Scientometric Analysis of Diesel Pollutions in Antarctic Territories: A Review of Causes and Potential Bioremediation Approaches
Abstract
:1. Introduction
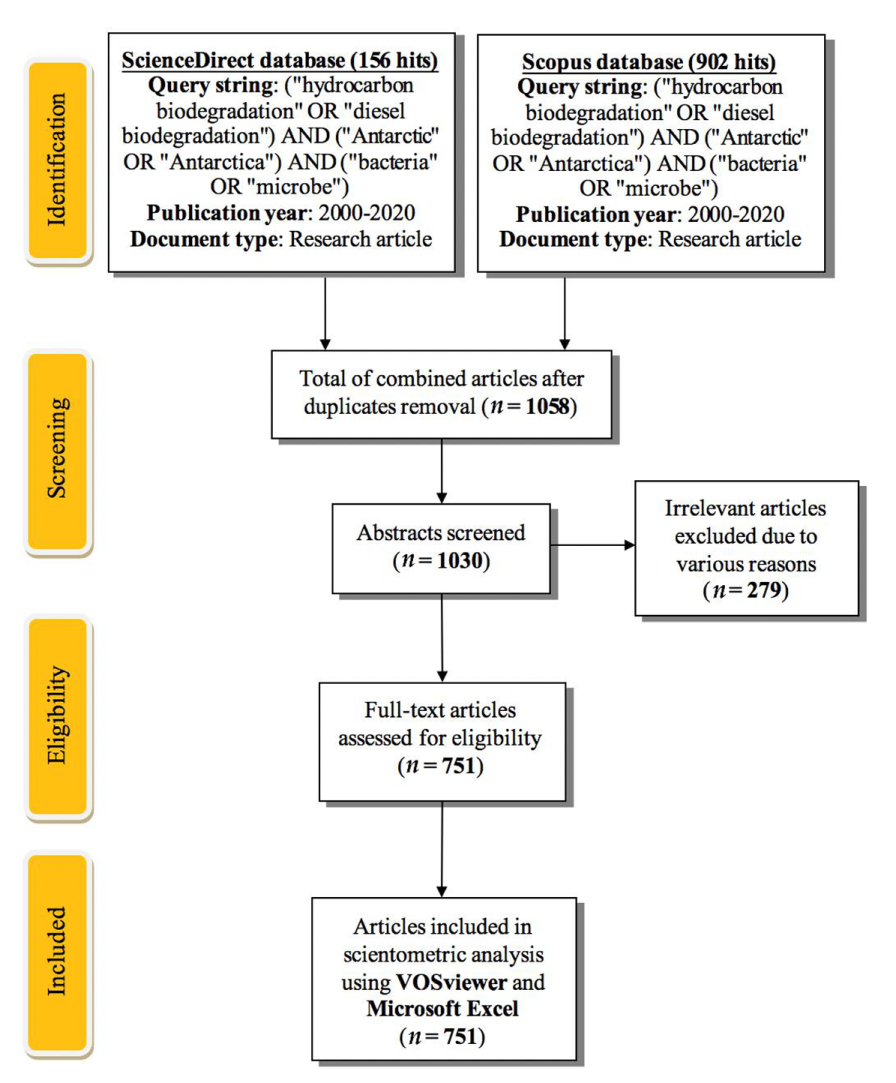
2. Methodology for Materials Collection
3. Scientometric Analysis
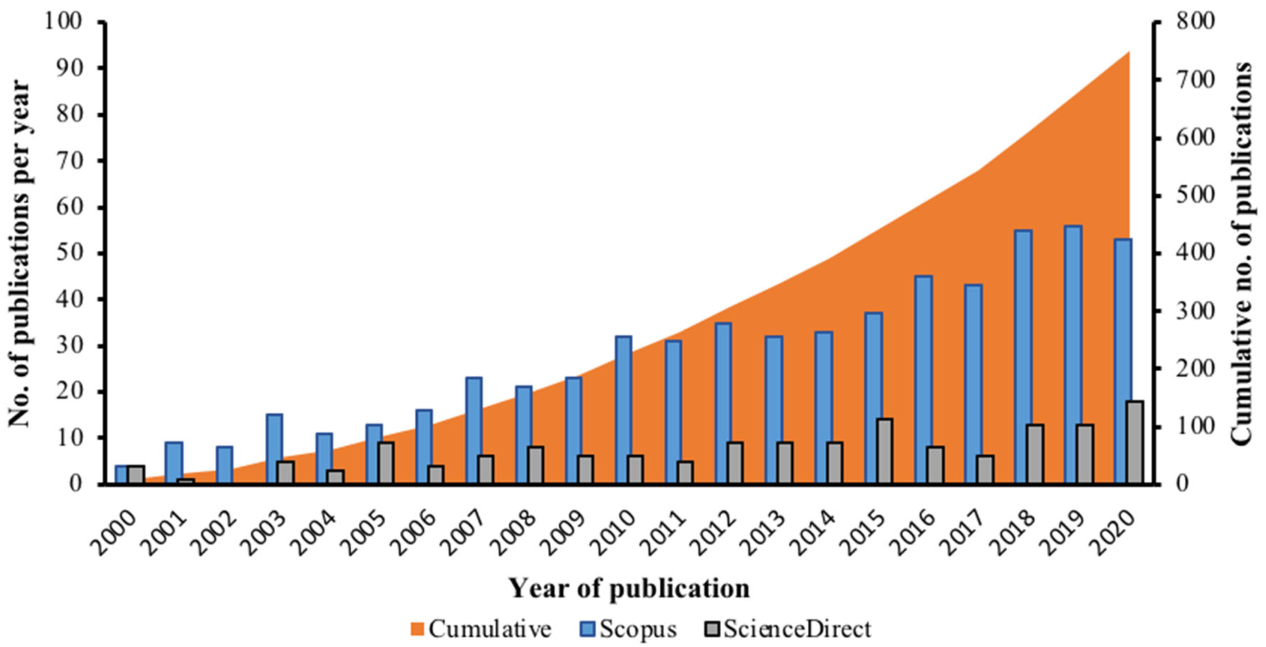
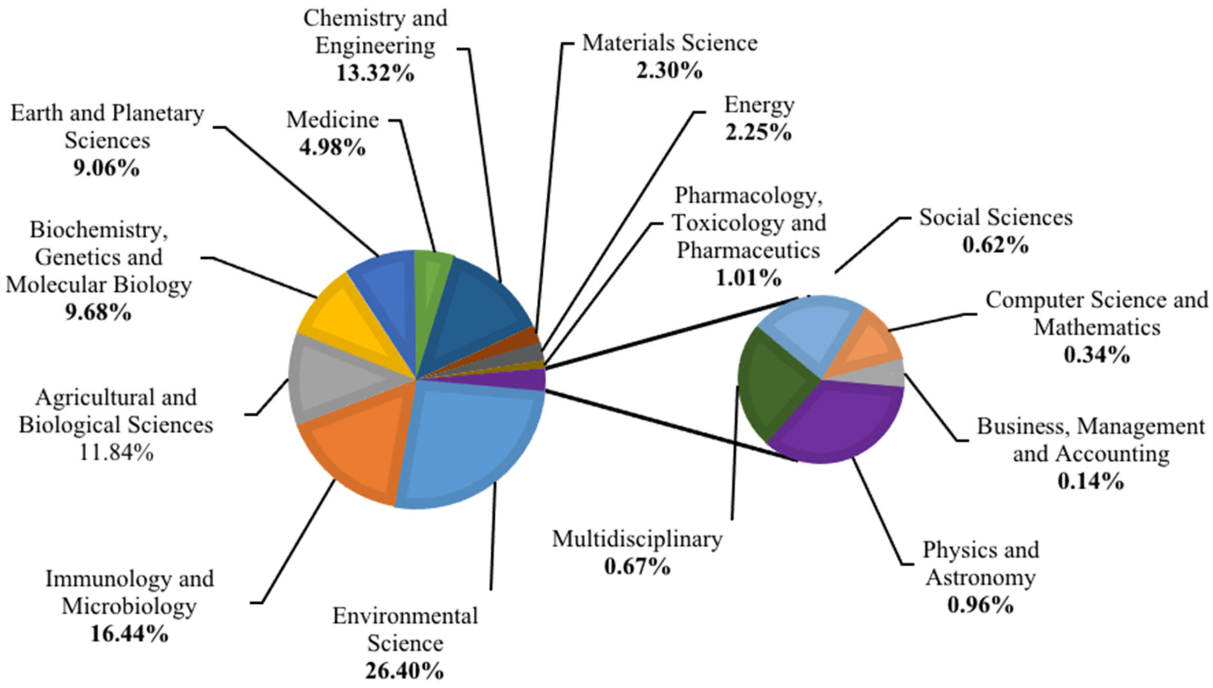
3.1. Research Trends and Driving Factors of Hydrocarbon Pollutions in Antarctica
3.2. Contribution of Member Countries toward Antarctic Research
3.3. Keywords Co-Occurrences Analysis for Publications in 20-Year Spans and Future Research Direction
3.4. Studies of Hydrocarbonoclastic Microbes for Low-Temperature Bioremediation
3.5. Genes Families Conferring to Hydrocarbons Catabolisms and Cold-Adaptation Features in Antarctic Microbes
3.6. Common Community Identification Techniques Applied in Bioremediation
3.6.1. Phospholipid Fatty Acid Analysis (PLFA) and Fatty Acyl Methyl Ester (FAME) Analysis
3.6.2. Non-PCR-Based Nucleic Acids Analyses
3.6.3. PCR-Based Nucleic Acids Analyses
3.6.4. Sequencing-Based Nucleic Acid Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, R.R.; Lim, Z.S.; Shaharuddin, N.A.; Zulkharnain, A.; Gomez-Fuentes, C.; Ahmad, S.A. Diesel in Antarctica and a bibliometric study on its indigenous microorganisms as remediation agent. Int. J. Environ. Res. Public Health 2021, 18, 1512. [Google Scholar] [CrossRef] [PubMed]
- Tin, T.; Fleming, Z.L.; Hughes, K.A.; Ainley, D.G.; Convey, P.; Moreno, C.A.; Pfeiffer, S.; Scott, J.; Snape, I. Review impacts of local human activities on the Antarctic environment. Antarct. Sci. 2009, 21, 3–33. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, N.N.; Roslee, A.F.A.; Gomez-Fuentes, C.; Zulkharnain, A.; Abdulrasheed, M.; Sabri, S.; Ramírez-Moreno, N.; Calisto-Ulloa, N.; Ahmad, S.A. Kinetic studies of marine psychrotolerant microorganisms capable of degrading diesel in the presence of heavy metals. Rev. Mex. Ing. Quím. 2020, 19, 1375–1388. [Google Scholar] [CrossRef]
- Zahri, K.N.M.; Zulkharnain, A.; Gomez-Fuentes, C.; Sabri, S.; Ahmad, S.A. Study of growth kinetics of Antarctic bacterial community for biodegradation of waste canola oil. Desalination Water Treat. 2021, 213, 128–138. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [Green Version]
- Meho, L.I.; Yang, K. Impact of data sources on citation counts and rankings of LIS faculty: Web of Science, Scopus and Google Scholar. J. Assoc. Inf. Sci. Technol. 2007, 58, 2105–2125. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339. [Google Scholar] [CrossRef] [Green Version]
