Sustainable Innovation in Membrane Technologies for Produced Water Treatment: Challenges and Limitations
Abstract
:1. Introduction
1.1. Produced Water from the Oil and Gas Industry
1.2. Produced Water Treatment Methods
- Minimal environmental impact, reduced energy costs, and highly automated installations.
- Widespread in oil exploration onshore and offshore.
- There is no requirement for chemical additives.
- During such processes, the membrane can be used to recycle the waste streams.
1.3. Research Prospective
2. Materials and Methods
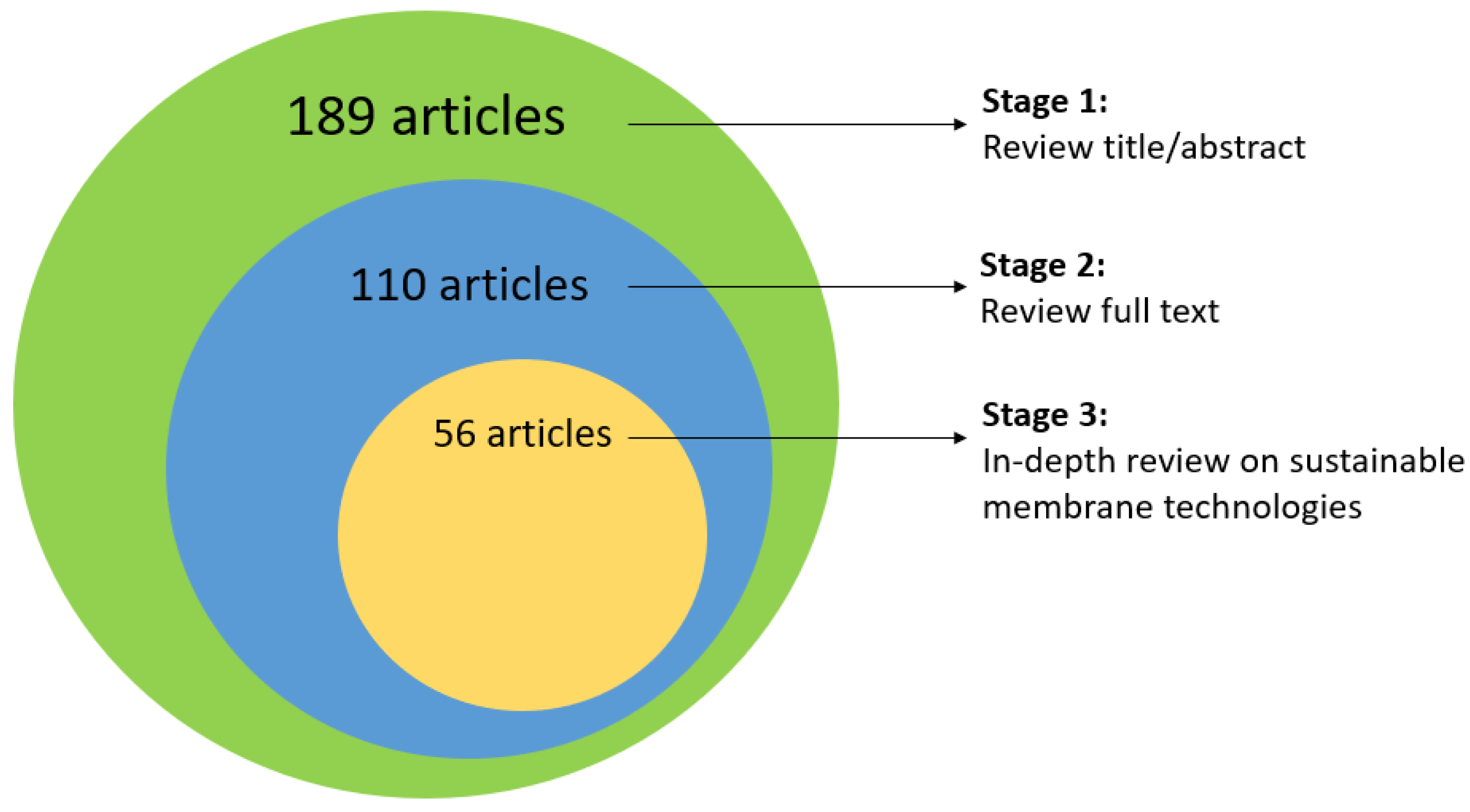
2.1. Review Protocol
- Technology-related terms: [innovative membrane technologies, water desalination, membrane distillation wastewater purification, industrial water discharge, wastewater treatment plants (WWTPS), produced wastewater, water processing].
- Sustainability-related terms: [environmental assessment, life cycle assessment, environmental sustainability, economic analysis].
2.2. Research Data
3. Results
3.1. Thermally-Driven Membrane Process
3.2. Pressure-Driven Membrane Process
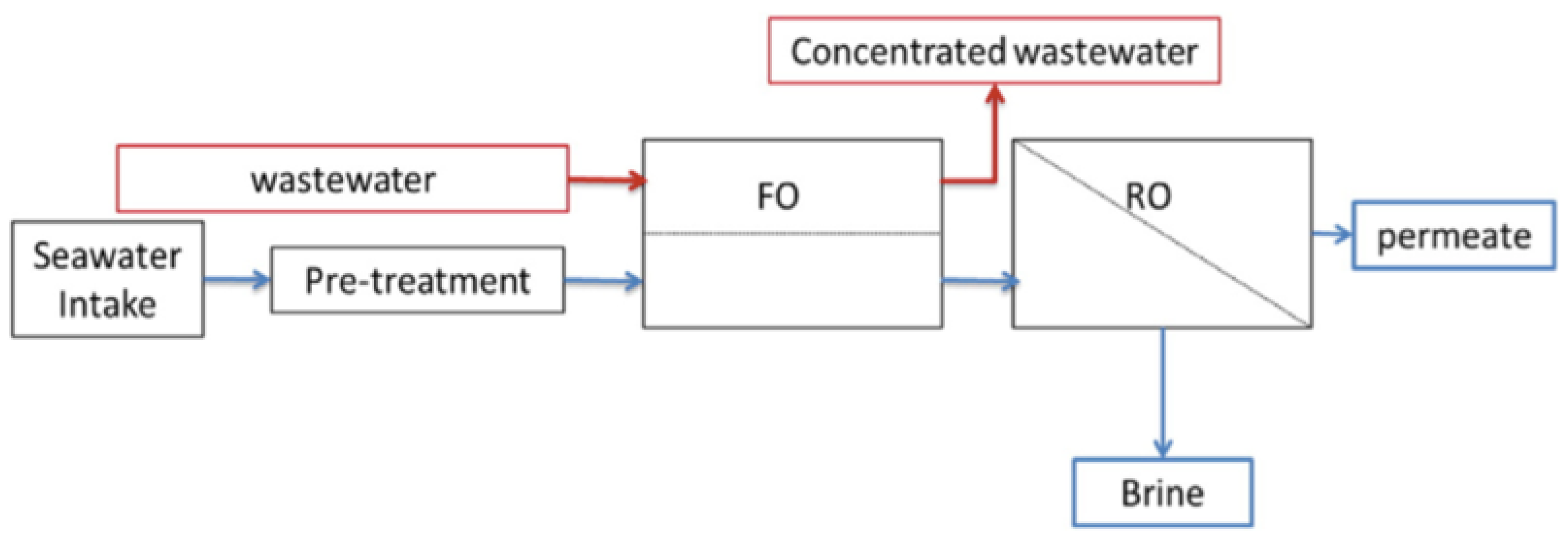
3.3. Hybrid Membrane Process
4. Discussion
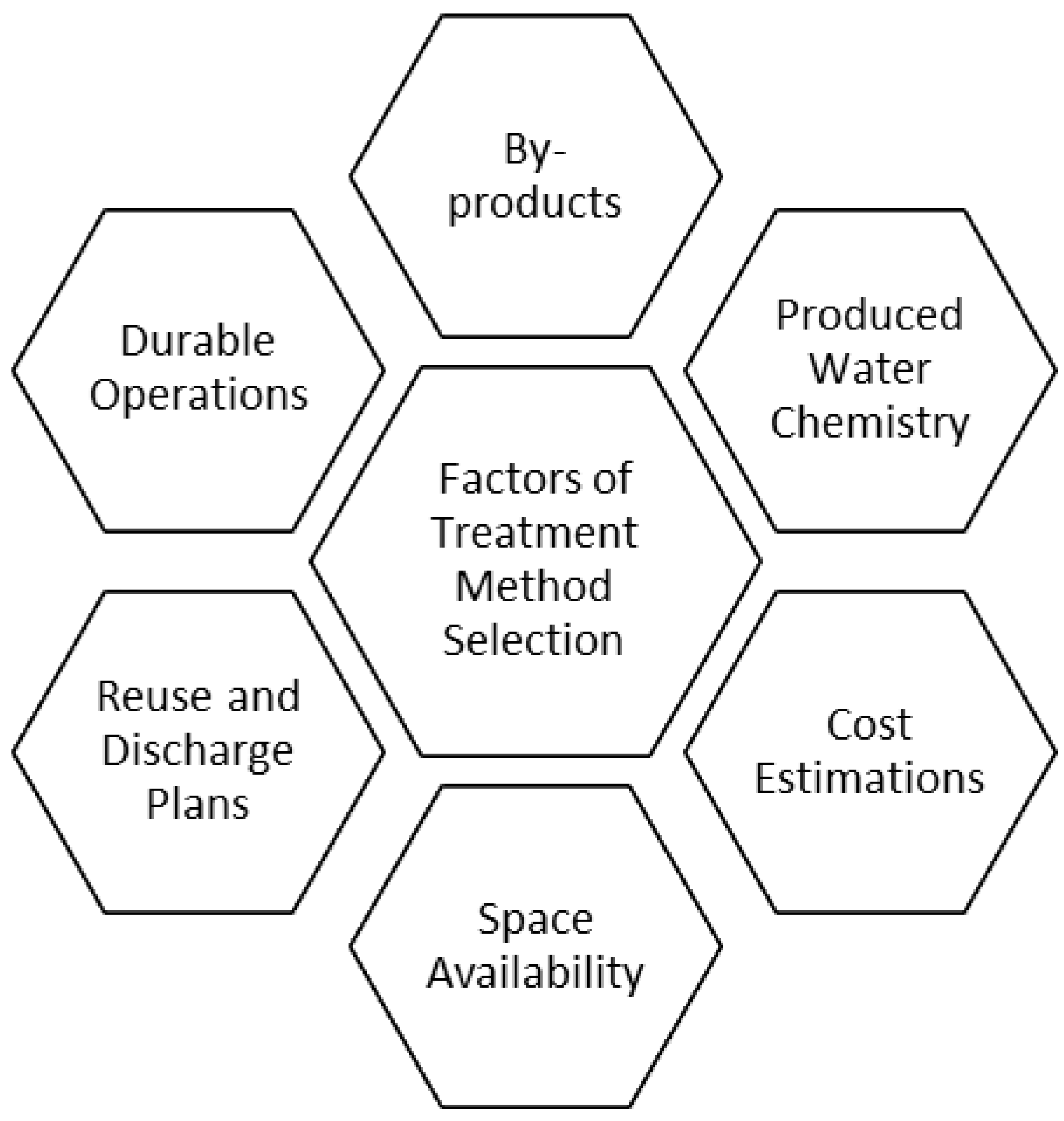
4.1. Grand Challenges in Membrane Technology
4.1.1. Operational Cost Analysis
4.1.2. Energy Consumption of Membrane Systems
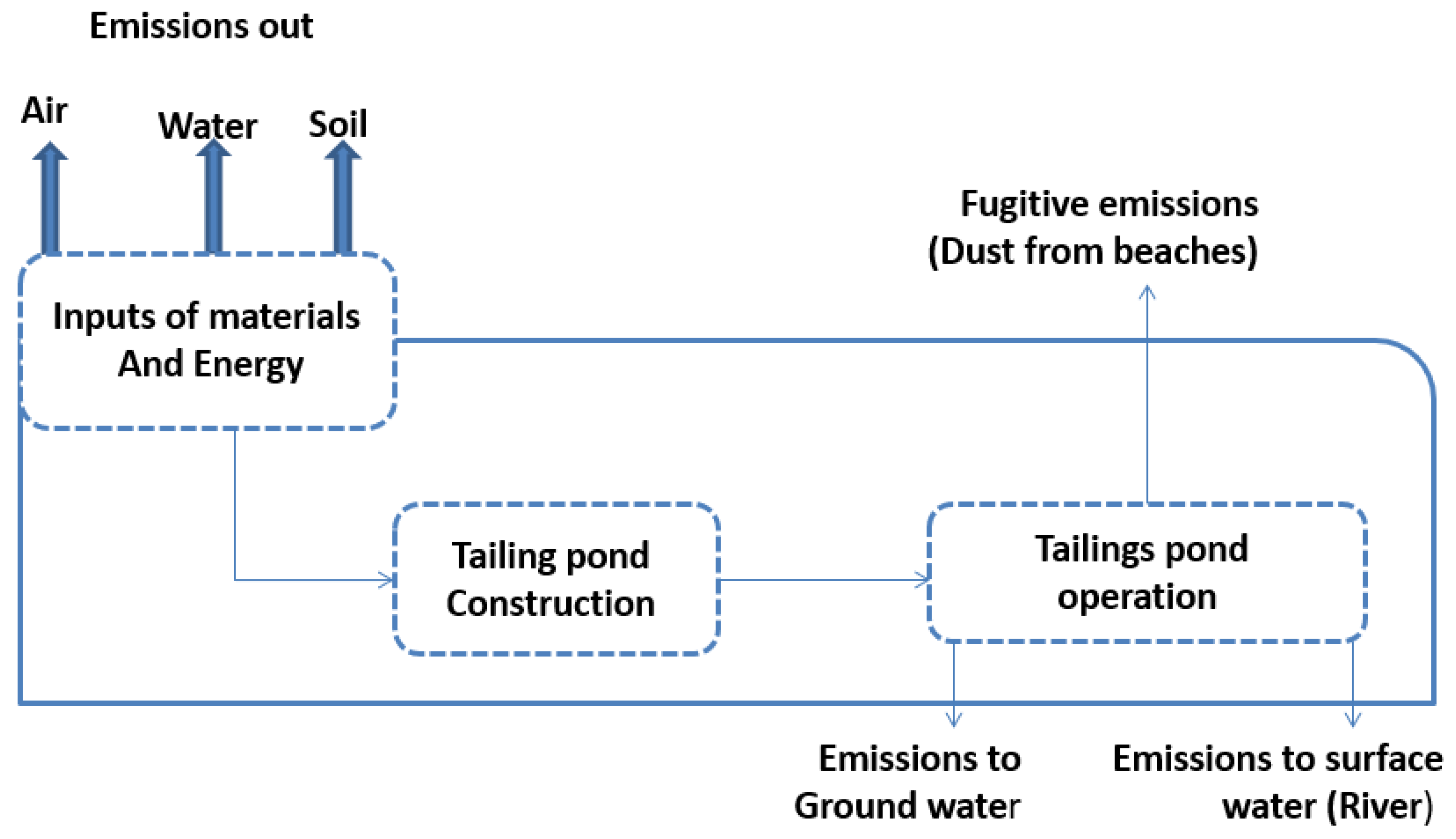
4.1.3. Environmental Analysis
4.2. Limitations and Constraints of Membrane Technology
4.2.1. Energy Usage and Costs
4.2.2. Environmental Constraints
4.3. Sustainable Innovation in Membrane Technology
4.3.1. Mechanism of Sustainable Technology
4.3.2. Feasibility of the Hybrid System
4.3.3. Future Potential Applications
5. Future Perspective
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahim, T.H.; Sabri, M.A.; Khamis, M.I. Application of multiwalled carbon nanotubes and its magnetite derivative for emulsified oil removal from produced water. Environ. Technol. 2019, 40, 3337–3350. [Google Scholar] [CrossRef]
- Estrada, J.M.; Bhamidimarri, R. A review of the issues and treatment options for wastewater from shale gas extraction by hydraulic fracturing. Fuel 2016, 182, 292–303. [Google Scholar] [CrossRef]
- Clay, L.; Pichtel, J. Treatment of Simulated Oil and Gas Produced Water via Pilot-Scale Rhizofiltration and Constructed Wetlands. Int. J. Environ. Res. 2019, 13, 185–198. [Google Scholar] [CrossRef]
