Synthesis of β-Ca2P2O7 as an Adsorbent for the Removal of Heavy Metals from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization of Synthesis Products
2.3. Adsorption Studies
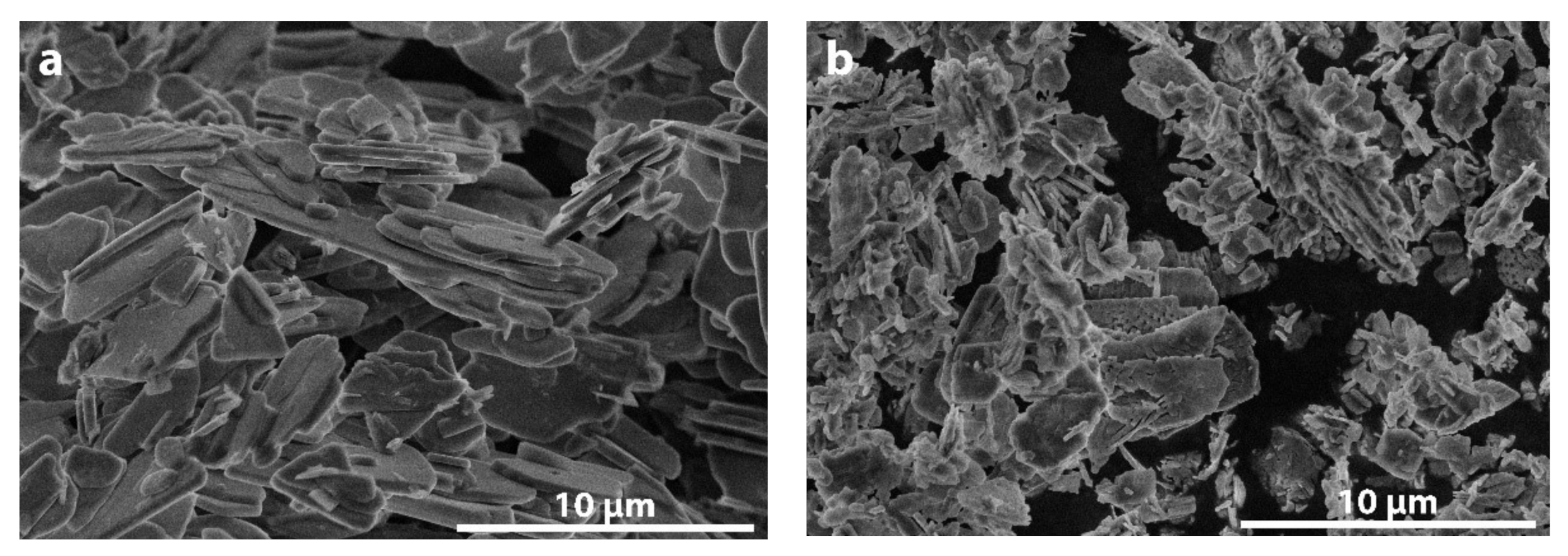
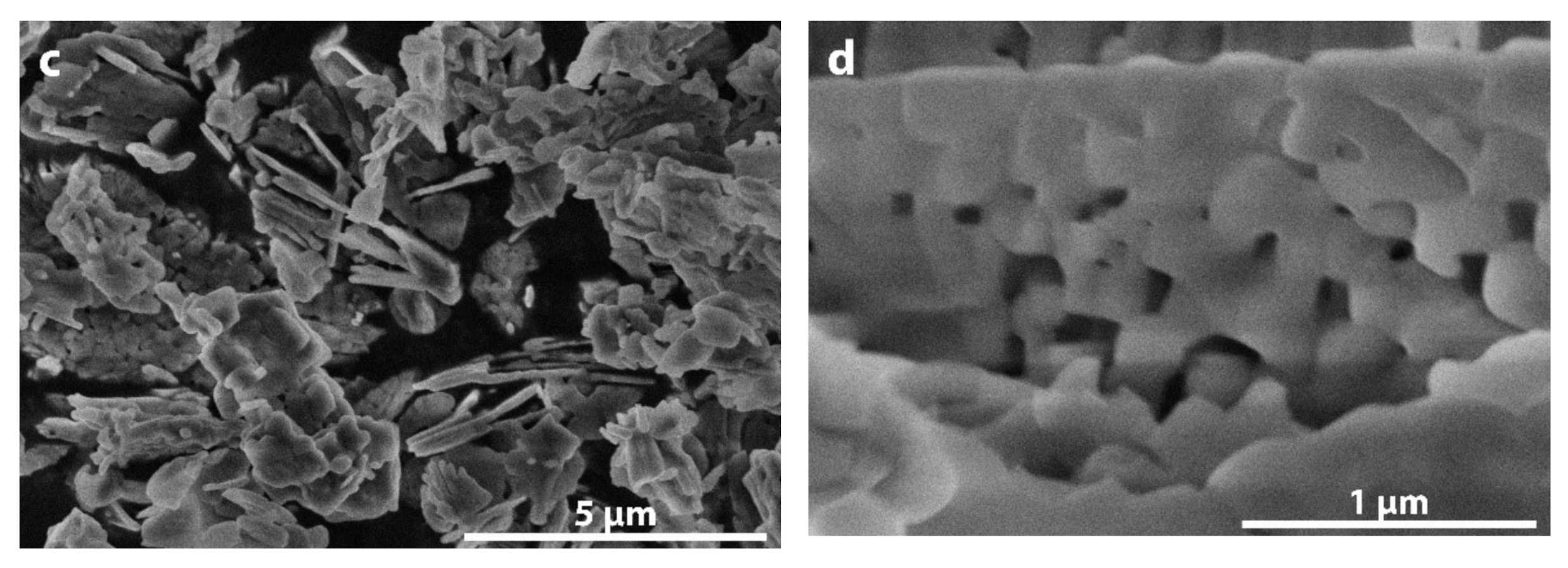
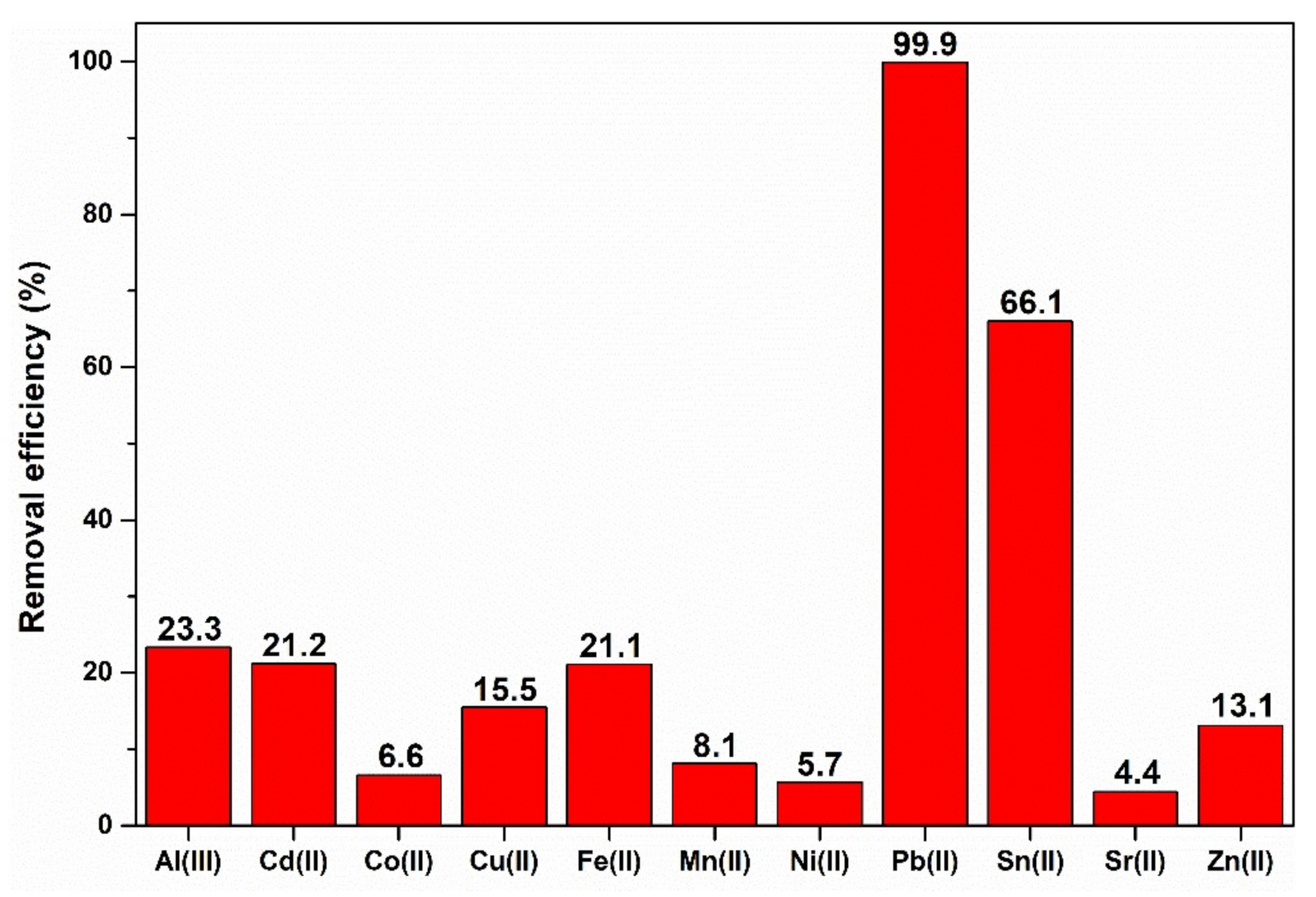
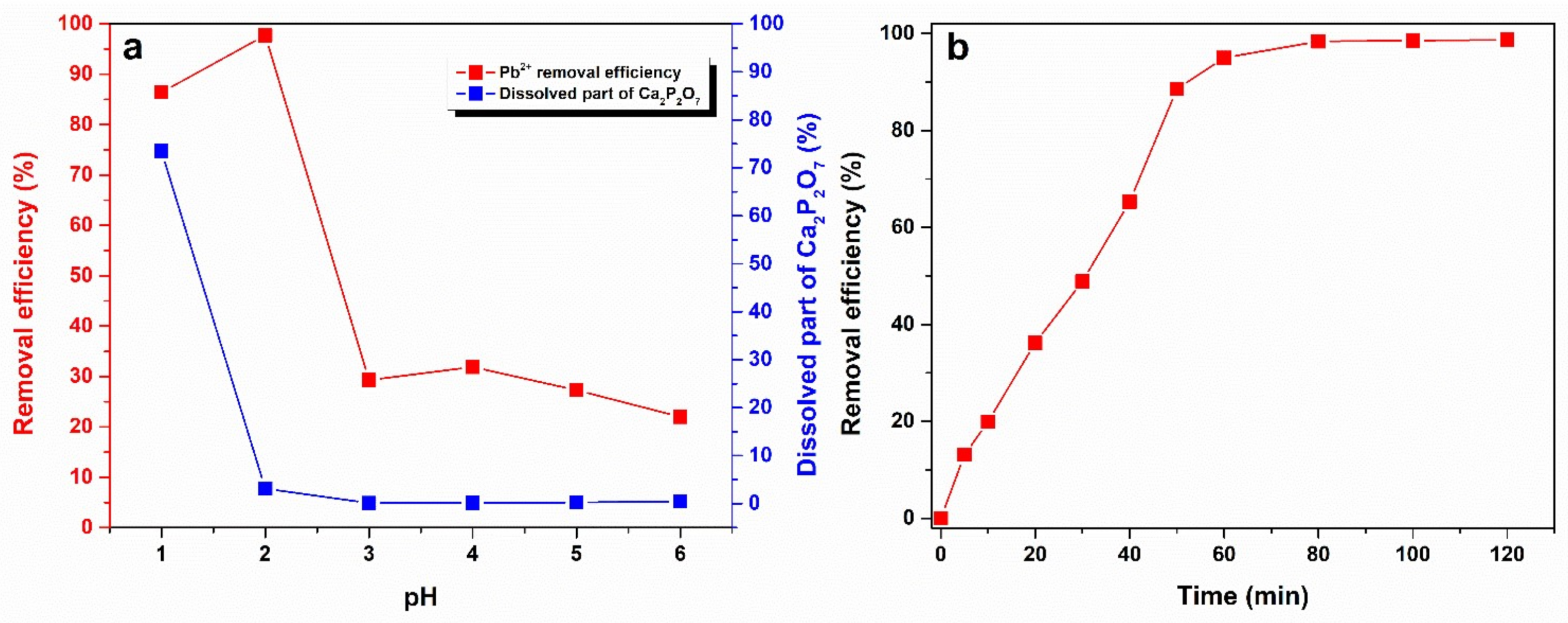
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ivanets, A.I.; Kitikova, N.V.; Shashkova, I.L.; Oleksiienko, O.V.; Levchuk, I.; Sillanpää, M. Removal of Zn2+, Fe2+, Cu2+, Pb2+, Cd2+, Ni2+ and Co2+ ions from aqueous solutions using modified phosphate dolomite. J. Environ. Chem. Eng. 2014, 2, 981–987. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Lee, S.J.; Karuppasamy, K.; Arulmani, S.; Veeralakshmi, S.; Ashokkumar, M.; Choi, M.Y. Application of advanced materials in sonophotocatalytic processes for the remediation of environmental pollutants. J. Hazard. Mater. 2021, 412, 125245. [Google Scholar] [CrossRef]
- Singh, S.; Wasewar, K.L.; Kansal, S.K. Low-cost adsorbents for removal of inorganic impurities from wastewater. In Inorganic Pollutants in Water; Devi, P., Singh, P., Kansal, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 173–203. [Google Scholar] [CrossRef]
- Kondo, Y.; Goto, T.; Sekino, T. Sorption capacity of seaweed-like sodium titanate mats for Co2+ removal. RSC Adv. 2020, 10, 41032–41040. [Google Scholar] [CrossRef]
- Ahmad, S.Z.N.; Wan Salleh, W.N.; Ismail, A.F.; Yusof, N.; Mohd Yusop, M.Z.; Aziz, F. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: Toxicity, roles of functional groups and mechanisms. Chemosphere 2020, 248, 126008. [Google Scholar] [CrossRef]
- Yao, N.; Li, C.; Yu, J.; Xu, Q.; Wei, S.; Tian, Z.; Yang, Z.; Yang, W.; Shen, J. Insight into adsorption of combined antibiotic-heavy metal contaminants on graphene oxide in water. Sep. Purif. Technol. 2020, 236, 116278. [Google Scholar] [CrossRef]