- de Jesus, H.E.; Peixoto, R.S.; Rosado, A.S. Bioremediation in Antarctic Soils. J. Pet. Environ. Biotechnol. 2015, 6, 248. [Google Scholar] [CrossRef] [Green Version]
- Antarctic Treaty Secretariat (ATS). 25 Years of the Protocol on Environmental Protection to the Antarctic Treaty. In Proceedings of the 39th Antarctic Treaty Consultative Meetings (ATCM), Buenos Aires, Argentina, 23 May–1 June 2016.
- Weber, M. Cooperation of the Antarctic Treaty System with the International Maritime Organization and the International Association of Antarctica Tour Operators. Polar J. 2012, 2, 372–390. [Google Scholar] [CrossRef]
- Stewart, E.J.; Draper, D. The sinking of the MS Explorer: Implications for cruise tourism in Arctic Canada. Arctic 2008, 61, 224. [Google Scholar]
- Kennicutt, M.C.; Sweet, S.T.; Fraser, W.R.; Stockton, W.L.; Culver, M. The Fate of diesel fuel spilled by the Bahia Paraiso in Arthur Harbour, Antarctica. In 1991 Oil Spill Conference; American Petroleum Institute: Washington, DC, USA, 1991; pp. 493–500. [Google Scholar]
- Lim, Z.S.; Wong, R.R.; Wong, C.; Zulkharnain, A.; Shaharuddin, N.A.; Ahmad, S.A. Bibliometric analysis of research on diesel pollution in Antarctica and a review on remediation techniques. Appl. Sci. 2021, 11, 1123. [Google Scholar] [CrossRef]
- Tin, T.; Liggett, D.; Maher, P.T. Antarctic Futures. In Antarctic Future: Human Engagement with the Antarctic Environments; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Bender, N.A.; Crosbie, K.; Lynch, H.J. Patterns of tourism in the Antarctic Peninsula region: A 20-year analysis. Antarct. Sci. 2016, 28, 194–203. [Google Scholar] [CrossRef] [Green Version]
- International Association of Antarctica Tour Operators (IAATO). Overview of Antarctic Tourism: 2018-19 Season and Preliminary Estimates for 2019-20 Season; IAATO ATCM XLI: Buenos Aires, Argentina, 2018. [Google Scholar]
- IAATO. IAATO overview of Antarctic tourism: 2018-19 season and preliminary estimates for 2019-20 Season. In Proceedings of the Information Paper 140, Antarctic Treaty Consultative Meeting XLII, Prague, Czech Republic, 27–28 June 2019. [Google Scholar]
- COMNAP. Council of Managers of National Antarctic Programs. Antarctic Station Catalogue (Issue August). 2017. Available online: www.comnap.aq (accessed on 25 May 2021).
- World Map: Advanced. Available online: https://mapchart.net/world-advanced.html (accessed on 5 March 2021).