- Mohammed, T.; Abbas, E.; Ahmed, T. Turbidity and oil removal from oilfield produced water, middle oil company by electrocoagulation technique. In Proceedings of the MATEC Web of Conferences. EDP Sci. 2018, 162, 5010. [Google Scholar]
- Li, L.; Al-Muntasheri, G.A.; Liang, F. A review of crosslinked fracturing fluids prepared with produced water. Petroleum 2016, 2, 313–323. [Google Scholar] [CrossRef]
- Faksness, L.G.; Grini, P.G.; Daling, P.S. Partitioning of semi-soluble organic compounds between the water phase and oil droplets in produced water. Mar. Pollut. Bull. 2004, 48, 731–742. [Google Scholar] [CrossRef]
- Frid, C. Bioaccumulation in Marine Organisms. Effect of Contaminants from Oil Well Produced Water. Org. Geochem. 2003, 34, 149. [Google Scholar] [CrossRef]
- Tibbetts, P.J.C.; Buchanan, I.T.; Gawel, L.J.; Large, R. A Comprehensive Determination of Produced Water Composition. Prod. Water 1992, 97–112. [Google Scholar] [CrossRef]
- Fathima, A.; Almohsin, A.; Michael, F.M.; Bataweel, M.; Alsharaeh, E.H. Polymer nanocomposites for water shutoff application-A review. Mater. Res. Express 2018, 6, 32001. [Google Scholar] [CrossRef]
- Esmaeilirad, N.; Carlson, K.; Ozbek, P.O. Influence of softening sequencing on electrocoagulation treatment of produced water. J. Hazard. Mater. 2015, 283, 721–729. [Google Scholar] [CrossRef]
- Sappington, E.N.; Rifai, H.S. Low-frequency electromagnetic treatment of oilfield produced water for reuse in agriculture: Effect on water quality, germination, and plant growth. Environ. Sci. Pollut. Res. 2018, 25, 34380–34391. [Google Scholar] [CrossRef]
- Smith, A.P.; Van De Ven, C.J.C.; Richardson, S.D. Current water management practices, challenges, and innovations for US unconventional oil and gas development. Curr. Sustain. Energy Rep. 2017, 4, 209–218. [Google Scholar] [CrossRef]
- Bakke, T.; Klungsøyr, J.; Sanni, S. Environmental impacts of produced water and drilling waste discharges from the Norwegian offshore petroleum industry. Mar. Environ. Res. 2013, 92, 154–169. [Google Scholar] [CrossRef] [Green Version]
- Giri, S.S.; Harshiny, M.; Sen, S.S.; Sukumaran, V.; Park, S.C. Production and characterization of a thermostable bioflocculant from Bacillus subtilis F9, isolated from wastewater sludge. Ecotoxicol. Environ. Saf. 2015, 121, 45–50. [Google Scholar] [CrossRef]
- He, K.; Borthwick, A.G.; Lin, Y.; Li, Y.; Fu, J.; Wong, Y.; Liu, W. Sale-based estimation of pharmaceutical concentrations and associated environmental risk in the Japanese wastewater system. Environ. Int. 2020, 139, 105690. [Google Scholar] [CrossRef]
