ye, H.; Gogotsi, Y.; Halverson, D.; Friedman, G.; Bradley, J.-C.; Kornev, K.G. Carbon Nanotubes Loaded with Magnetic Particles. Nano Lett. 2005, 5, 879–884. [Google Scholar] [CrossRef]
- Jia, C.L.; Mi, S.B.; Faley, M.; Poppe, U.; Schubert, J.; Urban, K. Oxygen octahedron reconstruction in the SrTiO3/LaAlO3 heterointerfaces investigated using aberration-corrected ultrahigh-resolution transmission electron microscopy. Phys. Rev. B 2009, 79, 081405. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Stading, M.; Wellner, N.; Parker, M.L.; Noel, T.R.; Mills, E.N.C.; Belton, P.S. Plasticization of a Protein-Based Film by Glycerol: A Spectroscopic, Mechanical, and Thermal Study. J. Agric. Food Chem. 2006, 54, 4611–4616. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Fu, K.; Lin, Y.; Huang, W. Functionalized Carbon Nanotubes: Properties and Applications. Acc. Chem. Res. 2002, 35, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Fallgren, P.H.; Morris, J.M.; Chen, Q. Removal of bacteria and viruses from waters using layered double hydroxide nanocomposites. Sci. Technol. Adv. Mater. 2007, 8, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Cao, Y.; Qin, B.; Zhong, G.; Zhang, J.; Yu, H.; Wang, H.; Peng, F. Highly efficient and acid-corrosion resistant nitrogen doped magnetic carbon nanotubes for the hexavalent chromium removal with subsequent reutilization. Chem. Eng. J. 2019, 361, 547–558. [Google Scholar] [CrossRef]
- Correa-Duarte, M.A.; Grzelczak, M.; Salgueiriño-Maceira, V.; Giersig, M.; Liz-Marzán, L.M.; Farle, M.; Sierazdki, K.; Diaz, R. Alignment of Carbon Nanotubes under Low Magnetic Fields through Attachment of Magnetic Nanoparticles. J. Phys. Chem. B 2005, 109, 19060–19063. [Google Scholar] [CrossRef]
- Rinzler, A.G.; Liu, J.; Dai, H.; Nikolaev, P.; Huffman, C.B.; Rodríguez-Macías, F.J.; Boul, P.J.; Lu, A.H.; Heymann, D.; Colbert, D.T.; et al. Large-scale purification of single-wall carbon nanotubes: Process, product, and characterization. Appl. Phys. A Mater. Sci. Process. 1998, 67, 29–37. [Google Scholar] [CrossRef]
- Gommes, C.; Blacher, S.; Masenelli-Varlot, K.; Bossuot, C.; McRae, E.; Fonseca, A.; Nagy, J.-B.; Pirard, J.-P. Image analysis characterization of multi-walled carbon nanotubes. Carbon N. Y. 2003, 41, 2561–2572. [Google Scholar] [CrossRef] [Green Version]
- Jimeno, A.; Goyanes, S.; Eceiza, A.; Kortaberria, G.; Mondragon, I.; Corcuera, M.A. Effects of Amine Molecular Structure on Carbon Nanotubes Functionalization. J. Nanosci. Nanotechnol. 2009, 9, 6222–6227. [Google Scholar] [CrossRef]
- Chai, S.-P.; Zein, S.H.S.; Mohamed, A.R. The effect of reduction temperature on Co-Mo/Al2O3 catalysts for carbon nanotubes formation. Appl. Catal. A Gen. 2007, 326, 173–179. [Google Scholar] [CrossRef]
- Kiang, C.-H.; Endo, M.; Ajayan, P.M.; Dresselhaus, G.; Dresselhaus, M.S. Size Effects in Carbon Nanotubes. Phys. Rev. Lett. 1998, 81, 1869–1872. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, C.; Chowdhuri, A.R.; Kumar, A.; Laha, D.; Garai, S.; Chakraborty, J.; Sahu, S.K. One pot synthesis of carbon dots decorated carboxymethyl cellulose- hydroxyapatite nanocomposite for drug delivery, tissue engineering and Fe3+ ion sensing. Carbohydr. Polym. 2018, 181, 710–718. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Bitter, J.L.; Howard Fairbrother, D. Chemical and structural characterization of carbon nanotube surfaces. Anal. Bioanal. Chem. 2010, 396, 1003–1014. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon N. Y. 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Liu, M.; Cowley, J.M. Structures of carbon nanotubes studied by HRTEM and nanodiffraction. Ultramicroscopy 1994, 53, 333–342. [Google Scholar] [CrossRef]
- Cao, A.; Zhang, X.; Xu, C.; Wei, B.; Wu, D. Tandem structure of aligned carbon nanotubes on Au and its solar thermal absorption. Sol. Energy Mater. Sol. Cells 2002, 70, 481–486. [Google Scholar] [CrossRef]
- Droppa, R.; Hammer, P.; Carvalho, A.C.; dos Santos, M.; Alvarez, F. Incorporation of nitrogen in carbon nanotubes. J. Non-Cryst. Solids 2002, 299–302, 874–879. [Google Scholar] [CrossRef]
- Chong, C.T.; Tan, W.H.; Lee, S.L.; Chong, W.W.F.; Lam, S.S.; Valera-Medina, A. Morphology and growth of carbon nanotubes catalytically synthesised by premixed hydrocarbon-rich flames. Mater. Chem. Phys. 2017, 197, 246–255. [Google Scholar] [CrossRef]
- Hammer, P.; Victoria, N.M.; Alvarez, F. Effects of increasing nitrogen concentration on the structure of carbon nitride films deposited by ion beam assisted deposition. J. Vac. Sci. Technol. A Vac. Surfaces Film. 2000, 18, 2277. [Google Scholar] [CrossRef]
- Pham-Huu, C.; Keller, N.; Roddatis, V.V.; Mestl, G.; Schlögl, R.; Ledoux, M.J. Large scale synthesis of carbon nanofibers by catalytic decomposition of ethane on nickel nanoclusters decorating carbon nanotubes. Phys. Chem. Chem. Phys. 2002, 4, 514–521. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cho, T.H.; Lee, B.K.; Rho, J.S.; An, K.H.; Lee, Y.H. Surface properties of fluorinated single-walled carbon nanotubes. J. Fluor. Chem. 2003, 120, 99–104. [Google Scholar] [CrossRef]
- Cao, A.; Xu, C.; Liang, J.; Wu, D.; Wei, B. X-ray diffraction characterization on the alignment degree of carbon nanotubes. Chem. Phys. Lett. 2001, 344, 13–17. [Google Scholar] [CrossRef]
