Optimizing of Microalgae Scenedesmus sp. Biomass Production in Wet Market Wastewater Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Wastewater Samples and Microalgae Inoculum
2.2. Microalgae Isolation and Identification of Scenedesmus sp.
2.3. Experimental Set Up
2.4. Analytical Methods
Determination of Microalgae Biomass Productivity and Specific Growth Rate
2.5. Biomass Characterization by GC-MS
Lipid Extraction Procedure
3. Results and Discussion
3.1. Characteristic of WMW
3.2. Isolation and Morophological
3.3. Optimization of Biomass Production
3.4. GC-MS Analysis of Scenedesmus sp.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rawat, I.; Kumar, R.R.; Mutanda, T.; Bux, F. Dual role of microalgae: Phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [Green Version]
- Hena, S.; Fatimah, S.; Tabassum, S. Cultivation of algae consortium in a dairy farm wastewater for biodiesel production. Water Resour. Ind. 2015, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Jena, J.; Pradhan, N.; Nayak, R.R.; Dash, B.P.; Sukla, L.B.; Panda, P.K.; Mishra, B.K. Microalga Scenedesmus sp.: A Potential Low-Cost Green Machine for Silver Nanoparticle Synthesis. J. Microbiol. Biotechnol. 2014, 24, 522–533. [Google Scholar] [CrossRef]
- Badwy, T.M.; Ibrahim, E.M.; Zeinhom, M.M. Partial replacement of fish meal with dried microalga (Chlorella spp. and Scenedesmus spp.) in nile tilapia (Oreochromis Niloticus) Diets. In Proceedings of the 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt, 12–14 October 2008; pp. 801–811. [Google Scholar]
- Garcia-Moscoso, J.L.; Obeid, W.; Kumar, S.; Hatcher, P.G. Flash hydrolysis of microalgae (Scenedesmus sp.) for protein extraction and production of biofuels intermediates. J. Supercrit. Fluids 2013, 82, 183–190. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Liu, B.-F.; Ma, C.; Zhao, L.; Ren, N. A new lipid-rich microalga Scenedesmus sp. strain R-16 isolated using Nile red staining: Effects of carbon and nitrogen sources and initial pH on the biomass and lipid production. Biotechnol. Biofuels 2013, 6, 143. [Google Scholar] [CrossRef] [Green Version]
- Pahazri, N.F.; Mohamed, R.; Al-Gheethi, A.; Kassim, A.H.M. Production and harvesting of microalgae biomass from wastewater: A critical review. Environ. Technol. Rev. 2016, 5, 39–56. [Google Scholar] [CrossRef]
- Chiu, S.-Y.; Kao, C.-Y.; Chen, T.-Y.; Chang, Y.-B.; Kuo, C.-M.; Lin, C.-S. Cultivation of microalgal Chlorella for biomass and lipid production using wastewater as nutrient resource. Bioresour. Technol. 2015, 184, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Al-Gheethi, A.; Mohamed, R.; Jais, N.M.; Efaq, A.N.; Halid, A.A.; Wurochekke, A.A.; Amir-Hashim, M.K. Influence of pathogenic bacterial activity on growth of Scenedesmus sp. and removal of nutrients from public market wastewater. J. Water Health 2017, 15, 741–756. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Latiffi, A.; Atikah, N.; Mohamed, R.; Saphira, R.M.; Apandi, N.M.; Tajuddin, R.M. Experimental assessment on effects of growth rates microalgae Scenedesmus sp. in different conditions of ph, temperature, light intensity and photoperiod. Key Eng. Mater. 2017, 744, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Markou, G.; Iconomou, D.; Muylaert, K. Applying raw poultry litter leachate for the cultivation of Arthrospira platensis and Chlorella vulgaris. Algal Res. 2016, 13, 79–84. [Google Scholar] [CrossRef]
- Kuo, C.M.; Chen, T.Y.; Lin, T.H.; Kao, C.Y.; Lai, J.T.; Chang, J.S.; Lin, C.S. Cultivation of Chlorella sp. GD using pig-gery wastewater for biomass and lipid production. Bioresour. Technol. 2015, 194, 326–333. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, W.; Min, M.; Ma, X.; Chandra, C.; Doan, Y.T.; Ma, Y.; Zheng, H.; Cheng, S.; Griffith, R.; et al. Growing Chlorella sp. on meat processing wastewater for nutrient removal and biomass production. Bioresour. Technol. 2015, 198, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Apandi, N.M.; Mohamed, R.M.S.R.; Al-Gheethi, A.A.S.; Kassim, A.H.M. Wastewater Phycoremediation by Microalgae for Sustainable Bioproduct Production. In Handbook of Algal Technologies and Phytochemicals; CRC Press: Boca Raton, FL, USA, 2019; pp. 3–12. [Google Scholar]
- Zhu, L.; Wang, Z.; Shu, Q.; Takala, J.; Hiltunen, E.; Feng, P.; Yuan, Z. Nutrient removal and biodiesel production by integration of freshwater algae cultivation with piggery wastewater treatment. Water Res. 2013, 47, 4294–4302. [Google Scholar] [CrossRef] [PubMed]
- Karpagam, R.; Raj, K.J.; AshokKumar, B.; Varalakshmi, P. Characterization and fatty acid profiling in two fresh water microalgae for biodiesel production: Lipid enhancement methods and media optimization using response surface methodology. Bioresour. Technol. 2015, 188, 177–184. [Google Scholar] [CrossRef]
- Skorupskaite, V.; Makareviciene, V.; Levisauskas, D. Optimization of mixotrophic cultivation of microalgae Chlorella sp. for biofuel production using response surface methodology. Algal Res. 2015, 7, 45–50. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- BLAST. Basic Local Alignment Search Tool; National Center for Biotechnology Information: Bethesda, MD, USA, 2011. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 12 October 2011).
