Utilization of Organic Mixed Biosludge from Pulp and Paper Industries and Green Waste as Carbon Sources in Blast Furnace Hot Metal Production
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Method
3. Results and Discussion
4. Conclusions
- Briquettes containing wastes from pulp and paper industries (such as a mixed paper sludge hydrochar (PSH) and a green waste hydrochar (GrH)) can partially be charged in a BF together with conventional briquettes during short term industrial trials.
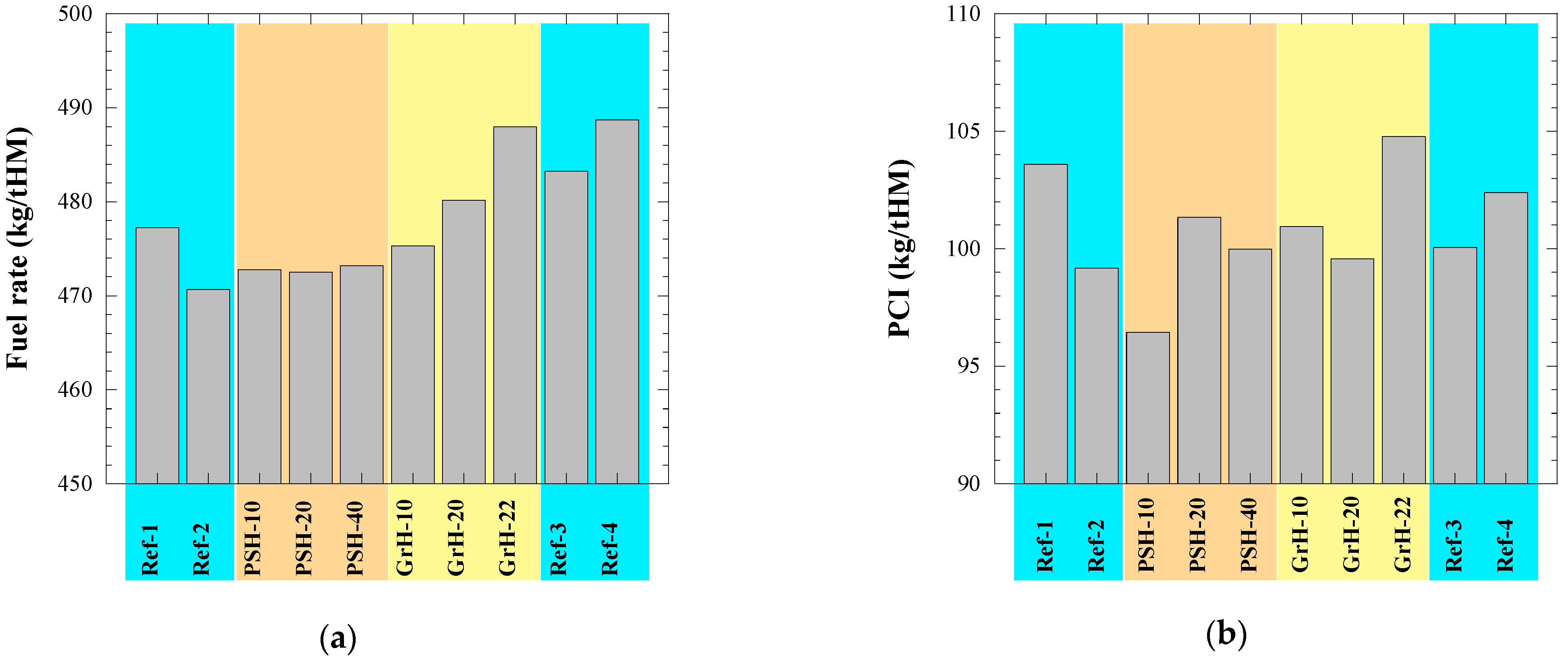
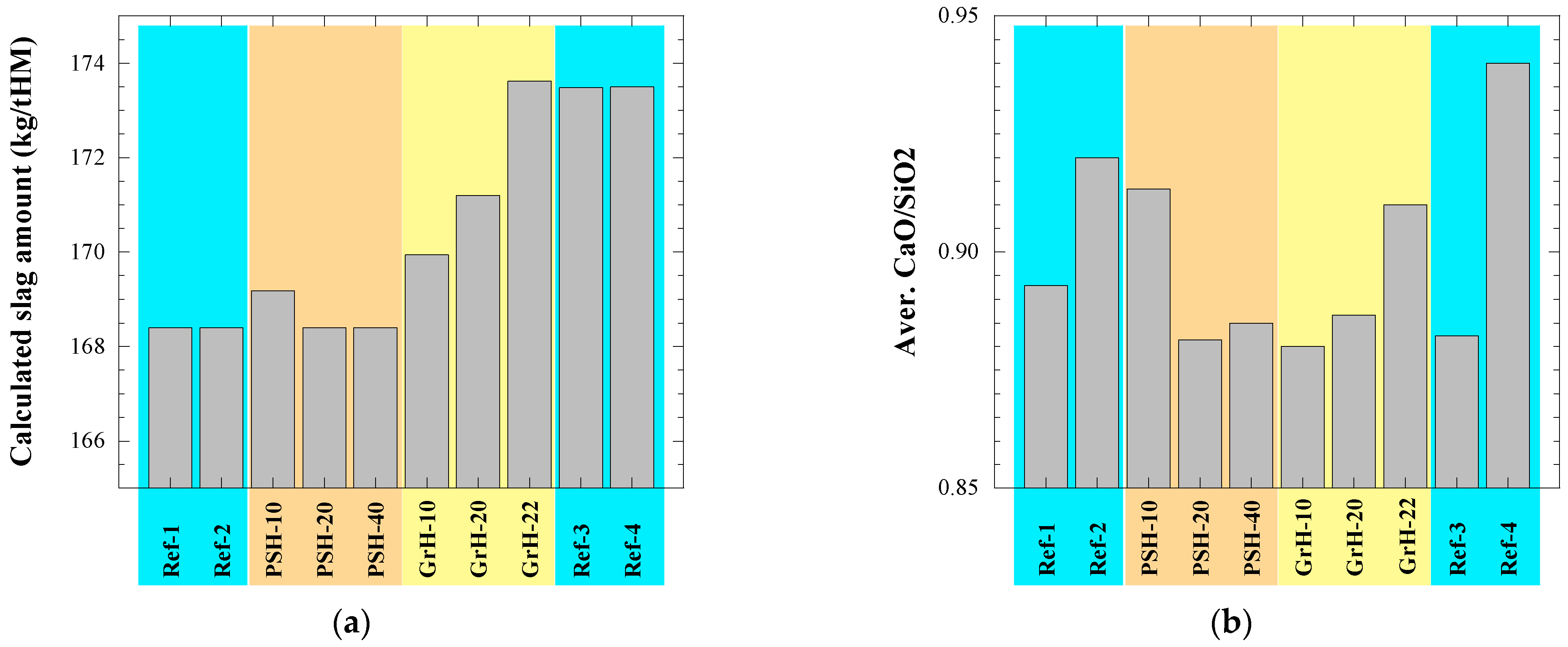
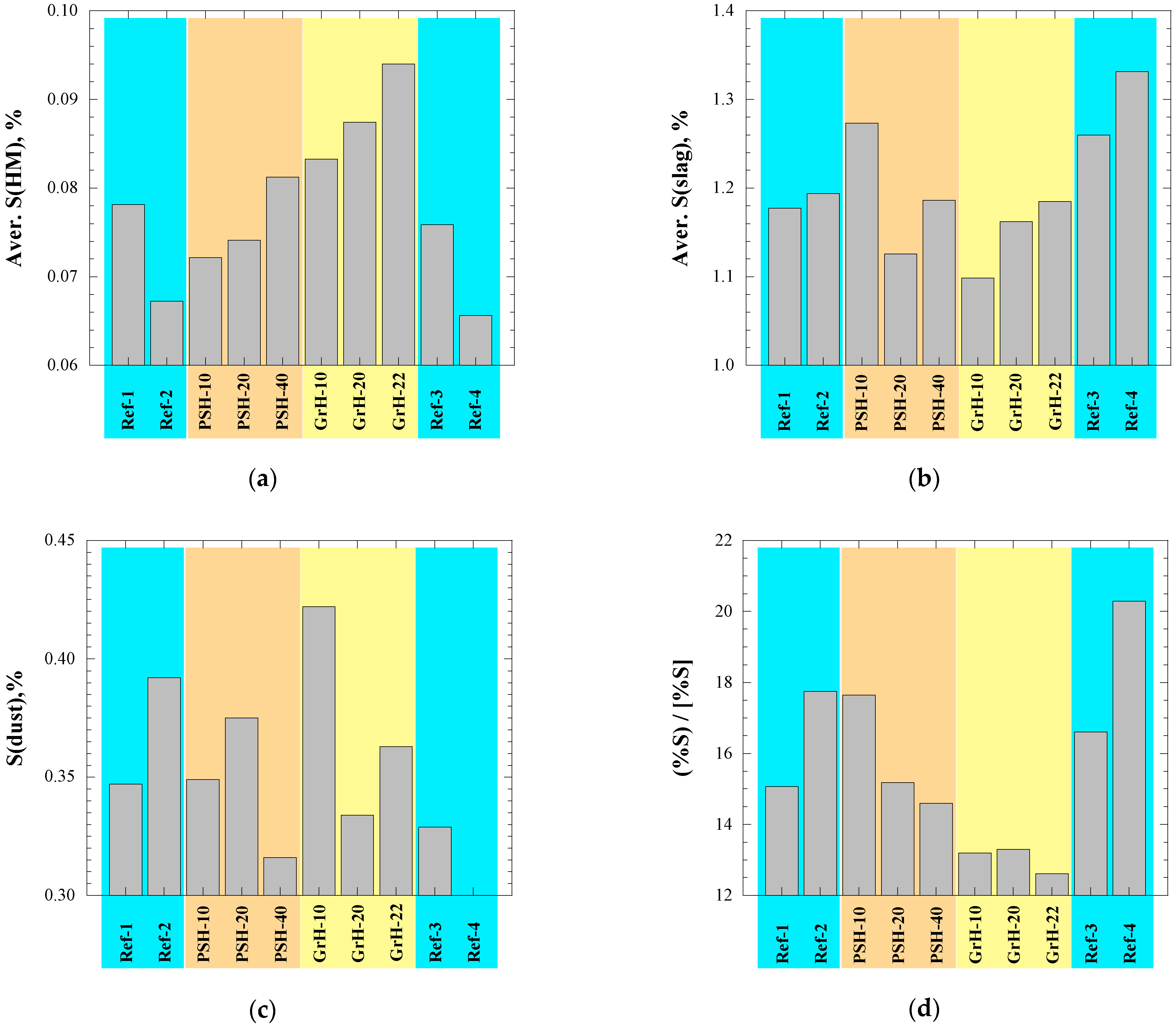
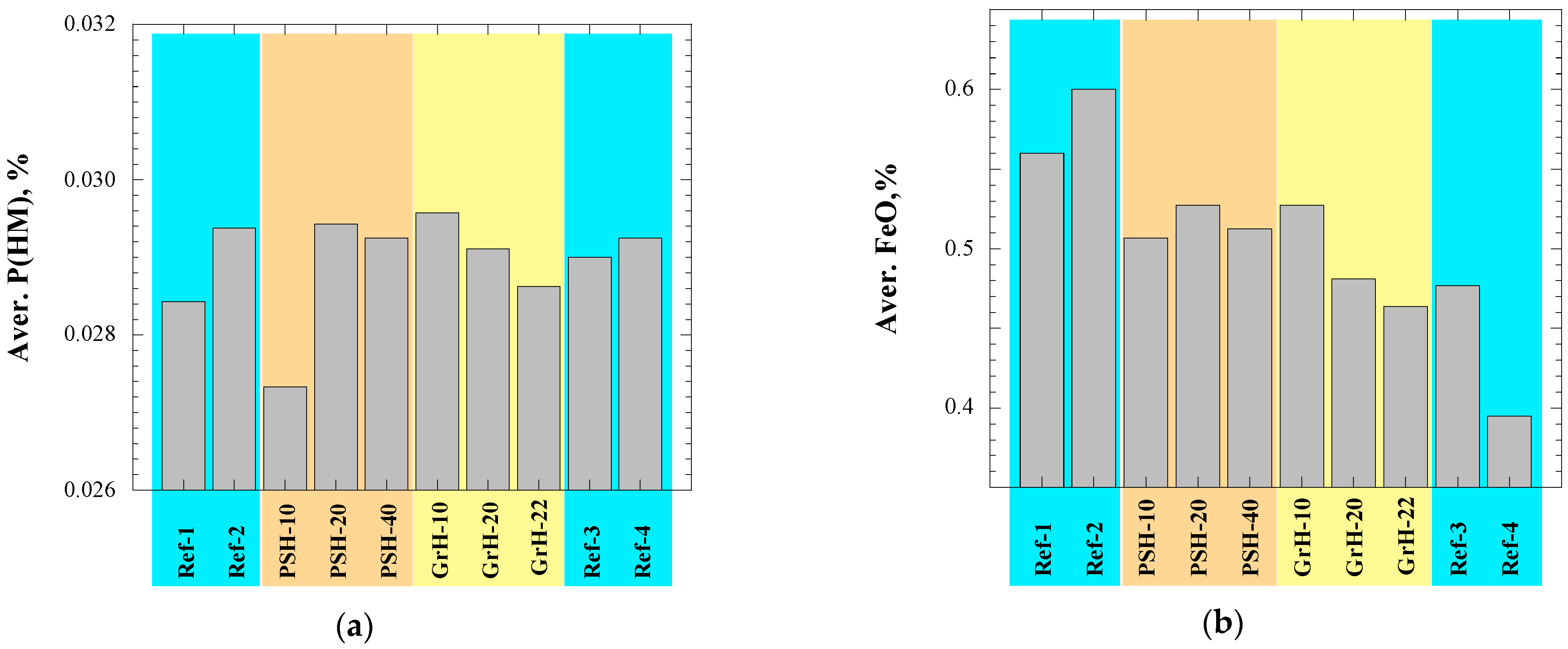
