The Effect of Blue-Green Infrastructure on Habitat Connectivity and Biodiversity: A Case Study in the Ōtākaro/Avon River Catchment in Christchurch, New Zealand
Abstract
:1. Introduction
2. Materials and Methods
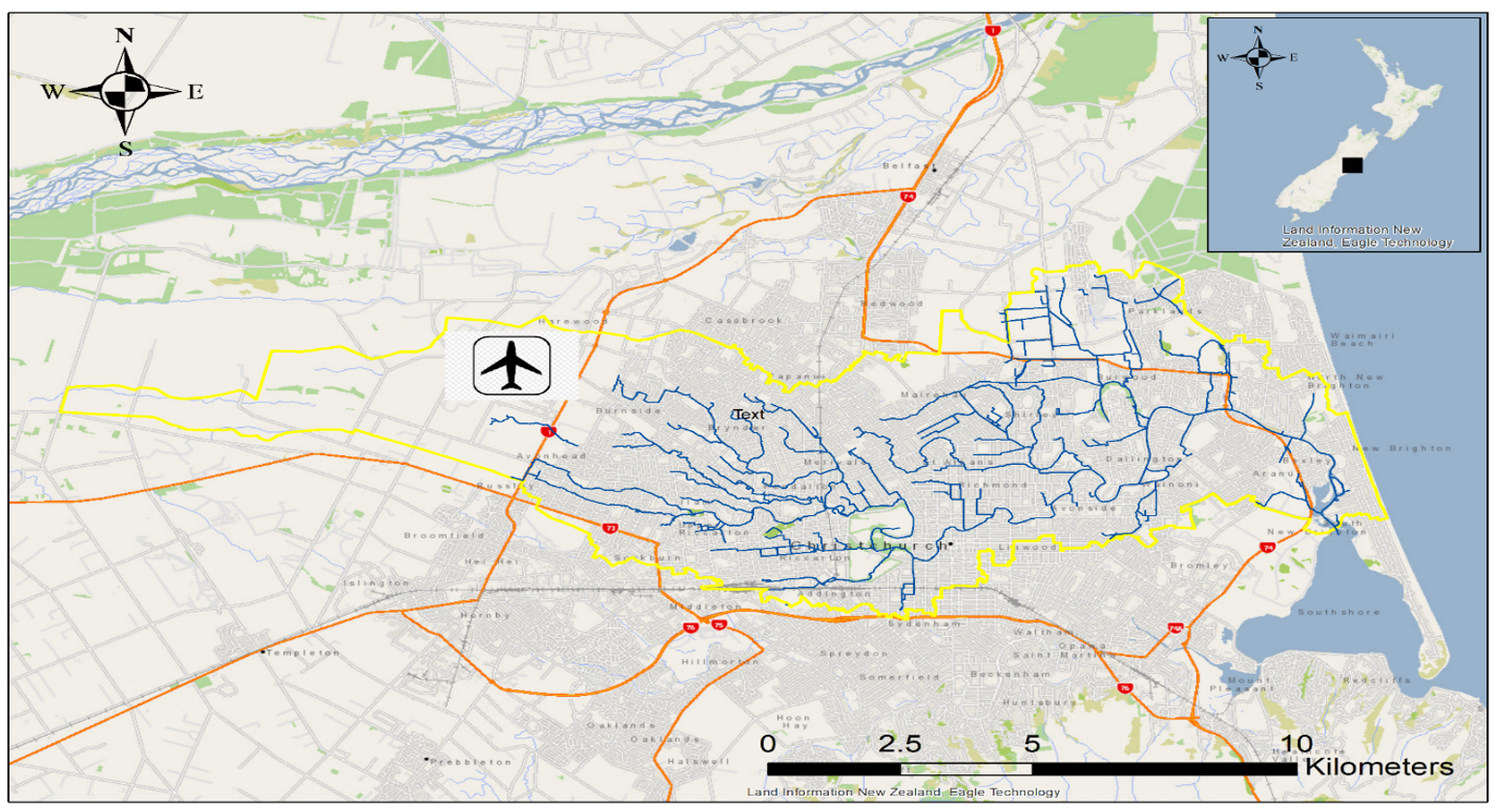
2.1. Study Area
2.2. Data and Methods
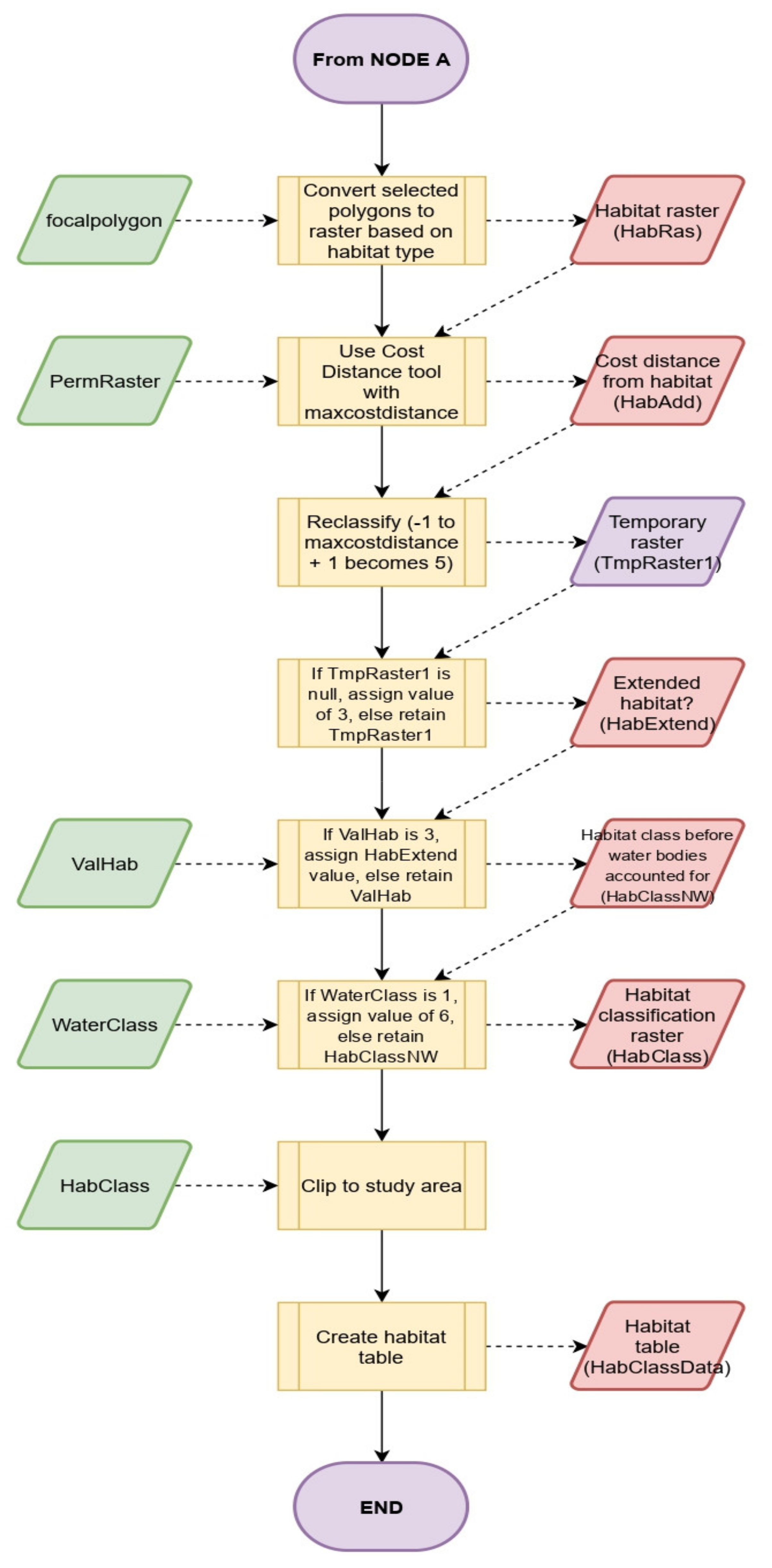
2.2.1. Input Data to Run LUCI Preprocessing
2.2.2. Parameterisation of the Selected Species
- The kereru/NZ wood pigeon
- The putakitaki/paradise shelduck
- The NZ gecko (Haplodactylis)
2.2.3. Land Use Scenarios
2.2.4. Habitat Connectivity Tool BEETLE
2.2.5. Distinct Habitat Connectivity Model Output Areas
3. Results
3.1. Kereru
3.1.1. Existing Potential Networks in and near the Ōtākaro/Avon River Catchment
3.1.2. Future Scenario and Gap Fillers
3.1.3. Changes in Habitat Patch Categories between Scenarios
3.1.4. Changes in Habitat Size
3.2. Paradise Shelduck
3.2.1. Existing Potential Networks in and near the Ōtākaro/Avon River Catchment
3.2.2. Planning Scenario and Gap Fillers
3.2.3. Changes in Habitat Patch Categories between Scenarios
3.3. Geckos
4. Discussion
- Wildlife movement (both transportive and digestive) and plant dispersal adaptations are fundamental to ecosystem integrity that generates a series of spatially determined feedback loops.
- These are all “chickens and eggs”, i.e., there is no beginning (animal?) or end (plant?) so long as the connectivity is maintained.
- Plant dispersal capability (especially in NZ) depends on wildlife (and to lesser extent air/water) movement, which in turn feed into evolving landscape patch growth and patterning, and in turn influence wildlife movement, foraging, and migration.
- Vegetation patterns across the landscape, reacting to hydro-topographic gradients, create physicochemical filtration, as well as food and fibre ecosystem services.
- Human culture is place based and interactive with local nature; local, regional, and planetary sustainability increasingly depends on a healthy understanding (ecological literacy) and relationship with (legible) nature.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


| Matrix Characteristics | Land Cover Types | Movement Cost-Low Cost Indicates High Permeability | |
|---|---|---|---|
| High permeability | Tall forest dominated by indigenous conifer, broadleaved, or beech species | Indigenous forest | Cost = 1 |