- Leydesdorff, L.; Rafols, I.; Chen, C. Interactive overlays of journals and the measurement of interdisciplinarity on the basis of aggregated journal-journal citations. J. Assoc. Inf. Sci. Technol. 2013, 64, 2573–2586. [Google Scholar] [CrossRef] [Green Version]
- Antarctic Treaty Inspection Programme Report 2014-15, ATCM (2015). In Report of the Antarctic Treaty Inspections undertaken jointly by the United Kingdom and the Czech Republic in accordance with Article VII of the Antarctic Treaty and Article 14 of the Environmental Protocol; Foreign and Commonwealth Office: London, UK; Ministry of Foreign Affairs: Prague, Czech Republic, 2014.
- Aislabie, J.; Saul, D.J.; Foght, J.M. Bioremediation of hydrocarbon–contaminated polar soils. Extremophiles 2006, 10, 171–179. [Google Scholar] [CrossRef]
- Chakraborty, R.; Wu, C.H.; Hazen, T.C. Systems biology approach to bioremediation. Curr. Opin. Biotechnol. 2012, 23, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Coulon, F.; Whelan, M.J.; Paton, G.I.; Semple, K.T.; Villa, R.; Pollard, S.J.T. Multimedia fate of petroleum hydrocarbons in the soil: Oil matrix of constructed biopiles. Chemosphere 2010, 81, 1454–1462. [Google Scholar] [CrossRef] [Green Version]
- Lim, M.W.; Lau, E.V.; Poh, P.E. A comprehensive guide of remediation technologies for oil contaminated soil–present works and future directions. Mar. Pollut. Bull. 2016, 109, 14–45. [Google Scholar] [CrossRef]
- Chandra, S.; Sharma, R.; Singh, K.; Sharma, A. Application of bioremediation technology in the environment contaminated with petroleum hydrocarbon. Ann. Microbiol. 2013, 63, 417–431. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Kumari, R. Bioremediation: A sustainable tool for environmental management—A review. Annu. Rev. Res. Biol. 2013, 3, 974–993. [Google Scholar]
- Chikere, C.B.; Okpokwasili, G.C.; Chikere, B.O. Monitoring of microbial hydrocarbon remediation in the soil. 3 Biotech 2011, 1, 117–138. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.S.; Keum, Y.S.; Li, Q.X. Bacterial degradation of aromatic compounds. Int. J. Environ. Res. Public Health 2009, 6, 278–309. [Google Scholar] [CrossRef]
- Mrozik, A.; Piotrowska–Seget, Z. Bioaugmentation as a strategy for cleaning up of soils contaminated with aromatic compounds. Microbiol. Res. 2010, 165, 363–375. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnol. Res. Int. 2011, 13. [Google Scholar] [CrossRef] [Green Version]
- Shankar, S.; Shikha. Management and remediation of problem soils, solid waste and soil pollution. In Principles and Applications of Environmental Biotechnology for a Sustainable Future, 2nd ed.; Singh, R.L., Ed.; Springer: Singapore, 2017; pp. 143–171. [Google Scholar]
- Roslee, A.F.A.; Zakaria, N.N.; Convey, P.; Zulkharnain, A.; Lee, G.L.Y.; Gomez-Fuentes, C.; Ahmad, S.A. Statistical optimisation of growth conditions and diesel degradation by the Antarctic bacterium, Rhodococcus sp. strain AQ5‒07. Extremophiles 2020, 24, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Abdulrasheed, M.; Zulkharnain, A.; Zakaria, N.N.; Roslee, A.F.A.; Khalil, K.A.; Napis, S.; Convey, P.; Gomez‒Fuentes, C.; Ahmad, S.A. Response surface methodology optimisation and kinetics of diesel degradation by cold‒adapted Antarctic bacterium, Arthrobacter sp. strain AQ5‒05. Sustainability 2020, 12, 6966. [Google Scholar] [CrossRef]
- Reyes-César, A.; Absalón, Á.E.; Fernández, F.J.; González, J.M.; Cortés-Espinosa, D.V. Biodegradation of a mixture of PAHs by non-ligninolytic fungal strains isolated from crude oil-contaminated soil. World J. Microbiol. Biotechnol. 2014, 30, 999–1009. [Google Scholar] [CrossRef]
- Al-Nasrawi, H. Biodegradation of crude oil by fungi isolated from Gulf of Mexico. J. Bioremediat. Biodegrad. 2012, 3, 1–6. [Google Scholar]
- Al-Disi, Z.; Al-Thani, D.; Al-Meer, S.; Zouari, N. Considering the specific impact of harsh conditions and oil weathering on diversity, adaptation, and activity of hydrocarbon–degrading bacteria in strategies of bioremediation of harsh oily–polluted soils. Biomed. Res. Int. 2017, 11. [Google Scholar] [CrossRef] [Green Version]
- van Hamme, J.D.; Singh, A.; Ward, O.P. Recent advances in petroleum microbiology. Microbiol. Mol. Biol. Rev. 2003, 67, 503–549. [Google Scholar] [CrossRef] [Green Version]
- Dadrasnia, A.; Agamuthu, P. Organic wastes to enhance phyto–treatment of diesel–contaminated soil. J. Waste Manag. Res. 2013, 31, 1133–1139. [Google Scholar] [CrossRef]
- Hamzah, A.; Phan, C.W.; Yong, P.H.; Ridzuan, N.H.M. Oil palm empty fruit bunch and sugarcane bagasse enhance the bioremediation of soil artificially polluted by crude oil. Soil Sediment Contam. 2014, 23, 751–762. [Google Scholar] [CrossRef]
- Antoniou, E.; Fodelianakis, S.; Korkakaki, E.; Kalogerakis, N. Biosurfactant production from marine hydrocarbon–degrading consortia and pure bacterial strains using crude oil as carbon source. Front. Microbiol. 2015, 6, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza Pereira Silva, D.; de Lima Cavalcanti, D.; de Melo, E.J.V.; dos Santos, P.N.F.; da Luz, E.L.P.; de Gusmão, N.B.; de Queiroz Sousa, M. Bio–removal of diesel oil through a microbial consortium isolated from a polluted environment. Int. Biodeterior. Biodegrad. 2015, 97, 85–89. [Google Scholar] [CrossRef]
- Kaczorek, E.; Pacholak, A.; Zdarta, A.; Smulek, W. The impact of biosurfactants on microbial cell properties leading to hydrocarbon bioavailability increase. J. Colloid Interface Sci. 2018, 2, 35. [Google Scholar] [CrossRef] [Green Version]
- Bezza, F.A.; Chirwa, E.M.N. Biosurfactant from Paenibacillus dendritiformis and its application in assisting polycyclic aromatic hydrocarbon (PAH) and motor oil sludge removal from contaminated soil and sand media. Process. Saf. Environ. Prot. 2015, 98, 354–364. [Google Scholar] [CrossRef]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation techniques–classification based on site of application: Principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016, 32, 180. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, M.; da Fonseca, M.M.R.; de Carvalho, C.C.C.R. Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegradation 2011, 22, 231–241. [Google Scholar] [CrossRef]
- Roy, A.S.; Baruah, R.; Borah, M.; Singh, A.K.; Deka Boruah, H.P.; Saikia, N.; Deka, M.; Dutta, N.; Bora, T.C. Bioremediation potential of native hydrocarbon degrading bacterial strains in crude oil contaminated soil under microcosm study. Int. Biodeterior. Biodegrad. 2014, 94, 79–89. [Google Scholar] [CrossRef]
- Antarctic Wildlife: Plants. British Antarctic Survey, BAS. 2015. Available online: https://www.bas.ac.uk/about/antarctica/wildlife/plants/ (accessed on 5 March 2021).
