Monomers, Materials and Energy from Coffee By-Products: A Review
Abstract
:1. Introduction
2. Challenges and Opportunities for Coffee Waste Utilization
3. Sustainable Polymers from Coffee Waste
3.1. Monomer Production
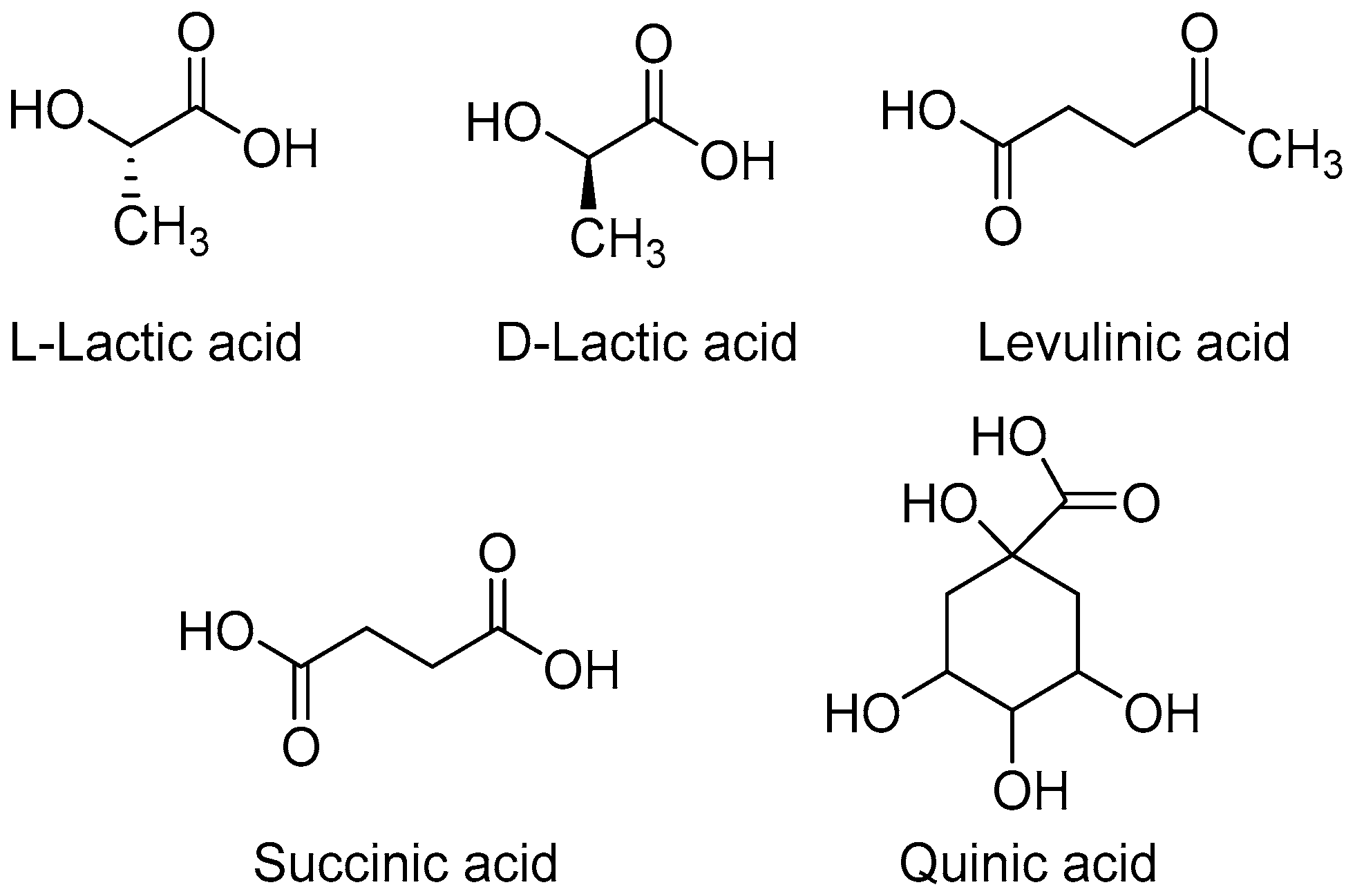
3.1.1. Lactic Acid
3.1.2. Succinic Acid
3.1.3. Levulinic Acid
3.1.4. Quinic Acid
3.1.5. n-Butanol, Isopropanol and Polyols
3.2. Polymer Production
3.3. Composite Materials Production
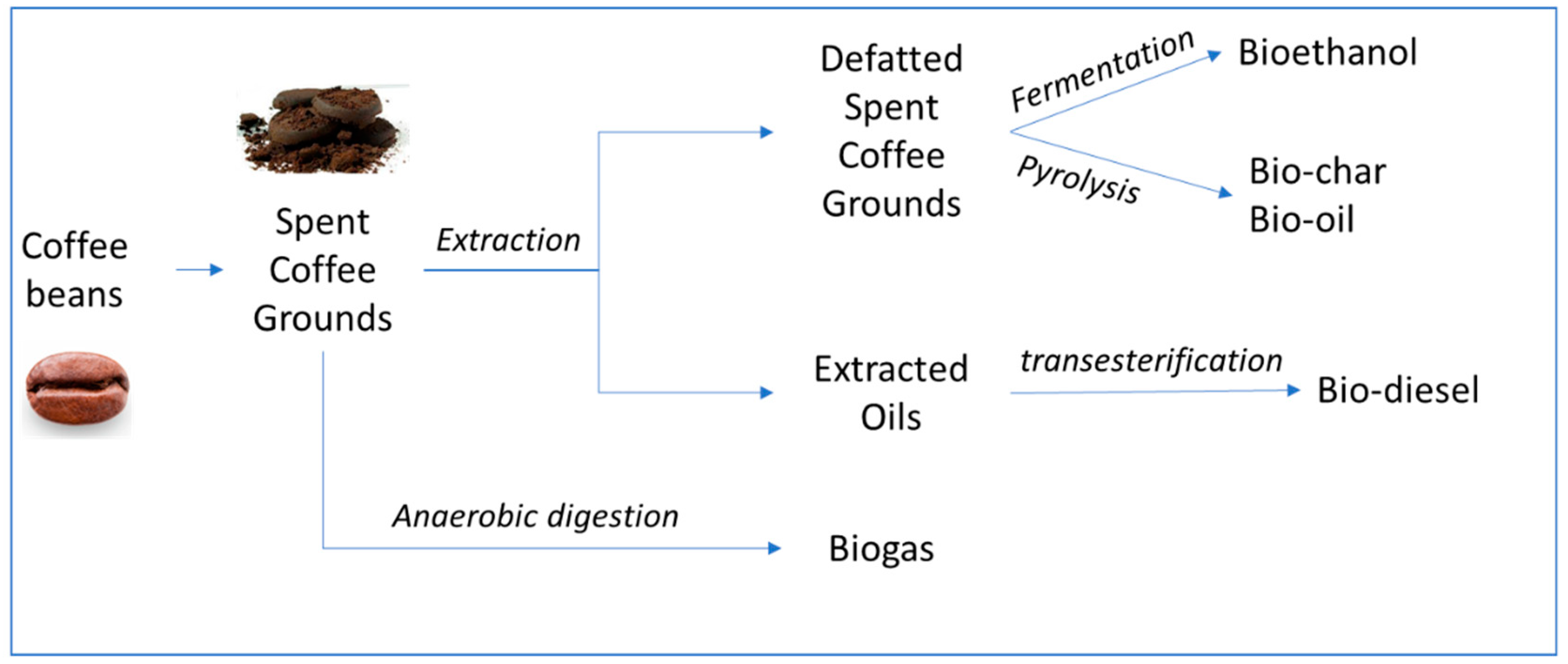
4. Biofuels Production from Coffee Waste
4.1. Biodiesel
4.2. Bioethanol
4.3. Bio-Oil
4.4. Biogas
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABE | mixture of acetone, butanol and ethanol |
| CH | coffee husk |
| CG | coffee grounds |
| CNCs | cellulose nanocrystals |
| CP | coffee pulp |
| CSS | coffee silver skin |
| DSCG | defatted spent coffee grounds |
| LA | lactic acid |
| PBAT | poly(butylene adipate-co-terephthalate) |
| PBS | poly(butylene succinate) |
| PE | poly(ethylene) |
| PEF | poly(ethylene 2,5-furandicarboxylate) |
| PET | poly(ethylene terephthalate) |
| PHA | polyhydroxyalkanoates |
| PHB-HV | poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| PLA | poly(lactic acid) |
| PLA-g-MA | maleic anhydride-grafted-polylactide |