- Medlin, L.; Elwood, H.J.; Stickel, S.; Sogin, M.L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 1988, 71, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Cullings, K.W. Design and testing of a plant-specific PCR primer for ecological and evolutionary studies. Mol. Ecol. 1992, 1, 233–240. [Google Scholar] [CrossRef]
- Main Page: Base Asia. Available online: https://base-asia.com/ (accessed on 13 February 2017).
- Bischoff, H.C. Some Soil Algae from Enchanted Rock and Related Algal Species; Phycological Studies IV; University of Texas Publ.: Austin, TX, USA, 1963; Volume 6318, pp. 1–95. [Google Scholar]
- Apandi, N.; Mohamed, R.; Al-Gheethi, A.; Gani, P.; Ibrahim, A.; Kassim, A.H.M. Scenedesmus Biomass Productivity and Nutrient Removal from Wet Market Wastewater, A Bio-kinetic Study. Waste Biomass Valorization 2018, 10, 2783–2800. [Google Scholar] [CrossRef]
- Yen, H.-W.; Hu, I.-C.; Chen, C.-Y.; Chang, J.-S. Design of Photobioreactors for Algal Cultivation. Biofuels Algae 2014, 23–45. [Google Scholar] [CrossRef]
- Gani, P.; Sunar, N.M.; Matias-Peralta, H.; Jamaian, S.S.; Latiff, A.A.A. Effects of different culture conditions on the phycoremediation efficiency of domestic wastewater. J. Environ. Chem. Eng. 2016, 4, 4744–4753. [Google Scholar] [CrossRef]
- Gani, P.; Sunar, N.M.; Matias-Peralta, H.; Mohamed, R.M.S.R.; Latiff, A.A.A.; Parjo, U.K. Extraction of hydrocarbons from freshwater green microalgae (Botryococcus sp.) biomass after phycoremediation of domestic wastewater. Int. J. Phytoremediat. 2017, 19, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Anjos, M.; Fernandes, B.D.; Vicente, A.A.; Teixeira, J.A.; Dragone, G. Optimization of CO2 bio-mitigation by Chlorella vulgaris. Bioresour. Technol. 2013, 139, 149–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, M. The Environmental Quality Act 1974: A significant legal instrument for implementing environmental policy directives of Malaysia. IIUM Law J. 2012, 19. [Google Scholar] [CrossRef]