- Michaud, L.; Lo Giudice, A.; Saitta, M.; De Dominico, M.; Bruni, V. The biodegradation efficiency on diesel oil by two psychrotrophic Antarctic marine bacteria during a two-month-long experiment. Mar. Pollut. Bull. 2004, 49, 405–409. [Google Scholar] [CrossRef]
- Saul, D.J.; Aislabie, J.M.; Brown, C.E.; Harris, L.; Foght, J.M. Hydrocarbon contamination changes the bacterial diversity of soil from around Scott Base, Antarctica. FEMS Microbiol. Ecol. 2005, 53, 141–155. [Google Scholar] [CrossRef] [Green Version]
- Pepi, M.; Cesaro, A.; Liut, G.; Baldi, F. An Antarctic psychrotrophic bacterium Halomonas sp. ANT-3b, growing on n-hexadecane, produces a new emulsyfying glycolipid. FEMS Microbiol. Ecol. 2005, 53, 157–166. [Google Scholar] [CrossRef]
- Stallwood, B.; Shears, J.; Williams, P.A.; Hughes, K.A. Low temperature bioremediation of oil-contaminated soil using biostimulation and bioaugmentation with a Pseudomonas sp. from maritime Antarctica. J. Appl. Microbiol. 2005, 99, 794–802. [Google Scholar] [CrossRef]
- Powell, S.M.; Ferguson, S.H.; Snape, I.; Siciliano, S.D. Fertilization stimulates anaerobic fuel degradation of antarctic soils by denitrifying microorganisms. Environ. Sci. Technol. 2006, 40, 2011–2017. [Google Scholar] [CrossRef]
- Pini, F.; Grossi, C.; Nereo, S.; Michaud, L.; Lo Giudice, A.; Bruni, V.; Baldi, F.; Fani, R. Molecular and physiological characterisation of psychrotrophic hydrocarbon-degrading bacteria isolated from Terra Nova Bay (Antarctica). Eur. J. Soil Biol. 2007, 43, 368–379. [Google Scholar] [CrossRef]
- Okere, U.V.; Cabrerizo, A.; Dachs, J.; Jones, K.C.; Semple, K.T. Biodegradation of phenanthrene by indigenous microorganisms in soils from Livingstone Island, Antarctica. FEMS Microbiol. Lett. 2012, 329, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, F.; Liang, Q.; He, B.; Miao, J. Low-temperature degradation mechanism analysis of petroleum hydrocarbon-degrading antarctic psychrophilic strains. J. Pure Appl. Microbiol. 2014, 8, 47–53. [Google Scholar]
- Martinez Alvarez, L.M.; Lo Balbo, A.; Mac Cormack, W.P.; Ruberto, L.A.M. Bioremediation of a petroleum hydrocarbon-contaminated Antarctic soil: Optimization of a biostimulation strategy using response-surface methodology (RSM). Cold Reg. Sci. Technol. 2015, 119, 61–67. [Google Scholar] [CrossRef]
- Vazquez, S.; Monien, P.; Minetti, R.P.; Jurgens, J.; Curtosi, A.; Villalba Primitz, J.; Frickenhaus, S.; Abele, D.; Mac Cormack, W.; Helmke, E. Bacterial communities and chemical parameters in soils and coastal sediments in response to diesel spills at Carlini Station, Antarctica. Sci. Total Environ. 2017, 605–606, 26–37. [Google Scholar] [CrossRef]
- Gran-Scheuch, A.; Fuentes, E.; Bravo, D.M.; Jiménez, J.C.; Pérez-Donoso, J.M. Isolation and characterization of phenanthrene degrading bacteria from diesel fuel-contaminated Antarctic soils. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, C.C.; Malavenda, R.; Gerçe, B.; Papale, M.; Syldatk, C.; Hausmann, R.; Bruni, V.; Michaud, L.; Lo Giudice, A.; Amalfitano, S. Effects of a simulated acute oil spillage on bacterial communities from Arctic and Antarctic marine sediments. Microorganisms 2019, 7, 632. [Google Scholar] [CrossRef] [Green Version]
- Villalba Primitz, J.; Vazquez, S.; Ruberto, L.A.M.; Lo Balbo, A.; Mac Cormack, W.P. Bioremediation of hydrocarbon-contaminated soil from Carlini Station, Antarctica: Effectiveness of different nutrient sources as biostimulation agents. Polar Biol. 2021, 44, 289–303. [Google Scholar] [CrossRef]
- Margesin, R.; Walder, G.; Schinner, F. Bioremediation assessment of a BTEX-contaminated soil. Acta Biotechnol. 2003, 23, 29–36. [Google Scholar] [CrossRef]
- Hua, Z.; Chen, J.; Lun, S.; Wang, X. Influence of biosurfactants produced by Candida antarctica on surface properties of microorganism and biodegradation of n-alkanes. Water Res. 2003, 37, 4143–4150. [Google Scholar] [CrossRef]
- Ferrari, B.C.; Zhang, C.; Van Dorst, J. Recovering greater fungal diversity from pristine and diesel fuel contaminated sub-antarctic soil through cultivation using both a high and a low nutrient media approach. Front. Microbiol. 2011, 2, 217. [Google Scholar] [CrossRef] [Green Version]
- Martorell, M.M.; Ruberto, L.A.M.; Fernández, P.M.; Castellanos de Figueroa, L.I.; Mac Cormack, W.P. Bioprospection of cold-adapted yeasts with biotechnological potential from Antarctica. J. Basic Microbiol. 2017, 57, 504–516. [Google Scholar] [CrossRef]
- Ghosal., D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): A review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef] [Green Version]
- Kostka, J.E.; Teske, A.P.; Joye, S.B.; Head, I.M. The metabolic pathways and environmental controls of hydrocarbon biodegradation in marine ecosystems. Front. Microbiol. 2014, 5, 471. [Google Scholar] [CrossRef] [Green Version]
- Rojo, F. Degradation of alkanes by bacteria. Environ. Microbiol. 2009, 11, 2477–2490. [Google Scholar] [CrossRef]
- Abbasian, F.; Lockington, R.; Mallavarapu, M.; Naidu, R.A. Comprehensive review of aliphatic hydrocarbon biodegradation by bacteria. J. Appl. Biochem. Biotechnol. 2015, 176, 670–699. [Google Scholar] [CrossRef]
- Mallick, S.; Chakraborty, J.; Dutta, T.K. Role of oxygenases in guiding diverse metabolic pathways in the bacterial degradation of low-molecular-weight polycyclic aromatic hydrocarbons: A review. Crit. Rev. Microbiol. 2011, 37, 64–90. [Google Scholar] [CrossRef]