| Medium permeability | Open, mainly grassed or sparsely treed, amenity, utility, and recreation areas Gardens or pockets of trees from residential houses, golf resorts, hospitals and schools | Urban parkland/open space | Cost = 4 |
| Low permeability | Artificial surfaces associated with transport Commercial, industrial and roads | Transport infrastructure Built-up area | Cost = 10 |
| Matrix Characteristics | Land Cover Types | Movement Cost-Low Cost Indicates High Permeability | |
|---|---|---|---|
| High permeability | Essentially permanent, open, freshwater without emerging vegetation including artificial features | Lake or pond | Cost = 1 |
| Flowing open freshwater, generally more than 30 m wide and without emerging vegetation | River | ||
| Herbaceous wetland communities occurring in freshwater habitats where the water table is above or just below the substrate surface for most of the year | Herbaceous Freshwater vegetation | ||
| Herbaceous wetland communities occurring in saline habitats subject to tidal inundation or saltwater intrusion | Herbaceous saline vegetation | ||
| Medium permeability | Exotic sward grassland and indigenous short tussock grassland of poor pastoral quality reflecting lower soil fertility and extensive grazing management or non-agricultural use | Low producing grassland | Cost = 3 |
| Exotic sward grassland of good pastoral quality and vigour reflecting relatively high soil fertility and intensive grazing management | High producing exotic grassland | ||
| Low permeability | Artificial surfaces associated with transport | Transport infrastructure | Cost = 10 |
| Commercial, industrial, and roads | Built-up area |
References
- Norbert, N. Differences in the standards living in rural and urban areas. Annu. Univ. Oradea 2020, 29, 79. [Google Scholar]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; Connell, C.O.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef] [Green Version]
- Given, D.R.; Meurk, C.D. Biodiversity of the urban environment: The importance of indigenous species and the role urban environments can play in their preservation. In Urban Biodiversity and Ecology as a Basis for Holistic Planning and Design, Proceedings of a Workshop held at the Lincoln University, Canterbury, New Zealand, 28–29 October 2000; Wickliffe Press: Dunedin, New Zealand, 2020. [Google Scholar]