| PP | poly(propylene) |
| PTT | poly(trimethylene terephthalate) |
| PUs | polyurethanes |
| SA | succinic acid |
| SCGO | spent coffee grounds oil |
| SFC | spent filter coffee |
| SCG | spent coffee grounds |
| TSCG | torrefied spent coffee grounds |
| VS | volatile solid |
References
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; van Ottedijk, R.; Meybeck, A. Global food losses and food waste: Extent, causes and prevention. In Save Food, Proceedings of the Interpack2011, Düsseldorf, Germany, 12–18 May 2011; FAO: Rome, Italy, 2011. [Google Scholar]
- Vermeulen, S.J.; Campbell, B.M.; Ingram, J.S.I. Climate change and food systems. Annu. Rev. Environ. Resour. 2012, 37, 195–222. [Google Scholar] [CrossRef] [Green Version]
- Scialabba, N. Food Wastage Footprint: Impacts on Natural Resources-Summary Report; FAO, Food and Agricultural Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Hatti-Kaul, R.; Nilsson, L.J.; Zhang, B.; Rehnberg, N.; Lundmark, S. Designing Biobased recyclable polymers for plastics. Trends Biotechnol. 2020, 38, 50–67. [Google Scholar] [CrossRef]
- European Bioplastics. Available online: https://www.european-bioplastics.org/market/ (accessed on 5 January 2021).
