Stormwater Runoff Treatment Using Pervious Concrete Modified with Various Nanomaterials: A Comprehensive Review
Abstract
:1. Introduction
2. The Impact of Polluted Stormwater Runoff and Contaminated Water on Public Health
2.1. Microbial Contamination
2.2. Heavy Metal Contamination
2.3. Organic Contaminants
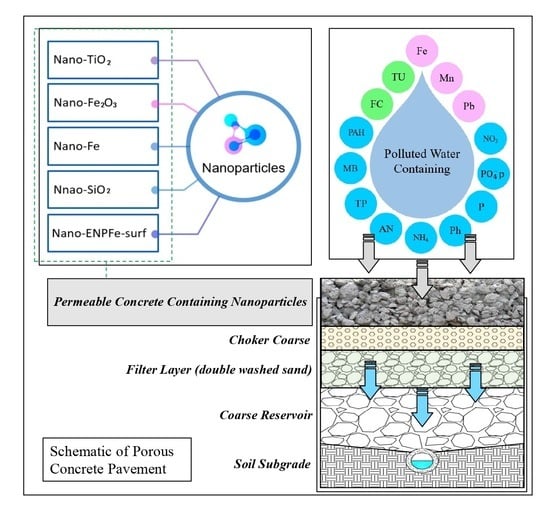
3. Porous Concrete
3.1. Materials
3.1.1. Coarse and Fine Aggregates
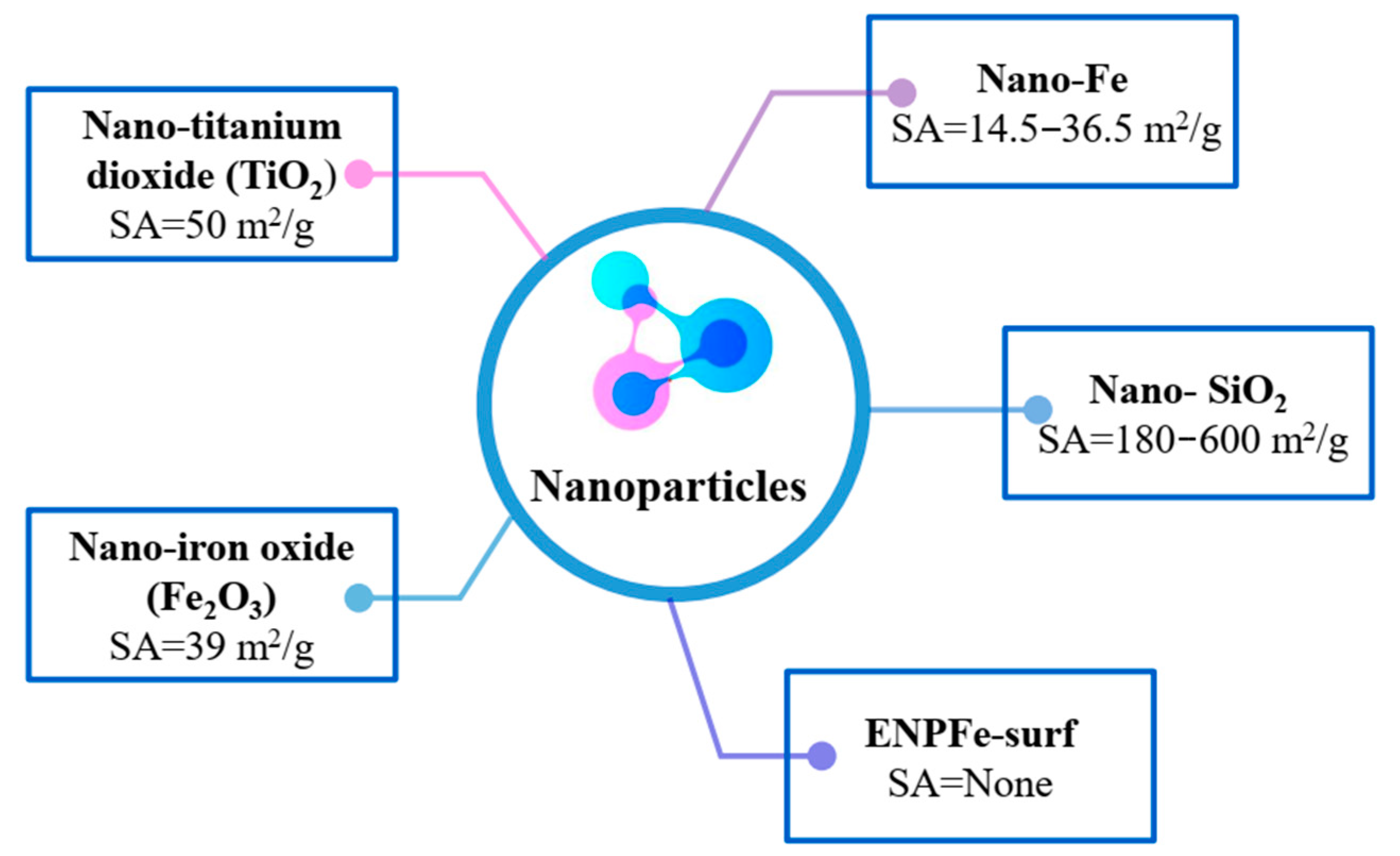
3.1.2. Nanoparticles Employed for the Improvement of PC and Pollutant Adsorption
3.2. Mix Design of Nanoparticle-Improved PC
3.3. Permeability, Compressive Strength, Adsorption Capacity and Regeneration Process of PC
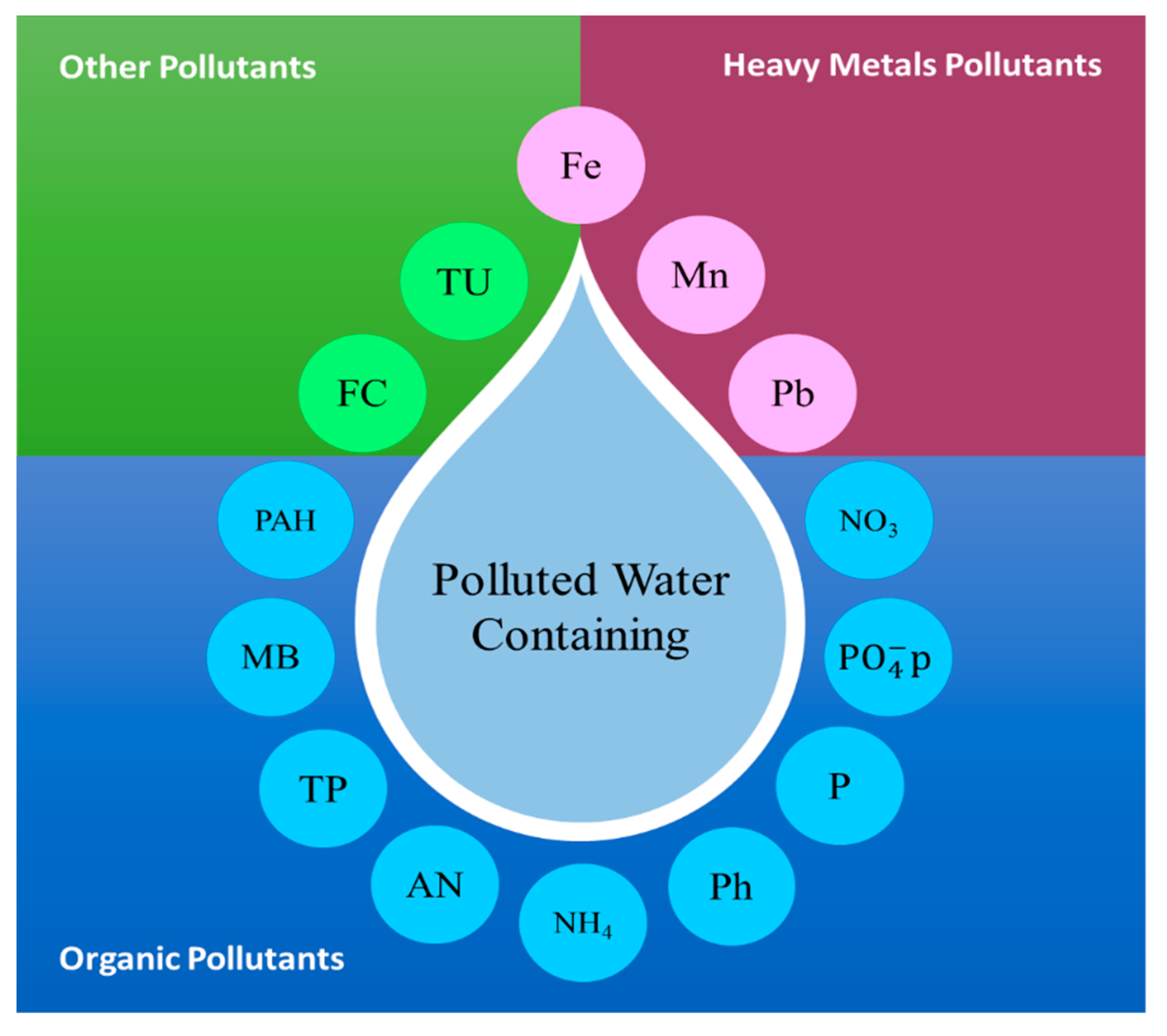
4. The Investigated Stormwater Contaminants
5. Effect of Nanoparticles on the Removal Percentage of Contaminants
5.1. The Process of Removing Pollutants Using PC
5.2. How Adding Nanomaterials Can Enhance PC (Structure, Stability, Morphology)
5.3. Impact of Nanomaterials on the Improvement of the Pollutants Removal
6. Conclusions
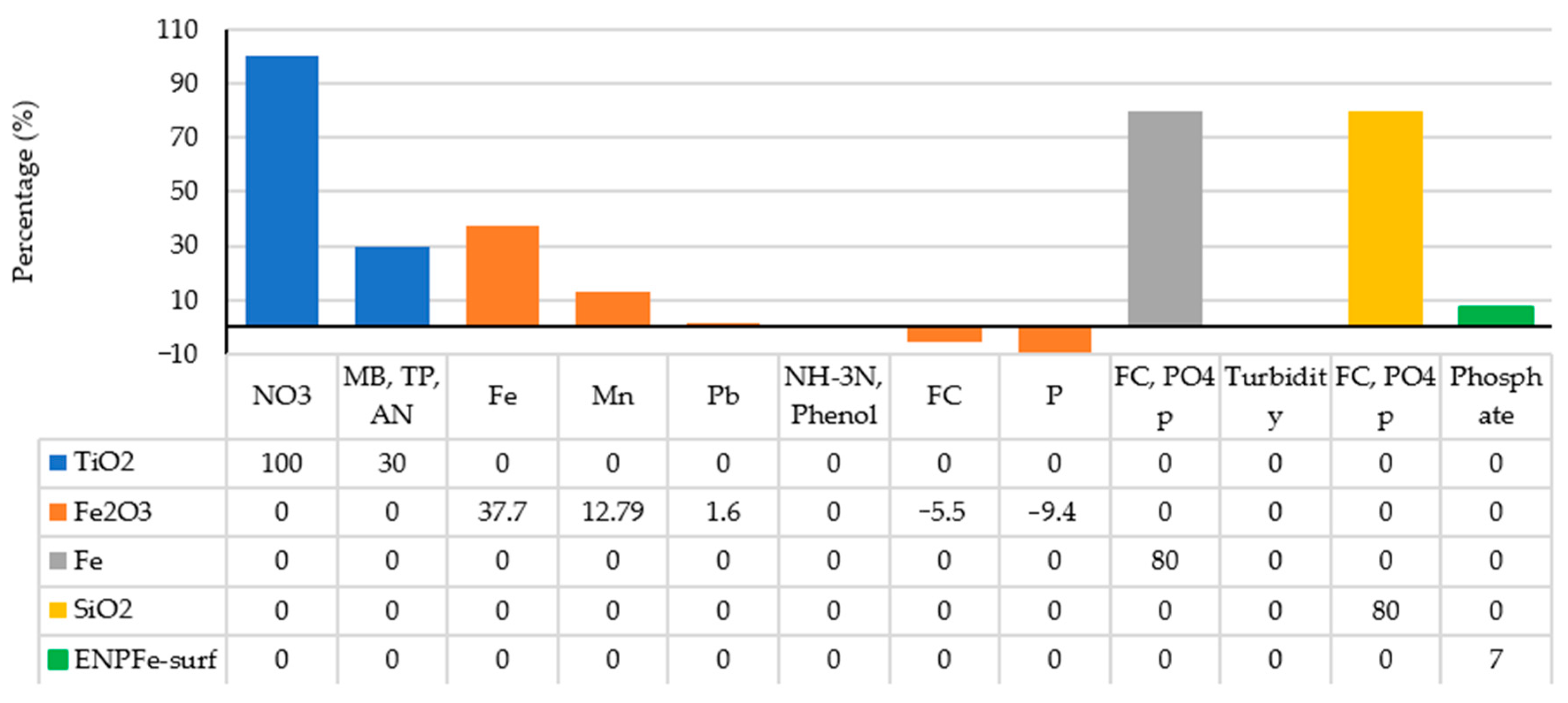
- All PC specimens improved with Fe and SiO2 nanoparticles were found to have satisfactory performance in removing FC and . However, while PC improved with Fe nanoparticles did not affect the turbidity (TU) removal, the PC modified with nSiO2 negatively affected the removal of TU.
- The addition of TiO2 nanoparticles to PC (i.e., TPC) imposed greater degradation on account of a larger pore area and a greater number of pores for a pore size of below 10 nm. This indicated that the nanoparticles altered the cement paste microstructure, resulting in the improvement of the removal of the organic contaminants, including ammonia nitrogen (AN), total phosphorus (TP), nitrate (NO3) and methylene blue (MB).
- The mean removal rates of heavy metals (Fe, Mn and Pb) significantly improved using PC specimens with Fe2O3 nanoparticles, but this type of concrete could not increase the removal of phenol (Ph) and ammonium (NH4) and also reduced the removal percentage of FC and P. Furthermore, PC containing Fe2O3 nanoparticles coated with surfactant (ENPFe-surf) increased phosphate removal in comparison with normal PC.
- Compared to normal PC, nanoparticles in modified PC significantly affected the compressive strength and permeability. Fe2O3, Fe, TiO2, ENPFe-surf and SiO2 nanoparticles generally increased the compressive strength of the PC, whereas the permeability of such concrete could be reduced by the incorporation of these nanomaterials; as an exception, ENPFe-surf increased the rate of permeability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alimohammadi, V.; Sedighi, M.; Jabbari, E. Response surface modeling and optimization of nitrate removal from aqueous solutions using magnetic multi-walled carbon nanotubes. J. Environ. Chem. Eng. 2016, 4, 4525–4535. [Google Scholar] [CrossRef]
- Shamshiri, A.; Alimohammadi, V.; Sedighi, M.; Jabbari, E.; Mohammadi, M. Enhanced removal of phosphate and nitrate from aqueous solution using novel modified natural clinoptilolite nanoparticles: Process optimization and assessment. Int. J. Environ. Anal. Chem. 2020, 1–20. [Google Scholar] [CrossRef]
- Tan, T.H.; Mo, K.H.; Ling, T.-C.; Lai, S.H. Current development of geopolymer as alternative adsorbent for heavy metal removal. Environ. Technol. Innov. 2020, 18, 100684. [Google Scholar] [CrossRef]
- Alimohammadi, V.; Sedighi, M. Reduction of TDS in Water by Using Magnetic Multiwalled Carbon Nanotubes and Optimizing with Response Surface Methodology. J. Environ. Eng. 2018, 144, 04017114. [Google Scholar] [CrossRef]
- Alimohammadi, V.; Sedighi, M.; Jabbari, E.; Nasrollahzadeh, M. Phosphate removal from aqueous solutions using magnetic multi-walled carbon nanotube; optimization by response surface methodology. Desalination Water Treat. 2017, 82, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Alimohammadi, V.; Sedighi, M.; Jabbari, E. Optimization of sulfate removal from wastewater using magnetic multi-walled carbon nanotubes by response surface methodology. Water Sci. Technol. 2017, 76, 2593–2602. [Google Scholar] [CrossRef] [PubMed]
- Alimohammadi, V.; Sedighi, M.; Jabbari, E. Experimental study on efficient removal of total iron from wastewater using magnetic-modified multi-walled carbon nanotubes. Ecol. Eng. 2017, 102, 90–97. [Google Scholar] [CrossRef]
- Li, X.; Wei, W.; Qin, H.; Hu, Y.H. Co-effects of graphene oxide sheets and single wall carbon nanotubes on mechanical properties of cement. J. Phys. Chem. Solids 2015, 85, 39–43. [Google Scholar] [CrossRef]
- Wang, J.; Iwasaki, R.; Bayen, A.M.; Harvey, J. Future road transportation technology. Int. J. Transp. Sci. Technol. 2016, 5, iii–iv. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Bach, P.M.; Mathios, J.; Dotto, C.B.; Deletic, A. Quantifying the benefits of stormwater harvesting for pollution mitigation. Water Res. 2020, 171, 115395. [Google Scholar] [CrossRef]
- Liang, X.; Cui, S.; Li, H.; Abdelhady, A.; Wang, H.; Zhou, H. Removal effect on stormwater runoff pollution of porous concrete treated with nanometer titanium dioxide. Transp. Res. Part D Transp. Environ. 2019, 73, 34–45. [Google Scholar] [CrossRef]