- Rols, S.; Almairac, R.; Henrard, L.; Anglaret, E.; Sauvajol, J.-L. Diffraction by finite-size crystalline bundles of single wall nanotubes. Eur. Phys. J. B 1999, 10, 263–270. [Google Scholar] [CrossRef]
- Kuzmany, H.; Plank, W.; Hulman, M.; Kramberger, C.; Grüneis, A.; Pichler, T.; Peterlik, H.; Kataura, H.; Achiba, Y. Determination of SWCNT diameters from the Raman response of the radial breathing mode. Eur. Phys. J. B 2001, 22, 307–320. [Google Scholar] [CrossRef]
- Santos, T.C.; dos Gates, R.S.; de Tinôco, I.F.F.; Zolnier, S.; da Baêta, F.C. Behavior of Japanese quail in different air velocities and air temperatures. Pesqui. Agropecuária Bras. 2017, 52, 344–354. [Google Scholar] [CrossRef]
- Cividanes, L.S.; Brunelli, D.D.; Antunes, E.F.; Corat, E.J.; Sakane, K.K.; Thim, G.P. Cure study of epoxy resin reinforced with multiwalled carbon nanotubes by Raman and luminescence spectroscopy. J. Appl. Polym. Sci. 2013, 127, 544–553. [Google Scholar] [CrossRef]
- Puangjan, A.; Chaiyasith, S.; Taweeporngitgul, W.; Keawtep, J. Application of functionalized multi-walled carbon nanotubes supporting cuprous oxide and silver oxide composite catalyst on copper substrate for simultaneous detection of vitamin B2, vitamin B6 and ascorbic acid. Mater. Sci. Eng. C 2017, 76, 383–397. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Francisco, W.; Menezes, B.R.C.; Brito, F.S.; Coutinho, A.S.; Cividanes, L.S.; Coutinho, A.R.; Thim, G.P. Correlation of surface treatment, dispersion and mechanical properties of HDPE/CNT nanocomposites. Appl. Surf. Sci. 2016, 389, 921–929. [Google Scholar] [CrossRef]
- Silambarasan, D.; Surya, V.J.; Iyakutti, K.; Asokan, K.; Vasu, V.; Kawazoe, Y. Gamma (γ)-ray irradiated multi-walled carbon nanotubes (MWCNTs) for hydrogen storage. Appl. Surf. Sci. 2017, 418, 49–55. [Google Scholar] [CrossRef]
- Arepalli, S.; Nikolaev, P.; Gorelik, O.; Hadjiev, V.G.; Holmes, W.; Files, B.; Yowell, L. Protocol for the characterization of single-wall carbon nanotube material quality. Carbon N. Y. 2004, 42, 1783–1791. [Google Scholar] [CrossRef]
- Alvarez, W.E.; Kitiyanan, B.; Borgna, A.; Resasco, D.E. Synergism of Co and Mo in the catalytic production of single-wall carbon nanotubes by decomposition of CO. Carbon N. Y. 2001, 39, 547–558. [Google Scholar] [CrossRef]
- Bahr, J.L.; Yang, J.; Kosynkin, D.V.; Bronikowski, M.J.; Smalley, R.E.; Tour, J.M. Functionalization of Carbon Nanotubes by Electrochemical Reduction of Aryl Diazonium Salts: A Bucky Paper Electrode. J. Am. Chem. Soc. 2001, 123, 6536–6542. [Google Scholar] [CrossRef]
- Liu, W.-W.; Aziz, A.; Chai, S.-P.; Mohamed, A.R.; Tye, C.-T. Preparation of iron oxide nanoparticles supported on magnesium oxide for producing high-quality single-walled carbon nanotubes. New Carbon Mater. 2011, 26, 255–261. [Google Scholar] [CrossRef]
- Anoshkin, I.V.; Nefedova, I.I.; Lioubtchenko, D.V.; Nefedov, I.S.; Räisänen, A.V. Single walled carbon nanotube quantification method employing the Raman signal intensity. Carbon N. Y. 2017, 116, 547–552. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, S.; Sun, Z.; Zhang, Y.; Jin, W. Alkaline electrochemical advanced oxidation process for chromium oxidation at graphitized multi-walled carbon nanotubes. Chemosphere 2017, 183, 156–163. [Google Scholar] [CrossRef]
- Faraji, S.; Yildiz, O.; Rost, C.; Stano, K.; Farahbakhsh, N.; Zhu, Y.; Bradford, P.D. Radial growth of multi-walled carbon nanotubes in aligned sheets through cyclic carbon deposition and graphitization. Carbon N. Y. 2017, 111, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Pillay, K.; Cukrowska, E.M.; Coville, N.J. Multi-walled carbon nanotubes as adsorbents for the removal of parts per billion levels of hexavalent chromium from aqueous solution. J. Hazard. Mater. 2009, 166, 1067–1075. [Google Scholar] [CrossRef]
- Yu, F.; Ma, J.; Wang, J.; Zhang, M.; Zheng, J. Magnetic iron oxide nanoparticles functionalized multi-walled carbon nanotubes for toluene, ethylbenzene and xylene removal from aqueous solution. Chemosphere 2016, 146, 162–172. [Google Scholar] [CrossRef]
- Usman Farid, M.; Luan, H.-Y.; Wang, Y.; Huang, H.; An, A.K.; Jalil Khan, R. Increased adsorption of aqueous zinc species by Ar/O 2 plasma-treated carbon nanotubes immobilized in hollow-fiber ultrafiltration membrane. Chem. Eng. J. 2017, 325, 239–248. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mota, J.P.B.; Rostam-Abadi, M.; Rood, M.J. Theoretical and Experimental Investigation of Morphology and Temperature Effects on Adsorption of Organic Vapors in Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2006, 110, 7640–7647. [Google Scholar] [CrossRef]
- Cho, H.-H.; Wepasnick, K.; Smith, B.A.; Bangash, F.K.; Fairbrother, D.H.; Ball, W.P. Sorption of Aqueous Zn[II] and Cd[II] by Multiwall Carbon Nanotubes: The Relative Roles of Oxygen-Containing Functional Groups and Graphenic Carbon. Langmuir 2010, 26, 967–981. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Luo, T.; Zhang, Y.-X.; Jia, Y.; Zhu, B.-J.; Fu, X.-C.; Liu, J.-H.; Huang, X.-J. Adsorption of Lead(II) on O2-Plasma-Oxidized Multiwalled Carbon Nanotubes: Thermodynamics, Kinetics, and Desorption. ACS Appl. Mater. Interfaces 2011, 3, 2585–2593. [Google Scholar] [CrossRef]