- Pica, N.E.; Carlson, K.; Steiner, J.J.; Waskom, R. Produced water reuse for irrigation of non-food biofuel crops: Effects on switchgrass and rapeseed germination, physiology and biomass yield. Ind. Crop. Prod. 2017, 100, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Dolan, F.C.; Cath, T.Y.; Hogue, T.S. Assessing the feasibility of using produced water for irrigation in Colorado. Sci. Total Environ. 2018, 640–641, 619–628. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Macedonio, F.; Drioli, E. Membrane technology for water production in agriculture: Desalination and wastewater reuse. Desalination 2015, 364, 17–32. [Google Scholar] [CrossRef]
- Lyu, S.; Chen, W.; Zhang, W.; Fan, Y.; Jiao, W. Wastewater reclamation and reuse in China: Opportunities and challenges. J. Environ. Sci. 2016, 39, 86–96. [Google Scholar] [CrossRef]
- Zoubeik, M.; Ismail, M.; Salama, A.; Henni, A. New developments in membrane technologies used in the treatment of produced water: A review. Arab. J. Sci. Eng. 2018, 43, 2093–2118. [Google Scholar] [CrossRef]
- Oshinowo, L.M.; Quintero, C.G.; Vilagines, R.D. CFD and population balance modeling of crude oil emulsions in batch gravity separation—comparison to ultrasound experiments. J. Dispers. Sci. Technol. 2016, 37, 665–675. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, Q.; Zhang, B.; Tian, F. Three-phase hydrocyclone separator–A review. Chem. Eng. Res. Des. 2015, 100, 554–560. [Google Scholar] [CrossRef]
- Ashoor, B.B.; Mansour, S.; Giwa, A.; Dufour, V.; Hasan, S.W. Principles and applications of direct contact membrane distillation (DCMD): A comprehensive review. Desalination 2016, 398, 222–246. [Google Scholar] [CrossRef]
- Jepsen, K.; Hansen, L.; Mai, C.; Yang, Z. Challenges of membrane filtration for produced water treatment in offshore oil & gas production. In Proceedings of the OCEANS 2016 MTS/IEEE Monterey, Monterey, CA, USA, 19–23 September 2016; pp. 1–8. [Google Scholar]
- Gurreri, L.; Cipollina, A.; Tamburini, A.; Micale, G. Electrodialysis for wastewater treatment—Part II: Industrial effluents. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 195–241. [Google Scholar]
- Azeez, R.A. Removal oil from produced water by using adsorption method with adsorbent a Papyrus reeds. Eng. Technol. J. 2019, 37, 157–165. [Google Scholar]
- Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baselines; World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF): Geneva, Switzerland, 2017.