- Murugan, M. Performance of Concrete Based Water Filtration System: Influence of Reduced Graphene Oxide And Accelerated Carbonation. Doctor of Philosophy Dissertation, Indian Institute of Technology Madras, University in Chennai, Chennai, India, 2017. [Google Scholar]
- El-Sabban, H.; Eid, M.; Moustafa, Y.; Abdel-Mottaleb, M. Pomegranate peel extract in situ assisted phytosynthesis of Silver Nanoparticles decorated Reduced Graphene Oxide as superior sorbents for Zn(II) and Lead(II). Egypt. J. Aquat. Biol. Fish. 2020, 24, 525–539. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Wang, H.; Song, A.; Hao, J. Superhydrogels of Nanotubes Capable of Capturing Heavy-Metal Ions. Chem. Asian J. 2013, 9, 245–252. [Google Scholar] [CrossRef]
- Fletcher, T.D.; Shuster, W.; Hunt, W.F.; Ashley, R.; Butler, D.; Arthur, S.; Trowsdale, S.; Barraud, S.; Semadeni-Davies, A.; Bertrand-Krajewski, J.-L.; et al. SUDS, LID, BMPs, WSUD and more. The evolution and application of terminology surrounding urban drainage. Urban Water J. 2015, 12, 525–542. [Google Scholar] [CrossRef]
- Zhang, K.; Yong, F.; McCarthy, D.T.; Deletic, A. Predicting long term removal of heavy metals from porous pavements for stormwater treatment. Water Res. 2018, 142, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.T.; Kayhanian, M.; Gulliver, J.S.; Khazanovich, L. Permeable pavement in northern North American urban areas: Research review and knowledge gaps. Int. J. Pavement Eng. 2019, 20, 143–162. [Google Scholar] [CrossRef]
- Dempsey, B.A.; Swisher, D.M. Evaluation of Porous Pavement and Infiltration in Centre County, PA. World Water Environ. Resour. Congress 2003, 1–11. [Google Scholar] [CrossRef]
- Legret, M.; Colandini, V. Effects of a porous pavement with reservoir structure on runoff water: Water quality and fate of heavy metals. Water Sci. Technol. 1999, 39, 111–117. [Google Scholar] [CrossRef]
- An alternative road construction for stormwater management in cold climates. Water Sci. Technol. 1995, 32, 79–84. [CrossRef]
- Xie, N.; Akin, M.; Shi, X. Permeable concrete pavements: A review of environmental benefits and durability. J. Clean. Prod. 2019, 210, 1605–1621. [Google Scholar] [CrossRef]
- Asadi, I.; Shafigh, P.; Hashemi, M.; Akhiani, A.R.; Maghfouri, M.; Sajadi, B.; Mahyuddin, N.; Esfandiari, M.; Talebi, H.R.; Metselaar, H.S.C. Thermophysical properties of sustainable cement mortar containing oil palm boiler clinker (OPBC) as a fine aggregate. Constr. Build. Mater. 2021, 268, 121091. [Google Scholar] [CrossRef]
- López-Carrasquillo, V.; Hwang, S. Comparative assessment of pervious concrete mixtures containing fly ash and nanomaterials for compressive strength, physical durability, permeability, water quality performance and production cost. Constr. Build. Mater. 2017, 139, 148–158. [Google Scholar] [CrossRef]
- Maghfouri, M.; Shafigh, P.; Alimohammadi, V.; Doroudi, Y.; Aslam, M. Appropriate drying shrinkage prediction models for lightweight concrete containing coarse agro-waste aggregate. J. Build. Eng. 2020, 29, 101148. [Google Scholar] [CrossRef]
- Maghfouri, M.; Shafigh, P.; Ibrahim, Z.B.; Alimohammadi, V. Quality control of lightweight aggregate concrete based on initial and final water absorption tests. In Proceedings of the IOP Conference Series: Materials Science and Engineering, University of Malaya, Kuala Lumpur, Malaysia, 5–6 April 2017; IOP Publishing: Kuala Lumpur, Malaysia, 2017; Volume 210, p. 12022. [Google Scholar]
- Rahman, K.; Barua, S.; Anwar, S.; Hasan, Z.; Islam, S. Removal of Heavy Metals from Stormwater Using Porous Concrete Pavement. J. Mod. Mater. 2020, 7, 37–44. [Google Scholar] [CrossRef]
- Hein, D.K.; Eng, P. Development of an ASCE standard for permeable interlocking concrete pavement. In Proceedings of the 2014 Conference and Exhibition of the Transportation Association of Canada, Québec, QC, Canada, 28 September–1 October 2014. [Google Scholar]
- Yusak, M.I.M.; Jaya, R.P.; Hainin, M.R.; Ibrahim, M.H.W. An overview on the performance of nano silica materials on the properties of porous concrete pavement. J. Adv. Sci. Res. 2014, 34–42. [Google Scholar]
- Maghfouri, M. Drying Shrinkage Strain Development of Agro-Waste Oil Palm Shell Lightweight Aggregate Concrete by Using the Experimental Result, ACI and Eurocode Prediction Models. Int. J. Integr. Eng 2019, 11, 255–263. [Google Scholar] [CrossRef]
- Bhutta, M.A.R.; Tsuruta, K.; Mirza, J. Evaluation of high-performance porous concrete properties. Constr. Build. Mater. 2012, 31, 67–73. [Google Scholar] [CrossRef]
- Drake, J.A.P.; Bradford, A.; Marsalek, J. Review of environmental performance of permeable pavement systems: State of the knowledge. Water Qual. Res. J. 2013, 48, 203–222. [Google Scholar] [CrossRef]
- Ortega-Villar, R.; Lizárraga-Mendiola, L.; Coronel-Olivares, C.; López, L.D.; Bigurra-Alzati, C.A.; Vázquez-Rodríguez, G.A. Effect of photocatalytic Fe2O3 nanoparticles on urban runoff pollutant removal by permeable concrete. J. Environ. Manag. 2019, 242, 487–495. [Google Scholar] [CrossRef]
- Jianming, G.; Xu, G.; Lu, X. Experimental study on eco-environmental effect of porous concrete. J. Southeast Univ. 2008, 38, 794–798. [Google Scholar]
