1. Introduction
According to the Food and Agriculture Organization’s (FAO) definition, sustainable diets have “low environmental impacts which contribute to food and nutrition security and to healthy life for present and future generations. Sustainable diets are protective and respectful of biodiversity and ecosystems, culturally acceptable, accessible, economically fair and affordable; nutritionally adequate, safe and healthy; while optimizing natural and human resources” [
1].
Unfortunately, food consumption and production have become more complex over time as food undergoes intricate processes, as well as longer and further transportation. These unsustainable patterns have led to the deterioration of the environment, accelerating climate change, and increasing natural resource degradation, compromising human health [
2,
3,
4]. Current food systems generate adverse outcomes in water and soil, biodiversity loss, and excessive greenhouse gas emissions [
5]. Other food production problems are the decrease in arable land and overfishing in the ocean [
6]. The FAO has reported that the percentage of fish stocks within a biologically sustainable level has progressively declined from 90% in 1990 to 65.8% in 2017 [
7].
The current food system has failed to protect the environment and has not assured access to sufficient and nutritionally adequate food for everyone. Despite world biodiversity and increase in food production and availability, there are still millions of people suffering from undernourishment, food insecurity, and hunger. At the same time, more than one billion are overweight or obese [
3,
5]. With the exponential global population growth, there is a general concern about achieving adequate nutrition; it is estimated that by 2030, the worldwide population will reach 8.5 billion, with 1.4 billion being over 60 years old [
1,
8]. The food production system will face serious challenges to meet the needs of both an aging and expanding population, mainly to cover the protein requirements [
1].
Protein plays a critical role in preventing protein-energy malnutrition and promotes healthy muscle aging, but animal-derived protein causes significant environmental damage, as most of it is produced inefficiently [
1,
4,
9]. Animal-derived protein requires large areas of dedicated land, water, nitrogen, and fossil energy for production and transportation, resulting in the emission of large amounts of greenhouse gases. Therefore, exploring alternative protein sources and transitioning towards more sustainable diets has been a recent research priority [
1]. As part of this quest, the nutritional and environmental profiles of alternative protein sources such as plant-based foods, insects, single-cell protein (mycoprotein or microalgae), and in vitro meat have been investigated [
10].
A sustainable and relatively novel food source of nutritional compounds are microalgae. These organisms can produce large amounts of high biological value proteins, long-chain polyunsaturated fatty acids, carotenoids, vitamins, minerals, and phenolic compounds, among others [
11,
12,
13]. Microalgae do not compete for arable land as other food sources do and, depending on their purpose, can even be grown using non-potable water, wastewater, or even salty water. Another advantage they present is their high biomass yield per unit area [
6,
13]. Microalgae include microscopic algae, eukaryotic, and photosynthetic bacteria, also known as prokaryotic cyanobacteria [
8]. Cyanobacteria, or blue-green algae, are considered part of this group due to their physiology similarities and their ability to perform photosynthesis. One of the most important genera of blue-green algae is
Arthrospira sp., with the most important species being
Spirulina platensis and
Spirulina maxima [
14,
15].
Spirulina platensis, or spirulina, is a planktonic, filamentous cyanobacterium found in tropical and subtropical alkaline warm lakes with high pH values and salt concentrations [
14,
15,
16]. One morphological feature of spirulina is the spiral shape of its multicellular cylindrical filaments in an open helix with lengths from 0.3 to 1.0 mm [
17].
These microalgae are extensively cultivated as they can have several applications in food, feed, nutraceuticals, pharmaceuticals, and cosmeceuticals [
18]. It is used in human nutrition not only because of its high protein content (up to 70% w/w) but also because of its desirable amino acid (AA) profile regarding the amount of essential AA and good digestibility, making it a potential alternative protein source [
19,
20,
21]. Additionally, microalgae proteins also have promising techno-functional properties, used as foaming, gelling, and emulsifying agents [
19,
20]. Some studies have shown that microalgal proteins can be competitive with some commercial proteins used as emulsifiers like sodium caseinate, whey protein, and soy protein [
19,
22].
Microalgae are also an essential source of minerals, principally Fe, Zn, Mn, and Cu [
23], as well as hydrosoluble vitamins (B complex and C) and vitamin E [
24].