- Li, X.; Lin, X.; Zhang, J.; Wu, Y.; Yin, R.; Feng, Y.; Wang, Y. Degradation of polycyclic aromatic hydrocarbons by crude extracts from spent mushroom substrate and its possible mechanisms. Curr. Microbiol. 2010, 60, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Geng, A.; Loh, K.-C. Induction of ortho- and meta-cleavage pathways in Pseudomonas in biodegradation of high benzoate concentration: MS identification of catabolic enzymes. Appl. Microbiol. Biotechnol. 2008, 81, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Brzeszcz, J.; Kaszycki, P. Aerobic bacteria degrading both n-alkanes and aromatic hydrocarbons: An undervalued strategy for metabolic diversity and flexibility. Biodegradation 2018, 29, 359–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caspi, R.; Billington, R.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Midford, P.E.; Ong, Q.; Ong, W.K.; et al. The MetaCyc database of metabolic pathways and enzymes. Nucleic Acids Res. 2018, 46, D633–D639. [Google Scholar] [CrossRef] [Green Version]
- Bosch, R.; Garcia-Valdes, E.; Moore, E.R. Genetic characterization and evolutionary implications of a chromosomally encoded naphthalene-degradation upper pathway from Pseudomonas stutzeri AN10. Gene 1999, 236, 149–157. [Google Scholar] [CrossRef]
- Kasak, L.; Horak, R.; Nurk, R.; Talvik, K.; Kivisaar, M. Regulation of the catechol 1,2-dioxygenase- and phenol monooxygenase-encoding pheBA operon in Pseudomonas putida PaW85. J. Bacteriol. 1993, 175, 8038–8042. [Google Scholar] [CrossRef] [Green Version]
- Bakermans, C.; Bergholz, P.W.; Rodrigues, D.F.; Vishnivetskaya, T.A.; Ayala-del-Rio, H.L.; Tiedje, J.M. Genomic and expression analyses of cold-adapted microorganisms. In Polar Microbiology: Life in a Deep Freeze; ASM Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Raymond-Bouchard, I.; Goordial, J.; Zolotarov, Y.; Ronholm, J.; Stromvik, M.; Bakermans, C.; Whyte, L.G. Conserved genomic and amino acid traits of cold adaptation in subzero-growing Arctic permafrost bacteria. FEMS Microbiol. Ecol. 2018, 94. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, M.K. Mechanism of bacterial adaptation to low temperature. J. Biosci. 2006, 31, 157–165. [Google Scholar] [CrossRef]
- Goordial, J.; Raymond-Bouchard, I.; Zolotarov, Y.; de Bethencourt, L.; Ronholm, J.; Shapiro, N.; Woyke, T.; Stromvik, M.; Greer, C.W.; Bakermans, C.; et al. Cold adaptive traits revealed by comparative genomic analysis of the eurypsychrophile Rhodococcus sp. JG3 isolated from high elevation McMurdo Dry Valley permafrost, Antarctica. FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef] [Green Version]
- Tribelli, P.M.; Lopez, N.I. Reporting key features in cold-adapted bacteria. Life 2018, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Kralova, S. Role of fatty acids in cold adaptation of antarctic psychrophilic Flavobacterium spp. Syst. Appl. Microbiol. 2017, 40, 329–333. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, M.P.; Shivaji, S. Molecular mechanisms of cold adaptation in bacteria. Aperito J. Cell Mol. Biol. 2015, 1. [Google Scholar] [CrossRef]
- Sarmiento, F.; Peralta, R.; Blamey, J.M. Cold and hot extremozymes: Industrial relevance and current trends. Front. Bioeng. Biotechnol. 2015, 3, 148. [Google Scholar] [CrossRef] [Green Version]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 108–115. [Google Scholar] [CrossRef] [Green Version]
- de Fatima Alves, L.; Westmann, C.A.; Lovate, G.L.; de Siqueira, G.M.V.; Borelli, T.C.; Guazzaroni, G.-E. Metagenomic approaches for understanding new concepts in microbial science. Int. J. Genom. 2018. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.Q.; Yoshigoe, K.; Yang, W.; Tong, W.; Qin, X.; Dunker, A.K.; Chen, Z.; Arbania, H.R.; Liu, J.S.; Niemierko, A.; et al. The emerging genomics and systems biology research lead to systems genomics studies. BMC Genom. 2014, 15. [Google Scholar] [CrossRef] [Green Version]
- Sohlenkamp, C.; Geiger, O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2015, 40, 40. [Google Scholar] [CrossRef] [Green Version]
- Moldoveanu, S.C.; David, V. Derivatization methods in GC and GC/MS. IntechOpen 2018. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, G.; Sani, R.K. Molecular techniques to assess microbial community structure, function, and dynamics in the environment. In Microbes and Microbial Technology: Agricultural and Environmental Applications; Ahmad, I., Ahmad, F., Pitchel, J., Eds.; Springer: New York, NY, USA, 2011; pp. 29–57. [Google Scholar]
- Lanekoff, I.; Karlsson, R. Analysis of intact ladderane phospholipids, originating from viable anammox bacteria, using RP-LC-ESI-MS. Anal. Bioanal. Chem. 2010, 397, 3543–3551. [Google Scholar] [CrossRef] [Green Version]
- Sabale, S.N.; Suryawanshi, P.P.; Krisnaraj, P.U. Soil metagenomics: Concepts and applications. IntechOpen 2019. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, P.; Petersen, S.O. Ester-linked polar lipid fatty acid profiles of soil microbial communities: A comparison of extraction methods and evaluation of interference from humic acids. Soil Biol. Biochem. 2000, 32, 1241–1249. [Google Scholar] [CrossRef]
- Smarda, P.; Bures, P.; Horova, L.; Leitch, I.J.; Mucina, L.; Pacini, E.; Tichy, L.; Grulich, V.; Rotreklova, O. Ecological and evolutionary significance of genomic GC content diversity in monocots. Proc. Natl. Acad. Sci. USA 2014. [Google Scholar] [CrossRef] [Green Version]
- Holben, W.E. GC fractionation allows comparative total microbial community analysis, enhances diversity assessment, and facilitates detection of minority populations of bacteria. In Handbook of Molecular Microbial Ecology, Volume I: Metagenomics and Complementary Approaches; de Bruijn, F.J., Ed.; Wiley-Blackwell, John Wiley and Sons: Hoboken, NJ, USA, 2011; pp. 183–196. [Google Scholar]
- Torsvik, V.; Daae, F.L.; Sandaa, R.-A.; Ovreas, L. Novel techniques for analysing microbial diversity in natural and perturbed environments. J. Biotechnol. 1998, 64, 53–62. [Google Scholar] [CrossRef]