- Kargari, A.; Shirazi, M.J.A. Direct contact membrane distillation for seawater desalination AU—Shirazi, Mohammad Mahdi A. Desalin. Water Treat. 2012, 49, 368–375. [Google Scholar] [CrossRef]
- Piesse, M. Global Water Supply and Demand Trends Point Towards Rising Water Insecurity; Future Directions International: Nedlands, Australia, 2020. [Google Scholar]
- Annual Report 2019; Qatar Electricity & Water Co.: Doha, Qatar, 2019.
- Mondal, S.; Wickramasinghe, S.R. Produced water treatment by nanofiltration and reverse osmosis membranes. J. Memb. Sci. 2008, 322, 162–170. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, R.; Tian, M.; Qiu, C.; Fane, A.G. Fabrication of polyvinylidene fluoride (PVDF) nanofiber membranes by electro-spinning for direct contact membrane distillation. J. Memb. Sci. 2013, 425–426, 30–39. [Google Scholar] [CrossRef]
- Lokare, O.R.; Tavakkoli, S.; Wadekar, S.; Khanna, V.; Vidic, R.D. Fouling in direct contact membrane distillation of produced water from unconventional gas extraction. J. Memb. Sci. 2017, 524, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kwon, H.; Lee, S.; Lee, S.; Hong, S. Membrane distillation (MD) integrated with crystallization (MDC) for shale gas produced water (SGPW) treatment. Desalination 2017, 403, 172–178. [Google Scholar] [CrossRef]
- Xiong, B.; Zydney, A.L.; Kumar, M. Fouling of microfiltration membranes by flowback and produced waters from the Marcellus shale gas play. Water Res. 2016, 99, 162–170. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, X.; Carlson, K.H.; Robbins, C.A.; Tong, T. Effective treatment of shale oil and gas produced water by membrane distillation coupled with precipitative softening and walnut shell filtration. Desalination 2019, 454, 82–90. [Google Scholar] [CrossRef]
- Abdelrazeq, H.; Khraisheh, M.; Al Momani, F.; McLeskey, J.T.; Hassan, M.K.; Gad-el-Hak, M.; Tafreshi, H.V. Performance of electrospun polystyrene membranes in synthetic produced industrial water using direct-contact membrane distillation. Desalination 2020, 493, 114663. [Google Scholar] [CrossRef]
- Esteves, R.J.A.; Gornick, V.; Alqurwani, D.S.; Koenig-Lovejoy, J.; Abdelrazeq, H.; Khraisheh, M.; Forzano, A.V.; Gad-el-Hak, M.; Tafreshi, H.V.; McLeskey, J.T. Activated carbon-doped polystyrene fibers for direct contact membrane desalination. Emergent Mater. 2020, 3, 807–814. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H.; Khraisheh, M. membranes Polydopamine Functionalized Graphene Oxide as Membrane Nanofiller: Spectral and Structural Studies. Membranes 2021, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Akyon, B.; Lipus, D.; Bibby, K. Glutaraldehyde inhibits biological treatment of organic additives in hydraulic fracturing produced water. Sci. Total Environ. 2019, 666, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Lutzu, G.A.; Dunford, N.T. Algal treatment of wastewater generated during oil and gas production using hydraulic fracturing technology. Environ. Technol. 2019, 40, 1027–1034. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Moore, F.; Mowla, D.; Keshavarzi, B. Improved waste-sourced biocomposite for simultaneous removal of crude oil and heavy metals from synthetic and real oilfield-produced water. Environ. Sci. Pollut. Res. 2018, 25, 31407–31420. [Google Scholar] [CrossRef]
- Cho, H.; Choi, Y.; Lee, S. Effect of pretreatment and operating conditions on the performance of membrane distillation for the treatment of shale gas wastewater. Desalination 2018, 437, 195–209. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Kim, W.; Francis, L.; Amy, G.; Ghaffour, N. Performance modeling of direct contact membrane distillation (DCMD) seawater desalination process using a commercial composite membrane. J. Memb. Sci. 2015, 478, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Ke, H.; Feldman, E.; Guzman, P.; Cole, J.; Wei, Q.; Chu, B.; Alkhudhiri, A.; Alrasheed, R.; Hsiao, B.S. Electrospun polystyrene nanofibrous membranes for direct contact membrane distillation. J. Memb. Sci. 2016, 515, 86–97. [Google Scholar] [CrossRef]
- Khayet, M.; Wang, R. Mixed Matrix Polytetrafluoroethylene/Polysulfone Electrospun Nanofibrous Membranes for Water Desalination by Membrane Distillation. ACS Appl. Mater. Interfaces 2018, 10, 24275–24287. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.S. Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. J. Memb. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- Cong, H.; Chuai, D.; Chul, Y.; Kyong, H.; Duc, L. A novel electrospun, hydrophobic, and elastomeric styrene-butadiene-styrene membrane for membrane distillation applications. J. Memb. Sci. 2018, 549, 420–427. [Google Scholar] [CrossRef]
- Wang, K.; Hou, D.; Wang, J.; Wang, Z.; Tian, B.; Liang, P. Applied Surface Science Hydrophilic surface coating on hydrophobic PTFE membrane for robust anti-oil-fouling membrane distillation. Appl. Surf. Sci. 2018, 450, 57–65. [Google Scholar] [CrossRef]
- Lee, E.J.; An, A.K.; Hadi, P.; Lee, S.; Woo, Y.C.; Shon, H.K. Advanced multi-nozzle electrospun functionalized titanium dioxide/polyvinylidene fluoride-co-hexafluoropropylene (TiO2/PVDF-HFP) composite membranes for direct contact membrane distillation. J. Memb. Sci. 2017, 524, 712–720. [Google Scholar] [CrossRef]
- Lalia, B.S.; Guillen-Burrieza, E.; Arafat, H.A.; Hashaikeh, R. Fabrication and characterization of polyvinylidenefluoride-co-hexafluoropropylene (PVDF-HFP) electrospun membranes for direct contact membrane distillation. J. Memb. Sci. 2013, 428, 104–115. [Google Scholar] [CrossRef]
- Lalia, B.S.; Guillen, E.; Arafat, H.A.; Hashaikeh, R. Nanocrystalline cellulose reinforced PVDF-HFP membranes for membrane distillation application. Desalination 2014, 332, 134–141. [Google Scholar] [CrossRef]
- Essalhi, M.; Khayet, M. Self-sustained webs of polyvinylidene fluoride electrospun nanofibers at different electrospinning times: 1. Desalination by direct contact membrane distillation. J. Memb. Sci. 2013, 433, 167–179. [Google Scholar] [CrossRef]
- Al-Obaidani, S.; Curcio, E.; Macedonio, F.; Di Profio, G.; Al-Hinai, H.; Drioli, E. Potential of membrane distillation in seawater desalination: Thermal efficiency, sensitivity study and cost estimation. J. Memb. Sci. 2008, 323, 85–98. [Google Scholar] [CrossRef]