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- European Commission. Press Release-Energy Union: Secure, Sustainable, Competitive, Affordable Energy for Every European; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee, the Committee of the Regions and the European Investment Bank; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Besset, C.J.; Lonnecker, A.T.; Streff, J.M.; Wooley, K.L. Polycarbonates from the polyhydroxy natural product quinic acid. Biomacromolecules 2011, 12, 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Shogren, R.; Wood, D.; Orts, W.; Glenn, G. Plant-based materials and transitioning to a circular economy. Sustain. Prod. Consum. 2019, 19, 194–215. [Google Scholar] [CrossRef]
- Monari, S.; Ferri, M.; Vannini, M.; Sisti, L.; Marchese, P.; Ehrnell, M.; Xanthakis, E.; Celli, A.; Tassoni, A. Cascade strategies for the full valorisation of Garganega white grape pomace towards bioactive extracts and bio-based materials. PLoS ONE 2020, 15, e0239629. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Weber, C.J.; Haugaard, V.; Festersen, R.; Bertelsen, G. Production and applications of biobased packaging materials for the food industry. Food Addit. Contam. 2002, 19, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Saccani, A.; Sisti, L.; Manzi, S.; Fiorini, M. PLA Composites formulated recycling residuals of the winery industry. Polym. Compos. 2019, 40, 1378–1383. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Ladas, D.; Mavromatis, A. Potential uses and applications of treated wine waste: A review. Int. J. Food Sci. Tech. 2006, 41, 475–487. [Google Scholar] [CrossRef]
- Ferri, M.; Vannini, M.; Ehrnell, M.; Eliasson, L.; Xanthakis, E.; Monari, S.; Sisti, L.; Marchese, P.; Celli, A.; Tassoni, A. From winery waste to bioactive compounds and new polymeric biocomposites: A contribution to the circular economy concept. J. Adv. Res. 2020, 24, 1–11. [Google Scholar] [CrossRef]
- Toschi, T.G.; Cardenia, V.; Bonaga, G.; Mandrioli, M.; Rodriguez-Estrada, M.T. Coffee silverskin: Characterization, possible uses, and safety aspects. J. Agric. Food Chem. 2014, 62, 10836–10844. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition-A Review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Bonilla-Hermosa, V.A.; Duarte, W.F.; Schwan, R.F. Utilization of coffee by-products obtained from semi-washed process for production of value-added compounds. Bioresour. Technol. 2014, 166, 142–150. [Google Scholar] [CrossRef]
- Janissen, B.; Huynh, T. Chemical composition and value adding applications of coffee industry by-products: A review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Michail, A.; Sigala, P.; Grigorakis, S.; Makris, D.P. Kinetics of ultrasound-assisted polyphenol extraction from spent filter coffee using aqueous glycerol. Chem. Eng. Commun. 2016, 203, 407–413. [Google Scholar] [CrossRef]
- Karmee, S.K. A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manag. 2018, 72, 240–254. [Google Scholar] [CrossRef]
- Dessie, W.; Zhu, J.; Xin, F.; Zhang, W.; Jiang, Y.; Wu, H.; Ma, J.; Jiang, M. Bio-succinic acid production from coffee husk treated with thermochemical and fungal hydrolysis. Bioprocess Biosyst. Eng. 2018, 41, 1461–1470. [Google Scholar] [CrossRef]
- Tukacs, J.M.; Holló, A.T.; Rétfalvi, N.; Cséfalvay, E.; Dibó, G.; Havasi, D.; Mika, L.T. Microwave-assisted valorization of biowastes to levulinic acid. Chem. Sel. 2017, 2, 1375–1380. [Google Scholar] [CrossRef]
- Obruca, S.; Benesova, P.; Kucera, D.; Petrik, S.; Marova, I. Biotechnological conversion of spent coffee grounds into polyhydroxyalkanoates and carotenoids. New Biotechnol. 2015, 32, 569–574. [Google Scholar] [CrossRef]
- Moustafa, H.; Guizani, C.; Dupont, C.; Martin, V.; Jeguirim, M.; Dufresne, A. Utilization of torrefied coffee grounds as reinforcing agent to produce high-quality biodegradable PBAT composites for food packaging applications. ACS Sustain. Chem. Eng. 2017, 5, 1906–1916. [Google Scholar] [CrossRef]
- Essabir, H.; Rajia, M.; Ait Laaziza, S.; Rodrique, D.; Bouhfid, R.; el kacem Qaiss, A. Thermo-mechanical performances of polypropylene biocomposites based on untreated, treated and compatibilized spent coffee grounds. Comp. Part B 2018, 149, 1–11. [Google Scholar] [CrossRef]