- Turco, M.; Brunetti, G.; Palermo, S.A.; Capano, G.; Grossi, G.; Maiolo, M.; Piro, P. On the environmental benefits of a permeable pavement: Metals potential removal efficiency and Life Cycle Assessment. Urban Water J. 2020, 17, 619–627. [Google Scholar] [CrossRef]
- Martin, W.; Sumanasooriya, M.; Kaye, N.B.; Putman, B. Design of Porous Pavements for Improved Water Quality and Reduced Runoff. Handb. Environ. Eng. 2018, 425–451. [Google Scholar] [CrossRef]
- Madkour, L.H. Ecotoxicology of Environmental Heavy Metal Ions and Free Radicals on Macromolecule Cell Organisms. In Bioengineering Applications of Carbon Nanostructures; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2020; pp. 1–46. [Google Scholar]
- Reza, R.; Singh, G. Heavy metal contamination and its indexing approach for river water. Int. J. Environ. Sci. Technol. 2010, 7, 785–792. [Google Scholar] [CrossRef] [Green Version]
- Welker, A.L.; Barbis, J.D.; Jeffers, P.A. A Side-by-Side Comparison of Pervious Concrete and Porous Asphalt1. JAWRA J. Am. Water Resour. Assoc. 2012, 48, 809–819. [Google Scholar] [CrossRef]
- Shabalala, A.N.; Ekolu, S.O.; Diop, S.; Solomon, F. Pervious concrete reactive barrier for removal of heavy metals from acid mine drainage—Column study. J. Hazard. Mater. 2017, 323, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Haselbach, L.; Poor, C.; Tilson, J. Dissolved zinc and copper retention from stormwater runoff in ordinary portland cement pervious concrete. Constr. Build. Mater. 2014, 53, 652–657. [Google Scholar] [CrossRef]
- Kuang, X.; Kim, J.-Y.; Gnecco, I.; Raje, S.; Garofalo, G.; Sansalone, J.J. Particle Separation and Hydrologic Control by Cementitious Permeable Pavement. Transp. Res. Rec. J. Transp. Res. Board 2007, 2025, 111–117. [Google Scholar] [CrossRef]
- Lee, M.-G.; Tia, M.; Chuang, S.-H.; Huang, Y.; Chiang, C.-L. Pollution and Purification Study of the Pervious Concrete Pavement Material. J. Mater. Civ. Eng. 2014, 26, 04014035. [Google Scholar] [CrossRef]
- Solpuker, U.; Sheets, J.; Kim, Y.; Schwartz, F. Leaching potential of pervious concrete and immobilization of Cu, Pb and Zn using pervious concrete. J. Contam. Hydrol. 2014, 161, 35–48. [Google Scholar] [CrossRef]
- Huang, J.; Valeo, C.; He, J.; Chu, A. Three Types of Permeable Pavements in Cold Climates: Hydraulic and Environmental Performance. J. Environ. Eng. 2016, 142, 04016025. [Google Scholar] [CrossRef]
- Jiang, A.; Cheng, Z.; Shen, Z.; Guo, W. QSAR study on the removal efficiency of organic pollutants in supercritical water based on degradation temperature. Chem. Central J. 2018, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Tian, W.; Qin, H.; Wang, X.; Zhao, W. Optimal design and control of Eastman organic wastewater treatment process. J. Clean. Prod. 2018, 198, 333–350. [Google Scholar] [CrossRef]
- Vithanage, M.; Mayakaduwa, S.; Herath, I.; Ok, Y.S.; Mohan, D. Kinetics, thermodynamics and mechanistic studies of carbofuran removal using biochars from tea waste and rice husks. Chemosphere 2016, 150, 781–789. [Google Scholar] [CrossRef]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef]
- Brown, R.A.; Line, D.E.; Hunt, W.F. LID Treatment Train: Pervious Concrete with Subsurface Storage in Series with Bioretention and Care with Seasonal High Water Tables. J. Environ. Eng. 2012, 138, 689–697. [Google Scholar] [CrossRef]
- Park, S.-B.; Tia, M. An experimental study on the water-purification properties of porous concrete. Cem. Concr. Res. 2004, 34, 177–184. [Google Scholar] [CrossRef]
- Jo, M.; Soto, L.; Arocho, M.; John, J.S.; Hwang, S. Optimum mix design of fly ash geopolymer paste and its use in pervious concrete for removal of fecal coliforms and phosphorus in water. Constr. Build. Mater. 2015, 93, 1097–1104. [Google Scholar] [CrossRef]
- Hwang, V.; Masters, A.; Arocho, M.; Hwang, S. Fly ash-amended pervious concrete pavement followed by bamboo bioretention basin with Dracaena sanderiana for urban stormwater runoff control. Constr. Build. Mater. 2017, 132, 161–169. [Google Scholar] [CrossRef]
- Karamalegos, A.M.; Barrett, M.E.; Lawler, D.F.; Malina, J.F. Particle Size Distribution of Highway Runoff and Modification Through Stormwater Treatment; Center for Research in Water Resources, University of Texas at Austin: Austin, TX, USA, 2005. [Google Scholar]
- Soto-Pérez, L.; Hwang, S. Mix design and pollution control potential of pervious concrete with non-compliant waste fly ash. J. Environ. Manag. 2016, 176, 112–118. [Google Scholar] [CrossRef]
- Monrose, J.; Tota-Maharaj, K.; Mwasha, A. Assessment of the physical characteristics and stormwater effluent quality of permeable pavement systems containing recycled materials. Road Mater. Pavement Des. 2019, 1–33. [Google Scholar] [CrossRef]
- American Water Works Association; Edzwald, J. Water Quality & Treatmenta Handbook on Drinking Water; McGraw-Hill Education: New York, NY, USA, 2011. [Google Scholar]
- Chowdhury, R.K.; Sharvelle, S.E.; Beecham, S. Greywater quality changes in a permeable pavement reservoir. Proc. Inst. Civ. Eng. Water Manag. 2016, 169, 190–198. [Google Scholar] [CrossRef]
- Tota-Maharaj, K.; Scholz, M. Efficiency of permeable pavement systems for the removal of urban runoff pollutants under varying environmental conditions. Environ. Prog. Sustain. Energy 2010, 29, 358–369. [Google Scholar] [CrossRef]