- Department of Environment Malaysia. DOE Environmental Requirements: A Guide for Investor, Appendix K1 & K2: Acceptable Condition of Sewage Discharge of Standard A and B. 2010; Department of Environment Malaysia: Putrajaya, Malaysia, 2008. [Google Scholar]
- Danial, O.; Salim, M.R.S.; Salmiati, S. Nutrient removal of grey water from wet market using sequencing batch reactor. Malays. J. Anal. Sci. 2016, 20, 142–148. [Google Scholar] [CrossRef]
- Rosmawanie, M.; Mohamed, R.; Al-Gheethi, A.; Pahazri, F.; Amir-Hashim, M.K.; Nur-Shaylinda, M.Z. Sequestering of pollutants from public market wastewater using Moringa oleifera and Cicer arietinum flocculants. J. Environ. Chem. Eng. 2018, 6, 2417–2428. [Google Scholar] [CrossRef]
- Jais, N.M.; Mohamed, R.; Al-Gheethi, A.; Hashim, M.K.A. The dual roles of phycoremediation of wet market wastewater for nutrients and heavy metals removal and microalgae biomass production. Clean Technol. Environ. Policy 2017, 19, 37–52. [Google Scholar] [CrossRef]
- Difusa, A.; Talukdar, J.; Kalita, M.C.; Mohanty, K.; Goud, V.V. Effect of light intensity and pH condition on the growth, biomass and lipid content of microalgae Scenedesmus species. Biofuels 2015, 6, 37–44. [Google Scholar] [CrossRef]
- França, S.; Vinagre, C.; Caçador, I.; Cabral, H.N. Heavy metal concentrations in sediment, benthic invertebrates and fish in three salt marsh areas subjected to different pollution loads in the Tagus Estuary (Portugal). Mar. Pollut. Bull. 2005, 50, 998–1003. [Google Scholar] [CrossRef]
- Alam, M.M.; Mumtaz, A.S.; Hallenbeck, P.C.; Shah, S.A.; Abidin, S.Z.U.; Inam, F. Morphological characterization, growth appraisal, and probing biofuels potential of newly isolated Scenedesmus sp. from desert Cholistan. Microsc. Res. Tech. 2019, 82, 2079–2088. [Google Scholar] [CrossRef]