- Most of technological parameters of the BF process in the experimental PSH and GrH trials were very similar compared to the values in the reference trials. For example, the differences between the average values of the reference days and the average values for the trial days were the following: (i) <1.5% with respect to the production rate of hot metal, fuel rate and amount of slag, (ii) 1.5–14% for the amount of dust, (iii) ~2% for the amount of injected pulverized coal, (iv) <3.5% for the FeO content in the slag, (v) 1.5% for the C content in the hot metal, (vi) 6–23% for the S content in the hot metal and (vii) <1.5% for the P content in the hot metal. Moreover, no noticeable influence on the blast furnace performance could be seen during the experimental trials in comparison to the reference trials.
- Overall, the addition of PSH briquettes show better operational results compared to the GrH briquettes.
- The strength of the briquettes needs to be improved before future long–time trials are carried out.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jernkontoret Klimatfärdplan. Available online: https://www.jernkontoret.se/globalassets/publicerat/stal-stalind/klimatfardplan2018-1-web.pdf (accessed on 15 April 2021).
- SSAB Sustainability. Available online: https://www.ssab.se/ssab-koncern/hallbarhet/hallbar-verksamhet/koldioxideffektivitet-ssab (accessed on 15 April 2021).
- Acharya, B.; Sule, I.O.; Dutta, A. A review on advances of torrefaction technologies for biomass processing. Biomass Convers. Biorefinery 2012, 2, 349–369. [Google Scholar] [CrossRef]
- Gavrilescu, D. Energy from biomass in pulp and paper mills. Environ. Eng. Manag. J. 2008, 7, 537–546. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration. Biomass Explained—Biomass and the Environment. Available online: https://www.eia.gov/energyexplained/biomass/biomass-and-the-environment.php (accessed on 12 April 2021).