- Liang, B.; Pan, K.; Li, L.; Giannelis, E.P.; Cao, B. High performance hydrophilic pervaporation composite membranes for water desalination. Desalination 2014, 347, 199–206. [Google Scholar] [CrossRef]
- Chew, N.G.P.; Zhao, S.; Malde, C.; Wang, R. Polyvinylidene fluoride membrane modification via oxidant-induced dopamine polymerization for sustainable direct-contact membrane distillation. J. Memb. Sci. 2018, 563, 31–42. [Google Scholar] [CrossRef]
- El-Bourawi, M.S.; Ding, Z.; Ma, R.; Khayet, M. A framework for better understanding membrane distillation separation process. J. Memb. Sci. 2006, 285, 4–29. [Google Scholar] [CrossRef]
- Wu, J.; Wang, L.; Dong, H.; Zhao, Y.; Jiang, L. Electrospun Porous Structure Fibrous Film with High Oil Adsorption Capacity. ACS Appl. Mater. Interfaces 2012, 4, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, C.; Yang, Y.; Wang, X.; Zhu, M.; Hsiao, B.S. Dual-biomimetic superhydrophobic electrospun polystyrene nanofibrous membranes for membrane distillation. ACS Appl. Mater. Interfaces 2014, 6, 2423–2430. [Google Scholar] [CrossRef]
- Li, X.; Deng, L.; Yu, X.; Wang, M.; Wang, X.; García-Payo, C.; Khayet, M. A novel profiled core-shell nanofibrous membrane for wastewater treatment by direct contact membrane distillation. J. Mater. Chem. A 2016, 4, 14453–14463. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, A.; Ciacchi, L.C.; Wei, G. Recent Advances in Nanoporous Membranes for Water Purification. Nanomaterials 2018, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- Dervin, S.; Dionysiou, D.D.; Pillai, S.C. 2D nanostructures for water purification: Graphene and beyond. Nanoscale 2016, 8, 15115–15131. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane materials for water purification: Design, development, and application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 1–16. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2014, 356, 15–30. [Google Scholar] [CrossRef]
- Ullah, R.; Khraisheh, M.; Esteves, R.J.; McLeskey, J.T.; AlGhouti, M.; Gad-el-Hak, M.; Vahedi Tafreshi, H. Energy efficiency of direct contact membrane distillation. Desalination 2018, 433, 56–67. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Marchand, E.A.; Childress, A.E. The forward osmosis membrane bioreactor: A low fouling alternative to MBR processes. Desalination 2009, 239, 10–21. [Google Scholar] [CrossRef]
- McGinnis, R.L.; Elimelech, M. Energy requirements of ammonia-carbon dioxide forward osmosis desalination. Desalination 2007, 207. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; McGinnis, R.L.; Elimelech, M. A novel ammonia-carbon dioxide forward (direct) osmosis desalination process. Desalination 2005, 174. [Google Scholar] [CrossRef]
- Martinetti, C.R.; Childress, A.E.; Cath, T.Y. High recovery of concentrated RO brines using forward osmosis and membrane distillation. J. Memb. Sci. 2009, 331. [Google Scholar] [CrossRef]
- Petrotos, K.B.; Lazarides, H.N. Osmotic concentration of liquid foods. J. Food Eng. 2001, 49, 201–206. [Google Scholar] [CrossRef]
- Sardari, K.; Fyfe, P.; Lincicome, D.; Wickramasinghe, S.R. Aluminum electrocoagulation followed by forward osmosis for treating hydraulic fracturing produced waters. Desalination 2018, 428, 172–181. [Google Scholar] [CrossRef]
- Kiss, Z.L.; Kovács, I.; Veréb, G.; Hodúr, C.; László, Z. Treatment of model oily produced water by combined pre-ozonation–microfiltration process. Desalin. Water Treat. 2016, 57, 23225–23231. [Google Scholar] [CrossRef] [Green Version]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination-Development to date and future potential. J. Memb. Sci. 2011, 370, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Chong, T.H.; Wong, F.S.; Fane, A.G. Implications of critical flux and cake enhanced osmotic pressure (CEOP) on colloidal fouling in reverse osmosis: Experimental observations. J. Memb. Sci. 2008, 314. [Google Scholar] [CrossRef]
- Ba-Alawi, A.H.; Ifaei, P.; Li, Q.; Nam, K.J.; Djeddou, M.; Yoo, C.K. Process assessment of a full-scale wastewater treatment plant using reliability, resilience, and econo-socio-environmental analyses (R2ESE). Process Saf. Environ. Prot. 2020, 133, 259–274. [Google Scholar] [CrossRef]
- Dudek, M.; Vik, E.A.; Aanesen, S.V.; Øye, G. Colloid chemistry and experimental techniques for understanding fundamental behaviour of produced water in oil and gas production. Adv. Colloid Interface Sci. 2020, 276, 102105. [Google Scholar] [CrossRef]
- Turková, J.; Korytárová, J. Methods for Evaluation of WWTPs Environmental Impacts: A Review. IOP Conf. Ser. Earth Environ. Sci. 2019, 222. [Google Scholar] [CrossRef]
- OSPAR. Background Document concerning Techniques for the Management of Produced Water from Offshore Installations; OSPAR Commission: Sintra, Portugal, 2013. [Google Scholar]
- Awad, H.; Gar Alalm, M.; El-Etriby, H.K. Environmental and cost life cycle assessment of different alternatives for improvement of wastewater treatment plants in developing countries. Sci. Total Environ. 2019, 660, 57–68. [Google Scholar] [CrossRef]
- Wenzlick, M.; Siefert, N. Techno-economic analysis of converting oil & gas produced water into valuable resources. Desalination 2020, 481, 114381. [Google Scholar] [CrossRef]
- Echchelh, A.; Hess, T.; Sakrabani, R. Reusing oil and gas produced water for irrigation of food crops in drylands. Agric. Water Manag. 2018, 206, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Arzate, S.; Pfister, S.; Oberschelp, C.; Sánchez-Pérez, J.A. Environmental impacts of an advanced oxidation process as tertiary treatment in a wastewater treatment plant. Sci. Total Environ. 2019, 694, 1–10. [Google Scholar] [CrossRef]
- Jackson, L.M.; Myers, J.E. Design and Construction of Pilot Wetlands for Produced-Water Treatment. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, Colorado, 5–8 October 2003; pp. 1–8. [Google Scholar]
- Echchelh, A.; Hess, T.; Sakrabani, R.; de Paz, J.M.; Visconti, F. Assessing the environmental sustainability of irrigation with oil and gas produced water in drylands. Agric. Water Manag. 2019, 223, 105694. [Google Scholar] [CrossRef]
- Environmental and Social Risk Briefing Oil & Gas; Barclays Bank PLC: London, UK, 2015; pp. 1–26.