- Addo Ntim, S.; Mitra, S. Removal of Trace Arsenic To Meet Drinking Water Standards Using Iron Oxide Coated Multiwall Carbon Nanotubes. J. Chem. Eng. Data 2011, 56, 2077–2083. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hu, J.; Shao, D.; Li, J.; Wang, X. Adsorption behavior of multiwall carbon nanotube/iron oxide magnetic composites for Ni(II) and Sr(II). J. Hazard. Mater. 2009, 164, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar Tarigh, G.; Shemirani, F. Magnetic multi-wall carbon nanotube nanocomposite as an adsorbent for preconcentration and determination of lead (II) and manganese (II) in various matrices. Talanta 2013, 115, 744–750. [Google Scholar] [CrossRef]
- Zhao, X.; Jia, Q.; Song, N.; Zhou, W.; Li, Y. Adsorption of Pb(II) from an Aqueous Solution by Titanium Dioxide/Carbon Nanotube Nanocomposites: Kinetics, Thermodynamics, and Isotherms. J. Chem. Eng. Data 2010, 55, 4428–4433. [Google Scholar] [CrossRef]
- Gupta, A.; Vidyarthi, S.R.; Sankararamakrishnan, N. Enhanced sorption of mercury from compact fluorescent bulbs and contaminated water streams using functionalized multiwalled carbon nanotubes. J. Hazard. Mater. 2014, 274, 132–144. [Google Scholar] [CrossRef]
- Gupta, V.K.; Moradi, O.; Tyagi, I.; Agarwal, S.; Sadegh, H.; Shahryari-Ghoshekandi, R.; Makhlouf, A.S.H.; Goodarzi, M.; Garshasbi, A. Study on the removal of heavy metal ions from industry waste by carbon nanotubes: Effect of the surface modification: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 93–118. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, D.; Wang, X. Removal of Pb(II) and Cu(II) from aqueous solution using multiwalled carbon nanotubes/iron oxide magnetic composites. Water Sci. Technol. 2011, 63, 917–923. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Z.; Sheng, G.; Wang, X. Investigation of the sequestration mechanisms of Cd(II) and 1-naphthol on discharged multi-walled carbon nanotubes in aqueous environment. Sci. Total Environ. 2012, 420, 214–221. [Google Scholar] [CrossRef]
- Vuković, G.D.; Marinković, A.D.; Čolić, M.; Ristić, M.Đ.; Aleksić, R.; Perić-Grujić, A.A.; Uskoković, P.S. Removal of cadmium from aqueous solutions by oxidized and ethylenediamine-functionalized multi-walled carbon nanotubes. Chem. Eng. J. 2010, 157, 238–248. [Google Scholar] [CrossRef]
- Stafiej, A.; Pyrzynska, K. Solid phase extraction of metal ions using carbon nanotubes. Microchem. J. 2008, 89, 29–33. [Google Scholar] [CrossRef]
- Lu, C.; Chiu, H. Adsorption of zinc(II) from water with purified carbon nanotubes. Chem. Eng. Sci. 2006, 61, 1138–1145. [Google Scholar] [CrossRef]
- Weng, C.-H.; Huang, C.P. Adsorption characteristics of Zn(II) from dilute aqueous solution by fly ash. Colloids Surf. A Physicochem. Eng. Asp. 2004, 247, 137–143. [Google Scholar] [CrossRef]
- Boehm, H. Surface oxides on carbon and their analysis: A critical assessment. Carbon N. Y. 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Sheng, G.; Hu, J.; Tan, X.; Wang, X. Effect of surfactants on Pb(II) adsorption from aqueous solutions using oxidized multiwall carbon nanotubes. Chem. Eng. J. 2011, 166, 551–558. [Google Scholar] [CrossRef]
- Atieh, M.A.; Bakather, O.Y.; Tawabini, B.S.; Bukhari, A.A.; Khaled, M.; Alharthi, M.; Fettouhi, M.; Abuilaiwi, F.A. Removal of Chromium (III) from Water by Using Modified and Nonmodified Carbon Nanotubes. J. Nanomater. 2010, 2010, 1–9. [Google Scholar] [CrossRef]
- Lu, C.; Liu, C.; Rao, G.P. Comparisons of sorbent cost for the removal of Ni2+ from aqueous solution by carbon nanotubes and granular activated carbon. J. Hazard. Mater. 2008, 151, 239–246. [Google Scholar] [CrossRef]
- Ali, I. Microwave assisted economic synthesis of multi walled carbon nanotubes for arsenic species removal in water: Batch and column operations. J. Mol. Liq. 2018, 271, 677–685. [Google Scholar] [CrossRef]
- Alijani, H.; Shariatinia, Z. Effective aqueous arsenic removal using zero valent iron doped MWCNT synthesized by in situ CVD method using natural α-Fe2O3 as a precursor. Chemosphere 2017, 171, 502–511. [Google Scholar] [CrossRef]
- Sankararamakrishnan, N.; Chauhan, D.; Dwivedi, J. Synthesis of functionalized carbon nanotubes by floating catalytic chemical vapor deposition method and their sorption behavior toward arsenic. Chem. Eng. J. 2016, 284, 599–608. [Google Scholar] [CrossRef]
- Ma, M.-D.; Wu, H.; Deng, Z.-Y.; Zhao, X. Arsenic removal from water by nanometer iron oxide coated single-wall carbon nanotubes. J. Mol. Liq. 2018, 259, 369–375. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, N.; He, M.; Chen, B.; Hu, B. Simultaneous speciation analysis of inorganic arsenic, chromium and selenium in environmental waters by 3-(2-aminoethylamino) propyltrimethoxysilane modified multi-wall carbon nanotubes packed microcolumn solid phase extraction and ICP-MS. Talanta 2015, 131, 266–272. [Google Scholar] [CrossRef]
- Veličković, Z.; Vuković, G.D.; Marinković, A.D.; Moldovan, M.-S.; Perić-Grujić, A.A.; Uskoković, P.S.; Ristić, M.Đ. Adsorption of arsenate on iron(III) oxide coated ethylenediamine functionalized multiwall carbon nanotubes. Chem. Eng. J. 2012, 181–182, 174–181. [Google Scholar] [CrossRef]