- Voordouw, G.; Voordouw, J.K.; Karkhoff-Schweizer, R.R.; Fedorak, P.M.; Westlake, D.W.S. Reverse sample genome probing, a new technique for identification of bacteria in environmental samples by DNA hybridization, and its application to the identification of sulfate-reducing bacteria in oil field samples. Appl. Environ. Microbiol. 1991, 57, 3070–3078. [Google Scholar] [CrossRef] [Green Version]
- Shanks, O.C.; Santo Domingo, J.W.; Lamendella, R.; Kelty, C.A.; Graham, J.E. Competitive metagenomic DNA hybridization identifies host-specific microbial genetic markers in cow fecal samples. Appl. Environ. Microbiol. 2006, 72, 4054–4060. [Google Scholar] [CrossRef] [Green Version]
- Bohme, K.; Barros-Velasquez, J.; Calo-Mata, P. Molecular tools to analyze microbial populations in red wines. In Red Wine Technology; Morata, A., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2019; pp. 115–123. [Google Scholar]
- Wu, Z.; Lin, W.; Li, B.; Wu, L.; Fang, C.; Zhang, Z. Terminal restriction fragment length polymorphism analysis of soil bacterial communities under different vegetation types in subtropical area. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Trent, R.J. DNA genetic testing. In Molecular Medicine: Genomics to Personalized Healthcare, 4th ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2012; pp. 81–114. [Google Scholar]
- Stone, A.; Bornhorst, J. An introduction to personalized medicine. In Therapeutic Drug Monitoring: Newer Drugs and Biomarkers; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2012; pp. 121–142. [Google Scholar]
- Samarajeewa, A.D.; Hammad, A.; Masson, L.; Khan, I.U.H.; Scroggins, R.; Beaudette, L.A. Comparative assessment of next-generation sequencing, denaturing gradient gel-electrophoresis, clonal restriction fragment length polymorphism and cloning-sequencing as methods for characterizing commercial microbial consortia. J. Microbioll. Methods 2015, 108, 103–111. [Google Scholar] [CrossRef]
- Enwall, K.; Hallin, S. Comparison of T-RFLP and DGGE techniques to assess denitrifier community composition in soil. Lett. Appl. Microbiol. 2009, 48, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Foroni, L.; Reid, A.G.; Gerrard, G.; Toma, S.; Hing, S. Molecular and cytogenetic analysis. In Dacie and Lewis Practical Haematology, 12th ed.; Bain, B.J., Bates, I., Laffan, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 126–164. [Google Scholar]
- Zhang, P.; Seth, A.; Fernandes, H. Other post-PCR detection technologies. In Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms; McManus, L.M., Mitchell, R.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 4074–4088. [Google Scholar]
- Lapidus, A.L. Genome sequence databases: Sequencing and assembly. In Encyclopedia of Microbiology; Schmidt, T.M., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 400–418. [Google Scholar] [CrossRef]
- Heidelberg, J.F.; Paulsen, I.T.; Nelseon, K.E.; Fraser, C.M. Microbial genomics. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 399–404. [Google Scholar]
- Clark, D.P.; Pazdernik, N.J. Genomics and systems biology. In Molecular Biology, 2nd ed.; Academic Cell: Cambridge, MA, USA, 2013; pp. e110–e117. [Google Scholar] [CrossRef]
- Perez-Cobas, E.; Gomez-Valero, L.; Buchrieser, C. Metagenomic approaches in microbial ecology: An update on whole-genome and marker gene sequencing analyses. Microb. Genom. 2020, 6, e000409. [Google Scholar] [CrossRef]
- Coupland, P.; Chandra, T.; Quail, M.; Reik, W.; Swerdlow, H. Direct sequencing of small genomes on the Pacific Biosciences RS without library preparation. BiotTechniques 2012, 53, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, A.; Au, K.F. PacBio sequencing and its applications. Genom. Proteom. Bioinform. 2015, 13, 278–289. [Google Scholar] [CrossRef] [Green Version]
- Quail, M.A.; Smith, M.; Coupland, P.; Otto, T.D.; Harris, S.R.; Connor, T.R.; Bertoni, A.; Swerdlow, H.P.; Gu, Y. A tale of three next generation sequencing platforms: Comparison of Ion Torrent, Pacific Biosciences and Illumina MiSeq sequencers. BMC Genom. 2012, 13, 341. [Google Scholar] [CrossRef] [Green Version]
- Reuter, J.A.; Spacek, D.; Snyder, M.P. High-throughput sequencing technologies. Mol. Cell 2015, 58, 586–597. [Google Scholar] [CrossRef] [Green Version]
- Berlin, K.; Koren, S.; Chin, C.-S.; Drake, J.; Landolin, J.M.; Phillippy, A.M. Assembling large genomes with single-molecule sequencing and locality sensitive hashing. Nat. Biotechnol. 2014, 33, 623–630. [Google Scholar] [CrossRef]




| Keyword | Occurences | Total Link Strength |
|---|---|---|
| Antarctica | 75 | 240 |
| Bioremediation | 35 | 122 |
| Diesel | 26 | 97 |
| Bacteria | 11 | 39 |
| Human impacts | 7 | 35 |
| Policy | 4 | 30 |
| Antarctic soils | 8 | 28 |
| Heavy metals | 7 | 28 |
| Pollution | 7 | 27 |
| Biostimulation | 7 | 26 |
| Antarctic Treaty | 4 | 24 |
| Conservation | 4 | 24 |
| Marine | 6 | 24 |
| Response surface methodology | 6 | 24 |
| Biosurfactants | 6 | 22 |
| Microorganism(s) | Substrate(s) Degraded | Initial Concentration | Maximum Efficiency (%) | Location of Study/Source of Sample | Method of Study | Reference |
|---|---|---|---|---|---|---|
| Bacteria | ||||||
| Pseudomonas Ant 5 and Sphingomonas Ant 17 | BTEX, naphthalene, JP-8 fuel | Various | NA | Scott Base (Ross Island, Antarctica) | Cell culture | [22] |
| Arthrobacter sp. E28 and Rhodococcus sp. E60 | Diesel | 1% v v−1 | 86% E28 and 89.2% E60, 160 days | Terra Nova Bay (Ross Sea, Antarctica) | Cell culture | [49] |
| Soil microbial community | n-Alkane, PAHs, toluene, | 5 μL ml−1 | NA | Scott Base (Ross Island, Antarctica) | RFLP | [50] |
| Halomonas sp. ANT 3b | Diesel, hexadecane | 2% v v−1 | NA | Terra Nova Bay station (Ross Sea, Antarctica) | Cell culture | [51] |
| Pseudomonas sp. ST41 | Polar Blend marine gasoil | 1% w dw−1 | NA | Signy Island (South Orkney Islands, Antarctica) | TGGE, bioaugmentation and biostimulation | [52] |