- Sung, S.H.; Chang, Y.; Han, J. Development of polylactic acid nanocomposite films reinforced with cellulose nanocrystals derived from coffee silverskin. Carbohydr. Polym. 2017, 169, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Sarasini, F.; Luzi, F.; Dominici, F.; Maffei, G.; Iannone, A.; Zuorro, A.; Lavecchia, R.; Torre, L.; Carbonell-Verdu, A.; Balart, R.; et al. Effect of Different Compatibilizers on Sustainable Composites Based on a PHBV/PBAT Matrix Filled with Coffee Silverskin. Polymers 2018, 10, 1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarasini, F.; Tirillò, J.; Zuorro, A.; Maffei, G.; Lavecchia, R.; Puglia, D.; Dominici, F.; Luzi, F.; Valente, T.; Torre, L. Recycling coffee silverskin in sustainable composites based on a poly(butylene adipate-co-terephthalate)/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) matrix. Ind. Crops Prod. 2018, 118, 311–320. [Google Scholar] [CrossRef]
- Dadi, D.; Beyene, A.; Simoens, K.; Soares, J.; Demeke, M.M.; Thevelein, J.M.; Bernaerts, K.; Luis, P.; Van der Bruggen, B. Valorization of coffee for bioethanol production using lignocellulosic yeast fermentation and pervaporation. Int. J. Environ. Sci. Technol. 2018, 15, 821–832. [Google Scholar] [CrossRef]
- Procentese, A.; Raganati, F.; Navarini, L.; Olivieri, G.; Russo, M.E.; Marzocchella, A. Coffee silver skin as a renewable resource to produce butanol and isopropanol. Chem Eng. Trans. 2018, 64, 139–144. [Google Scholar]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- European Commission, Eurostat: Some Statistics for Coffee Time. 2019. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/EDN-20191001-1 (accessed on 15 January 2021).
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- Cherubini, F.; Ulgiati, S. Crop residues as raw materials for biorefinery systems. Appl. Energy 2010, 87, 47–57. [Google Scholar] [CrossRef]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and challenges of biomass as a suitable feedstock for bioenergy and biochemical production: A review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, A.C.; Lima, M.; Sousa, A.F.; Silvestre, A.J.; Coelho, J.F.J.; Serra, A.C. Cinnamic acid derivatives as promising building blocks for advanced polymers: Synthesis, properties and applications. Polym. Chem. 2019, 10, 1696–1723. [Google Scholar] [CrossRef]
- Alexandri, M.; Schneider, R.; Mehlmann, K.; Venus, J. Recent advances in D-Lactic Acid production from renewable resources: Case studies on agro-industrial waste streams. Food Technol. Biotech. 2019, 57, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Tarraran, L.; Mazzoli, R. Alternative strategies for lignocellulose fermentation through lactic acid bacteria: The state of the art and perspectives. FEMS Microbiol. Lett. 2018, 365, 1–14. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Kim, J.S.; Nguyen, T.N.; Kim, S.K.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Kim, J.C. Production of L- and D-lactic acid from waste Curcuma longa biomass through simultaneous saccharification and cofermentation. Bioresour. Technol. 2013, 146, 35–43. [Google Scholar] [CrossRef]
- De la Torre, I.; Ladero, M.; Santos, V.E. Production of D-lactic acid by Lactobacillus delbrueckii ssp. delbrueckii from orange peel waste: Techno-economical assessment of nitrogen sources. Appl. Microbiol. Biotechnol. 2018, 102, 10511–10521. [Google Scholar] [CrossRef]
- Hartmann, H. High molecular weight polylactic acid polymers. In Biopolymers from Renewable Resources, 1st ed.; Kaplan, D.L., Ed.; Springer: Berlin, Germany, 1998; pp. 367–411. [Google Scholar]
- Hudeckova, H.; Neureiter, M.; Obruca, S.; Frühauf, S.; Marova, I. Biotechnological conversion of spent coffee grounds into lactic acid. Lett. Appl. Microbiol. 2018, 66, 306–312. [Google Scholar] [CrossRef]
- Bretón-Toral, A.; Trejo-Estrada, S.R.; McDonald, A.G. Lactic acid production from potato peel waste, spent coffee grounds and almond shells with undefined mixed cultures isolated from coffee mucilage from Coatepec Mexico. Ferment. Technol. 2017, 6, 1000139–1000145. [Google Scholar] [CrossRef] [Green Version]
- Neu, A.K.; Pleissner, D.; Mehlmann, K.; Schneider, R.; Puerta-Quintero, G.I.; Venus, J. Fermentative utilization of coffee mucilage using Bacillus coagulans and investigation of down-stream processing of fermentation broth for optically pure L(+)-lactic acid production. Bioresour. Technol. 2016, 211, 398–405. [Google Scholar] [CrossRef]