- Gour, R.S.; Chawla, A.; Singh, H.; Chauhan, R.S.; Kant, A. Characterization and Screening of Native Scenedesmus sp. Isolates Suitable for Biofuel Feedstock. PLoS ONE 2016, 11, e0155321. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Meena, H.; Chakraborty, S.; Meikap, B.C. Application of response surface methodology (RSM) for optimiza-tion of leaching parameters for ash reduction from low-grade coal. Int. J. Mining Sci. Technol. 2018, 28, 621–629. [Google Scholar] [CrossRef]
- Alipourzadeh, A.; Mehrnia, M.; Sani, A.H.; Babaei, A. Application of response surface methodology for investigation of membrane fouling behaviours in microalgal membrane bioreactor: The effect of aeration rate and biomass concentration. RSC Adv. 2016, 6, 111182–111189. [Google Scholar] [CrossRef]
- Chen, J.; Lee, Y.; Lai, W.; Chen, S.; Chiang, T. Optimization of simultaneous biomass production and nutrient removal by mixotrophic Chodatella sp. using Box-Behnken experimental design. Int. Proc. Chem. Biol. Environ. Eng. 2015, 84, 83–87. [Google Scholar] [CrossRef]
- Imamoglu, E.; Demirel, Z.; Dalay, M.C. A response surface methodology study for the protein production of Chlorella sp. Int’l. J. Adv. Chem. Engg. Biol. Sci. 2014, 1, 76–79. [Google Scholar]
- Chang, L.; Hagel, J.M.; Facchini, P.J. Isolation and Characterization of O-methyltransferases Involved in the Biosynthesis of Glaucine in Glaucium flavum1. Plant. Physiol. 2015, 169, 1127–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Moraes, J.; de Oliveira, R.N.; Costa, J.P.; Junior, A.L.G.; de Sousa, D.P.; Freitas, R.M.; Allegretti, S.M.; Pinto, P.L.S. Phytol, a Diterpene Alcohol from Chlorophyll, as a Drug against Neglected Tropical Disease Schistosomiasis Mansoni. PLoS Negl. Trop. Dis. 2014, 8, e2617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
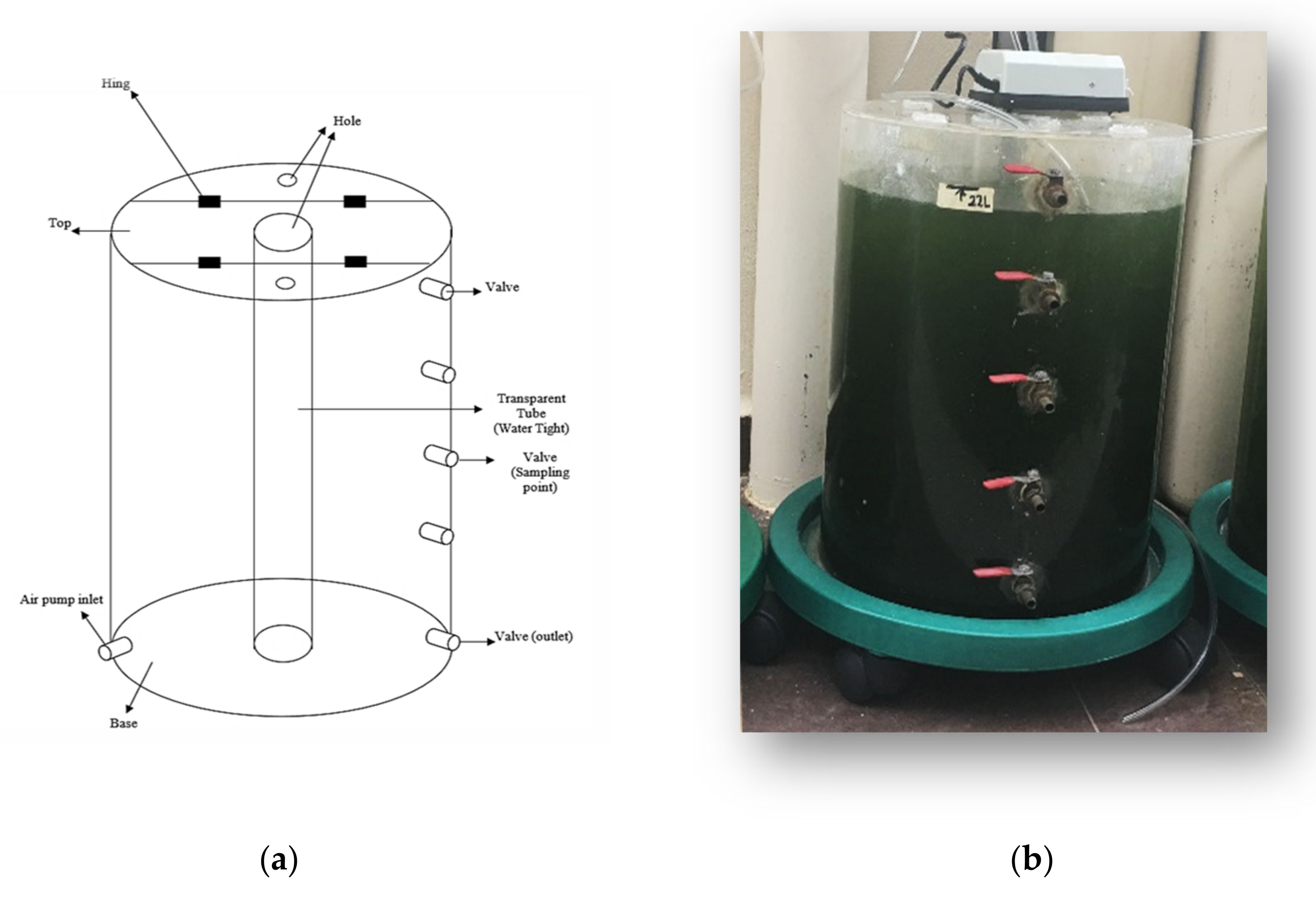


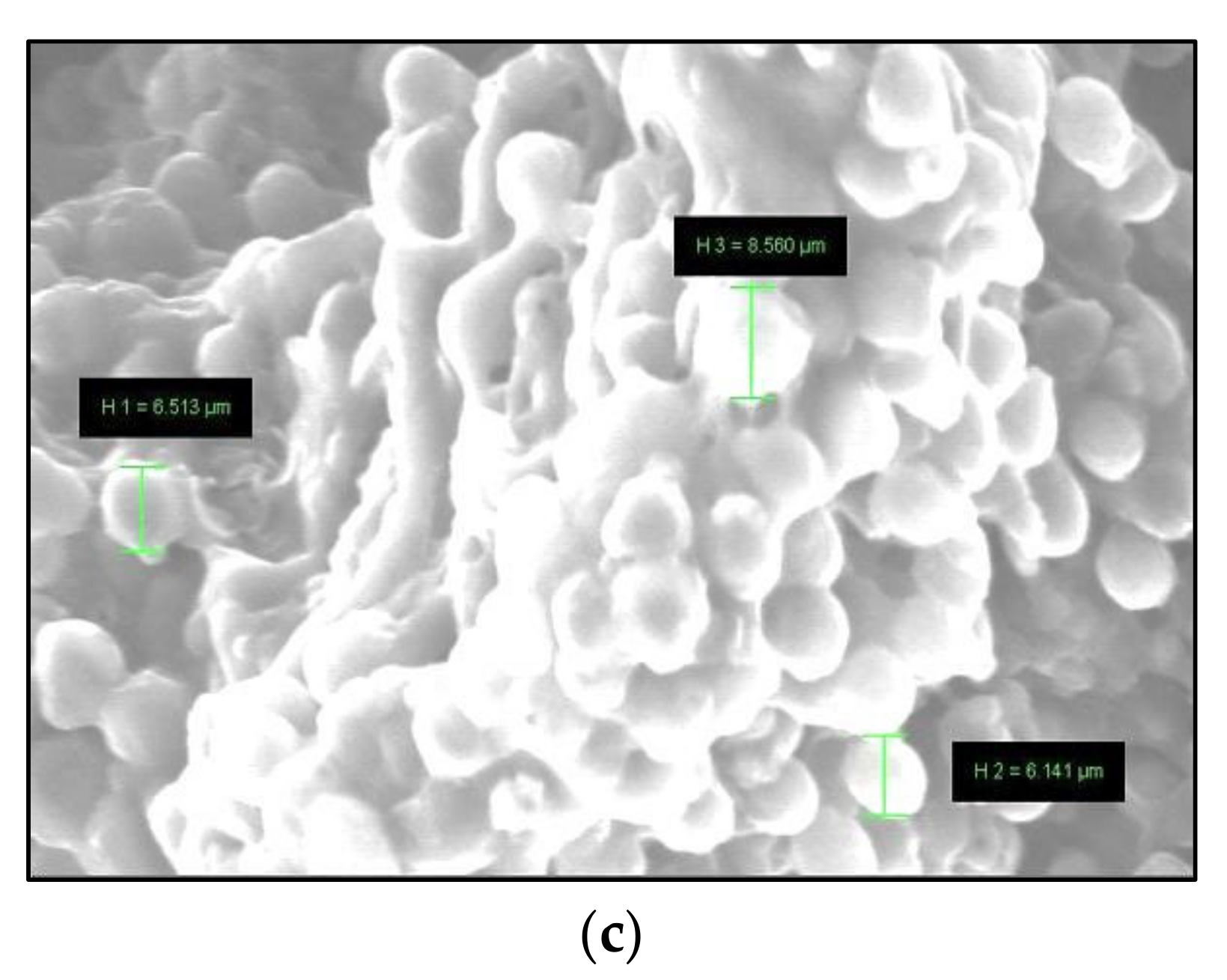



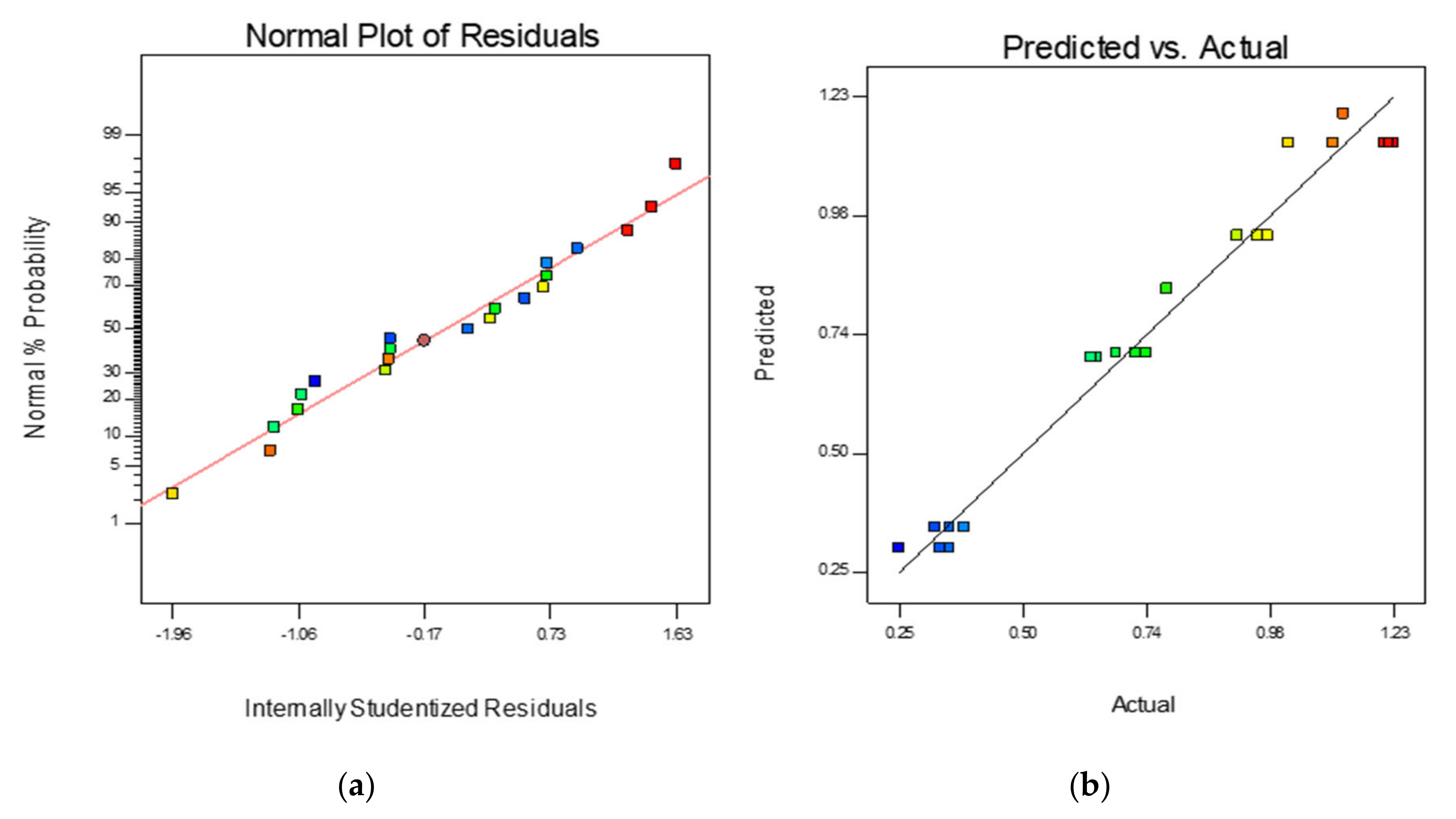