- Pilon, B.S.; Tyner, J.S.; Yoder, D.C.; Buchanan, J.R. The Effect of Pervious Concrete on Water Quality Parameters: A Case Study. Water 2019, 11, 263. [Google Scholar] [CrossRef] [Green Version]
- Boutilier, L.; Jamieson, R.; Gordon, R.; Lake, C.; Hart, W. Adsorption, sedimentation, and inactivation of E. coli within wastewater treatment wetlands. Water Res. 2009, 43, 4370–4380. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hall, G.; Champagne, P. The role of algae in the removal and inactivation of pathogenic indicator organisms in wastewater stabilization pond systems. Algal Res. 2020, 46, 101777. [Google Scholar] [CrossRef]
- Rusciano, G.M.; Obropta, C.C. Bioretention Column Study: Fecal Coliform and Total Suspended Solids Reductions. Trans. ASABE 2007, 50, 1261–1269. [Google Scholar] [CrossRef]
- Xu, L.; Xian, F.; Chen, Y.; Miao, J.; Su, J.; Pei, S.; Gu, F.; Qian, L. Tunable nanoporosity in Sr-doped ZnO thin films by thermal annealing treatment and its effect on photocatalytic activity. J. Optoelectron. Adv. Mater. J. 2017, 19, 96–101. [Google Scholar]
- Che, H.; Liu, C.; Hu, W.; Hu, H.; Li, J.; Dou, J.; Shi, W.; Li, C.; Dong, H. NGQD active sites as effective collectors of charge carriers for improving the photocatalytic performance of Z-scheme g-C3N4/Bi2WO6 heterojunctions. Catal. Sci. Technol. 2017, 8, 622–631. [Google Scholar] [CrossRef]
- Bolt, J.R.; Zhuge, Y.; Bullen, F. The Impact of Photocatalytic on Degradation of Poly Aromatic Hydrocarbons through Permeable Concrete. 2014. Available online: http://eprints.usq.edu.au/id/eprint/27541 (accessed on 29 December 2014).
- Liang, Z.; Ni, J. Improving the ammonium ion uptake onto natural zeolite by using an integrated modification process. J. Hazard. Mater. 2009, 166, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Hamilton, K.; Toze, S.; Cook, S.; Page, D. A review on microbial contaminants in stormwater runoff and outfalls: Potential health risks and mitigation strategies. Sci. Total Environ. 2019, 692, 1304–1321. [Google Scholar] [CrossRef]
- Azizullah, A.; Khattak, M.N.K.; Richter, P.; Häder, D.-P. Water pollution in Pakistan and its impact on public health—A review. Environ. Int. 2011, 37, 479–497. [Google Scholar] [CrossRef]
- Leclerc, H.; Schwartzbrod, L.; Dei-Cas, E. Microbial Agents Associated with Waterborne Diseases. Crit. Rev. Microbiol. 2002, 28, 371–409. [Google Scholar] [CrossRef]
- Ali, M.; Emch, M.; Donnay, J.; Yunus, M.; Sack, R. Identifying environmental risk factors for endemic cholera: A raster GIS approach. Health Place 2002, 8, 201–210. [Google Scholar] [CrossRef]
- Nelson, E.J.; Harris, J.B.; Morris, J.G., Jr.; Calderwood, S.B.; Camilli, A. Cholera transmission: The host, pathogen and bacteriophage dynamic. Nat. Rev. Genet. 2009, 7, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Dewan, A.; Corner, R.; Hashizume, M.; Ongee, E.T. Typhoid Fever and Its Association with Environmental Factors in the Dhaka Metropolitan Area of Bangladesh: A Spatial and Time-Series Approach. PLoS Negl. Trop. Dis. 2013, 7, e1998. [Google Scholar] [CrossRef] [Green Version]
- A Crump, J. Progress in Typhoid Fever Epidemiology. Clin. Infect. Dis. 2019, 68, S4–S9. [Google Scholar] [CrossRef] [Green Version]
- Heymann, D.L. Control of Communicable Diseases Manual; American Public Health Association: Washington, DC, USA, 2008. [Google Scholar]
- Ryu, S.; A Won, S.; Uh, J.; Song, J.Y. Hepatitis A Virus Infection from a Contaminated Tap of Ground Water Facility in a Neighborhood Park, Republic of Korea. Infect. Chemother. 2019, 51, 62–66. [Google Scholar] [CrossRef]
- Hooshyar, H.; Rostamkhani, P.; Arbabi, M.; Delavari, M. Giardia lamblia infection: Review of current diagnostic strategies. Gastroenterol. Hepatol. Bed Bench 2019, 12, 3–12. [Google Scholar]
- Gerace, E.; Presti, V.D.M.L.; Biondo, C. Cryptosporidium infection: Epidemiology, pathogenesis, and differential diagnosis. Eur. J. Microbiol. Immunol. 2019, 9, 119–123. [Google Scholar] [CrossRef]
- Acrylamide, O. National Primary Drinking Water Regulations. Kidney 2009, 2, 7. [Google Scholar]
- Haq, M. Surface and ground water contamination in NWFP and Sindh provinces with respect to trace elements. Int J. Agric. Biol. 2005, 7, 214–217. [Google Scholar]
- Santos, D.; Vieira, R.; Luzio, A.; Félix, L. Zebrafish Early Life Stages for Toxicological Screening: Insights From Molecular and Biochemical Markers. Adv. Mol. Toxicol. 2018, 12, 151–179. [Google Scholar] [CrossRef]
- Vhahangwele, M.; Khathutshelo, L. Environmental contamination by heavy metals. Heavy Met. 2018, 10, 115–132. [Google Scholar]
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and Health Effects of Heavy Metal Toxicity in Humans. In Poisoning in the Modern World-New Tricks for an Old Dog; IntechOpen: London, UK, 2019; pp. 77–100. [Google Scholar] [CrossRef] [Green Version]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Prashanth, T.; Anura, K. Summary on Adverse Effects of Excess Iron. Indian J. Community Health 2018, 30, 103–107. Available online: https://www.iapsmupuk.org/journal/index.php/IJCH/article/view/822 (accessed on 1 July 2018).