- Pleissner, D.; Neu, A.K.; Mehlmann, K.; Schneider, R.; Puerta-Quintero, G.I.; Venus, J. Fermentative lactic acid production from coffee pulp hydrolysate using Bacillus coagulans at laboratory and pilot scales. Bioresour. Technol. 2016, 218, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Jang, J.H.; Yeo, H.J.; Seol, J.; Kim, S.R.; Jung, Y.H. Lactic Acid Production from a whole slurry of acid-pretreated spent coffee grounds by engineered Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2019, 189, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Sisti, L.; Totaro, G.; Marchese, P. PBS makes its entrance into the family of biobased plastics. In Biodegradable and Biobased, Polymers for Environmental and Biomedical Applications; Kalia, S., Averous, L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 225–285. [Google Scholar]
- Li, C.; Ong, K.L.; Yang, X.; Lin, C.S.K. Bio-refinery of waste streams for green and efficient succinic acid production by engineered Yarrowia lipolytica without pH control. Chem. Eng. J. 2019, 371, 804–812. [Google Scholar] [CrossRef]
- Li, C.; Ong, K.L.; Cui, Z.; Sang, Z.; Li, X.; Patria, R.D.; Qi, Q.; Fickers, P.; Yan, J.; Lin, C.S.K. Promising advancement in fermentative succinic production by yeast hosts. J. Hazard. Mater. 2021, 401, 123414. [Google Scholar] [CrossRef] [PubMed]
- Niglio, S.; Procentese, A.; Russo, M.E.; Sannia, G.; Marzocchella, A. Investigation of enzymatic hydrolysis of coffee silverskin aimed at the production of butanol and succinic acid by fermentative processes. Bioenergy Res. 2019, 12, 312–324. [Google Scholar] [CrossRef]
- Pileidis, F.D.; Titirici, M.M. Levulinic acid biorefineries: New challenges for efficient utilization of biomass. ChemSusChem 2016, 9, 562–582. [Google Scholar] [CrossRef]
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Qin, K.; Yan, Y.; Zhang, Y.; Tang, Y. Direct production of levulinic acid in high yield from cellulose: Joint effect of high ion strength and microwave field. RSC Adv. 2016, 6, 39131–39136. [Google Scholar] [CrossRef]
- Im, H.; Kim, B.; Lee, J.W. Concurrent production of biodiesel and chemicals through wet in situ transesterification of microalgae. Bioresour. Technol. 2015, 193, 386–392. [Google Scholar] [CrossRef]
- Kim, B.; Yang, J.; Kim, M.; Lee, J.W. One-pot selective production of levulinic acid and formic acid from spent coffee grounds in a catalyst-free biphasic system. Bioresour. Technol. 2020, 303, 122898–122906. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Youn, S.H.; Lee, K.M.; Kim, K.Y.; Lee, S.M.; Woo, H.M.; Um, Y. Effective isopropanol–butanol (IB) fermentation with high butanol content using a newly isolated Clostridium sp. A1424. Biotechnol. Biofuels 2016, 9, 230–245. [Google Scholar] [CrossRef] [Green Version]
- Soares, B.; Gama, N.; Freire, C.S.R.; Barros-Timmons, A.; Brandão, I.; Silva, R.; Neto, C.P.; Ferreira, A. Spent coffee grounds as a renewable source for ecopolyols production. J. Chem. Technol. Biotechnol. 2015, 90, 1480–1488. [Google Scholar] [CrossRef]
- Yang, Y.; Hoogewind, A.; Moon, Y.H.; Day, D. Bioprocess Production of butanol and isopropanol with an immobilized Clostridium. Biosyst. Eng. 2016, 39, 421–428. [Google Scholar]
- Gama, N.V.; Soares, B.; Freire, C.S.R.; Silva, R.; Neto, C.P.; Barros-Timmons, A.; Ferreira, A. Bio-based polyurethane foams toward applications beyond thermal insulation. Mater. Des. 2015, 76, 77–85. [Google Scholar] [CrossRef]
- Kovalcik, A.; Kucera, D.; Matouskova, P.; Pernicova, I.; Obruca, S.; Kalina, M.; Enev, K.; Marova, I. Influence of removal of microbial inhibitors on PHA production from spent coffee grounds employing Halomonas halophila. J. Environ. Chem. Eng. 2018, 6, 3495–3501. [Google Scholar] [CrossRef]
- Bathia, S.K.; Kim, J.H.; Kim, M.S.; Kim, J.; Hong, J.W.; Hong, Y.G.; Kim, H.J.; Jeon, J.M.; Kim, S.H.; Ahn, J.; et al. Production of (3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymer from coffee waste oil using engineered Ralstonia eutropha. Bioprocess Biosyst. Eng. 2018, 41, 229–235. [Google Scholar]