- Addo Ntim, S.; Mitra, S. Adsorption of arsenic on multiwall carbon nanotube–zirconia nanohybrid for potential drinking water purification. J. Colloid Interface Sci. 2012, 375, 154–159. [Google Scholar] [CrossRef] [Green Version]
- De Marques Neto, J.O.; Bellato, C.R.; de Silva, D.C. Iron oxide/carbon nanotubes/chitosan magnetic composite film for chromium species removal. Chemosphere 2019, 218, 391–401. [Google Scholar] [CrossRef]
- Shin, K.-Y.; Hong, J.-Y.; Jang, J. Heavy metal ion adsorption behavior in nitrogen-doped magnetic carbon nanoparticles: Isotherms and kinetic study. J. Hazard. Mater. 2011, 190, 36–44. [Google Scholar] [CrossRef]
- Huang, Y.; Lee, X.; Macazo, F.C.; Grattieri, M.; Cai, R.; Minteer, S.D. Fast and efficient removal of chromium (VI) anionic species by a reusable chitosan-modified multi-walled carbon nanotube composite. Chem. Eng. J. 2018, 339, 259–267. [Google Scholar] [CrossRef]
- Lu, W.; Li, J.; Sheng, Y.; Zhang, X.; You, J.; Chen, L. One-pot synthesis of magnetic iron oxide nanoparticle-multiwalled carbon nanotube composites for enhanced removal of Cr(VI) from aqueous solution. J. Colloid Interface Sci. 2017, 505, 1134–1146. [Google Scholar] [CrossRef]
- Salam, M.A. Preparation and characterization of chitin/magnetite/multiwalled carbon nanotubes magnetic nanocomposite for toxic hexavalent chromium removal from solution. J. Mol. Liq. 2017, 233, 197–202. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, X.; Yang, D. Adsorption of Cr(VI) in wastewater using magnetic multi-wall carbon nanotubes. Water Sci. Eng. 2015, 8, 226–232. [Google Scholar] [CrossRef] [Green Version]
- Di, Z.-C.; Ding, J.; Peng, X.-J.; Li, Y.-H.; Luan, Z.-K.; Liang, J. Chromium adsorption by aligned carbon nanotubes supported ceria nanoparticles. Chemosphere 2006, 62, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sui, J.; Li, J.; Tang, Y.; Cai, W. Efficient removal of heavy metal ions by thiol-functionalized superparamagnetic carbon nanotubes. Chem. Eng. J. 2012, 210, 45–52. [Google Scholar] [CrossRef]
- Wang, G.; Gao, Z.; Tang, S.; Chen, C.; Duan, F.; Zhao, S.; Lin, S.; Feng, Y.; Zhou, L.; Qin, Y. Microwave Absorption Properties of Carbon Nanocoils Coated with Highly Controlled Magnetic Materials by Atomic Layer Deposition. ACS Nano 2012, 6, 11009–11017. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Zhou, L.; Bai, X.; Shao, Y.; Zhao, G.; Qu, Y.; Wang, C.; Li, Y. Facile synthesis of multiwall carbon nanotubes/iron oxides for removal of tetrabromobisphenol A and Pb(ii). J. Mater. Chem. 2012, 22, 15853. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.; Hu, Z. Boron-Doped Carbon Nanotubes as Metal-Free Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2011, 50, 7132–7135. [Google Scholar] [CrossRef] [PubMed]
- Tofighy, M.A.; Mohammadi, T. Adsorption of divalent heavy metal ions from water using carbon nanotube sheets. J. Hazard. Mater. 2011, 185, 140–147. [Google Scholar] [CrossRef]
- Ren, X.; Shao, D.; Zhao, G.; Sheng, G.; Hu, J.; Yang, S.; Wang, X. Plasma Induced Multiwalled Carbon Nanotube Grafted with 2-Vinylpyridine for Preconcentration of Pb(II) from Aqueous Solutions. Plasma Process. Polym. 2011, 8, 589–598. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Z. Role of multi-wall carbon nanotube network in composites to crystallization of isotactic polypropylene matrix. Polymer 2008, 49, 330–338. [Google Scholar] [CrossRef]
- Wang, S.; Gong, W.; Liu, X.; Yao, Y.; Gao, B.; Yue, Q. Removal of lead(II) from aqueous solution by adsorption onto manganese oxide-coated carbon nanotubes. Sep. Purif. Technol. 2007, 58, 17–23. [Google Scholar] [CrossRef]
- Wang, H.J.; Zhou, A.L.; Peng, F.; Yu, H.; Chen, L.F. Adsorption characteristic of acidified carbon nanotubes for heavy metal Pb(II) in aqueous solution. Mater. Sci. Eng. A 2007, 466, 201–206. [Google Scholar] [CrossRef]
- Liang, J.; Liu, J.; Yuan, X.; Dong, H.; Zeng, G.; Wu, H.; Wang, H.; Liu, J.; Hua, S.; Zhang, S.; et al. Facile synthesis of alumina-decorated multi-walled carbon nanotubes for simultaneous adsorption of cadmium ion and trichloroethylene. Chem. Eng. J. 2015, 273, 101–110. [Google Scholar] [CrossRef]
- Al-Khaldi, F.A.; Abusharkh, B.; Khaled, M.; Atieh, M.A.; Nasser, M.S.; Saleh, T.A.; Agarwal, S.; Tyagi, I.; Gupta, V.K. Adsorptive removal of cadmium(II) ions from liquid phase using acid modified carbon-based adsorbents. J. Mol. Liq. 2015, 204, 255–263. [Google Scholar] [CrossRef]
- Salam, M.A.; Makki, M.S.I.; Abdelaal, M.Y.A. Preparation and characterization of multi-walled carbon nanotubes/chitosan nanocomposite and its application for the removal of heavy metals from aqueous solution. J. Alloys Compd. 2011, 509, 2582–2587. [Google Scholar] [CrossRef]
- Bandaru, N.M.; Reta, N.; Dalal, H.; Ellis, A.V.; Shapter, J.; Voelcker, N.H. Enhanced adsorption of mercury ions on thiol derivatized single wall carbon nanotubes. J. Hazard. Mater. 2013, 261, 534–541. [Google Scholar] [CrossRef]
- Hadavifar, M.; Bahramifar, N.; Younesi, H.; Li, Q. Adsorption of mercury ions from synthetic and real wastewater aqueous solution by functionalized multi-walled carbon nanotube with both amino and thiolated groups. Chem. Eng. J. 2014, 237, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Pillay, K.; Cukrowska, E.M.; Coville, N.J. Improved uptake of mercury by sulphur-containing carbon nanotubes. Microchem. J. 2013, 108, 124–130. [Google Scholar] [CrossRef]