- Mimet, A.; Kerbiriou, C.; Simon, L.; Julien, J.-F.; Raymond, R. Contribution of private gardens to habitat availability, connectivity and conservation of the common pipistrelle in Paris. Landsc. Urban Plan. 2020, 193, 103671. [Google Scholar] [CrossRef]
- Pellissier, V.; Mimet, A.; Fontaine, C.; Svenning, J.C.; Couvet, D. Relative importance of the land-use composition and intensity for the bird community composition in anthropogenic landscapes. Ecol. Evol. 2017, 7, 10513–10535. [Google Scholar] [CrossRef] [Green Version]
- Wu, J. Urban ecology and sustainability: The state-of-the-science and future directions. Landsc. Urban Plan. 2014, 125, 209–221. [Google Scholar] [CrossRef]
- Bregman, T.P.; Sekercioglu, C.H.; Tobias, J.A. Global patterns and predictors of bird species responses to forest fragmentation: Implications for ecosystem function and conservation. Biol. Conserv. 2014, 169, 372–383. [Google Scholar] [CrossRef]
- Fagan, W.F.; Calabrese, J.M. Quantifying connectivity: Balancing metric performance with data requirements. Connect. Conserv. 2006, 297–317. [Google Scholar] [CrossRef]
- Tischendorf, L.; Fahrig, L. On the usage and measurement of landscape connectivity. Oikos 2000, 90, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Watts, K.; Eycott, A.E.; Handley, P.; Ray, D.; Humphrey, J.W.; Quine, C.P. Targeting and evaluating biodiversity conservation action within fragmented landscapes: An approach based on generic focal species and least-cost networks. Landsc. Ecol. 2010, 25, 1305–1318. [Google Scholar] [CrossRef]
- Xu, X.; Xie, Y.; Qi, K.; Luo, Z.; Wang, X. Detecting the response of bird communities and biodiversity to habitat loss and fragmentation due to urbanization. Sci. Total Environ. 2018, 624, 1561–1576. [Google Scholar] [CrossRef] [PubMed]
- Mayani-Parás, F.; Botello, F.; Castañeda, S.; Munguía-Carrara, M.; Sánchez-Cordero, V. Cumulative habitat loss increases conservation threats on endemic species of terrestrial vertebrates in Mexico. Biol. Conserv. 2021, 253, 108864. [Google Scholar] [CrossRef]
- Møller, A.P.; Diaz, M.; Flensted-Jensen, E.; Grim, T.; Ibáñez-Álamo, J.D.; Jokimäki, J.; Mänd, R.; Markó, G.; Tryjanowski, P. High urban population density of birds reflects their timing of urbanization. Oecologia 2012, 170, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; De Vries, W.; De Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, E.C.; Thorne, C.R.; Yeakley, J.A.; Chan, F.K.S. Sustainable flood risk and stormwater management in blue-green cities; an interdisciplinary case study in Portland, Oregon. JAWRA J. Am. Water Resour. Assoc. 2020, 56, 757–775. [Google Scholar] [CrossRef]