- Obruca, S.; Petrik, S.; Benesova, P.; Svoboda, Z.; Eremka, L.; Marova, I. Utilization of oil extracted from spent coffee grounds for sustainable production of polyhydroxyalkanoates. Appl. Microbiol. Biotechol. 2014, 98, 5883–5890. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.V.; Paiva, A.; Lisboa, P.; Freitas, F.; Alves, V.D.; Simões, P.; Barreiros, S.; Reis, M.A.M. Production of polyhydroxyalkanoates from spent coffee grounds oil obtained by supercritical fluid extraction technology. Bioresour. Technol. 2014, 157, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef] [Green Version]
- Iriondo-De Hond, A.; Haza, A.I.; Ávalos, A.; del Castillo, M.D.; Morales, P. Validation of coffee silverskin extract as a food ingredient by the analysis of cytotoxicity and genotoxicity. Food Res. Int. 2017, 100, 791–797. [Google Scholar] [CrossRef] [Green Version]
- Trache, D.; Donnot, A.; Khimeche, K.; Benelmir, R.; Brosse, N. Physico-chemical properties and thermal stability of microcrystalline cellulose isolated from Alfa fibres. Carbohydr. Polym. 2014, 104, 223–230. [Google Scholar] [CrossRef]
- Zarrinbakhsh, N.; Wang, T.; Rodriguez-Uribe, A.; Misra, M.; Mohanty, A.K. Characterization of wastes and coproducts from the coffee industry for composite material production. BioResources 2016, 11, 7637–7653. [Google Scholar] [CrossRef] [Green Version]
- Dominici, F.; García García, D.; Fombuena, V.; Luzi, F.; Puglia, D.; Torre, L.; Balart, R. Bio-Polyethylene-based composites reinforced with alkali and palmitoyl chloride-treated coffee Silverskin. Molecules 2019, 24, 3113. [Google Scholar] [CrossRef] [Green Version]
- Gigante, V.; Seggiani, M.; Cinelli, P.; Signori, F.; Vania, A.; Navarini, L.; Amato, G.; Lazzeri, A. Utilization of coffee silverskin in the production of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) biopolymer-based thermoplastic biocomposites for food contact applications. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106172–106182. [Google Scholar] [CrossRef]
- Wu, C.S. Renewable resource-based green composites of surface-treated spent coffee grounds and polylactide: Characterisation and biodegradability. Polym. Deg. Stab. 2015, 121, 51–59. [Google Scholar] [CrossRef]
- Totaro, G.; Sisti, L.; Fiorini, M.; Lancellotti, M.; Andreola, F.N.; Saccani, A. Formulation of green particulate composites from PLA and PBS matrix and wastes deriving from the coffee production. J. Polym. Environm. 2019, 27, 1488–1496. [Google Scholar] [CrossRef]
- Food and Agricultural Organization of the United Nations, “Ubet Unified Bioenergy Terminology”. 2004. Available online: http://www.fao.org/3/j4504E/j4504e07.htm#P731_40655 (accessed on 1 June 2020).
- Simell, P.; Hannula, S.; Toumi, S.; Nieminen, M.; Kurkela, E.; Hiltunen, I. Clean syngas from biomass-Process development and concept assessment. Biomass Convers. Biorefinery 2014, 4, 357–370. [Google Scholar] [CrossRef]
- Scully, D.S.; Jalswal, A.K.; Abu-Ghannam, N. An investigation into spent coffee waste as a renewable source of bioactive compounds and industrially important sugars. Bioengineering 2016, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Atabani, A.E.; Al-Muhtaseb, A.H.; Kumar, G.; Saratale, G.D.; Aslam, M.; Khan, H.A.; Said, Z.; Mahmoud, E. Valorization of spent coffee grounds into biofuels and value-added products: Pathway towards integrated bio-refinery. Fuel 2019, 254, 115640–115660. [Google Scholar] [CrossRef]
- Banu, J.R.; Kavitha, S.; Yukesh Kannah, R.; Dinesh Kumar, M.; Preethi; Atabani, A.E.; Kumar, G. Biorefinery of spent coffee grounds waste: Viable pathway towards circular bioeconomy. Bioresour. Technol. 2020, 302, 122821–122836. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.W. Renewable Liquid Transport Fuels from Microbes and Waste Resources. Ph.D. Thesis, Centre for Sustainable Chemical Technologies, Department of Chemical Engineering, University of Bath, Bath, UK, 2014; p. 252. [Google Scholar]
- Ferrario, V.; Veny, H.; De Angelis, E.; Navarini, L.; Ebert, C.; Gardossi, L. Lipases immobilization for effective synthesis of biodiesel starting from coffee waste oils. Biomolecules 2013, 3, 514. [Google Scholar] [CrossRef]
- Haile, M. Integrated valorization of spent coffee grounds to biofuels. Biofuel Res. J. 2014, 1, 65–69. [Google Scholar] [CrossRef]