- El-Sheikh, A.H.; Al-Degs, Y.S.; Al-As’ad, R.M.; Sweileh, J.A. Effect of oxidation and geometrical dimensions of carbon nanotubes on Hg(II) sorption and preconcentration from real waters. Desalination 2011, 270, 214–220. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Alicia, R.F.; Abdullah, E.C.; Sahu, J.N.; Haslija, A.B.A.; Tan, J. Statistical optimization and kinetic studies on removal of Zn2+ using functionalized carbon nanotubes and magnetic biochar. J. Environ. Chem. Eng. 2013, 1, 486–495. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Z.; Xiao, D.; Xiong, P.; Ye, N. Sulfonated multi-walled carbon nanotubes for the removal of copper (II) from aqueous solutions. J. Ind. Eng. Chem. 2014, 20, 1765–1771. [Google Scholar] [CrossRef]
- Salehi, E.; Madaeni, S.S.; Rajabi, L.; Vatanpour, V.; Derakhshan, A.A.; Zinadini, S.; Ghorabi, S.; Ahmadi Monfared, H. Novel chitosan/poly(vinyl) alcohol thin adsorptive membranes modified with amino functionalized multi-walled carbon nanotubes for Cu(II) removal from water: Preparation, characterization, adsorption kinetics and thermodynamics. Sep. Purif. Technol. 2012, 89, 309–319. [Google Scholar] [CrossRef]
- Shao, D.; Hu, J.; Chen, C.; Sheng, G.; Ren, X.; Wang, X. Polyaniline Multiwalled Carbon Nanotube Magnetic Composite Prepared by Plasma-Induced Graft Technique and Its Application for Removal of Aniline and Phenol. J. Phys. Chem. C 2010, 114, 21524–21530. [Google Scholar] [CrossRef]
- Chen, H.; Li, J.; Shao, D.; Ren, X.; Wang, X. Poly(acrylic acid) grafted multiwall carbon nanotubes by plasma techniques for Co(II) removal from aqueous solution. Chem. Eng. J. 2012, 210, 475–481. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Chen, C.; Ren, X.; Hu, J.; Wang, X. Removal of cobalt from aqueous solution by magnetic multiwalled carbon nanotube/iron oxide composites. Chem. Eng. J. 2011, 174, 126–133. [Google Scholar] [CrossRef]
- Mobasherpour, I.; Salahi, E.; Ebrahimi, M. Removal of divalent nickel cations from aqueous solution by multi-walled carbon nano tubes: Equilibrium and kinetic processes. Res. Chem. Intermed. 2012, 38, 2205–2222. [Google Scholar] [CrossRef]
- Kandah, M.I.; Meunier, J.-L. Removal of nickel ions from water by multi-walled carbon nanotubes. J. Hazard. Mater. 2007, 146, 283–288. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X. Adsorption of Ni(II) from Aqueous Solution Using Oxidized Multiwall Carbon Nanotubes. Ind. Eng. Chem. Res. 2006, 45, 9144–9149. [Google Scholar] [CrossRef]
- Abdel-Ghani, N.T.; El-Chaghaby, G.A.; Helal, F.S. Individual and competitive adsorption of phenol and nickel onto multiwalled carbon nanotubes. J. Adv. Res. 2015, 6, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Deb, A.K.S.; Ilaiyaraja, P.; Ponraju, D.; Venkatraman, B. Diglycolamide functionalized multi-walled carbon nanotubes for removal of uranium from aqueous solution by adsorption. J. Radioanal. Nucl. Chem. 2012, 291, 877–883. [Google Scholar] [CrossRef]
- Chen, C.; Hu, J.; Xu, D.; Tan, X.; Meng, Y.; Wang, X. Surface complexation modeling of Sr(II) and Eu(III) adsorption onto oxidized multiwall carbon nanotubes. J. Colloid Interface Sci. 2008, 323, 33–41. [Google Scholar] [CrossRef]
- Tousova, Z.; Oswald, P.; Slobodnik, J.; Blaha, L.; Muz, M.; Hu, M.; Brack, W.; Krauss, M.; Di Paolo, C.; Tarcai, Z.; et al. European demonstration program on the effect-based and chemical identification and monitoring of organic pollutants in European surface waters. Sci. Total Environ. 2017, 601–602, 1849–1868. [Google Scholar] [CrossRef]
- Sillanpää, M. Natural Organic Matter in Water, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780128015032. [Google Scholar]
- Zare, K.; Gupta, V.K.; Moradi, O.; Makhlouf, A.S.H.; Sillanpää, M.; Nadagouda, M.N.; Sadegh, H.; Shahryari-ghoshekandi, R.; Pal, A.; Wang, Z.; et al. A comparative study on the basis of adsorption capacity between CNTs and activated carbon as adsorbents for removal of noxious synthetic dyes: A review. J. Nanostruct. Chem. 2015, 5, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Ghaedi, M.; Khajehsharifi, H.; Yadkuri, A.H.; Roosta, M.; Asghari, A. Oxidized multiwalled carbon nanotubes as efficient adsorbent for bromothymol blue. Toxicol. Environ. Chem. 2012, 94, 873–883. [Google Scholar] [CrossRef]
- Mahmoodian, H.; Moradi, O.; Shariatzadeha, B.; Salehf, T.A.; Tyagi, I.; Maity, A.; Asif, M.; Gupta, V.K. Enhanced removal of methyl orange from aqueous solutions by poly HEMA–chitosan-MWCNT nano-composite. J. Mol. Liq. 2015, 202, 189–198. [Google Scholar] [CrossRef]
- Duman, O.; Tunç, S.; Polat, T.G.; Bozoğlan, B.K. Synthesis of magnetic oxidized multiwalled carbon nanotube-κ-carrageenan-Fe 3 O 4 nanocomposite adsorbent and its application in cationic Methylene Blue dye adsorption. Carbohydr. Polym. 2016, 147, 79–88. [Google Scholar] [CrossRef]
- Sadegh, H.; Zare, K.; Maazinejad, B.; Shahryari-ghoshekandi, R.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Synthesis of MWCNT-COOH-Cysteamine composite and its application for dye removal. J. Mol. Liq. 2016, 215, 221–228. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Wei, X. Influence of wastewater precoagulation on adsorptive filtration of pharmaceutical and personal care products by carbon nanotube membranes. Chem. Eng. J. 2018, 333, 66–75. [Google Scholar] [CrossRef]