- Bose, B.K. Global Warming: Energy, Environmental Pollution, and the Impact of Power Electronics. IEEE Ind. Electron. Mag. 2010, 4, 6–17. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Lee, J.-Y.; Mao, X.; Ye, L.; Xu, W.; Ning, X.; Zhang, N.; Teng, H.; Wang, C. Hydrothermal carbonization of maize straw for hydrochar production and its injection for blast furnace. Appl. Energy 2020, 266, 114818. [Google Scholar] [CrossRef]
- Mousa, E.; Lundgren, M.; Ökvist, L.S.; From, L.-E.; Robles, A.; Hällsten, S.; Sundelin, B.; Friberg, H.; El-Tawil, A. Reduced Carbon Consumption and CO2 Emission at the Blast Furnace by Use of Briquettes Containing Torrefied Sawdust. J. Sustain. Met. 2019, 5, 391–401. [Google Scholar] [CrossRef] [Green Version]
- Miedema, J.H.; Benders, R.M.; Moll, H.C.; Pierie, F. Renew, reduce or become more efficient? The climate contribution of biomass co-combustion in a coal-fired power plant. Appl. Energy 2017, 187, 873–885. [Google Scholar] [CrossRef] [Green Version]
- Merzari, F.; Lucian, M.; Volpe, M.; Andreottola, G.; Fiori, L. Hydrothermal Carbonization of Biomass: Design of a Bench Scale Reactor for Evaluating the Heat of Reaction. Chem. Eng. Trans. 2018, 65, 43–48. [Google Scholar]
- Hitzl, M.; Corma, A.; Pomares, F.; Renz, M. The hydrothermal carbonization (HTC) plant as a decentral biorefinery for wet biomass. Catal. Today 2015, 257, 154–159. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Balasubramanian, R. Preparation and characterization of fuel pellets from woody biomass, agro-residues and their corresponding hydrochars. Appl. Energy 2014, 113, 1315–1322. [Google Scholar] [CrossRef]
- Skogsindustrierna Miljödatabas. Available online: https://miljodatabas.skogsindustrierna.org/simdb/Web/main/reportselect.aspx?l1=report&l1=report (accessed on 15 April 2021).
- Reckamp, J.M.; Garrido, R.A.; Satrio, J.A. Selective pyrolysis of paper mill sludge by using pretreatment pro-cesses to enhance the quality of bio-oil and biochar products. Biomass Bioenergy 2014, 71, 235–244. [Google Scholar] [CrossRef]
- Abdullah, R.; Ishak, C.F.; Kadir, W.R.; Abu Bakar, R. Characterization and Feasibility Assessment of Recycled Paper Mill Sludges for Land Application in Relation to the Environment. Int. J. Environ. Res. Public Health 2015, 12, 9314–9329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EURELCO. Data Launched on the Landfill Situation in the EU-28 2019:1–4. Available online: https://eurelco.org/infographic/ (accessed on 22 June 2021).
- Official Journal of the European Communities. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31999L0031&from=EN (accessed on 29 March 2021).
- Meyer, T.; Amin, P.; Allen, D.G.; Tran, H. Dewatering of pulp and paper mill biosludge and primary sludge. J. Environ. Chem. Eng. 2018, 6, 6317–6321. [Google Scholar] [CrossRef]
- Sveriges Riksdag. Available online: https://www.riksdagen.se/sv/dokument-lagar/dokument/svensk-forfattningssamling/forordning-2001512-om-deponering-av-avfall_sfs-2001-512 (accessed on 23 June 2021).
- Stoica, A.; Sandberg, M.; Holby, O. Energy use and recovery strategies within wastewater treatment and sludge handling at pulp and paper mills. Bioresour. Technol. 2009, 100, 3497–3505. [Google Scholar] [CrossRef] [PubMed]
- Sveriges Riksdag. Available online: https://www.riksdagen.se/sv/dokument-lagar/dokument/svensk-forfattningssamling/lag-20191274-om-skatt-pa-avfall-som-forbranns_sfs-2019-1274 (accessed on 23 June 2021).