- Pauleit, S.; Ambrose-Oji, B.; Andersson, E.; Anton, B.; Buijs, A.; Haase, D.; Elands, B.; Elands, B.; Hansen, R.; Kronenberg, J.; et al. Advancing urban green infrastructure in Europe: Outcomes and reflections from the GREEN SURGE project. Urban For. Urban Green. 2019, 40, 4–16. [Google Scholar] [CrossRef]
- Ghofrani, Z.; Sposito, V.; Faggian, R. A comprehensive review of blue-green infrastructure concepts. Int. J. Environ. Sustain. 2017, 6. [Google Scholar] [CrossRef]
- Ignatieva, M.; Meurk, C.D.; Van Roon, M.; Simcock, R.; Stewart, G.H. How to Put Nature into Our Neighbourhoods: Application of Low Impact Urban Design and Development (LIUDD) Principles, with a Biodiversity Focus, for New Zealand Developers and Homeowners; Manaaki Whenua Press, Landcare Research: Lincoln, New Zealand, 2008. [Google Scholar]
- Cohen-Shacham, E.; Walters, G.; Janzen, C.; Maginnis, S. Nature-Based Solutions to Address Global Societal Challenges; LUCN: Gland, Switzerland, 2016; p. 97. [Google Scholar] [CrossRef] [Green Version]
- Colls, A.; Ash, N.; Ikkala, N. Ecosystem-Based Adaptation: A Natural Response to Climate Change; IUCN: Gland, Switzerland, 2009; Volume 21. [Google Scholar]
- Federal Emergency Management Agency. Building Community Resilience with Nature-Based Solutions: A Guide for Local Communities; Federal Emergency Management Agency: Washington, DC, USA, 2020. [Google Scholar]
- Stefanakis, A.I. The role of constructed wetlands as green infrastructure for sustainable urban water management. Sustainability 2019, 11, 6981. [Google Scholar] [CrossRef] [Green Version]
- Washington Department of Ecology. Floodplains by Design: Report to the Legislature; Publication No. 19-06-004; Washington Department of Ecology: Washington, DC, USA, 2019. [Google Scholar]
- Davies, R. Landscapes for Life (Stage 2)—An Investigation of Opportunities for Aesthetic Improvement and Biodiversity Enhancement for Living Roofs in New Zealand; Unitec Institute of Technology: Auckland, New Zealand, 2012. [Google Scholar]
- Francis, R.A. Wall ecology: A frontier for urban biodiversity and ecological engineering. Prog. Phys. Geogr. 2011, 35, 43–63. [Google Scholar] [CrossRef]