- Kondamudi, N.; Mohapatra, S.K.; Misra, M. Spent coffee grounds as a versatile source of green energy. J. Agric. Food Chem. 2008, 56, 11757–11760. [Google Scholar] [CrossRef]
- Abdullah, M.; Koc, A.B. Oil removal from waste coffee grounds using two-phase solvent extraction enhanced with ultrasonication. Renew. Energy 2013, 50, 965–970. [Google Scholar] [CrossRef]
- Rocha, M.V.P.; de Matos, L.J.B.L.; de Lima, L.P.; da Silva Figueiredo, P.M.; Lucena, I.L.; Fernandes, F.A.N.; Gonçalves, L.R.B. Ultrasound-assisted production of biodiesel and ethanol from spent coffee grounds. Bioresour. Technol. 2014, 167, 343–348. [Google Scholar] [CrossRef] [PubMed]
- de Melo, M.M.R.; Barbosa, H.M.A.; Passos, C.P.; Silva, C.M. Supercritical fluid extraction of spent coffee grounds: Measurement of extraction curves, oil characterization and economic analysis. J. Supercrit. Fluids 2014, 86, 150–159. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, Q.; Knothe, G.; Lu, M. Direct transesterification of spent coffee grounds for biodiesel production. Fuel 2017, 199, 157–161. [Google Scholar] [CrossRef]
- Tongcumpou, C.; Usapein, P.; Tuntiwiwattanapun, N. Complete utilization of wet spent coffee grounds waste as a novel feedstock for antioxidant, biodiesel, and biochar production. Ind. Crops Prod. 2019, 138, 111484–111491. [Google Scholar] [CrossRef]
- Kwon, E.E.; Yi, H.; Jeon, Y.J. Sequential co-production of biodiesel and bioethanol with spent coffee grounds. Bioresour. Technol. 2013, 136, 475–480. [Google Scholar] [CrossRef]
- Go, Y.W.; Yeom, S.H. Statistical analysis and optimization of biodiesel production from waste coffee grounds by a two-step process. Biotechnol. Bioprocess Eng. 2017, 22, 440–449. [Google Scholar] [CrossRef]
- Juarez, G.F.Y.; Pabiloña, K.B.C.; Manlangit, K.B.L.; Go, A.W. Direct dilute acid hydrolysis of Spent Coffee Grounds: A new approach in sugar and lipid recovery. Waste Biomass Valor. 2018, 9, 235–246. [Google Scholar] [CrossRef]
- Haile, M.; Asfaw, A.; Asfaw, N. Investigation of waste coffee ground as a potential raw material for biodiesel production. Int. J. Renew. Energy Res. 2013, 3, 854–860. [Google Scholar]
- Primaz, C.T.; Schena, T.; Lazzari, E.; Caramãoa, E.B.; Jacques, R.A. Influence of the temperature in the yield and composition of the bio-oil from the pyrolysis of spent coffee grounds: Characterization by comprehensive two dimensional gas chromatography. Fuel 2018, 232, 572–580. [Google Scholar] [CrossRef]
- Kelkar, S.; Saffron, C.M.; Chai, L.; Bovee, J.; Stuecken, T.R.; Garedew, M.; Li, Z.; Kriegeld, R.M. Pyrolysis of spent coffee grounds using a screw-conveyor reactor. Fuel Process Technol. 2015, 137, 170–178. [Google Scholar] [CrossRef]
- Zanella, E.; Della Zassa, M.; Navarini, L.; Canu, P. Low-temperature co-pyrolysis of polypropylene and coffee wastes to fuels. Energy Fuels 2013, 27, 1357–1364. [Google Scholar] [CrossRef]
- Yang, L.; He, Q.; Havard, P.; Corscadden, K.; Xu, C.; Wang, X. Co-liquefaction of spent coffee grounds and lignocellulosic feedstocks. Bioresour. Technol. 2017, 237, 108–121. [Google Scholar] [CrossRef]
- Vítez, T.; Koutny, T.; Sotnar, M.; Chovanec, J. On the spent coffee grounds biogas production. Acta Univ. Agric. Silvicult. Mendel. Brun. 2016, 64, 1279–1282. [Google Scholar] [CrossRef] [Green Version]
- Girotto, F.; Lavagnolo, M.C.; Pivato, A. Spent coffee grounds alkaline pre-treatment as biorefinery option to enhance their anaerobic digestion yield. Waste Biomass Valor. 2018, 9, 2565–2570. [Google Scholar] [CrossRef]
- Lee, M.; Yang, M.; Choi, S.; Shin, J.; Park, C.; Cho, S.K.; Kim, Y.M. Sequential production of lignin, fatty acid methyl esters and biogas from Spent Coffee Grounds via an integrated physicochemical and biological process. Energies 2019, 12, 2360. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yang, H.; Yang, Q.; Hao, H.; Zhu, B.; Chen, H. Torrefaction of agriculture straws and its application on biomass pyrolysis poly-generation. Bioresour. Technol. 2014, 156, 70–77. [Google Scholar] [CrossRef] [PubMed]

| Polymer | Chemical Structure | |
|---|---|---|
| Non-Biodegradable | Bio-poly(ethylene) (bio-PE) | 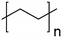 |
| Bio-poly(propylene) (bio-PP) |  | |
| Bio-poly(ethylene terephthalate) (bio-PET) | 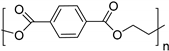 | |