- Wang, S.; Liang, S.; Liang, P.; Zhang, X.; Sun, J.; Wu, S.; Huang, X. In-situ combined dual-layer CNT/PVDF membrane for electrically-enhanced fouling resistance. J. Memb. Sci. 2015, 491, 37–44. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Huang, H.; Cho, H.-H. Carbon nanotube composite membranes for microfiltration of pharmaceuticals and personal care products: Capabilities and potential mechanisms. J. Memb. Sci. 2015, 479, 165–174. [Google Scholar] [CrossRef]
- Jahangiri-Rad, M.; Nadafi, K.; Mesdaghinia, A.; Nabizadeh, R.; Younesian, M.; Rafiee, M. Sequential study on reactive blue 29 dye removal from aqueous solution by peroxy acid and single wall carbon nanotubes: Experiment and theory. Iran. J. Environ. Health Sci. Eng. 2013, 10, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, M.; Chefetz, B. Adsorption and desorption of dissolved organic matter by carbon nanotubes: Effects of solution chemistry. Environ. Pollut. 2016, 213, 90–98. [Google Scholar] [CrossRef]
- Ajmani, G.S.; Goodwin, D.; Marsh, K.; Fairbrother, D.H.; Schwab, K.J.; Jacangelo, J.G.; Huang, H. Modification of low pressure membranes with carbon nanotube layers for fouling control. Water Res. 2012, 46, 5645–5654. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lee, J.; Yuan, L.; Chae, S.-R.; Peterson, V.K.; Minett, A.I.; Yin, Y.; Harris, A.T. Removal of natural organic matter in water using functionalised carbon nanotube buckypaper. Carbon N. Y. 2013, 59, 160–166. [Google Scholar] [CrossRef]
- Deng, J.; Shao, Y.; Gao, N.; Deng, Y.; Tan, C.; Zhou, S.; Hu, X. Multiwalled carbon nanotubes as adsorbents for removal of herbicide diuron from aqueous solution. Chem. Eng. J. 2012, 193–194, 339–347. [Google Scholar] [CrossRef]
- Hamdi, H.; De La Torre-Roche, R.; Hawthorne, J.; White, J.C. Impact of non-functionalized and amino-functionalized multiwall carbon nanotubes on pesticide uptake by lettuce (Lactuca sativa L.). Nanotoxicology 2015, 9, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.-D.R.; Rogers, R.E.; Dichiara, A.B.; Capasse, R.C. Emerging investigators series: Highly effective adsorption of organic aromatic molecules from aqueous environments by electronically sorted single-walled carbon nanotubes. Environ. Sci. Water Res. Technol. 2017, 3, 203–212. [Google Scholar] [CrossRef]
- Dichiara, A.B.; Harlander, S.F.; Rogers, R.E. Fixed bed adsorption of diquat dibromide from aqueous solution using carbon nanotubes. RSC Adv. 2015, 5, 61508–61512. [Google Scholar] [CrossRef]
- Srivastava, A.; Srivastava, O.N.; Talapatra, S.; Vajtai, R.; Ajayan, P.M. Carbon nanotube filters. Nat. Mater. 2004, 3, 610–614. [Google Scholar] [CrossRef]
- Tahaikt, M.; El Habbani, R.; Ait Haddou, A.; Achary, I.; Amor, Z.; Taky, M.; Alami, A.; Boughriba, A.; Hafsi, M.; Elmidaoui, A. Fluoride removal from groundwater by nanofiltration. Desalination 2007, 212, 46–53. [Google Scholar] [CrossRef]
- Fornasiero, F.; Park, H.G.; Holt, J.K.; Stadermann, M.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Ion exclusion by sub-2-nm carbon nanotube pores. Proc. Natl. Acad. Sci. USA 2008, 105, 17250–17255. [Google Scholar] [CrossRef] [Green Version]
- Noy, A.; Park, H.G.; Fornasiero, F.; Holt, J.K.; Grigoropoulos, C.P.; Bakajin, O. Nanofluidics in carbon nanotubes. Nano Today 2007, 2, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Hummer, G.; Rasaiah, J.C.; Noworyta, J.P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 2001, 414, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Moradi, O. Adsorption Behavior of Basic Red 46 by Single-Walled Carbon Nanotubes Surfaces. Fuller. Nanotub. Carbon Nanostructures 2013, 21, 286–301. [Google Scholar] [CrossRef]
- Yang, S.; Wang, L.; Zhang, X.; Yang, W.; Song, G. Enhanced adsorption of Congo red dye by functionalized carbon nanotube/mixed metal oxides nanocomposites derived from layered double hydroxide precursor. Chem. Eng. J. 2015, 275, 315–321. [Google Scholar] [CrossRef]
- Konicki, W.; Pełech, I.; Mijowska, E.; Jasińska, I. Adsorption of anionic dye Direct Red 23 onto magnetic multi-walled carbon nanotubes-Fe3C nanocomposite: Kinetics, equilibrium and thermodynamics. Chem. Eng. J. 2012, 210, 87–95. [Google Scholar] [CrossRef]
- Setareh Derakhshan, M.; Moradi, O. The study of thermodynamics and kinetics methyl orange and malachite green by SWCNTs, SWCNT-COOH and SWCNT-NH2 as adsorbents from aqueous solution. J. Ind. Eng. Chem. 2014, 20, 3186–3194. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, W.; Chen, C.; Wang, X. Adsorption of Methyl Orange Dye Onto Multiwalled Carbon Nanotubes. Procedia Environ. Sci. 2013, 18, 890–895. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Razali, M.H.; Mamat, M.; Mehamod, F.S.B.; Anuar Mat Amin, K. Adsorption of methyl orange by synthesized and functionalized-CNTs with 3-aminopropyltriethoxysilane loaded TiO2 nanocomposites. Chemosphere 2017, 168, 474–482. [Google Scholar] [CrossRef]
- Robati, D.; Mirza, B.; Ghazisaeidi, R.; Rajabi, M.; Moradi, O.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Adsorption behavior of methylene blue dye on nanocomposite multi-walled carbon nanotube functionalized thiol (MWCNT-SH) as new adsorbent. J. Mol. Liq. 2016, 216, 830–835. [Google Scholar] [CrossRef]