- Francis, R.A.; Lorimer, J. Urban reconciliation ecology: The potential of living roofs and walls. J. Environ. Manag. 2011, 92, 1429–1437. [Google Scholar] [CrossRef]
- Murray, C.G.; Kasel, S.; Loyn, R.H.; Hepworth, G.; Hamilton, A.J. Waterbird use of artificial wetlands in an Australian urban landscape. Hydrobiologia 2013, 716, 131–146. [Google Scholar] [CrossRef]
- Christchurch City Council. Ōtākaro/Avon River Stormwater Management Plan; Christchurch City Council: Christchurch, New Zealand, 2015. [Google Scholar]
- Dymond, J.R. Ecosystem Services in New Zealand: Conditions and Trends; Manaaki Whenua Press, Landcare Research: Lincoln, New Zealand, 2013. [Google Scholar]
- Wei, Q. Vegetation Change and Native Forest Restoration in Urban Environments: Management Options for Post-Earthquake Christchurch. Ph.D. Thesis, Lincoln University, Canterbury, New Zealand, 2020. [Google Scholar]
- Regenerate Christchurch. The Ōtākaro/Avon River Corridor Regeneration Plan; Regenerate Christchurch: Christchurch, New Zealand, 2019. [Google Scholar]
- Watts, K.; Humphrey, J.W.; Griffiths, M.; Quine, C.; Ray, D. Evaluating biodiversity in fragmented landscapes: Principles. Inf. Note For. Comm. 2005, 73, 8. [Google Scholar]
- Ranius, T.; Johansson, V.; Fahrig, L. A comparison of patch connectivity measures using data on invertebrates in hollow oaks. Ecography 2010, 33, 971–978. [Google Scholar] [CrossRef]
- Chen, C.; Meurk, C.D.; Ignatieva, M.; Stewart, G.H.; Wu, S. Identifying and evaluating functional connectivity for building urban ecological networks. Acta Ecol. Sin. 2015, 35, 18–35. [Google Scholar] [CrossRef] [Green Version]
- Adriaensen, F.; Chardon, J.; De Blust, G.; Swinnen, E.; Villalba, S.; Gulinck, H.; Matthysen, E. The application of ‘least-cost’ modelling as a functional landscape model. Landsc. Urban Plan. 2003, 64, 233–247. [Google Scholar] [CrossRef]
- Balbi, M.; Petit, E.J.; Croci, S.; Nabucet, J.; Georges, R.; Madec, L.; Ernoult, A. Ecological relevance of least cost path analysis: An easy implementation method for landscape urban planning. J. Environ. Manag. 2019, 244, 61–68. [Google Scholar] [CrossRef]