S. platensis is rich in vitamin B
12, as it has been reported that it can contain between 1.6 and 3.2 μg of this vitamin per gram of dried mass, which can cover between 25 and 133% of the daily intake recommendations [
25]. In the last few years, microalgae biomass has been successfully applied as an additive for food products, giving innovative and healthy alternatives such as vegetarian cookies, pasta, biscuits, mayonnaise, sausages, and gelled desserts [
12,
26,
27]. Some results have shown that the addition of
S. platensis at different levels improved some physicochemical and sensorial attributes in wheat pasta such as water absorption capacity, texture, color, as well as antioxidant capacity [
28]. Meanwhile, subjecting
S. platensis to pre-treatments with lactic acid fermentation has shown to enhance the aromatic characteristics and decrease off-flavors to extend its applications in food products [
29].
Therefore, this study aimed to characterize food prototypes (pasta and mayonnaise) added with Spirulina platensis protein (SPP) for developing highly nutritional products for specific population requirements.
2. Materials and Methods
First, the proximate composition, fatty acid profile, and mineral content of the Spirulina platensis biomass were determined. Next, protein from
S. platensis was isolated, characterized, and studied for its use as a food additive in two food product prototypes (pasta and mayonnaise). Additionally, the developed products were characterized.
Spirulina platensis biomass (frozen) was purchased from “Galtec Algae Technologies, Guadalupe, Mexico”, amaranth seeds (
Amaranthus cruentus) were obtained from “Mesoamérica para el Mundo S.C. de R.L,” the brown algae (
Macrocystis pyrifera) were purchased from “Algas Pacific, Ensenada, Mexico”, and chia seeds (
Salvia hispanica) were supplied by “El Shaddai, Oaxaca, Mexico”. Soybean (SB) oil and wheat flour were purchased in local supermarkets. Sigma-Aldrich Co. (Saint Louis, MO, USA), Bio-rad (Hercules, CA, USA), and Merck (Darmstadt, Germany) were suppliers of soybean protein (SBP), sodium alginate, amino acid standard mix, and all reagents. A summary of the methodology followed in this study is shown in
Figure 1.
2.1. Proximate Analysis
The proximate composition of the raw materials and pasta was determined following the AOAC procedures [
30]. Nitrogen was determined by the Kjeldahl method (954.01), approximately 1 g of sample was hydrolyzed mixing 15 mL of sulfuric acid and copper tablets (as catalyzer) at 420 °C for 2 h; then, the samples were neutralized and titrated for calculating the protein content using a 6.25 value as nitrogen conversion factor.
The fat content was determined by Soxhlet extraction (920.39) using n-hexane as the solvent. The solvent was heated up until boiling and refluxed to extract the fat. After the full extraction, the fat content was calculated by weight difference.
The ash content (923.03) was determined gravimetrically after incineration at 600 °C for 2 h. For the crude fiber determination (962.09), the samples were digested with sulfuric acid (1.25% v/v) and rinsed with distilled water for neutralization, followed by alkaline digestion with NaOH (1.25% w/v). After neutralization, the sample was dried in a convection oven (135 °C) and incinerated at 600 °C. The fiber content was calculated by weight difference. The moisture content was determined gravimetrically by drying in a convection oven at 135 °C until constant weight (925.09). Finally, the carbohydrate content was estimated by difference.
2.2. Fatty Acid Profile
The fatty acid profile was determined by gas chromatography coupled with a flame ionization detector (GC-FID/capillary column, Thermo Scientific Trace 1310; Waltham, MA, USA). A split/splitless injector and a Phenomenex Zebron ZB-FAME column (30 m × 0.25 mm, 0.2 µm; Torrance, CA, USA) were used. The methodology followed was the AOAC 996.06 procedure and the fatty acids identification was carried out by comparing the relative retention times of the fatty acid methyl esters (FAME) peaks from samples with commercial standards and quantified with an internal standard. The results were analyzed using the software Chromeleon v7.2.1 (Thermo Scientific, Waltham, MA, USA) and expressed in relative percentages. The commercial standard for the fatty acid profile was Supelco 37 FAME Mix (CRM47885, Sigma-Aldrich), and undecanoic acid (Sigma-Aldrich) was used as an internal standard.
2.3. Protein Extraction
Spirulina platensis protein (SPP) was extracted using an alkaline solution, followed by isoelectric precipitation. Defatted microalga powder was dispersed in a NaOH solution (0.05 M) adjusted to pH 9 using HCl (0.1 M) in a ratio of 1:10. The dispersion was pre-treated with mechanical lysis using an ultrasound device (EW-04711–70 Cole-Parmer, Vernon Hills, IL, USA) at 70% of amplitude and 100 μm of wavelength and two intervals of 2.5 min. Then, the microalgae dispersion was stirred and heated in a water bath (Cimarec™ Series, Thermo Fisher, Waltham, MA, USA) at 60 °C for 60 min. The mixture was centrifuged at 6000 rpm for 25 min at 4 °C and the supernatant was acidified to pH 3 using HCl (1 M). The precipitated protein was neutralized, dialyzed (Spectra/Por MWCO: 12–14 kDa, Fisher Scientific, Waltham, MA) against distilled water for 24 h and freeze-dried [
31].