| Variables | Symbol | Level | ||
|---|---|---|---|---|
| −1 (Min) | 0 (Medium) | 1 (Max) | ||
| Wet Market Wastewater (WMW) Concentration (%) | A | 10 | 45 | 75 |
| Aeration rate (L/min) | B | 0.02 | 2.01 | 4.00 |
| Run | Actual Factor Values | Response Values | ||||
|---|---|---|---|---|---|---|
| Biomass Productivity (mg/L/d) | Maximum Growth Rate (µmax/d) | |||||
| Wet Market Wastewater Concentration (%) | Aeration Rate (L/min) | Actual (Experiment) | Predicted (RSM Model) | Actual (Experiment) | Predicted (RSM Model) | |
| 1 | 10 | 2 | 21.33 | 21.7 | 0.35 | 0.3 |
| 2 | 10 | 2 | 21.45 | 21.7 | 0.25 | 0.3 |
| 3 | 10 | 2 | 23.56 | 21.7 | 0.33 | 0.3 |
| 4 | 75 | 2 | 71.23 | 69.35 | 0.72 | 0.7 |
| 5 | 75 | 2 | 62.36 | 69.35 | 0.68 | 0.7 |
| 6 | 75 | 2 | 71.26 | 69.35 | 0.74 | 0.7 |
| 7 | 10 | 4.5 | 11.32 | 15.06 | 0.32 | 0.34 |
| 8 | 10 | 4.5 | 11.26 | 15.06 | 0.48 | 0.34 |
| 9 | 10 | 4.5 | 32.56 | 15.06 | 0.35 | 0.34 |
| 10 | 75 | 4.5 | 22.52 | 22.83 | 0.96 | 0.94 |
| 11 | 75 | 4.5 | 23.45 | 22.83 | 0.92 | 0.94 |
| 12 | 75 | 4.5 | 22.31 | 22.83 | 0.98 | 0.94 |
| 13 | 10 | 3.25 | 35.63 | 41.25 | 0.64 | 0.69 |
| 14 | 75 | 3.25 | 72.23 | 68.96 | 1.13 | 1.19 |
| 15 | 42.5 | 2 | 58.32 | 56.33 | 0.63 | 0.69 |
| 16 | 42.5 | 4.5 | 25.41 | 29.75 | 0.78 | 0.83 |
| 17 | 42.5 | 3.25 | 63.25 | 65.91 | 1.23 | 1.13 |
| 18 | 42.5 | 3.25 | 74.33 | 65.91 | 1.11 | 1.13 |
| 19 | 42.5 | 3.25 | 63.75 | 65.91 | 1.02 | 1.13 |
| 20 | 42.5 | 3.25 | 68.45 | 65.91 | 1.21 | 1.13 |
| 21 | 42.5 | 3.25 | 62.12 | 65.9 | 1.22 | 1.13 |
| Parameter | Concentration (mg/L) | Effluent Standard, mg/L EQA, (1974) | |
|---|---|---|---|
| A | B | ||
| Physiochemical | |||
| Chemical Oxygen Demand, COD | 3506 ± 259 | 80 | 200 |
| Biological Oxygen Demand, BOD | 1784 ± 85.87 | 20 | 50 |
| Total Phosphorus, TP | 169.75 ± 4.03 | 5 | 10 |
| Total Nitrogen, TN | 961.38 ± 1.5 | na | na |
| Total Organic Carbon, TOC | 1539 ± 47.4 | na | na |