| Soil microbial community | Special Antarctic Blend (SAB) | 10,000–47,000 ± 630 mg kg−1 | NA | Casey Station (Bailey Peninsula, Antarctica) | DGGE | [53] |
| Rhodococcus sp. and Alcaligenes sp. co-culture | Diesel, n-alkane | 2% v v−1 | NA | Terra Nova Bay (Ross Sea, Antarctica) | ARDRA and RAPD | [54] |
| Soil microbial community | Phenanthrene | 0.14–1.47 ng g−1 dw | 47.93%, 7 days | Livingstone Island (South Shetlands Islands, Antarctica) | Cell culture | [55] |
| Planococcus sp. NJ41 and Shewanella sp. NJ49 | Diesel, naphthalene, hexadecane | 50 mg L−1 | NA | Antarctic Ocean | Cell culture | [56] |
| Soil microbial community | Diesel | 2180 mg kg−1 dw | 75.79% BP and 49.54% BS, 50 days | Carlini station (South Shetlands Islands, Antarctica) | RSM, Biopile (BP) and biostimulation (BS) | [57] |
| Soil and sediment microbial communities | Diesel | 200–1000 μg g−1 dw | NA | Carlini station (South Shetlands Islands, Antarctica) | DGGE | [58] |
| Sphingobium xenophagum D43FB | Phenanthrene | 2000 ppm | 95%, 5 days | King George Island, South Shetland Islands, Antarctica | Cell culture | [59] |
| Marine sediment microbial communities | Crude oil and diesel | 1.5% v v−1 | NA | Livingston Island (Byers Peninsula, Antarctica) | T-RFLP and DGGE | [60] |
| Arthrobacter sp. AQ5-05 | Diesel | 3% v v−1 | 56.32%, 10 days | King George Island (South Shetland Islands, Antarctica) | RSM, cell culture | [34] |
| Rhodococcus sp. AQ5-07 | Diesel | 1% v v−1 | 90.39%, 7 days | King George Island (South Shetland Islands, Antarctica) | RSM, cell culture | [33] |
| Soil microbial communities | Antarctic Gasoil, AGO | 7620 ± 680 mg kg−1 dw | 87 ± 13%, 1 year | Carlini station (South Shetlands, Antarctica) | DGGE | [61] |
| Fungi/Yeast | ||||||
| Various yeast strains | Hexadecane dodecane, | 1 g L−1 | 39.9% and 8 days | Continental glacier, Antarctica | Cell culture | [62] |
| Candida antarctica T-34 | Undecane | 83.9 ± 1.2 % | Ohridski Base, Livingston Island, Antarctica | Cell culture | [63] | |
| Exophiala sp. and Pseudeurotium bakeri | Special Antarctic Blend (SAB) | 50–20,000 mg kg−1 | NA | Australian Research station (Macquarie Island, Antarctica) | RFLP | [64] |
| Pichia caribbicia | n-alkanes and diesel fuel | 1 g L−1 | NA | Carlini Station (South Shetlands Islands, Antarctica) | Cell culture | [65] |
| Gene Name | EC Number | Enzyme Name |
|---|---|---|
| Cyclohexane (via β-oxidation) | ||
| bmoX | - | cyclohexane hydroxylase |
| chnA | 1.1.1.245 | cyclohexanol dehydrogenase |
| chnB | 1.14.13.22 | cyclohexanone-NADPH monooxygenase |
| chnC | 3.1.1.- | caprolactone hydrolase |
| chnD | 1.1.1.258 | hydroxyhexanoate-NAD+ dehydrogenase |
| chnE | 1.2.1.63 | oxohexanoate dehydrogenase |
| Ethylbenzene (Via procathecuate) | ||
| ebdA | 1.17.99.2 | ethylbenzene dehydrogenase-α |
| ebdB | ethylbenzene dehydrogenase-β | |
| ebdC | ethylbenzene dehydrogenase-γ | |
| ped | 1.1.1.311 | (S)-1-phenylethanol dehydrogenase |
| apcA | 6.4.1.8 | acetophenone carboxylase |
| apcB | acetophenone carboxylase | |
| apcC | acetophenone carboxylase | |
| apcD | acetophenone carboxylase | |
| apcE | acetophenone carboxylase | |
| bal | - | benzoylacetate-CoA ligase |
| fadA | - | 3-keto-acyl-CoA-thiolase |
| Shikimate/quinate degradation (via procatechuate) | ||
| quiA | 1.1.5.8 | shikimate dehydrogenase |
| quiA | quinate dehydrogenase | |
| quiB | 4.2.1.10 | dehydroquinate dehydratase |
| quiC | 4.2.1.118 | dehydroshikimate dehydratase |
| Hydroxymandelate degradation (via procatechuate) | ||
| mdlA | 5.1.2.2 | (S)-4-hydroxymandelate racemase |
| mdlA | mandelate racemase | |
| mdlB | 1.1.5.- | (S)-mandelate dehydrogenase |
| mdlB | (S)-2-hydroxy-2-(4-hydroxyphenyl)acetate:acceptor 2-oxidoreductase | |
| mdlC | 4.1.1.7 | p-hydroxybenzoylformate carboxy-lyase |
| mdlD | 1.2.1.96 | NADP+-4-hydroxybenzaldehyde dehydrogenase |
| pobA | 1.14.13.2 | p-hydroxybenzoate hydroxylase |
| pobA | p-hydroxybenzoate hydroxylase | |
| Procatechuate degradation (via 2-hydroxypenta-2,4-dienoate) | ||
| praA | 1.13.11.- | protocatechuate 2,3-dioxygenase |
| praH | - | 5-carboxy-2-hydroxymuconate-6-semialdehyde decarboxylase |
| praB | 1.2.1.85 | 2-hydroxymuconate-6-semialdehyde dehydrogenase |
| xylG | 2-hydroxymuconate semialdehyde dehydrogenase | |
| praC | 5.3.2.6 | 4-oxalocrotonate tautomerase |
| xylH | 2-hydroxymuconate tautomerase | |
| praD | 4.1.1.77 | 2-oxo-3-hexenedioate decarboxylase |
| xylI | 2-oxo-3-hexenedioate decarboxylase | |
| Toluene degradation (via 2-hydroxypenta-2,4-dienoate degradation) | ||
| todC2 | 1.14.12.11 | toluene 1,2-dioxygenase |
| todC1 | toluene 1,2-dioxygenase | |
| todA | toluene 1,2-dioxygenase | |
| todB | toluene 1,2-dioxygenase | |
| todD | 1.3.1.19 | cis-toluene dihydrodiol dehydrogenase |
| todE | 1.13.11.2 | 3-methylcatechol 2,3-dioxygenase |
| todF | 3.7.1.25 | 2-hydroxy-6-oxohepta-2,4-dienoate hydrolase |
| Mandelate degradation (via catechol) | ||
| mdlA | 5.1.2.2 | mandelate racemase |
| mdlB | 1.1.99.31 | (S)-2-hydroxy-2-phenylacetate:acceptor 2-oxidoreductase |
| mdlC | 4.1.1.7 | benzoylformate carboxy-lyase |
| mdlD | 1.2.1.7 | NADP+-benzaldehyde dehydrogenase |
| ntnC | 1.2.1.28 | benzaldehyde dehydrogenase |
| xylC | benzaldehyde dehydrogenase | |
| xylX | 1.14.12.10 | benzoate 1,2-dioxygenase |
| xylY | benzoate 1,2-dioxygenase | |
| xylZ | benzoate 1,2-dioxygenase | |
| xylL | 1.3.1.25 | 1,2-dihyroxy-3,5-cyclohexadiene-1-carboxylate dehydrogenase |
| Naphthalene degradation (via catechol) | ||
| nahAd | 1.14.12.12 | naphthalene 1,2-dioxygenase |
| nahAc | naphthalene 1,2-dioxygenase | |
| nahAb | naphthalene 1,2-dioxygenase | |
| nahAa | naphthalene 1,2-dioxygenase | |
| ndoA | naphthalene 1,2-dioxygenase | |
| ndoC | naphthalene 1,2-dioxygenase | |