- Novoseltseva, V.; Yankovych, H.; Kovalenko, O.; Vaclavikova, M.; Melnyk, I. Production of high-performance lead(II) ions adsorbents from pea peels waste as a sustainable resource. Waste Manag. Res. 2021, 39, 584–593. [Google Scholar] [CrossRef] [PubMed]
- El-Amier, Y.A.; Elsayed, A.; El-Esawi, M.A.; Noureldeen, A.; Darwish, H.; Fakhry, H. Optimizing the Biosorption Behavior of Ludwigia stolonifera in the Removal of Lead and Chromium Metal Ions from Synthetic Wastewater. Sustainability 2021, 13, 6390. [Google Scholar] [CrossRef]
- Pelalak, R.; Heidari, Z.; Khatami, S.M.; Kurniawan, T.A.; Marjani, A.; Shirazian, S. Oak wood ash/GO/Fe3O4 adsorption efficiencies for cadmium and lead removal from aqueous solution: Kinetics, equilibrium and thermodynamic evaluation. Arab. J. Chem. 2021, 14, 102991. [Google Scholar] [CrossRef]
- Iqbal, T.; Iqbal, S.; Batool, F.; Thomas, D.; Iqbal, M.M.H. Utilization of a Newly Developed Nanomaterial Based on Loading of Biochar with Hematite for the Removal of Cadmium Ions from Aqueous Media. Sustainability 2021, 13, 2191. [Google Scholar] [CrossRef]
- Melnyk, I.V.; Pogorilyi, R.P.; Zub, Y.L.; Vaclavikova, M.; Gdula, K.; Dabrowski, A.; Seisenbaeva, G.A.; Kessler, V.G. Protection of Thiol Groups on the Surface of Magnetic Adsorbents and Their Application for Wastewater Treatment. Sci. Rep. 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.K.; Mansour, S.F.; Ramadan, R.; Afifi, M.; Mostafa, M.S.; El-dek, S.I.; Uskoković, V. Tuning the composition of new brushite/vivianite mixed systems for superior heavy metal removal efficiency from contaminated waters. J. Water Process Eng. 2020, 34, 101090. [Google Scholar] [CrossRef]
- Hoang, T.T.T.L.; Unob, F.; Suvokhiaw, S.; Sukpirom, N. One-pot synthesis of amorphous calcium phosphate/Fe3O4 composites and the application in the removal of cadmium. J. Environ. Chem. Eng. 2020, 8, 103653. [Google Scholar] [CrossRef]