- EA Bioenergy. Will the Tax on Waste Incineration Increase Recycling in Sweden? Available online: https://task36.ieabioenergy.com/news/tax-on-waste-incineration-increase-recycling-sweden/ (accessed on 23 June 2021).
- Doherty, J. Sweden Confirms EfW Tax from April 2020. LetsrecycleCom 2019. Available online: https://www.letsrecycle.com/news/latest-news/sweden-confirmsefw-tax-from-april-2020/ (accessed on 23 June 2021).
- United Nations Climate Change, Sweden’s Plan to Be Carbon Neutral by 2045. Available online: https://unfccc.int/news/sweden-plans-to-be-carbon-neutral-by-2045 (accessed on 23 June 2021).
- Jarnerud, T.; Hu, X.; Karasev, A.V.; Wang, C.; Jönsson, P.G. Application of Fly Ash from Pulp and Paper Industries as Slag Formers in Electric Arc Furnace Stainless Steel Production. Steel Res. Int. 2020, 91. [Google Scholar] [CrossRef]
- Hu, X.; Jarnerud, T.; Karasev, A.; Jönsson, P.G.; Wang, C. Utilization of fly ash and waste lime from pulp and paper mills in the Argon Oxygen Decarburization process. J. Clean. Prod. 2020, 261, 121182. [Google Scholar] [CrossRef]
- Tsai, W.-T. An Analysis of Waste Management Policies on Utilizing Biosludge as Material Resources in Taiwan. Sustainability 2012, 4, 1879–1887. [Google Scholar] [CrossRef] [Green Version]
- Nomura, S.; Kitaguchi, H.; Yamaguchi, K.; Naito, M. The Characteristics of Catalyst-coated Highly Reactive Coke. ISIJ Int. 2007, 47, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Matsui, Y.; Sawayama, M.; Kasai, A.; Yamagata, Y.; Noma, F. Reduction Behavior of Carbon Composite Iron Ore Hot Briquette in Shaft Furnace and Scope on Blast Furnace Performance Reinforcement. ISIJ Int. 2003, 43, 1904–1912. [Google Scholar] [CrossRef]
- Fruehan, R.J. The rate of reduction of iron oxides by carbon. Met. Mater. Trans. A 1977, 8, 279–286. [Google Scholar] [CrossRef]
- Hara, Y.; Tsuchiya, M.; Kondo, S.-I. Intraparticle Temperature of Iron-Oxide Pellet during the Reduction. Tetsu-to-Hagane 1974, 60, 1261–1270. [Google Scholar] [CrossRef] [Green Version]
- Ueda, S.; Watanabe, K.; Yanagiya, K.; Inoue, R.; Ariyama, T. Improvement of Reactivity of Carbon Iron Ore Composite with Biomass Char for Blast Furnace. ISIJ Int. 2009, 49, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- European Standards. Available online: https://www.en-standard.eu/search/?q=UNE+32004 (accessed on 14 April 2021).
- European Standards. Available online: https://www.en-standard.eu/une-32019-1984-combustibles-minerales-solidos-determinacion-del-contenido-en-materias-volatiles/ (accessed on 14 April 2021).
- Su, F.; Lampinen, H.-O.; Robinson, R. Recycling of Sludge and Dust to the BOF Converter by Cold Bonded Pelletizing. ISIJ Int. 2004, 44, 770–776. [Google Scholar] [CrossRef] [Green Version]
- The International Organization for Standardization (ISO). Standard 3271. Available online: https://www.iso.org/standard/62135.html (accessed on 29 April 2021).