- Island, P.E.; Scotia, N.; Territories, N. Manganese in Drinking Water; Federal-Provincial-Territorial Committee on Drinking Water; Government of Canada: Ottawa, ON, Canada, 2016.
- Röllin, H.; Nogueira, C. Manganese: Environmental Pollution and Health Effects. Encycl. Environ. Health 2011, 617–629. [Google Scholar]
- Idrees, N.; Tabassum, B.; Abd_Allah, E.; Hashem, A.; Sarah, R.; Hashim, M. Groundwater contamination with cadmium concentrations in some West U.P. Regions, India. Saudi J. Biol. Sci. 2018, 25, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Burke, F.; Hamza, S.; Naseem, S.; Nawaz-Ul-Huda, S.; Azam, M.; Khan, I. Impact of Cadmium Polluted Groundwater on Human Health. SAGE Open 2016, 6, 2158244016634409. [Google Scholar] [CrossRef] [Green Version]
- Tsang, D.C.; Yu, I.K.; Xiong, X. Novel Application of Biochar in Stormwater Harvesting. Biochar Biomass Waste 2019, 319–347. [Google Scholar] [CrossRef]
- Chowdhury, S.; Khan, N.; Kim, G.-H.; Harris, J.; Longhurst, P.; Bolan, N.S. Zeolite for Nutrient Stripping From Farm Effluents. In Environmental Materials and Waste; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 569–589. [Google Scholar]
- Xue, Y.; Song, J.; Zhang, Y.; Kong, F.; Wen, M.; Zhang, G. Nitrate Pollution and Preliminary Source Identification of Surface Water in a Semi-Arid River Basin, Using Isotopic and Hydrochemical Approaches. Water 2016, 8, 328. [Google Scholar] [CrossRef]
- Parvizishad, M.; Dalvand, A.; Mahvi, A.H.; Goodarzi, F. A Review of Adverse Effects and Benefits of Nitrate and Nitrite in Drinking Water and Food on Human Health. Health Scope 2017, in press. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.L. Nitrate and phosphate contamination in water and possible remedial measures. Environ. Problems Plant. 2016, 3, 44–56. [Google Scholar]
- Calvo, M.S.; Uribarri, J. Public health impact of dietary phosphorus excess on bone and cardiovascular health in the general population. Am. J. Clin. Nutr. 2013, 98, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Shafy, H.I.; Mansour, M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Marc, R. Asian textile dye makers are a growing power in changing market, C EN Northeast. News Bureau 1996, 73, 10–12. [Google Scholar]
- Fayazi, M.; Taher, M.A.; Afzali, D.; Mostafavi, A. Enhanced Fenton-like degradation of methylene blue by magnetically activated carbon/hydrogen peroxide with hydroxylamine as Fenton enhancer. J. Mol. Liq. 2016, 216, 781–787. [Google Scholar] [CrossRef]
- Khataeeabc, A.R.; Kasiri, M.B. Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: Influence of the chemical structure of dyes. J. Mol. Catal. A Chem. 2010, 328, 8–26. [Google Scholar] [CrossRef]
- Monib, S.; Mohamed, A.; I Abdelaziz, M. Methylene Blue Spray for Identification of Parathyroid Glands During Thyroidectomy. Cureus 2020, 12. [Google Scholar] [CrossRef]
- Hassanpour, M.; Safardoust-Hojaghan, H.; Salavati-Niasari, M. Degradation of methylene blue and Rhodamine B as water pollutants via green synthesized Co3O4/ZnO nanocomposite. J. Mol. Liq. 2017, 229, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Waller, R.M. Ground water and the rural homeowner. Gen. Interest Publ. 1988. [Google Scholar] [CrossRef]
- Taylor, A.A.; Tsuji, J.S.; Garry, M.R.; McArdle, M.E.; Goodfellow, W.L.; Adams, W.J.; Menzie, C.A. Critical Review of Exposure and Effects: Implications for Setting Regulatory Health Criteria for Ingested Copper. Environ. Manag. 2020, 65, 131–159. [Google Scholar] [CrossRef] [Green Version]
- Ayotte, J.D.; Medalie, L.; Qi, S.L.; Backer, L.C.; Nolan, B. Estimating the High-Arsenic Domestic-Well Population in the Conterminous United States. Environ. Sci. Technol. 2017, 51, 12443–12454. [Google Scholar] [CrossRef]
- Hong, Y.-S.; Song, K.-H.; Chung, J.-Y. Health Effects of Chronic Arsenic Exposure. J. Prev. Med. Public Health 2014, 47, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Basu, H.; Saha, S.; Mahadevan, I.A.; Pimple, M.V.; Singhal, R.K. Humic acid coated cellulose derived from rice husk: A novel biosorbent for the removal of Ni and Cr. J. Water Process. Eng. 2019, 32, 100892. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [Green Version]
- Deda, A.; Alushllari, M.; Mico, S. Measurement of Heavy Metal Concentrations in Groundwater; AIP Publishing: Birmingham, UK, 2017. [Google Scholar]
- Musa, O.K.; Shaibu, M.M.; Kudamnya, E.A. Heavy metal concentration in groundwater around Obajana and its environs, Kogi State, North Central Nigeria. Am. Int J. Contemp Res. 2013, 3, 170–177. [Google Scholar]
- Maghfouri, M.; Shafigh, P.; Aslam, M. Optimum oil palm shell content as coarse aggregate in concrete based on me-chanical and durability properties. Adv. Mater. Sci. Eng. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- National Ready Mixed Concrete Association. Freeze-Thaw Resistance Of Pervious Concrete; National Ready Mixed Concrete Association: Silver Spring, MD, USA, 2004. [Google Scholar]
- ACI Committee 522. Report on Pervious Concrete; American Concrete Institute: Indianapolis, IN, USA, 2010. [Google Scholar]
- C/CM-14a. Standard Test Method for Density and Void Content of Freshly Mixed Pervious Concrete; ASTM International West: Conshohocken, PA, USA, 2014. [Google Scholar]
- Neithalath, N.; Weiss, W.; Olek, J. Characterizing Enhanced Porosity Concrete using electrical impedance to predict acoustic and hydraulic performance. Cem. Concr. Res. 2006, 36, 2074–2085. [Google Scholar] [CrossRef]
- Muñoz, A.M. Evaluation of Sustainability, Durability and the Effect of Specimen Type in Pervious Concrete Mixtures; Texas State University-San Marcos: San Marcos, TX, USA, 2012. [Google Scholar]
- Matsuo, Y.; Morino, K.; Iwatsuki, E. A study of porous concrete using electric arc furnace oxidizing slag aggregate. Bull. Aichi Inst. Technol. Part. B 2005, 40, 167–217. [Google Scholar]
- Aoki, Y. Development of Pervious Concrete. Master’s Thesis, Technical University of Sydney, Sydney, Australia, 2009. [Google Scholar]
- Lee, M.J.; Huang, Y.; Chiang, C.L. Purification Study of Pervious Concrete Pavement. Int. J. Eng. Technol. 2013, 5, 532–535. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, V.M. No-fines concrete-its properties and applications. J. Proc. 1974, 17, 628–644. [Google Scholar] [CrossRef]
- Ghafoori, N.; Dutta, S. Building and Nonpavement Applications of No-Fines Concrete. J. Mater. Civ. Eng. 1995, 7, 286–289. [Google Scholar] [CrossRef]
- Barnhouse, P.W.; Srubar, W.V. Material characterization and hydraulic conductivity modeling of macroporous recycled-aggregate pervious concrete. Constr. Build. Mater. 2016, 110, 89–97. [Google Scholar] [CrossRef]
- Kim, G.; Jang, J.; Khalid, H.R.; Lee, H. Water purification characteristics of pervious concrete fabricated with CSA cement and bottom ash aggregates. Constr. Build. Mater. 2017, 136, 1–8. [Google Scholar] [CrossRef]
- Luck, J.D.; Workman, S.R.; Coyne, M.S.; Higgins, S.F. Solid material retention and nutrient reduction properties of pervious concrete mixtures. Biosyst. Eng. 2008, 100, 401–408. [Google Scholar] [CrossRef]