- Ebrahimi, M.; Kovacs, Z.; Schneider, M.; Mund, P.; Bolduan, P.; Czermak, P. Multistage filtration process for efficient treatment of oil-field produced water using ceramic membranes. Desalin. Water Treat. 2012, 42, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Memon, S.; Kim, Y.D.; Soomro, S.; Soomro, M.I.; Kim, W.S. A new approach for freshwater production and energy recovery from an oil field. J. Water Process Eng. 2020, 34, 101145. [Google Scholar] [CrossRef]
- Hoogmartens, R.; Van Passel, S.; Van Acker, K.; Dubois, M. Bridging the gap between LCA, LCC and CBA as sustainability assessment tools. Environ. Impact Assess. Rev. 2014, 48, 27–33. [Google Scholar] [CrossRef]
- Risch, E.; Loubet, P.; Núñez, M.; Roux, P. How environmentally significant is water consumption during wastewater treatment?: Application of recent developments in LCA to WWT technologies used at 3 contrasted geographical locations. Water Res. 2014, 57, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Molinos-Senante, M.; Hernández-Sancho, F.; Sala-Garrido, R. Economic feasibility study for new technological alternatives in wastewater treatment processes: A review. Water Sci. Technol. 2012, 65, 898–906. [Google Scholar] [CrossRef]
- Al-Dosary, S.; Galal, M.M.; Abdel-Halim, H. Environmental Impact Assessment of Wastewater Treatment Plants. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 953–964. [Google Scholar]
- Dixon, A.; Simon, M.; Burkitt, T. Assessing the environmental impact of two options for small-scale wastewater treatment: Comparing a reedbed and an aerated biological filter using a life cycle approach. Ecol. Eng. 2003, 20, 297–308. [Google Scholar] [CrossRef]
- McLaughlin, M.C.; Blotevogel, J.; Watson, R.A.; Schell, B.; Blewett, T.A.; Folkerts, E.J.; Goss, G.G.; Truong, L.; Tanguay, R.L.; Argueso, J.L.; et al. Mutagenicity assessment downstream of oil and gas produced water discharges intended for agricultural beneficial reuse. Sci. Total Environ. 2020, 715, 136944. [Google Scholar] [CrossRef]
- Beylot, A.; Villeneuve, J. Accounting for the environmental impacts of sulfidic tailings storage in the Life Cycle Assessment of copper production: A case study. J. Clean. Prod. 2017, 153, 139–145. [Google Scholar] [CrossRef]
- EPA Life Cycle Assessment: Principles and Practice. Vasa 2008, 88, 1–6. [CrossRef]
- Planning and Statistics Authority. Infographic Indicators: Qatar’s Sustainable Development Goals 2018; National Development Strategy: Doha, Qatar, 2018. [Google Scholar]
- Hao, X.; Wang, X.; Liu, R.; Li, S.; van Loosdrecht, M.C.M.; Jiang, H. Environmental impacts of resource recovery from wastewater treatment plants. Water Res. 2019, 160, 268–277. [Google Scholar] [CrossRef]
- Barjoveanu, G.; Comandaru, I.M.; Teodosiu, C. Life Cycle Assessment of Water and Wastewater Treatment Systems: An overview. Bull. Polytech. Inst. Iasi Chem. Chem. Eng. Sect. 2010, LVI, 73–86. [Google Scholar]
- Adham, S.; Hussain, A.; Minier-Matar, J.; Janson, A.; Sharma, R. Membrane applications and opportunities for water management in the oil & gas industry. Desalination 2018, 440, 2–17. [Google Scholar] [CrossRef]
- Memon, F.A.; Zheng, Z.; Butler, D.; Shirley-Smith, C.; Lui, S.; Makropoulos, C.; Avery, L. Life cycle impact assessment of greywater recycling technologies for new developments. Environ. Monit. Assess. 2007, 129, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, J.; Koo-Oshima, S. Water desalination for agricultural applications. In Proceedings of the FAO Expert Consultation on Water Desalination for Agricultural Applications, Rome, Italy, 26–27 April 2006. [Google Scholar]
- Barron, O.; Ali, R.; Hodgson, G.; Smith, D.; Qureshi, E.; McFarlane, D.; Campos, E.; Zarzo, D. Feasibility assessment of desalination application in Australian traditional agriculture. Desalination 2015, 364, 33–45. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marĩas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Feitelson, E.; Rosenthal, G. Desalination, space and power: The ramifications of Israel’s changing water geography. Geoforum 2012, 43, 272–284. [Google Scholar] [CrossRef]
- March, H.; Saurí, D.; Rico-Amorós, A.M. The end of scarcity? Water desalination as the new cornucopia for Mediterranean Spain. J. Hydrol. 2014, 519, 2642–2651. [Google Scholar] [CrossRef] [Green Version]
- Semiat, R. Energy issues in desalination processes. Environ. Sci. Technol. 2008, 42, 8193–8201. [Google Scholar] [CrossRef]