- Shih, M.-W.; Chin, C.-J.M.; Yu, Y.-L. The role of oxygen-containing groups on the adsorption of bisphenol-A on multi-walled carbon nanotube modified by HNO3 and KOH. Process Saf. Environ. Prot. 2017, 112, 308–314. [Google Scholar] [CrossRef]
- Kuo, C.-Y. Comparison with as-grown and microwave modified carbon nanotubes to removal aqueous bisphenol A. Desalination 2009, 249, 976–982. [Google Scholar] [CrossRef]
- Liao, Q.; Sun, J.; Gao, L. Adsorption of chlorophenols by multi-walled carbon nanotubes treated with HNO3 and NH3. Carbon N. Y. 2008, 46, 553–555. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Sazila, N.; Nizamuddin, S.; Abdullah, E.C.; Sahu, J.N. Adsorptive removal of phenol from aqueous solution by using carbon nanotubes and magnetic biochar. Nano World J. 2017, 3, 32–37. [Google Scholar] [CrossRef]
- Yao, Y.-X.; Li, H.-B.; Liu, J.-Y.; Tan, X.-L.; Yu, J.-G.; Peng, Z.-G. Removal and Adsorption of p-Nitrophenol from Aqueous Solutions Using Carbon Nanotubes and Their Composites. J. Nanomater. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Flores, P.E.; López-Urías, F.; Terrones, M.; Rangel-Mendez, J.R. Simultaneous adsorption of Cd2+ and phenol on modified N-doped carbon nanotubes: Experimental and DFT studies. J. Colloid Interface Sci. 2009, 334, 124–131. [Google Scholar] [CrossRef]
- Tóth, A.; Törőcsik, A.; Tombácz, E.; László, K. Competitive adsorption of phenol and 3-chlorophenol on purified MWCNTs. J. Colloid Interface Sci. 2012, 387, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Arasteh, R.; Masoumi, M.; Rashidi, A.M.; Moradi, L.; Samimi, V.; Mostafavi, S.T. Adsorption of 2-nitrophenol by multi-wall carbon nanotubes from aqueous solutions. Appl. Surf. Sci. 2010, 256, 4447–4455. [Google Scholar] [CrossRef]
- Kassem, A.; Ayoub, G.M.; Malaeb, L. Antibacterial activity of chitosan nano-composites and carbon nanotubes: A review. Sci. Total Environ. 2019, 668, 566–576. [Google Scholar] [CrossRef]
- Smith, S.C.; Rodrigues, D.F. Carbon-based nanomaterials for removal of chemical and biological contaminants from water: A review of mechanisms and applications. Carbon N. Y. 2015, 91, 122–143. [Google Scholar] [CrossRef]
- Brady-Estévez, A.S.; Kang, S.; Elimelech, M. A Single-Walled-Carbon-Nanotube Filter for Removal of Viral and Bacterial Pathogens. Small 2008, 4, 481–484. [Google Scholar] [CrossRef]
- Lu, C.; Su, F. Adsorption of natural organic matter by carbon nanotubes. Sep. Purif. Technol. 2007, 58, 113–121. [Google Scholar] [CrossRef]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef]
- Ihsanullah; Asmaly, H.A.; Saleh, T.A.; Laoui, T.; Gupta, V.K.; Atieh, M.A. Enhanced adsorption of phenols from liquids by aluminum oxide/carbon nanotubes: Comprehensive study from synthesis to surface properties. J. Mol. Liq. 2015, 206, 176–182. [Google Scholar] [CrossRef]
- Bohonak, D.; Zydney, A. Compaction and permeability effects with virus filtration membranes. J. Memb. Sci. 2005, 254, 71–79. [Google Scholar] [CrossRef]
- Mostafavi, S.T.; Mehrnia, M.R.; Rashidi, A.M. Preparation of nanofilter from carbon nanotubes for application in virus removal from water. Desalination 2009, 238, 271–280. [Google Scholar] [CrossRef]
- Savage, N.; Diallo, M.S. Nanomaterials and Water Purification: Opportunities and Challenges. J. Nanopart. Res. 2005, 7, 331–342. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef]
- Nepal, D.; Balasubramanian, S.; Simonian, A.L.; Davis, V.A. Strong Antimicrobial Coatings: Single-Walled Carbon Nanotubes Armored with Biopolymers. Nano Lett. 2008, 8, 1896–1901. [Google Scholar] [CrossRef]
- Cortes, P.; Deng, S.; Smith, G.B. The Adsorption Properties of Bacillus atrophaeus Spores on Single-Wall Carbon Nanotubes. J. Sens. 2009, 2009, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ong, Y.T.; Ahmad, A.L.; Zein, S.H.S.; Tan, S.H. A review on carbon nanotubes in an environmental protection and green engineering perspective. Braz. J. Chem. Eng. 2010, 27, 227–242. [Google Scholar] [CrossRef]
- Yuan, W.; Jiang, G.; Che, J.; Qi, X.; Xu, R.; Chang, M.W.; Chen, Y.; Lim, S.Y.; Dai, J.; Chan-Park, M.B. Deposition of Silver Nanoparticles on Multiwalled Carbon Nanotubes Grafted with Hyperbranched Poly(amidoamine) and Their Antimicrobial Effects. J. Phys. Chem. C 2008, 112, 18754–18759. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Ihsanullah; Laoui, T.; Al-Amer, A.M.; Khalil, A.B.; Abbas, A.; Khraisheh, M.; Atieh, M.A. Novel anti-microbial membrane for desalination pretreatment: A silver nanoparticle-doped carbon nanotube membrane. Desalination 2015, 376, 82–93. [Google Scholar] [CrossRef]
- Nie, C.; Yang, Y.; Cheng, C.; Ma, L.; Deng, J.; Wang, L.; Zhao, C. Bioinspired and biocompatible carbon nanotube-Ag nanohybrid coatings for robust antibacterial applications. Acta Biomater. 2017, 51, 479–494. [Google Scholar] [CrossRef]
- Morsi, R.E.; Alsabagh, A.M.; Nasr, S.A.; Zaki, M.M. Multifunctional nanocomposites of chitosan, silver nanoparticles, copper nanoparticles and carbon nanotubes for water treatment: Antimicrobial characteristics. Int. J. Biol. Macromol. 2017, 97, 264–269. [Google Scholar] [CrossRef]