| Dissolve oxygen, DO | 3.72 ± 1.1 | na | na |
| Turbidity (NTU) | 125 ± 9.6 | na | na |
| pH | 7.18 ± 0.26 | 6.0–9.0 | 5.5–9.0 |
| Oil and Grease | 1169 ± 105.91 | 20 | 20 |
| Total Suspended Solids | 225 ± 13.14 | 50 | 100 |
| Heavy metals | |||
| Ferum, Fe | 5.05 ± 0.1 | 1 | 5 |
| Cadmium Cd | 0.0172 ± 0.43 | 0.01 | 0.02 |
| Chromium, Cr | 0.194 ± 1.52 | 0.2 | 1 |
| Zinc, Zn | 4.85 ± 1.73 | 1 | 1 |
| Arsenic, As | 0.015 ± 2.31 | 0.05 | 0.1 |
| Copper, Cu | 0.598 ± 5.47 | 0.2 | 1 |
| Lead, Pb | 0.0764 ± 6.06 | 0.1 | 0.5 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 10,657.14 | 5 | 2131.43 | 106.8 | <0.0001 |
| A—WMW | 2688.29 | 1 | 2688.29 | 134.7 | <0.0001 |
| B—Aeration rate | 2473.53 | 1 | 2473.53 | 123.94 | <0.0001 |
| AB | 1192.41 | 1 | 1192.41 | 59.75 | <0.0001 |
| A2 | 366.83 | 1 | 366.83 | 18.38 | 0.0563 |
| B2 | 1643.63 | 1 | 1643.63 | 82.36 | 0.4584 |
| Residual | 299.36 | 15 | 19.96 | ||
| Lack of Fit | 76.5 | 3 | 25.5 | ||
| Pure Error | 222.86 | 12 | 18.57 | 1.37 | 0.2981 |
| Cor Total | 10,956.5 | 20 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 2.17 | 5 | 0.43 | 105.53 | <0.0001 |
| A-WMW | 0.88 | 1 | 0.88 | 213.64 | <0.0001 |
| B-Aeration rate | 0.07 | 1 | 0.07 | 17 | <0.0009 |
| AB | 0.03 | 1 | 0.03 | 7.28 | <0.0165 |
| A2 | 0.12 | 1 | 0.12 | 27.94 | <0.0001 |
| B2 | 0.43 | 1 | 0.43 | 105.22 | <0.0001 |
| Residual | 0.062 | 15 | 0.004 | ||
| Lack of Fit | 0.018 | 3 | 0.006 | ||
| Pure Error | 0.044 | 12 | 0.004 | 1.59 | 0.2434 |
| Cor Total | 2.24 | 20 |
| Face Centred Central Composite Design (FCCCD) Result | Validation Experiments | ||||||
|---|---|---|---|---|---|---|---|
| WMW (%) | Aeration rate (L/min) | Predicted Biomass Productivity (mg/L/d) | Predicted Maximum Growth Rate (day−1) | Biomass Productivity (mg/L/d) | Error, % | Maximum Growth Rate (day−1) | Error, % |
| 64.26 | 3.08 | 72.69 | 1.19 | 68.32 | 6 | 1.16 | 2.25 |