- Goto, T.; Cho, S.H.; Ohtsuki, C.; Sekino, T. Selective adsorption of dyes on TiO2-modified hydroxyapatite photocatalysts morphologically controlled by solvothermal synthesis. J. Environ. Chem. Eng. 2021, 9, 105738. [Google Scholar] [CrossRef]
- Zhu, J.; Shu, J.; Yue, X.; Su, Y. Hollow and porous octacalcium phosphate superstructures mediated by the polyelectrolyte PSS: A superior removal capacity for heavy metal and antibiotics. J. Mater. Sci. 2020, 55, 7502–7517. [Google Scholar] [CrossRef]
- Hernández-Cocoletzi, H.; Salinas, R.A.; Águila-Almanza, E.; Rubio-Rosas, E.; Chai, W.S.; Chew, K.W.; Mariscal-Hernández, C.; Show, P.L. Natural hydroxyapatite from fishbone waste for the rapid adsorption of heavy metals of aqueous effluent. Environ. Technol. Innov. 2020, 20, 101109. [Google Scholar] [CrossRef]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Ishikawa, K.; Garskaite, E.; Kareiva, A. Sol-gel synthesis of calcium phosphate-based biomaterials—A review of environmentally benign, simple and effective synthesis routes. J. Sol-Gel Sci. Technol. 2020, 94, 551–572. [Google Scholar] [CrossRef]
- Sinusaite, L.; Popov, A.; Antuzevics, A.; Mažeika, K.; Baltrunas, D.; Yang, J.-C.; Horng, J.-L.; Shi, S.; Sekino, T.; Ishikawa, K.; et al. Fe and Zn co-substituted beta-tricalcium phosphate (β-TCP): Synthesis, structural, magnetic, mechanical and biological properties. Mater. Sci. Eng. C 2020, 112, 110918. [Google Scholar] [CrossRef]
- Sinusaite, L.; Kareiva, A.; Zarkov, A. Thermally induced crystallization and phase evolution of amorphous calcium phosphate substituted with divalent cations having different size. Cryst. Growth Des. 2021, 21, 1242–1248. [Google Scholar] [CrossRef]