- Chi, M.-F.; Wu, W.-L.; Du, Y.; Chin, C.-J.M.; Lin, C.-C. Inactivation of Escherichia coli planktonic cells by multi-walled carbon nanotubes in suspensions: Effect of surface functionalization coupled with medium nutrition level. J. Hazard. Mater. 2016, 318, 507–514. [Google Scholar] [CrossRef]
- Al-Hakami, S.M.; Khalil, A.B.; Laoui, T.; Atieh, M.A. Fast Disinfection of Escherichia coli Bacteria Using Carbon Nanotubes Interaction with Microwave Radiation. Bioinorg. Chem. Appl. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]









| Properties | SWNTs | MWCNTs |
|---|---|---|
| Layer type | Single graphene layer | Multiple graphene layer |
| Catalyst requirement | Essential during synthesis | No need during synthesis |
| Bulk or massive production | Difficult | Easy |
| Purity level | Low | Large |
| Defect’s level | High | Low |
| Characterization | Easy | Difficult |
| Manage | Easily twisted | Cannot be twisted easily |
| Specific gravity | About 0.8 g/cm3 | Less than 1.8 g/cm3 |
| Elastic modulus | About 1.4 TPa | Ranging from 0.3 to 1 TPa |
| Strength | Ranging from 50 to 500 GPa | Ranging from 10 to 60 GPa |
| Electrical conductivity | Ranging from 102 to 106 S/cm | Ranging from 103 to 105 S/cm |
| Electron mobility | About 105 cm2/(V s) | Ranging from 104 to 105 cm2/(V s) |
| Thermal conductivity | About 6000 W/(m K) | About 2000 W/(m K) |
| Coefficient of thermal expansion | Greater than 1.1 × 10−3 K−1 | About −1.37 × 10−3 K−1 |
| Thermal stability in air | Ranging from 600 to 800 °C | Ranging from 600 to 800 °C |
| Resistivity | Ranging from 50 to 500 μΩ cm | Ranging from 50 to 500 μΩ cm |
| Specific Surface Area | Ranging from 400 to 900 m2/g | Ranging from 200 to 400 m2/g |
| CNTs | Adsorption Capacity (mg/g) | Surface Area (m2/g) |
|---|---|---|
| Pristine | 1.1 | 82.2 |
| H2O2 oxidized | 2.6 | 130.0 |
| HNO3 oxidized | 5.1 | 84.3 |
| KMnO4 oxidized | 11.0 | 128. |
| NaOCl oxidized | 47.4 | 94.9 |
| Methods | Benefit(s) | Limitation(s) |
|---|---|---|
| Covalent functionalization | Highly stable bonds are formed | Intrinsic characteristics are damaged Structural defects CNTs Aggregation of CNTs |
| Non-covalent functionalization | Simple and easy procedure CNTs structure is maintained with minimum defects Electronic characteristics of CNTs are not affected | Stability of bonds is weak |
| Characterization Techniques | Used for Studying |
|---|---|
| Microscopy and diffraction techniques | [157,158,159] |
| SEM | Morphological analysis (diameter and length), aggregation state |
| TEM/HR-TEM | Morphological analysis of internal structure (diameter, number of layers and distance between them) |
| AFM | Morphological analysis of internal structure (diameter, number of layers and distance between them) |
| Scanning tunneling microscopy | Morphological analysis of internal structure (diameter, number of layers and distance between them) |
| Neutron diffraction | Morphological analysis of bulk samples |
| XRD | Morphological analysis of bulk samples |
| Spectroscopic techniques | [139,160,161] |
| Raman spectroscopy | Purity and presence of by-products, diameter distribution, (n, m) chirality |
| IR and FT-IR | Purity, functionalization by attaching functional groups to the sidewall |
| UV–vis and NIR | Dispersion efficiency, diameter and length distribution, purity |
| Fluorescence spectroscopy | Size, dispersion efficiency, (n, m) chirality |
| XPS and EDS | Elemental composition, functionalization (covalent and non-covalent) |
| Thermal techniques | [162] |
| TGA | Purity and presence of by-products, quality control of synthesis and manufacture processes |
| Separation techniques | |
| Size exclusion chromatography | Purification, separation by size (length) |
| Capillary electrophoresis | Purification, separation by size (length, diameter, and cross-section) |
| Field flow fractionation | Fractionation by size (length) |
| Ultracentrifugation | Separation by chirality, electronic type, length, and enantiomeric identity |
| Magnetic techniques | [158,163,164] |
| Vibrating sample magnetometry | Magnetic properties |
| Alternating gradient magnetometry | Magnetic properties |
| Superconducting quantum interference device | Magnetic properties |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslam, M.M.-A.; Kuo, H.-W.; Den, W.; Usman, M.; Sultan, M.; Ashraf, H. Functionalized Carbon Nanotubes (CNTs) for Water and Wastewater Treatment: Preparation to Application. Sustainability 2021, 13, 5717. https://doi.org/10.3390/su13105717
Aslam MM-A, Kuo H-W, Den W, Usman M, Sultan M, Ashraf H. Functionalized Carbon Nanotubes (CNTs) for Water and Wastewater Treatment: Preparation to Application. Sustainability. 2021; 13(10):5717. https://doi.org/10.3390/su13105717
Chicago/Turabian StyleAslam, Mian Muhammad-Ahson, Hsion-Wen Kuo, Walter Den, Muhammad Usman, Muhammad Sultan, and Hadeed Ashraf. 2021. "Functionalized Carbon Nanotubes (CNTs) for Water and Wastewater Treatment: Preparation to Application" Sustainability 13, no. 10: 5717. https://doi.org/10.3390/su13105717
APA StyleAslam, M. M.-A., Kuo, H.-W., Den, W., Usman, M., Sultan, M., & Ashraf, H. (2021). Functionalized Carbon Nanotubes (CNTs) for Water and Wastewater Treatment: Preparation to Application. Sustainability, 13(10), 5717. https://doi.org/10.3390/su13105717