2.4. Characterization of Spirulina Platensis Protein (SPP)
2.4.1. Protein Content Determination
The protein content was determined using the Peterson modification of Lowry’s method [
32]. Protein solutions were prepared at a concentration of 1 mg/mL using a solution of NaOH (0.05 N, pH 13) and stirred until fully dissolved. An aliquot of 500 µL of protein solution was used and it was mixed with 50 µL of deoxycholate solution (0.15%) and allowed to stand for 10 min. Next, each sample was mixed with 50 µL of trichloroacetic acid (72%) for 10 min. Afterwards, the samples were centrifuged at 5000 rpm at room temperature for 5 min. The supernatant was eliminated, and the precipitated protein was dissolved in a solution made of the Lowry reagent and distilled water (1:1). The Lowry reagent was prepared by mixing sodium carbonate (2%
w/v), cupric sulfate (1%
w/v), and sodium potassium tartrate (2%
w/v) solutions (100:1:1 ratio) [
33].
After the addition of the Lowry reagent/water solution, the samples were allowed to stand for 20 min at room temperature. Next, 250 µL of Folin–Ciocalteu reagent was added and allowed to stand for 30 min at room temperature in the dark. After the reaction took place, the samples were homogenized using a vortex and 250 µL of the samples were transferred into a 96 wells microplate. The absorbance was measured using a UV-Vis spectrophotometer model Multiskan Sky Microplate (Thermo Fischer Scientific, Waltham, MA, USA) at 720 nm. A calibration curve was prepared using Bovine Serum Albumin (0–1000 µg/mL) to calculate the protein content in the samples. The protein content was calculated using Equation (1) and expressed as a percentage (%).
where Ps is the protein solubilized in the sample (μg) and Sw is the weight of the sample (μg).
2.4.2. Molecular Mass (SDS-PAGE) and Secondary Structure (FTIR) of Spirulina Platensis Protein (SPP)
Sodium Dodecyl-Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE) was used to determine the molecular structure of SPP. Protein bands separation was carried out under reducing conditions using a double cell Mini-Protean II system (Bio-Rad Laboratories, Hercules, CA, USA). First, a polyacrylamide gel using 4.5% (stacking) and 12.5% (separation) of polyacrylamide mixed with ammonium persulfate at 10%
w/v (20 and 50 µL for stacking and separation gels, respectively) and TEMED (2 µL) until its polymerization. Protein solutions (4 mg/mL of protein concentration) using distilled water were mixed in a ratio of 1:1 with Laemmli buffer (5% glycerol, 20% SDS, 0.1% bromophenol blue, and 2% β-mercaptoethanol), heated at 95 °C for 5 min and centrifuged at 2000 rpm for 5 min (room temperature). Afterwards, 5 μL of molecular marker Biorad Pretrained Precision Plus Protein Dual Xtra (2–250 kDa) and 15 μL of the sample were loaded into the gel wells running at 90 V for 100 min [
34]. The gels were stained using ethanol: acetic acid: water solution (70:5:20) with 0.1% of Coomassie Blue G-205 for 45 min and destained with an ethanol: acetic acid: water solution (70:5:20) until no further color was released from the gel.
2.4.3. Secondary Structure Determination of SPP
The secondary structure of the protein was observed by Fourier Transform Infrared spectroscopy (FTIR). Spectra at a 400–4000 cm
−1 wavelength range were measured using a spectrophotometer Agilent Cary 630 coupled to an ATR ZNSe. The results were analyzed with Microlab PC (Agilent Technologies Inc., Santa Clara, CA, USA) software [
35]. The enthalpy and denaturation temperatures were studied by Differential Scanning Calorimeter (DSC) analysis. Approximately 10 mg of protein isolate were placed in hermetically sealed aluminum pans. The analysis was carried out between 25 and 40 °C with a heating rate of 10 °C/min, using a TA Instruments Q2000 V24.11 (TA Instruments Inc., New Castle, DE, USA) calorimeter, and the data were analyzed using a Universal Analysis 2000 software (TA Instruments Inc., New Castle, DE, USA). An empty, hermetically sealed aluminum pan was used as a reference [
36].