| Feed Stock | C * | H * | N * | S * | Ash Content (%dry) ** | Volatiles (%dry) ** | Fixed C (%dry) ** |
|---|---|---|---|---|---|---|---|
| Mixed biosludge | 62.0 | N/A | N/A | 0.3 | 16.0 | 65.0 | 20.0 |
| Green waste | 61.4 ± 1.6 | 6.3 ± 0.3 | 1.3 ± 0.09 | 0.1 ± 0.1 | 10.2 ± 2.3 | 64.7 ± 5.6 | 25.0 ± 5.1 |
| Feed Stock | Na2O | K2O | MgO | CaO | Al2O3 | Fe2O3 | SiO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|
| Mixed biosludge | 1.3 | 5.8 | 1.3 | 12.9 | 10.7 | 2.5 | 52.4 | 4.5 |
| Green waste | 0.9 ± 0.4 | 4.3 ± 1.7 | 3.9 ± 0.5 | 42.7 ± 4.9 | 6.3 ± 1.0 | 4.6 ± 0.7 | 31.5 ± 1.9 | 4.6 ± 0.7 |
| Test Day | BF Productivity (ton HM/day) | Fuel Rate (kg/tHM) | PCI (kg/tHM) | Dust (kg/tHM) | Temp. (HM) (°C) | Slag (kg/tHM) | CaO/SiO2 | (%S)/[%S] |
|---|---|---|---|---|---|---|---|---|
| Ref-1 | 2845.0 | 477.2 | 103.6 | 20 | 1463 | 168.4 | 0.89 | 15.1 |
| Ref-2 | 2909.2 | 470.6 | 99.2 | 13 | 1474 | 168.4 | 0.92 | 17.8 |
| PSH-10 | 2913.6 | 472.8 | 96.4 | 18 | 1472 | 169.2 | 0.91 | 17.6 |
| PSH-20 | 2842.2 | 472.5 | 101.3 | 16 | 1469 | 168.4 | 0.88 | 15.2 |
| PSH-40 | 2894.9 | 473.2 | 100.0 | 14 | 1469 | 168.4 | 0.89 | 14.6 |
| GrH-10 | 2860.7 | 475.3 | 101.0 | 15 | 1454 | 169.9 | 0.88 | 13.2 |
| GrH-20 | 2855.9 | 480.2 | 99.6 | 12 | 1457 | 171.2 | 0.89 | 13.3 |
| GrH-22 | 2811.9 | 488.0 | 104.8 | 15 | 1458 | 173.6 | 0.91 | 12.6 |
| Ref-3 | 2835.7 | 483.2 | 100.1 | 12 | 1473 | 173.5 | 0.88 | 16.6 |
| Ref-4 | 2787.0 | 488.7 | 102.4 | 20 | 1474 | 173.5 | 0.94 | 20.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarnerud, T.; Karasev, A.V.; Wang, C.; Bäck, F.; Jönsson, P.G. Utilization of Organic Mixed Biosludge from Pulp and Paper Industries and Green Waste as Carbon Sources in Blast Furnace Hot Metal Production. Sustainability 2021, 13, 7706. https://doi.org/10.3390/su13147706
Jarnerud T, Karasev AV, Wang C, Bäck F, Jönsson PG. Utilization of Organic Mixed Biosludge from Pulp and Paper Industries and Green Waste as Carbon Sources in Blast Furnace Hot Metal Production. Sustainability. 2021; 13(14):7706. https://doi.org/10.3390/su13147706
Chicago/Turabian StyleJarnerud, Tova, Andrey V. Karasev, Chuan Wang, Frida Bäck, and Pär G. Jönsson. 2021. "Utilization of Organic Mixed Biosludge from Pulp and Paper Industries and Green Waste as Carbon Sources in Blast Furnace Hot Metal Production" Sustainability 13, no. 14: 7706. https://doi.org/10.3390/su13147706
APA StyleJarnerud, T., Karasev, A. V., Wang, C., Bäck, F., & Jönsson, P. G. (2021). Utilization of Organic Mixed Biosludge from Pulp and Paper Industries and Green Waste as Carbon Sources in Blast Furnace Hot Metal Production. Sustainability, 13(14), 7706. https://doi.org/10.3390/su13147706