| ndoB | naphthalene 1,2-dioxygenase | |
| ndoR | naphthalene 1,2-dioxygenase | |
| nahB | 1.3.1.29 | cis-1,2-dihydro-1,2-dihydroxynaphthalene-1, 2-dehydrogenase |
| nahC | 1.3.11.56 | 1,2-dihydroxynaphthalene dioxygenase |
| nahD | 5.99.1.4 | 2-hydroxychromene-2-carboxylate isomerase |
| nahE | 4.1.2.45 | trans-o-hydroxybenzylidenepyruvate hydratase-aldolase |
| nahF | 1.2.1.65 | salicylaldehyde dehydrogenase |
| alkH | aldehyde dehydrogenase | |
| Salicylate degradation (via catechol) | ||
| salA | salicylate 1-hydroxylase | |
| nahW | salicylate hydroxylase | |
| nahG | salicylate hydroxylase | |
| Anthranilate degradation (via catechol) | ||
| kyn | 3.7.1.3 | kynureninase |
| antB | 1.14.12.1 | anthranilate dioxygenase |
| antA | anthranilate dioxygenase | |
| antC | anthranilate dioxygenase | |
| Catechol degradation (via 2-hydroxypenta-2,4-dienoate) | ||
| xylE | 1.13.11.2 | catechol 2,3-dioxygenase |
| xylF | 3.7.1.9 | 2-hydroxymuconic semialdehyde hydrolase |
| 2-Hydroxypenta-2,4-dienoate degradation (via acetyl-CoA) | ||
| todG | 4.2.1.80 | 2-oxopent-4-enoate hydratase |
| cmtF | 2-oxopent-4-enoate hydratase | |
| xylJ | 2-oxopent-4-enoate hydratase | |
| cmtG | 4.1.3.39 | 4-hydroxy-2-oxovalerate aldolase |
| todH | 4-hydroxy-2-oxovalerate aldolase | |
| xylK | 4-hydroxy-2-oxovalerate aldolase | |
| todI | 1.2.1.0 | acylating aldehyde dehydrogenase |
| cmtH | acetaldehyde dehydrogenase | |
| Heat-Shock (Hsp) and Cold-Shock (Csp) Proteins |
| Molecular chaperone GrpE (heat shock protein) |
| Molecular chaperone DnaK (HSP70) |
| Molecular chaperone IbpA, HSP20 family |
| Ribosomal 50S subunit-recycling heat shock protein |
| Chaperonin GroEL (HSP60 family) |
| Co-chaperonin GroES (HSP10) |
| Cold shock protein, CspA family |
| Universal stress protein, UspA family |
| Membrane and peptidoglycan modification |
| 3-oxoacyl-[acyl-carrier-protein] reductase |
| Glycosyltransferase involved in cell wall biosynthesis |
| Fatty-acid desaturase |
| D-alanyl-D-alanine carboxypeptidase |
| Polysaccharide envelope |
| Capsule polysaccharide export protein |
| Capsular polysaccharide biosynthesis protein, EpsC |
| Exopolysaccharide biosynthesis protein |
| Carotenoid biosynthesis |
| Phytoene/squalene synthetase |
| Phytoene dehydrogenase-related protein |
| Osmotic and oxidative stress response |
| ABC proline/glycine betaine transport, ATPase and permease |
| Osmoprotectant binding protein |
| Choline dehydrogenase or related flavoprotein |
| Trehalose-6-phosphate synthase |
| Na+/proline symporter |
| Na+/H+ antiporters |
| Catalase |
| Glutathione peroxidase |
| Glyoxylase or related hydrolase, β-lactamase superfamily II |
| Translation and transcription factors |
| Translation elongation factor EF-Tu/G, GTPase |
| Translation initiation factor IF-2/3, GTPase |
| Transcription antitermination factor NusA |
| Transcription termination factor NusB |
| Superfamily II DNA or RNA helicase, SNF2 |
| Method | Gen. | Read Length (bp) | No. of Reads per Run | Error Rate per Run (%) | Read Time |
|---|---|---|---|---|---|
| Sanger 3730×l | 1st | 600–1000 | 96 | 0.001 | 0.3–3 h |
| Ion Torrent semiconductor | 2nd | 200 | 8.2 × 107 | 1 | 2–4 h |
| Roche 454 Pyrosequencing | 2nd | 700 | 1 × 106 | 1 | 1 d |
| Illumina HiSeq 3000/4000 (High throughput) | 2nd | 2 × 150 | 8 × 109 (paired) | 0.1 | 1–4 d |
| SOliD 5500×l | 2nd | 2 × 60 | 8 × 108 | 5 | 6 d |
| PacBio RSII SMRT | 3rd | ~1.0–1.5 × 104 | 3.5–7.5 × 104 | 13 | 0.5–4 h |
| Oxford Nanopore MinION | 3rd | 2–5 × 103 | 1.1–4.7 × 104 | 38 | 2 d |
| Cluster | Prospects |
|---|---|
| Remediation strategies | Discovery of autochthonous hydrocarbonoclastic microorganisms; development of immediate response frameworks towards hydrocarbon pollutions; optimization of existing clean-up methods currently being employed in Antarctica. |
| Sustainability | Repurposing of general wastes in Antarctic stations and proper managements; partial incorporation of more renewable power sources for daily routines; safeguarding of both scientific and public interests (biota and wilderness areas) in Antarctica. |
| Anthropogenic impacts and management | Revision of transportation routes throughout Antarctic regions to minimize foreign contaminations by vessels; revamping or bolstering of existing laws in the Annex of Environmental Protocol to the Antarctic Treaty; establishment of proactive approaches that limit the capacity of tourists per annum as well as the type of tourisms. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roslee, A.F.A.; Ahmad, S.A.; Gomez-Fuentes, C.; Shaharuddin, N.A.; Khalil, K.A.; Zulkharnain, A. Scientometric Analysis of Diesel Pollutions in Antarctic Territories: A Review of Causes and Potential Bioremediation Approaches. Sustainability 2021, 13, 7064. https://doi.org/10.3390/su13137064
Roslee AFA, Ahmad SA, Gomez-Fuentes C, Shaharuddin NA, Khalil KA, Zulkharnain A. Scientometric Analysis of Diesel Pollutions in Antarctic Territories: A Review of Causes and Potential Bioremediation Approaches. Sustainability. 2021; 13(13):7064. https://doi.org/10.3390/su13137064
Chicago/Turabian StyleRoslee, Ahmad Fareez Ahmad, Siti Aqlima Ahmad, Claudio Gomez-Fuentes, Noor Azmi Shaharuddin, Khalilah Abdul Khalil, and Azham Zulkharnain. 2021. "Scientometric Analysis of Diesel Pollutions in Antarctic Territories: A Review of Causes and Potential Bioremediation Approaches" Sustainability 13, no. 13: 7064. https://doi.org/10.3390/su13137064
APA StyleRoslee, A. F. A., Ahmad, S. A., Gomez-Fuentes, C., Shaharuddin, N. A., Khalil, K. A., & Zulkharnain, A. (2021). Scientometric Analysis of Diesel Pollutions in Antarctic Territories: A Review of Causes and Potential Bioremediation Approaches. Sustainability, 13(13), 7064. https://doi.org/10.3390/su13137064