- McCarty, P.L.; Bae, J.; Kim, J. Domestic wastewater treatment as a net energy producer-can this be achieved? Environ. Sci. Technol. 2011, 45, 7100–7106. [Google Scholar] [CrossRef]
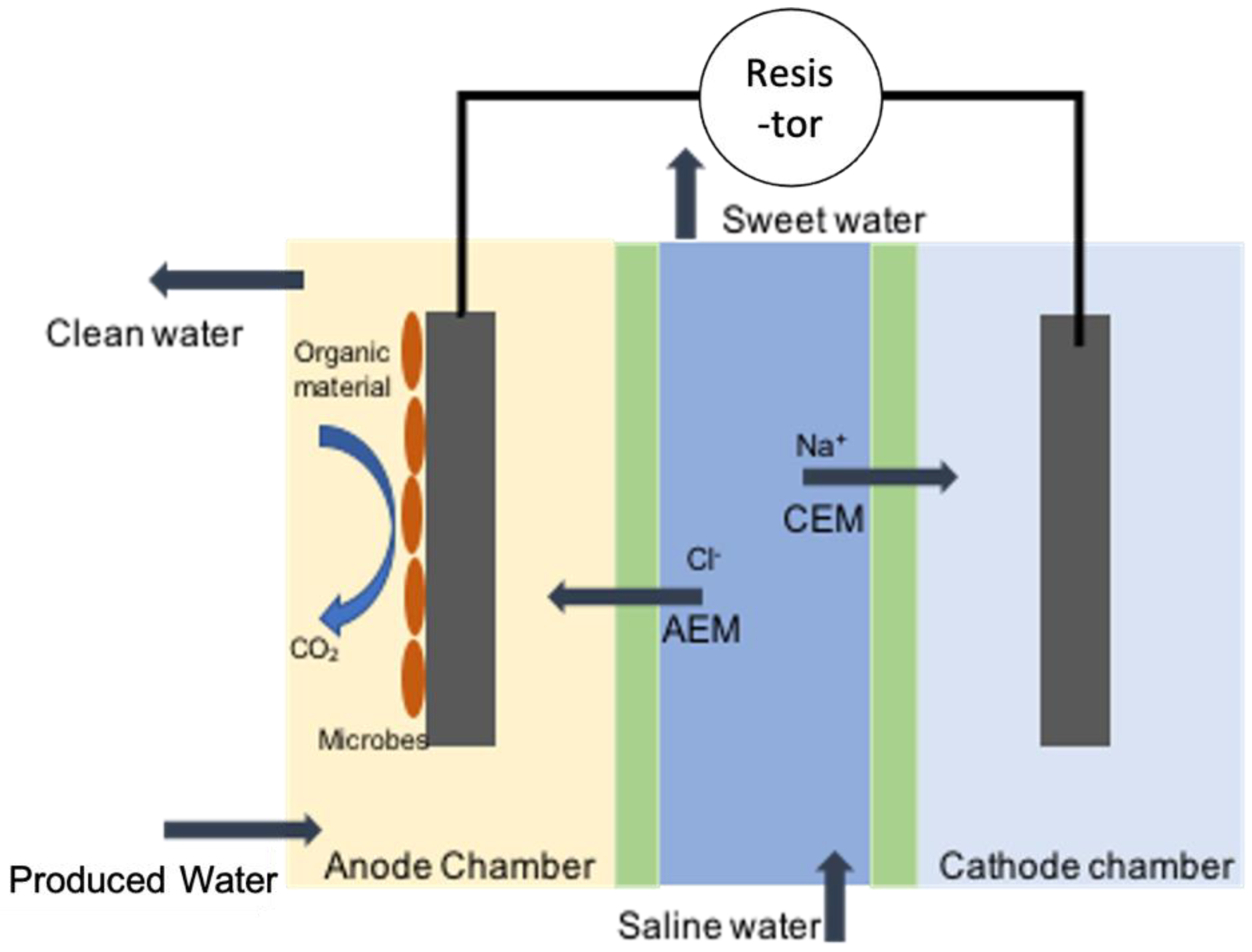
- Cao, X.; Huang, X.; Liang, P.; Xiao, K.; Zhou, Y.; Zhang, X.; Logan, B.E. A new method for water desalination using microbial desalination cells. Environ. Sci. Technol. 2009, 43, 7148–7152. [Google Scholar] [CrossRef]
- Kokabian, B.; Gude, V.G. Sustainable photosynthetic biocathode in microbial desalination cells. Chem. Eng. J. 2015, 262, 958–965. [Google Scholar] [CrossRef]
- Kim, Y.; Logan, B.E. Microbial desalination cells for energy production and desalination. Desalination 2013, 308, 122–130. [Google Scholar] [CrossRef]
- Chen, X.; Xia, X.; Liang, P.; Cao, X.; Sun, H.; Huang, X. Stacked microbial desalination cells to enhance water desalination efficiency. Environ. Sci. Technol. 2011, 45, 2465–2470. [Google Scholar] [CrossRef] [PubMed]
- Ping, Q.; He, Z. Improving the flexibility of microbial desalination cells through spatially decoupling anode and cathode. Bioresour. Technol. 2013, 144, 304–310. [Google Scholar] [CrossRef]
- Forrestal, C.; Xu, P.; Ren, Z. Sustainable desalination using a microbial capacitive desalination cell. Energy Environ. Sci. 2012, 5, 7161–7167. [Google Scholar] [CrossRef]
- Kim, Y.; Logan, B.E. Series assembly of microbial desalination cells containing stacked electrodialysis cells for partial or complete seawater desalination. Environ. Sci. Technol. 2011, 45, 5840–5845. [Google Scholar] [CrossRef]
- Liu, G.; Luo, H.; Tang, Y.; Chen, S.; Zhang, R.; Hou, Y. Tetramethylammonium hydroxide production using the microbial electrolysis desalination and chemical-production cell. Chem. Eng. J. 2014, 258, 157–162. [Google Scholar] [CrossRef]
- Zhang, Y.; Angelidaki, I. A new method for in situ nitrate removal from groundwater using submerged microbial desalination-denitrification cell (SMDDC). Water Res. 2013, 47, 1827–1836. [Google Scholar] [CrossRef]
- Zuo, K.; Cai, J.; Liang, S.; Wu, S.; Zhang, C.; Liang, P.; Huang, X. A ten liter stacked microbial desalination cell packed with mixed ion-exchange resins for secondary effluent desalination. Environ. Sci. Technol. 2014, 48, 9917–9924. [Google Scholar] [CrossRef]
- Cath, T.Y.; Childress, A.E.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Memb. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.; Tang, C.Y.; Mulcahy, D. Recent developments in forward osmosis: Opportunities and challenges. J. Memb. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- Lutchmiah, K.; Verliefde, A.R.D.; Roest, K.; Rietveld, L.C.; Cornelissen, E.R. Forward osmosis for application in wastewater treatment: A review. Water Res. 2014, 58, 179–197. [Google Scholar] [CrossRef]
- Liu, Z.; Bai, H.; Lee, J.; Sun, D.D. A low-energy forward osmosis process to produce drinking water. Energy Environ. Sci. 2011, 4, 2582–2585. [Google Scholar] [CrossRef]
- Qin, M.; He, Z. Self-Supplied Ammonium Bicarbonate Draw Solute for Achieving Wastewater Treatment and Recovery in a Microbial Electrolysis Cell-Forward Osmosis-Coupled System. Environ. Sci. Technol. Lett. 2014, 1. [Google Scholar] [CrossRef]
- Lu, Y.; Qin, M.; Yuan, H.; Abu-Reesh, I.M.; He, Z. When bioelectrochemical systems meet forward osmosis: Accomplishing wastewater treatment and reuse through synergy. Water 2015, 7, 38–50. [Google Scholar] [CrossRef]
- Zhang, B.; He, Z. Improving water desalination by hydraulically coupling an osmotic microbial fuel cell with a microbial desalination cell. J. Memb. Sci. 2013, 441, 18–24. [Google Scholar] [CrossRef]
- Konieczny, K.H.; Wszelaka-Rylik, M.; Macherzyński, B. Membrane processes innovation in environmental protection: Review. Arch. Environ. Prot. 2019, 45, 20–29. [Google Scholar] [CrossRef]
- Ping, Q.; Zhang, C.; Chen, X.; Zhang, B.; Huang, Z.; He, Z. Mathematical model of dynamic behavior of microbial desalination cells for simultaneous wastewater treatment and water desalination. Environ. Sci. Technol. 2014, 48, 13010–13019. [Google Scholar] [CrossRef]
- Ping, Q.; He, Z. Effects of inter-membrane distance and hydraulic retention time on the desalination performance of microbial desalination cells. Desalin. Water Treat. 2014, 52, 1324–1331. [Google Scholar] [CrossRef]
- Ping, Q.; Cohen, B.; Dosoretz, C.; He, Z. Long-term investigation of fouling of cation and anion exchange membranes in microbial desalination cells. Desalination 2013, 325, 48–55. [Google Scholar] [CrossRef]