- Sheth, P.D.K.; Patel, M. Pervious Concrete As Environmentally Friendly Material-A review. Int. J. Eng. Sci. 2015, 1, 1–6. [Google Scholar]
- Fu, T.C.; Yeih, W.; Chang, J.J.; Huang, R. The Influence of Aggregate Size and Binder Material on the Properties of Pervious Concrete. Adv. Mater. Sci. Eng. 2014, 2014, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Sata, V.; Wongsa, A.; Chindaprasirt, P. Properties of pervious geopolymer concrete using recycled aggregates. Constr. Build. Mater. 2013, 42, 33–39. [Google Scholar] [CrossRef]
- Sun, Z.; Lin, X.; Vollpracht, A. Pervious concrete made of alkali activated slag and geopolymers. Constr. Build. Mater. 2018, 189, 797–803. [Google Scholar] [CrossRef]
- Marolf, A.; Neithalath, N.; Sell, E.; Wegner, K.; Weiss, J.; Olek, J. Influence of Aggregate Size and Gradation on Acoustic Absorption of Enhanced Porosity Concrete. ACI Mater. J. Am. Concr. Inst. 2004, 101, 82–91. [Google Scholar]
- Deo, O.; Sumanasooriya, M.; Neithalath, N. Permeability Reduction in Pervious Concretes due to Clogging: Experiments and Modeling. J. Mater. Civ. Eng. 2010, 22, 741–751. [Google Scholar] [CrossRef]
- Dierkes, C.; Benze, W.; Wells., J. Sustainable urban drainage and pollutant source control by infiltration. In Proceedings of the 6th Regional Conference on Urban Stormwater, Stormwater Industry Association, Orange, Australia,, 22–26 April 2002. [Google Scholar]
- Kevern, J.T.; Wang, K.; Schaefer, V.R. Effect of Coarse Aggregate on the Freeze-Thaw Durability of Pervious Concrete. J. Mater. Civ. Eng. 2010, 22, 469–475. [Google Scholar] [CrossRef]
- Maghfouri, M.; Alimohammadi, V.; Azarsa, P.; Asadi, I.; Doroudi, Y.; Balakrishnan, B. Impact of Fly Ash on Time-Dependent Properties of Agro-Waste Lightweight Aggregate Concrete. J. Compos. Sci. 2021, 5, 156. [Google Scholar] [CrossRef]
- Wan, D.S.L.Y.; Aslani, F.; Ma, G. Lightweight Self-Compacting Concrete Incorporating Perlite, Scoria, and Polystyrene Aggregates. J. Mater. Civ. Eng. 2018, 30, 04018178. [Google Scholar] [CrossRef]
- Harker, K.T.; Mahar, J. Use of Porous Concrete and Scoria Bases to Clean Groundwater Recharge; Missouri University of Science and Technology: Rolla, MI, USA, 2003; Paper 41; Available online: http://scholarsmine.mst.edu/icchge/7icchge/session_06/41 (accessed on 1 July 2021).
- Reddy, K.R.; Anderson, S.; Beena, K.S.; Burken, J.; Gingery, J.R.; Kolev, C.; Rosyidi, S.A.P.; Yano, Y. General Report-Session 6; Missouri University of Science and Technology: Rolla, MI, USA, 2013. [Google Scholar]
- Holmes, R.R.; Hart, M.L.; Kevern, J.T. Heavy metal removal capacity of individual components of permeable reactive concrete. J. Contam. Hydrol. 2017, 196, 52–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochmah, N.; Sarya, G.; Setiawan, F. The Use of The Semanding Tuban Limestone as A Partial Replacement of Coarse Aggregate in Concrete Mixes. In Proceedings of the 1st Asian Conference on Humanities, Industry, and Technology for Society, ACHITS 2019, Surabaya Indonesia, 30–31 July 2019. [Google Scholar]
- Vázquez-Rivera, N.I.; Soto-Pérez, L.; John, J.N.S.; Molina-Bas, O.I.; Hwang, S.S. Optimization of pervious concrete containing fly ash and iron oxide nanoparticles and its application for phosphorus removal. Constr. Build. Mater. 2015, 93, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Sandoval, G.F.B.; Reyes, I.G.; Schwantes-Cezario, N.; Moura, A.C.; Toralles, B.M. Correlation between Permeability and Porosity for Pervious Concrete (PC). DYNA 2019, 86, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, G.; Wang, B.; Wu, M. Mechanical strengths and durability properties of pervious concretes with blended steel slag and natural aggregate. J. Clean. Prod. 2020, 271, 122590. [Google Scholar] [CrossRef]
- Islam, A.B.M.S.; Al-Kutti, W.; Alsaidan, M.; Alharbi, F.; Nasir, M.; Anwar, F. Potential of volcanic waste Scoria as an eco-friendly aggregate to produce Lightweight Concrete. Smart Cities Symp. 2018, 28. [Google Scholar] [CrossRef]
- Thomas, C.; Setién, J.; Polanco, J. Structural recycled aggregate concrete made with precast wastes. Constr. Build. Mater. 2016, 114, 536–546. [Google Scholar] [CrossRef] [Green Version]
- Ozbakkaloglu, T.; Gu, L.; Gholampour, A. Short-Term Mechanical Properties of Concrete Containing Recycled Polypropylene Coarse Aggregates under Ambient and Elevated Temperature. J. Mater. Civ. Eng. 2017, 29, 04017191. [Google Scholar] [CrossRef]
- Moore, J.G. Density of basalt core from Hilo drill hole, Hawaii. J. Volcanol. Geotherm. Res. 2001, 112, 221–230. [Google Scholar] [CrossRef]
- Hydzik-Wiśniewska, J.; Wilk, A.; Bednarek, Ł.; Olesiak, S. Mixture of Crushed- Stone Aggregate as Material For Substructure Layers. Stud. Geotech. Mech. 2018, 40, 154–162. [Google Scholar] [CrossRef]
- American Association of State Highway; Transportations Officials. AASHTO Guide for Design of Pavement Structures; The American Association of State Highway and Transportation Officials: Washington, DC, USA, 1993. [Google Scholar]
- Chen, X.; Niu, Z.; Zhang, H.; Guo, Y.; Liu, M.; Zhou, M. Study on the metakaolin-based geopolymer pervious concrete (MKGPC) and its removal capability of heavy metal ions. Int. J. Pavement Eng. 2019, 1–12. [Google Scholar] [CrossRef]
- Weiss, P.T.; Kayhanian, M.; Khazanovich, L.; Gulliver, J.S. Permeable Pavements in Cold Climates: State of The Art And Cold Climate Case Studies; Center for Transportation Studies, University of Minnesota: Minneapolis, MN, USA, 2015. [Google Scholar]
- Ramadhansyah, P.; Ibrahim, M.H.W.; Rosli, H.M.; Warid, M.N.M.; Hainin, M.R. Porous Concrete Pavement Containing Nano-Silica: Pre-Review. Adv. Mater. Res. 2014, 911, 454–458. [Google Scholar] [CrossRef] [Green Version]
- Mehrabi, P.; Shariati, M.; Kabirifar, K.; Jarrah, M.; Rasekh, H.; Trung, N.T.; Shariati, A.; Jahandari, S. Effect of pumice powder and nano-clay on the strength and permeability of fiber-reinforced pervious concrete incorporating recycled concrete aggregate. Constr. Build. Mater. 2021, 287, 122652. [Google Scholar] [CrossRef]
- Salemi, N.; Behfarnia, K. Effect of nano-particles on durability of fiber-reinforced concrete pavement. Constr. Build. Mater. 2013, 48, 934–941. [Google Scholar] [CrossRef]
- Roychand, R.; Li, J.; De Silva, S.; Saberian, M.; Law, D.; Pramanik, B.K. Development of zero cement composite for the protection of concrete sewage pipes from corrosion and fatbergs. Resour. Conserv. Recycl. 2021, 164, 105166. [Google Scholar] [CrossRef]
- Kawashima, S.; Hou, P.; Corr, D.; Shah, S.P. Modification of cement-based materials with nanoparticles. Cem. Concr. Compos. 2013, 36, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Torgal, F.; Jalali, S. Nanotechnology: Advantages and drawbacks in the field of construction and building materials. Constr. Build. Mater. 2011, 25, 582–590. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, F.; Sobolev, K. Nanotechnology in concrete—A review. Constr. Build. Mater. 2010, 24, 2060–2071. [Google Scholar] [CrossRef]