- Hamad, A.A.; Hassouna, M.S.; Shalaby, T.I.; Elkady, M.F.; Abd Elkawi, M.A.; Hamad, H.A. Electrospun cellulose acetate nanofiber incorporated with hydroxyapatite for removal of heavy metals. Int. J. Biol. Macromol. 2020, 151, 1299–1313. [Google Scholar] [CrossRef]
- Pai, S.; Kini, S.M.; Selvaraj, R.; Pugazhendhi, A. A review on the synthesis of hydroxyapatite, its composites and adsorptive removal of pollutants from wastewater. J. Water Process Eng. 2020, 38, 101574. [Google Scholar] [CrossRef]
- Ma, K.; Cui, H.; Zhou, A.; Wu, H.; Dong, X.; Zu, F.; Yi, J.; Wang, R.; Xu, Q. Mesoporous hydroxyapatite: Synthesis in molecular self-assembly and adsorption properties. Microporous Mesoporous Mater. 2021, 323, 111164. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B. Hydroxyapatite as an advanced adsorbent for removal of heavy metal ions from water: Focus on its applications and limitations. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Ayodele, O.; Olusegun, S.J.; Oluwasina, O.O.; Okoronkwo, E.A.; Olanipekun, E.O.; Mohallem, N.D.S.; Guimarães, W.G.; Gomes, B.L.F.d.M.; Souza, G.d.O.; Duarte, H.A. Experimental and theoretical studies of the adsorption of Cu and Ni ions from wastewater by hydroxyapatite derived from eggshells. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100439. [Google Scholar] [CrossRef]
- Tran, T.N.; Kim, J.; Park, J.-S.; Chung, Y.; Han, J.; Oh, S.; Kang, S. Novel Hydroxyapatite Beads for the Adsorption of Radionuclides from Decommissioned Nuclear Power Plant Sites. Appl. Sci. 2021, 11, 1746. [Google Scholar] [CrossRef]
- Goto, T.; Sasaki, K. Synthesis of morphologically controlled hydroxyapatite from fish bone by urea-assisted hydrothermal treatment and its Sr2+ sorption capacity. Powder Technol. 2016, 292, 314–322. [Google Scholar] [CrossRef]