| Microalgae | Medium Source | Medium Concentration | Aeration Rate | Biomass Productivity | Maximum Growth Rate | References |
|---|---|---|---|---|---|---|
| Chodatella sp. | Piggery wastewater | 40% | 40 L/h.L | na | 0.31 day−1 | Chen et al., [42] |
| Chlorella sp. | Wastewater sludge | 11.4% | na | 2300 mg/L/d | na | Skorupskaite, Makareviciene & Levisauskas, [19] |
| Coelastrella sp. | Sugar industry effluent | 31.25% | na | 61.5 mg/L/d | na | Karpagam et al., [18] |
| Chlorella sp. | F/2 medium | na | 2.35L/min | na | 0.21 day−1 | Imamoglu, Demirel & Dalay, [43] |
| Chlorella vulgaris | BG 11 | na | 3.1 L/min | 0.65 g/L | Na | Alipourzadeh et al., [41] |
| Scenedesmus sp. | Wet market wastewater | 64.26% | 3.08 L/min | 72.70 mg/L/d | 1.19 day−1 | This study |
| Peak No. | Retention Time, RT (min) | Compound | Chemical Formula | Compound Nature | Molecular Weight (g/mol) | Area (%) | Biological Activity |
|---|---|---|---|---|---|---|---|
| 1 | 32.2365 | Glaucine | C21H25NO4 | Alkaloid | 355.434 | 16.1627 | Anti-inflammator, Antitussive |
| 2 | 33.0176 | Undecane | C11H24 | Alkane | 156.31 | 4.2863 | Anti-inflammator, Antimicrobial |
| 3 | 38.8833 | Hexadecane | C16H34 | Alkane | 226.44 | 24.8907 | Antimicrobial Antifungal |
| 4 | 39.8269 | Furan, 3-methyl- | C5H6O | Aromatic organic | 82.102 | 5.2256 | Antioxidant, Antibacterial, |
| 5 | 40.4035 | Spiro[4.5]decan-1-one | C15H24O2 | 236 | 9.5345 | Anti-inflammatory | |
| 6 | 44.0729 | Cyclohexasiloxane, dodecamethyl- | C12H36O6Si6 | Aromatic | 444.924 | 8.8785 | Antioxidant, Antimicrobial Antifungal |
| 7 | 48.104 | Phytol | C20H40O | Diterpene | 296 | 8.3319 | Anti-inflammator, Anticancer, Antioxidant Diuretic Fragrance |
| 8 | 48.6124 | Heptasiloxane, 1,1,3,3,5,5,7,7,9,9,11,11,13,13-tetradecamethyl- | C14H44O6Si7 | 504 | 5.2457 | Antimicrobial, Anti-inflammatory Antiseptic | |