2.4.4. Emulsifying Capacity (EC) and Emulsion Stability (ES)
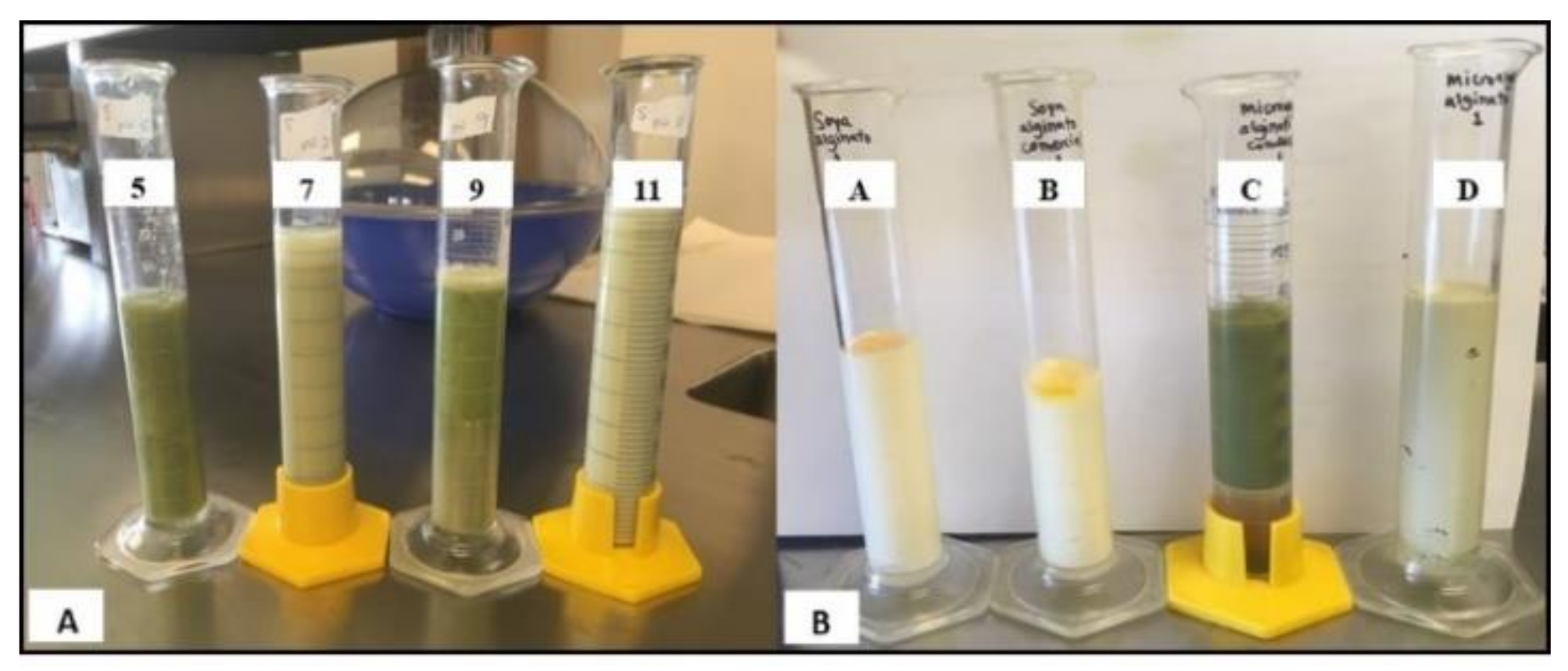
The emulsifying capacity (EC) and emulsion stability (ES) of the protein-emulsions were determined according to the method described by Wang et al. [
37]. Oil-in-water (o/w) emulsions were prepared by the homogenization of SBP and SPP protein solutions (0.5%
w/v) at different pH values (5, 7, 9, and 11) at room temperature. Next, chia seed oil was slowly incorporated (1:1
v/v) to the aqueous phase (protein solution) under constant stirring. An Oster Immersion blender (model FPSTHB Sunbeam Products, Inc, Boca Raton, FL, USA) at the maximum speed for 1 min was used as a homogenizer. The EC was calculated using Equation (2). The physical ES was calculated with Equation (3) by measuring the phase separation after 1 and 14 days of storage at room temperature (25 °C):
where Eh is the height of the emulsified liquid in cm, Hs is the total height of the system in cm, and Hi the height of the initial emulsion in cm.