- Ivanets, A.I.; Kitikova, N.V.; Shashkova, I.L.; Roshchina, M.Y.; Srivastava, V.; Sillanpää, M. Adsorption performance of hydroxyapatite with different crystalline and porous structure towards metal ions in multicomponent solution. J. Water Process Eng. 2019, 32, 100963. [Google Scholar] [CrossRef]
- Sekine, Y.; Motokawa, R.; Kozai, N.; Ohnuki, T.; Matsumura, D.; Tsuji, T.; Kawasaki, R.; Akiyoshi, K. Calcium-deficient Hydroxyapatite as a Potential Sorbent for Strontium. Sci. Rep. 2017, 7, 2064. [Google Scholar] [CrossRef] [PubMed]
- Steinhauser, G.; Schauer, V.; Shozugawa, K. Concentration of Strontium-90 at Selected Hot Spots in Japan. PLoS ONE 2013, 8, e57760. [Google Scholar] [CrossRef] [Green Version]
- Koarai, K.; Kino, Y.; Takahashi, A.; Suzuki, T.; Shimizu, Y.; Chiba, M.; Osaka, K.; Sasaki, K.; Fukuda, T.; Isogai, E.; et al. 90Sr in teeth of cattle abandoned in evacuation zone: Record of pollution from the Fukushima-Daiichi Nuclear Power Plant accident. Sci. Rep. 2016, 6, 24077. [Google Scholar] [CrossRef] [Green Version]
- Boanini, E.; Silingardi, F.; Gazzano, M.; Bigi, A. Synthesis and Hydrolysis of Brushite (DCPD): The Role of Ionic Substitution. Cryst. Growth Des. 2021, 21, 1689–1697. [Google Scholar] [CrossRef]
- Tortet, L.; Gavarri, J.R.; Nihoul, G.; Dianoux, A.J. Study of Protonic Mobility in CaHPO4·2H2O (Brushite) and CaHPO4(Monetite) by Infrared Spectroscopy and Neutron Scattering. J. Solid State Chem. 1997, 132, 6–16. [Google Scholar] [CrossRef]
- Tas, A.C.; Bhaduri, S.B. Chemical Processing of CaHPO4·2H2O. J. Am. Ceram. Soc. 2004, 87, 2195–2200. [Google Scholar] [CrossRef] [Green Version]
- Bolarinwa, A.; Gbureck, U.; Purnell, P.; Bold, M.; Grover, L.M. Cement casting of calcium pyrophosphate based bioceramics. Adv. Appl. Ceram. 2010, 109, 291–295. [Google Scholar] [CrossRef]
- Fowler, B.O.; Moreno, E.C.; Brown, W.E. Infra-red spectra of hydroxyapatite, octacalcium phosphate and pyrolysed octacalcium phosphate. Arch. Oral Biol. 1966, 11, 477–492. [Google Scholar] [CrossRef]