- Yuan, L.; Yang, X.; Liang, P.; Wang, L.; Huang, Z.H.; Wei, J.; Huang, X. Capacitive deionization coupled with microbial fuel cells to desalinate low-concentration salt water. Bioresour. Technol. 2012, 110, 735–738. [Google Scholar] [CrossRef]
- Morel, A.; Zuo, K.; Xia, X.; Wei, J.; Luo, X.; Liang, P.; Huang, X. Microbial desalination cells packed with ion-exchange resin to enhance water desalination rate. Bioresour. Technol. 2012, 118, 243–248. [Google Scholar] [CrossRef]
- Lin, H.; Peng, W.; Zhang, M.; Chen, J.; Hong, H.; Zhang, Y. A review on anaerobic membrane bioreactors: Applications, membrane fouling and future perspectives. Desalination 2013, 314, 169–188. [Google Scholar] [CrossRef]
- Kim, S.L.; Paul Chen, J.; Ting, Y.P. Study on feed pretreatment for membrane filtration of secondary effluent. Sep. Purif. Technol. 2002, 29, 171–179. [Google Scholar] [CrossRef]
- Alzahrani, S.; Mohammad, A.W. Challenges and trends in membrane technology implementation for produced water treatment: A review. J. Water Process Eng. 2014, 4, 107–133. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Da’na, D.A. Produced water characteristics, treatment and reuse: A review. J. Water Process Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]




| Density | pH | TOC * | TSS * | COD * | Total Oils | Volatiles |
|---|---|---|---|---|---|---|
| [kg/m3] | [mg/L] | [mg/L] | [mg/L] | [mg/L] | [mg/L] | |
| 1014–1140 | 4.3–10 | 0–1500 | 1.2–1000 | 1220 | 2–565 | 0.35–0.39 |
| Chloride | Bicarbonate | Sulfate | Sulphide | Ammoniacal nitrogen | Higher Acids | Phenols |
| [mg/L] | [mg/L] | [mg/L] | [mg/L] | [mg/L] | [mg/L] | [mg/L] |
| 80–200,000 | 77–3990 | <2–1650 | 10 | 10–300 | <1–63 | 0.009–23 |
| Produced Water Treatment Methods | |||||
|---|---|---|---|---|---|
| Microfiltration | Ultrafiltration | Reverse Osmosis | Adsorption | Ion-Exchange | |
| Advantages | ►High recovery of fresh water | ►High recovery of fresh water | ►Compact module ►Removes dossilved contaminants | ►Cheap ►Efficient ►Compact | ►Low energy required ►Continuous treatment possible |
| Drawbacks | ►High energy required ►Low effeciency | ►High energy required ►High membrane fouling | ►Requires high pressure ►Small traces of grease may cause memb/oil causes membrane fouling | ►Low effeciency at high feed concentrations ►High retention time | ►Requires pre-treatment ►Requires post-treatment |
| Type of Wastewater | Main Contaminants | Treatment Objectives | Membrane Technologies | Limitations |
|---|---|---|---|---|
| Produced water |
|
|
|
|
| Municipal wastewater |
|
|
|
|
| Method | Cost ($/bbl) | Limitations | Benefits |
|---|---|---|---|
| Surface discharge | 0.01–0.08 | Energy costs | Livestock, irrigation |
| Secondary recovery | 0.05–1.25 | Infrastructure | Increase production |
| Commercial water hauling | 0.01–5.50 | Distance | - |
| Shallow reinjection | 0.10–1.33 | Energy and maintenance | Recharge aquifer |
| Evaporation pits | 0.01–0.80 | Soil contamination | Livestock impoundment |
| Constructed wetland | 0.001–2.00 | Land area | Communities, education |
| Cost Parameter | Membrane Technology | |
|---|---|---|
| Thermally-Driven | Pressure-Driven | |
| Capital cost (US $/m3) | 0.449 | 0.301 |
| Energy (US $/m3) | 0.555 | 0.25–0.27 |
| Labour (US $/m3) | 0.128 | 0.128 |
| Chemicals (US $/m3) | 0.024–0.045 | 0.018–0.054 |
| Membrane replacement (US $/m3) | 0 | 0.001–0.072 |
| Maintenance (US $/m3) | 0.018–0.032 | 0.018–0.032 |
| Total costs (US $/m3) | 1.10–1.15 | 0.45–0.877 |
| Cell | COD Removal % | Water Recovery (mL) | The rate of Decrease in TDS (g/L.d) |
|---|---|---|---|
| MDC-FO | 43.7 ± 1.3 | 153.6 ± 6.7 | 22.7 ± 5.3 |
| MDC | 40.2 ± 2.5 | - | 11.2 ± 0.3 |
| FO | - | 166.4 ± 6.2 | 20.1 ± 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrazeq, H.; Khraisheh, M.; Ashraf, H.M.; Ebrahimi, P.; Kunju, A. Sustainable Innovation in Membrane Technologies for Produced Water Treatment: Challenges and Limitations. Sustainability 2021, 13, 6759. https://doi.org/10.3390/su13126759
Abdelrazeq H, Khraisheh M, Ashraf HM, Ebrahimi P, Kunju A. Sustainable Innovation in Membrane Technologies for Produced Water Treatment: Challenges and Limitations. Sustainability. 2021; 13(12):6759. https://doi.org/10.3390/su13126759
Chicago/Turabian StyleAbdelrazeq, Haneen, Majeda Khraisheh, Hafsa Mohammed Ashraf, Parisa Ebrahimi, and Ansaruddin Kunju. 2021. "Sustainable Innovation in Membrane Technologies for Produced Water Treatment: Challenges and Limitations" Sustainability 13, no. 12: 6759. https://doi.org/10.3390/su13126759