2.5. Food Prototypes Elaboration
Microalgae emulsions were prepared by the homogenization of 0.5% SPP solutions, chia oil (mechanically extracted with a press), and 0.5% sodium alginate as a stabilizer. Protein solutions and sodium alginate (neutral pH 7) were stirred for approximately 2 h until solubilization. Then, the oily phase was slowly added in a ratio of 1:1 (v/v) and mixed with an Oster Immersion blender (model FPSTHB, Sunbeam Products, Inc, Boca Raton, FL, USA) at full speed for 1 min. Additionally, commercial SBP was used as a control emulsion.
Fettuccine dough was made with commercial wheat flour (control) and a blend of 50:50 wheat flour: amaranth flour (AWP) and eggs. Three different AWP pastas were enriched with 6, 8, and 10% of SPP (
Table 1). First, the amaranth seeds were ground in a coffee grinder for flour obtention; then, it was blended in equal proportions with wheat flour and the adequate proportion of SPP, then the blend was sieved (270–300 μm). Afterwards, both the battered eggs and the pre-heated water (40 °C) were gradually added to the mixture. The blend was manually kneaded until a homogeneous dough was obtained [
38]. The pasta dough was pressed and cut using a household pasta maker Metaltex model 8002522517402 (Metaltex Co., Genestrerio, Switzerland) and dried for 48 h at room temperature. The final pasta (fettuccine) dimensions were 1.5 mm thick × 6 mm width × 30 ± 5 cm in length.
2.6. Viscosity, Particle Size, and Color of Mayonnaise (Emulsions)
The sample preparation for the following measurements is shown in
Table 2. The viscosity of the emulsions was determined using a Brookfield Viscometer DV II (AMETEK Inc., Berwyn, PA, USA) equipped with an RV-4 spindle at 100 rpm for 45 s at a temperature of 25 °C. The particle size distribution was measured using dynamic light scattering with a particle size analyzer, Bluewave Nanotrac Wave II (Microtrac Inc., Montgomeryville, PA USA), with a refractive index of 1.47 for oil droplets and 1.33 for water. The results are given as the mean of the particle size distribution [
39,
40]. Konica Minolta model CR-400 colorimeter (Konica Minolta Holdings, Inc., Tokio, Japan) was used for color determination and expressed in CIELab system coordinates (L*, a*, b*). Additionally, chroma (C) and hue angle (H) were calculated using Equation (4) and Equation (5), respectively [
41]:
where
a* and
b* are CIELab system parameters (“*” in
a* and
b* are part of the variable).
2.7. Texture, Color, and Nutritional Composition of Pasta
The texture of dried pasta was measured using a Texturometer Shimadzu EZ-SX Texture Analyzer (Shimadzu Co., Kioto, Japan) equipped with a load cell adjusted to 50 kg and coupled to an aluminum probe. The break force test was performed at a compression speed rate of 3 mm/s (at room temperature) [
38]. The software used for data analysis was Trapezium X (Shimadzu Co., Kioto, Japan). The pasta color parameters were determined with a colorimeter previously described in
Section 2.6. (mayonnaise color measurements).
Proximate analyses were performed by AOAC methods [
30]. The mineral content (Ca, Fe, Mg, Mn, and P) was determined using an Agilent 5110 ICP-OES based on AOAC Method 2011.4. Briefly, samples (2 mL) were digested on a CEM Mars 6 microwave system (Charlotte, NC, USA) with concentrated nitric acid. Afterwards, samples were filtered and diluted. The spectra reading was carried out at specific wavelengths for each mineral. For the amino acid profile, first, the samples (0.3 g) were acid hydrolyzed for 24 h at 110 °C in a nitrogen atmosphere using 30 mL of HCl solution (6 M) containing 0.02% of phenol and diluted 25 mL using distilled water. The samples were then stored at 4 °C, filtered with 0.45 μm filter, and analyzed using the Agilent amino acid analysis protocol in an Agilent 1290 Infinity II (Agilent Technologies Inc., USA) HPLC coupled to a diode array detector (DAD) with a ZORBAX Eclipse Plus C18 column (Agilent Technologies Inc., Santa Clara, CA, USA). Mobile phase A and B consisted in 10 mM of Na
2HPO
4:10 mM Na
2B
4O
7 (pH 8.4) buffer and an acetonitrile: methanol: water (45:45:10) solution, respectively. Primary amino acids derivatization was carried out with o-phthalaldehyde (OPA) and the secondary amino acids were derivatized using 9-fluorenylmethyl chloroformate (FMOC). Amino acid detection was performed at 263 and 338 nm. The quantification was done using norvaline as an internal standard [
42].