- Valeikiene, L.; Grigoraviciute-Puroniene, I.; Kareiva, A. Alkaline earth metal substitution effects in sol-gel derived mixed-metal oxides and Mg2-xMx/Al1 (M = Ca, Sr, Ba)-layered double hydroxides. J. Aust. Ceram. Soc. 2020, 56, 1531–1541. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, A.; Peng, F.; Yu, H.; Yang, J. Mechanism study on adsorption of acidified multiwalled carbon nanotubes to Pb(II). J. Colloid Interface Sci. 2007, 316, 277–283. [Google Scholar] [CrossRef]
- Momčilović, M.; Purenović, M.; Bojić, A.; Zarubica, A.; Ranđelović, M. Removal of lead(II) ions from aqueous solutions by adsorption onto pine cone activated carbon. Desalination 2011, 276, 53–59. [Google Scholar] [CrossRef]
- Mouflih, M.; Aklil, A.; Sebti, S. Removal of lead from aqueous solutions by activated phosphate. J. Hazard. Mater. 2005, 119, 183–188. [Google Scholar] [CrossRef]
- Mobasherpour, I.; Salahi, E.; Pazouki, M. Comparative of the removal of Pb2+, Cd2+ and Ni2+ by nano crystallite hydroxyapatite from aqueous solutions: Adsorption isotherm study. Arab. J. Chem. 2012, 5, 439–446. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griesiute, D.; Gaidukevic, J.; Zarkov, A.; Kareiva, A. Synthesis of β-Ca2P2O7 as an Adsorbent for the Removal of Heavy Metals from Water. Sustainability 2021, 13, 7859. https://doi.org/10.3390/su13147859
Griesiute D, Gaidukevic J, Zarkov A, Kareiva A. Synthesis of β-Ca2P2O7 as an Adsorbent for the Removal of Heavy Metals from Water. Sustainability. 2021; 13(14):7859. https://doi.org/10.3390/su13147859
Chicago/Turabian StyleGriesiute, Diana, Justina Gaidukevic, Aleksej Zarkov, and Aivaras Kareiva. 2021. "Synthesis of β-Ca2P2O7 as an Adsorbent for the Removal of Heavy Metals from Water" Sustainability 13, no. 14: 7859. https://doi.org/10.3390/su13147859