- Keshavarzian, F.; Saberian, M.; Li, J. Investigation on mechanical properties of steel fiber reinforced reactive powder concrete containing nano-SiO2: An experimental and analytical study. J. Build. Eng. 2021, 44, 102601. [Google Scholar] [CrossRef]
- Said, A.; Zeidan, M.; Bassuoni, M.; Tian, Y. Properties of concrete incorporating nano-silica. Constr. Build. Mater. 2012, 36, 838–844. [Google Scholar] [CrossRef]
- Tyson, B.M.; Abu Al-Rub, R.; Yazdanbakhsh, A.; Grasley, Z.C. Carbon Nanotubes and Carbon Nanofibers for Enhancing the Mechanical Properties of Nanocomposite Cementitious Materials. J. Mater. Civ. Eng. 2011, 23, 1028–1035. [Google Scholar] [CrossRef]
- Yazdanbakhsh, A.; Grasley, Z.; Tyson, B.; Abu Al-Rub, R. Distribution of Carbon Nanofibers and Nanotubes in Cementitious Composites. Transp. Res. Rec. J. Transp. Res. Board 2010, 2142, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Toghroli, A.; Mehrabi, P.; Shariati, M.; Trung, N.T.; Jahandari, S.; Rasekh, H. Evaluating the use of recycled concrete aggregate and pozzolanic additives in fiber-reinforced pervious concrete with industrial and recycled fibers. Constr. Build. Mater. 2020, 252, 118997. [Google Scholar] [CrossRef]
- Lv, S.; Ma, Y.; Qiu, C.; Sun, T.; Liu, J.; Zhou, Q. Effect of graphene oxide nanosheets of microstructure and mechanical properties of cement composites. Constr. Build. Mater. 2013, 49, 121–127. [Google Scholar] [CrossRef]
- Makar, J.M.; Chan, G.W. Growth of Cement Hydration Products on Single-Walled Carbon Nanotubes. J. Am. Ceram. Soc. 2009, 92, 1303–1310. [Google Scholar] [CrossRef]
- Metaxa, Z.S.; Konsta-Gdoutos, M.S.; Shah, S.P. Carbon nanofiber cementitious composites: Effect of debulking procedure on dispersion and reinforcing efficiency. Cem. Concr. Compos. 2013, 36, 25–32. [Google Scholar] [CrossRef]
- Chuah, S.; Pan, Z.; Sanjayan, J.; Wang, C.; Duan, W.H. Nano reinforced cement and concrete composites and new perspective from graphene oxide. Constr. Build. Mater. 2014, 73, 113–124. [Google Scholar] [CrossRef]
- Xu, G.; Shi, X. Environmentally Friendly Pervious Concrete for Treating Deicer-Laden Stormwater (Phase II Final Report); Center for Environmentally Sustainable Transportation in Cold Climates, The University of Alaska: Fairbanks, AK, USA, 2018. [Google Scholar]
- Ayanda, O.S.; Nelana, S.M.; Naidoo, E.B. Ultrasonic degradation of aqueous phenolsulfonphthalein (PSP) in the presence of nano-Fe/H2O2. Ultrason. Sonochem. 2018, 47, 29–35. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, R.; Pang, L.X.; Zhang, W.; Tan, X. Study on Photo-catalytic Efficiency and Durability of Nano-TiO2 in Permeable Concrete Pavement Structure. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Beijing, China, 20–22 September 2019; Volume 371. [Google Scholar]
- Tota-Maharaj, K.; Coleman, N. Developing Novel Photocatalytic Cementitious Permeable Pavements for Depollution of Contaminants and Impurities in Urban Cities. In Proceedings of the 10th International Conference Environmental Engineering, Vilnius, Lithuania, 27–28 April 2017. [Google Scholar]
- AlShareedah, O.; Nassiri, S. Pervious concrete mixture optimization, physical, and mechanical properties and pavement design: A review. J. Clean. Prod. 2021, 288, 125095. [Google Scholar] [CrossRef]
- ASTM, C. Standard Specification For Concrete Aggregates; American Society for Testing and Materials: Philadelphia, PA, USA, 2003. [Google Scholar]
- Meininger, R.C. No-fines pervious concrete for paving. Concr. Eng. Int. 1988, 10, 20–27. [Google Scholar]
- Standard, A. C150-07. Standard Specification for Portland Cement; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- ASTM, E. 647: Standard test method for measurement of fatigue crack growth rates. In Annual Book of ASTM Standards; West ASTM International: Conshohocken, PA, USA, 2011.
- ASTM. Standard Test Method for Ash in Biomass; ASTM International West: Conshohocken, PA, USA, 2001. [Google Scholar]
- Huang, B.; Wu, H.; Shu, X.; Burdette, E.G. Laboratory evaluation of permeability and strength of polymer-modified pervious concrete. Constr. Build. Mater. 2010, 24, 818–823. [Google Scholar] [CrossRef]
- Kayhanian, M.; Anderson, D.; Harvey, J.T.; Jones, D.; Muhunthan, B. Permeability measurement and scan imaging to assess clogging of pervious concrete pavements in parking lots. J. Environ. Manag. 2012, 95, 114–123. [Google Scholar] [CrossRef]
- Sonebi, M.; Bassuoni, M. Investigating the effect of mixture design parameters on pervious concrete by statistical modelling. Constr. Build. Mater. 2013, 38, 147–154. [Google Scholar] [CrossRef]
- Sumanasooriya, M.S.; Deo, O.; Neithalath, N. Particle Packing-Based Material Design Methodology for Pervious Concretes. ACI Mater. J. 2012, 109. [Google Scholar]
- Sumanasooriya, M.S.; Neithalath, N. Stereology-and Morphology-Based Pore Structure Descriptors of Enhanced Po-rosity (Pervious) Concretes. ACI Mater. J. 2009, 106. [Google Scholar]
- Mahboub, K.C.; Canler, J.; Rathbone, R.; Robl, T.; Davis, B. Pervious concrete: Compaction and aggregate gradation. ACI Mater. J. 2009, 106, 523. [Google Scholar]
- Tan, L.; Wang, J.; Liu, Q.; Sun, Y.; Jing, X.; Liu, L.; Liu, J.; Song, D. The synthesis of a manganese dioxide–iron oxide–graphene magnetic nanocomposite for enhanced uranium(vi) removal. New J. Chem. 2014, 39, 868–876. [Google Scholar] [CrossRef]
- Putman, B.J.; Neptune, A.I. Comparison of test specimen preparation techniques for pervious concrete pavements. Constr. Build. Mater. 2011, 25, 3480–3485. [Google Scholar] [CrossRef]
- Mousavi, S.-F.; Karami, H.; Farzin, S.; Teymouri, E. Effects of adding mineral adsorbents to porous concrete for enhancing the quality performance of urban runoff systems. World J. Eng. 2018, 15, 489–497. [Google Scholar] [CrossRef]
- Montes, F.; Haselbach, L. Measuring Hydraulic Conductivity in Pervious Concrete. Environ. Eng. Sci. 2006, 23, 960–969. [Google Scholar] [CrossRef]
- Rezania, M.; Panahandeh, M.; Razavi, S.; Berto, F. Experimental study of the simultaneous effect of nano-silica and nano-carbon black on permeability and mechanical properties of the concrete. Theor. Appl. Fract. Mech. 2019, 104, 102391. [Google Scholar] [CrossRef]
- Vázquez-Rivera, N.I. Statistical Optimization Of Pervious Concrete Pavement Containing Fly Ash And Engineered Iron Oxide Nanoparticles For Runoff Quality And Quantity Controls. Ph.D. Thesis, University of Puerto Rico Mayagüez Campus, Mayagüez, PR, USA, 2014. [Google Scholar]
- Beecham, S.; Pezzaniti, D.; Kandasamy, J. Stormwater treatment using permeable pavements. In Proceedings of the Water Management; Thomas Telford Ltd.: London, UK, 2012; Volume 165, pp. 161–170. [Google Scholar]
- Imran, H.; Akib, S.; Karim, M.R. Permeable pavement and stormwater management systems: A review. Environ. Technol. 2013, 34, 2649–2656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, S.; Yanbe, M. Adsorption by and artificial release of zinc and lead from porous concrete for recycling of adsorbed zinc and lead and of porous concrete to reduce urban non-point heavy metal runoff. Chemosphere 2018, 197, 451–456. [Google Scholar] [CrossRef]