- Piemontese, M.; Onal, M.; Xiong, J.; Wang, Y.; Almeida, M.; Thostenson, J.D.; Weinstein, R.S.; Manolagas, S.C.; O’Brien, C.A. Suppression of autophagy in osteocytes does not modify the adverse effects of glucocorticoids on cortical bone. Bone 2015, 75, 18–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, B.; Pagella, T.; Sinclair, F.; Orellana, B.; Henshaw, A.; Reynolds, B.; Mcintyre, N.; Wheater, H.; Eycott, A. Polyscape: A GIS mapping framework providing efficient and spatially explicit landscape-scale valuation of multiple ecosystem services. Landsc. Urban Plan. 2013, 112, 74–88. [Google Scholar] [CrossRef]
- Emmett, B.E.; The GMEP Team. Glastir Monitoring & Evaluation Programme; Final Report to Welsh Government—Executive Summary (Contract Reference: C147/2010/11); NERC/Centre for Ecology & Hydrology (CEH Projects: NEC04780/NEC05371/NEC05782): Wallingford, UK, 2017. [Google Scholar]
- McGann, R. The Climate of Christchurch; Ministry of Transport, New Zealand Meteorological Service: Wellington, New Zealand, 1983; Volume 167.
- Potter, S.H.; Becker, J.S.; Johnston, D.M.; Rossiter, K.P. An overview of the impacts of the 2010–2011 Canterbury earthquakes. Int. J. Disaster Risk Reduct. 2015, 14, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, B.; Bryan, C.; Clarkson, F. Reconstructing hamilton’s indigenous ecosystems: The waiwhakareke natural heritage park. City Green 2012, 4, 60–67. [Google Scholar] [CrossRef]
- Sullivan, J.J.; Meurk, C.; Whaley, K.J.; Simcock, R. Restoring native ecosystems in urban Auckland: Urban soils, isolation, and weeds as impediments to forest establishment. N. Z. J. Ecol. 2009, 33, 60–71. [Google Scholar]
- Stewart, G.H.; Ignatieva, M.E.; Meurk, C.D.; Earl, R.D. The re-emergence of indigenous forest in an urban environment, Christchurch, New Zealand. Urban For. Urban Green. 2004, 2, 149–158. [Google Scholar] [CrossRef]
- Morgenroth, J.A. Tree Canopy Cover in Christchurch, New Zealand; Report prepared for the Christchurch City Council; Christchurch City Council: Christchurch, New Zealand, 2017. [Google Scholar]
- Land Information New Zealand. Canterbury—Christchurch and Ashley River LiDAR 1m DEM (2018–2019). 2020. Available online: https://data.linz.govt.nz/layer/104497-canterbury-christchurch-and-ashley-river-lidar-1m-dem-2018-2019/ (accessed on 20 December 2020).
- Landcare Research New Zealand. LCDB v5.0—Land Cover Database Version 5.0, Mainland New Zealand. 2020. Available online: https://lris.scinfo.org.nz/layer/104400-lcdb-v50-land-cover-database-version-50-mainland-new-zealand/ (accessed on 15 February 2021).