| Bio-poly(trimethylene terephthalate) (bio-PTT) | 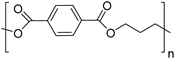 | |
| Poly(ethylene 2,5-furandicarboxylate) (PEF) 1 | 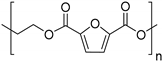 | |
| Bio-polyamides |  | |
| Biodegradable | Starch blends | 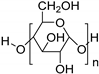 |
| Poly(hydroxyalkanoate)s (PHA) | 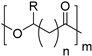 | |
| Poly(lactic acid) (PLA) | 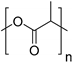 | |
| Poly(butylene succinate) (PBS) | 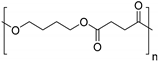 | |
| Poly(butylene adipate-co-terephthalate) (PBAT) | 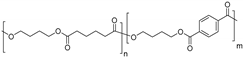 |
| Monomer | Coffee By-Product | Organism | Yield | Ref. |
|---|---|---|---|---|
| Lactic acid | Spent coffee ground | Saccharomices cerevisiae | 0.11 g/gSCG | [49] |
| Bacillus coagulans | 98.0% | [45] | ||
| Coffee mucilage | Bacillus coagulans | 99.8% | [47] | |
| Coffee pulp | Bacillus coagulans | 99.7% | [48] | |
| Succinic acid | Coffee husk | Actinobacillus succinogens | 0.95 g/greducing sugars | [23] |
| Coffee Silver skin | Actinobacillus succinogens | 84% | [53] | |
| Levulinic acid | Spent coffee ground | / | 13–15% | [24] |
| / | 47% | [58] | ||
| n-Butanol | Coffee Silver skin | Clostridium beijerinckii | 0.0086 g/gCSS | [32] |
| Isopropanol | Coffee Silver skin | Clostridium beijerinckii | 0.0066 g/gCSS | [32] |
| Polyols | Spent coffee ground | / | 70% | [37] |
| Polymer Matrix | Coffee By-Product | Bio-Based | Compostable/ Biodegradable | Ref. |
|---|---|---|---|---|
| PP | Coffee Silver skin/ spent coffee ground | No/Yes Possible with bioPP | No | [71] |
| PP | Spent coffee ground 1 | No/Yes Possible with bioPP | No | [27] |
| bioPE | Coffee Silver skin | Yes | No | [72] |
| Grafted- -bioPE | Coffee Silver skin 2 | Yes | No | [72] |
| PBAT | Coffee grounds | No/Yes | Yes | [26] |
| PBAT/PHBV | Coffee Silver skin | Partly (PBAT No, PHBV and CSS Yes) | Yes | [30,73] |
| PLA | Spent coffee ground 3 | Yes | Yes | [74] |
| PLA | Nano cellulose produced from coffee silver skin | Yes | Yes | [28] |
| PLA/PBS | Coffee Silver skin | Yes | Yes | [75] |
| Biomass Type | Derived from Plants Type |
|---|---|
| Sugar/Starch | Crops mainly devoted to the production of ethanol. Fuel mainly used in transport (alone or blended with gasoline). Produced by fermentation of glucose derived from sugar-containing plants (e.g., sugarcane) or starchy materials after hydrolysis. |
| Oil | Oleaginous plants (e.g., sunflower and rape) planted for direct energy use of vegetable oil extracted, or as raw material for further conversion into a diesel substitute, using transesterification processes. |
| Other | Plants and specialised crops more recently considered for energy use, such as: elephant grass (Miscanthus), cordgrass and galingale (Spartina spp. and Cyperus longus), giant reed (Arundo donax) and reed canary grass (Phalaris arundinacea). |
| Coffee By-Product | Methods | Fuel | Secondary Products |
|---|---|---|---|
| Spent coffee ground (SCG) | Hydrolysis/fermentation | Bioethanol | Fuel pellets (soil amendment) |
| Pyrolysis | Bio-oil | Biochar, syngas | |
| Spent coffee grounds oil (SCGO) | Chemical conversion/enzymatic/in situ | Biodiesel | Glycerin to bio-hydrogen |
| Enzymatic conversion | Biodiesel | Glycerin to bio-hydrogen | |
| Defatted spent coffee grounds (DSCG) | Hydrolysis/fermentation | Bioethanol | Fuel pellets |
| Pyrolysis | Bio-oil | Biochar |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sisti, L.; Celli, A.; Totaro, G.; Cinelli, P.; Signori, F.; Lazzeri, A.; Bikaki, M.; Corvini, P.; Ferri, M.; Tassoni, A.; et al. Monomers, Materials and Energy from Coffee By-Products: A Review. Sustainability 2021, 13, 6921. https://doi.org/10.3390/su13126921
Sisti L, Celli A, Totaro G, Cinelli P, Signori F, Lazzeri A, Bikaki M, Corvini P, Ferri M, Tassoni A, et al. Monomers, Materials and Energy from Coffee By-Products: A Review. Sustainability. 2021; 13(12):6921. https://doi.org/10.3390/su13126921
Chicago/Turabian StyleSisti, Laura, Annamaria Celli, Grazia Totaro, Patrizia Cinelli, Francesca Signori, Andrea Lazzeri, Maria Bikaki, Philippe Corvini, Maura Ferri, Annalisa Tassoni, and et al. 2021. "Monomers, Materials and Energy from Coffee By-Products: A Review" Sustainability 13, no. 12: 6921. https://doi.org/10.3390/su13126921