| 9 | 56.3915 | 1,1,1,5,7,7,7-Heptamethyl-3,3-bis(trimethylsiloxy)tetrasiloxane | C13H40O5Si6 | Oxygenated diterpene | 444.97 | 2.6927 | Antimicrobial, Anti-inflammatory |
| 10 | 59.8722 | Hexa-t-butyl-3,5-dioxa-1,2,4-trisilolane | - | - | - | 3.325 | - |
| 11 | 62.3412 | 2-Methyl-6-(5-methyl-2-thiazolin-2-ylamino)pyridine | C10H13N3S | - | 207.3 | 2.4042 | Antioxidant |
| 12 | 63.117 | Hexasiloxane, tetradecamethyl- | C14H42O5Si6 | Organic silicon | 458 | 6.5538 | Antimicrobial Anti-inflammatory |
| 13 | 64.3384 | Methyl (5-hydroxy-1H-benzimidazol-2-yl)carbamate | C9H9N3O3 | Benomyl | 207.2 | 2.4685 | Anticancer Antiviral infections, Antifungal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Apandi, N.; Muhamad, M.S.; Radin Mohamed, R.M.S.; Mohamed Sunar, N.; Al-Gheethi, A.; Gani, P.; A. Rahman, F. Optimizing of Microalgae Scenedesmus sp. Biomass Production in Wet Market Wastewater Using Response Surface Methodology. Sustainability 2021, 13, 2216. https://doi.org/10.3390/su13042216
Mohd Apandi N, Muhamad MS, Radin Mohamed RMS, Mohamed Sunar N, Al-Gheethi A, Gani P, A. Rahman F. Optimizing of Microalgae Scenedesmus sp. Biomass Production in Wet Market Wastewater Using Response Surface Methodology. Sustainability. 2021; 13(4):2216. https://doi.org/10.3390/su13042216
Chicago/Turabian StyleMohd Apandi, Najeeha, Mimi Suliza Muhamad, Radin Maya Saphira Radin Mohamed, Norshuhaila Mohamed Sunar, Adel Al-Gheethi, Paran Gani, and Fahmi A. Rahman. 2021. "Optimizing of Microalgae Scenedesmus sp. Biomass Production in Wet Market Wastewater Using Response Surface Methodology" Sustainability 13, no. 4: 2216. https://doi.org/10.3390/su13042216
APA StyleMohd Apandi, N., Muhamad, M. S., Radin Mohamed, R. M. S., Mohamed Sunar, N., Al-Gheethi, A., Gani, P., & A. Rahman, F. (2021). Optimizing of Microalgae Scenedesmus sp. Biomass Production in Wet Market Wastewater Using Response Surface Methodology. Sustainability, 13(4), 2216. https://doi.org/10.3390/su13042216