- Meurk, C.D.; Hall, G.M. Options for enhancing forest biodiversity across New Zealand’s managed landscapes based on ecosystem modelling and spatial design. N. Z. J. Ecol. 2006, 30, 131–146. [Google Scholar]
- Daglish, L. City Pigeons: Kererū (Hemiphaga novaeseelandiae) in the Urban Dunedin Environment: Abundance, Habitat Selection and Rehabilitation Outcomes. Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2005. [Google Scholar]
- Wotton, D.M.; Drake, D.R.; Powlesland, R.G.; Ladley, J.J. The role of lizards as seed dispersers in New Zealand. J. R. Soc. N. Z. 2016, 46, 40–65. [Google Scholar] [CrossRef]
- Walker, S.; Cieraad, E.; Barringer, J. The Threatened Environment Classification for New Zealand 2012: A Guide for Users; Report LC2184; Landcare Research: Dunedin, New Zealand, 2015. [Google Scholar]
- Clarkson, B.; Kirby, C.; Wallace, K. Restoration Targets for Biodiversity Depleted Environments in New Zealand; Environmental Research Institute Te Pūtahi Rangahau Taiao, The University of Waikato: Hamilton, New Zealand, 2018. [Google Scholar]
- Schotborgh, H.M. An Analysis of Home Ranges, Movements, Foods, and Breeding of Kereru (Hemiphaga novaeseelandiae) in a Rural-Urban Landscape on Banks Peninsula, New Zealand; Lincoln University: Canterbury, New Zealand, 2005. [Google Scholar]
- Wilson, D.J.; Mulvey, R.L.; Clarke, D.A.; Reardon, J.T. Assessing and comparing population densities and indices of skinks under three predator management regimes. N. Z. J. Ecol. 2017, 41, 84–97. [Google Scholar] [CrossRef] [Green Version]
- Woolley, C.K.; Hartley, S.; Hitchmough, R.A.; Innes, J.G.; Van Heezik, Y.; Wilson, D.J.; Nelson, N.J. Reviewing the past, present and potential lizard faunas of New Zealand cities. Landsc. Urban Plan. 2019, 192, 103647. [Google Scholar] [CrossRef]
- Riccarton Bush TRUST. Annual Report 2016. Available online: https://riccartonhouse.co.nz/wp-content/uploads/2017/04/Riccarton-Bush-Trust-RBT-Annual-Report-2016-Signed-with-Audit-Report.pdf (accessed on 3 March 2021).
- Meurk, C.D. Evergreen Broadleaved Forests of New Zealand and Their Bioclimatic Definition; Box, E.O., Peet, R.K., Masuzawa, T., Yamada, I., Eds.; Landcare Research New Zealand Inc.: Lincoln, New Zealand, 1995; pp. 151–197. [Google Scholar]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.; Meurk, C.; Benavidez, R.; Jackson, B.; Pahlow, M. The Effect of Blue-Green Infrastructure on Habitat Connectivity and Biodiversity: A Case Study in the Ōtākaro/Avon River Catchment in Christchurch, New Zealand. Sustainability 2021, 13, 6732. https://doi.org/10.3390/su13126732
Nguyen TT, Meurk C, Benavidez R, Jackson B, Pahlow M. The Effect of Blue-Green Infrastructure on Habitat Connectivity and Biodiversity: A Case Study in the Ōtākaro/Avon River Catchment in Christchurch, New Zealand. Sustainability. 2021; 13(12):6732. https://doi.org/10.3390/su13126732
Chicago/Turabian StyleNguyen, Thuy Thi, Colin Meurk, Rubianca Benavidez, Bethanna Jackson, and Markus Pahlow. 2021. "The Effect of Blue-Green Infrastructure on Habitat Connectivity and Biodiversity: A Case Study in the Ōtākaro/Avon River Catchment in Christchurch, New Zealand" Sustainability 13, no. 12: 6732. https://doi.org/10.3390/su13126732