- Harada, S.; Komuro, Y. Decrease of non-point zinc runoff using porous concrete. Chemosphere 2010, 78, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Kara, I.; Tunc, D.; Sayin, F.; Akar, S.T. Study on the performance of metakaolin based geopolymer for Mn(II) and Co(II) removal. Appl. Clay Sci. 2018, 161, 184–193. [Google Scholar] [CrossRef]
- Ge, Y.; Cui, X.; Kong, Y.; Li, Z.; He, Y.; Zhou, Q. Porous geopolymeric spheres for removal of Cu(II) from aqueous solution: Synthesis and evaluation. J. Hazard. Mater. 2015, 283, 244–251. [Google Scholar] [CrossRef]
- Kara, I.; Yilmazer, D.; Akar, S.T. Metakaolin based geopolymer as an effective adsorbent for adsorption of zinc(II) and nickel(II) ions from aqueous solutions. Appl. Clay Sci. 2017, 139, 54–63. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.S.; Al Zboon, K.; Al-Makhadmeh, L.; Hararah, M.; Mahasneh, M. Fly ash based geopolymer for heavy metal removal: A case study on copper removal. J. Environ. Chem. Eng. 2015, 3, 1669–1677. [Google Scholar] [CrossRef]
- Neithalath, N.; Sumanasooriya, M.S.; Deo, O. Characterizing pore volume, sizes, and connectivity in pervious concretes for permeability prediction. Mater. Charact. 2010, 61, 802–813. [Google Scholar] [CrossRef]
- Sansalone, J.; Kuang, X.; Ranieri, V. Permeable Pavement as a Hydraulic and Filtration Interface for Urban Drainage. J. Irrig. Drain. Eng. 2008, 134, 666–674. [Google Scholar] [CrossRef]
- Chen, X.; Niu, Z.; Zhang, H.; Lu, M.; Lu, Y.; Zhou, M.; Li, B. Design of a chitosan modifying alkali-activated slag pervious concrete with the function of water purification. Constr. Build. Mater. 2020, 251, 118979. [Google Scholar] [CrossRef]
- Selbig, W.R.; Buer, N.; Danz, M.E. Stormwater-quality performance of lined permeable pavement systems. J. Environ. Manag. 2019, 251, 109510. [Google Scholar] [CrossRef]
- Jang, Y.-I.; Lee, B.-J.; Lee, J.-W. Strength and Water Purification Properties of Environment-Friendly Construction Material Produced with the (D)PAOs and Zeolite. Appl. Sci. 2019, 9, 972. [Google Scholar] [CrossRef] [Green Version]
- Muthu, M.; Santhanam, M.; Kumar, M. Pb removal in pervious concrete filter: Effects of accelerated carbonation and hydraulic retention time. Constr. Build. Mater. 2018, 174, 224–232. [Google Scholar] [CrossRef]
- Kandra, H.; McCarthy, D.T.; Deletic, A.; Zhang, K. Modelling the clogging of a field filtration system used for stormwater harvesting. Environ. Sci. Water Res. Technol. 2020, 6, 993–1003. [Google Scholar] [CrossRef]
- Debnath, B.; Sarkar, P.P. Pervious concrete as an alternative pavement strategy: A state-of-the-art review. Int. J. Pavement Eng. 2018, 21, 1516–1531. [Google Scholar] [CrossRef]
- Ferguson, B. Porous Pavements; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- ACI Committee 522R. Report on Pervious Concrete, American Concrete Institute Committee 522. In Technical Committee Document; American Concrete Institute: Farmington Hills, MI, USA, 2010. [Google Scholar]
- Chopra, M.; Kakuturu, S.; Ballock, C.; Spence, J.; Wanielista, M. Effect of Rejuvenation Methods on the Infiltration Rates of Pervious Concrete Pavements. J. Hydrol. Eng. 2010, 15, 426–433. [Google Scholar] [CrossRef]
- Schaefer, V.R.; Wang, K. Mix design development for pervious concrete in cold weather climates. Sponsored by Iowa Department of Transportation. Highway Division. 2006. Available online: https://www.perviouspavement.org/downloads/Iowa.pdf (accessed on 1 July 2021).
- Tong, B. Clogging Effects of Portland Cement Pervious Concrete. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2018. [Google Scholar]
- Li, X.; Wang, B.; Cao, Y.; Zhao, S.; Wang, H.; Feng, X.; Zhou, J.; Ma, X. Water Contaminant Elimination Based on Metal–Organic Frameworks and Perspective on Their Industrial Applications. ACS Sustain. Chem. Eng. 2019, 7, 4548–4563. [Google Scholar] [CrossRef]
- Wang, C.; Feng, Y.; Zhao, S.; Li, B.-L. A dynamic contaminant fate model of organic compound: A case study of Nitrobenzene pollution in Songhua River, China. Chemosphere 2012, 88, 69–76. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da’Na, E. Adsorption of heavy metals on functionalized-mesoporous silica: A review. Microporous Mesoporous Mater. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Show, P.-L.; Lau, B.F.; Chang, J.-S.; Ling, T.C. New Prospects for Modified Algae in Heavy Metal Adsorption. Trends Biotechnol. 2019, 37, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Parihar, R.; Sharma, A.; Bakshi, P.; Sidhu, G.P.S.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.; Gyasi-Agyei, Y.; et al. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef]
- Smith, V.; Tilman, G.; Nekola, J. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef]
- Hathaway, J.M.; Hunt, W.F.; Graves, A.K.; Bass, K.L.; Caldwell, A. Exploring fecal indicator bacteria in a constructed stormwater wetland. Water Sci. Technol. 2011, 63, 2707–2712. [Google Scholar] [CrossRef] [PubMed]
- Vitro, K.A.; BenDor, T.K.; Jordanova, T.V.; Miles, B. A geospatial analysis of land use and stormwater management on fecal coliform contamination in North Carolina streams. Sci. Total Environ. 2017, 603-604, 709–727. [Google Scholar] [CrossRef]
- Senzia, M.; Mashauri, D.; Mayo, A. Suitability of constructed wetlands and waste stabilisation ponds in wastewater treatment: Nitrogen transformation and removal. Phys. Chem. Earth Parts A/B/C 2003, 28, 1117–1124. [Google Scholar] [CrossRef]
- Dodds, W.K.; Whiles, M.R. Quality and Quantity of Suspended Particles in Rivers: Continent-Scale Patterns in the United States. Environ. Manag. 2004, 33, 355–367. [Google Scholar] [CrossRef]
- Farrell, C.; Hassard, F.; Jefferson, B.; Leziart, T.; Nocker, A.; Jarvis, P. Turbidity composition and the relationship with microbial attachment and UV inactivation efficacy. Sci. Total Environ. 2018, 624, 638–647. [Google Scholar] [CrossRef]
- Kehoe, S.C. Effect of agitation, turbidity, aluminium foil reflectors and container volume on the inactivation efficiency of batch-process solar disinfectors. Water Res. 2001, 35, 1061–1065. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Fach, S.; Geiger, W. Effective pollutant retention capacity of permeable pavements for infiltrated road runoffs determined by laboratory tests. Water Sci. Technol. 2005, 51, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Aldea, C.-M.; Song, W.-J.; Popovics, J.S.; Shah, S.P. Extent of Healing of Cracked Normal Strength Concrete. J. Mater. Civ. Eng. 2000, 12, 92–96. [Google Scholar] [CrossRef]
- Andersen, J. An ignition method for determination of total phosphorus in lake sediments. Water Res. 1976, 10, 329–331. [Google Scholar] [CrossRef]
- Abbott, C.L.; Comino-Mateos, L. In-situ hydraulic performance of a permeable pavement sustainable urban drainage system. Water Environ. J. 2003, 17, 187–190. [Google Scholar] [CrossRef]
- Qian Chun-xiang, L. Study on the kinetic model of photocatalytic reaction of nano-titanium dioxide loaded on ce-ment-based materials. J. Saf. Environ. Eng. Sci. 2005, 25, 60–64. [Google Scholar]
- Pacheco-Torgal, F.; Miraldo, S.; Ding, Y.; Labrincha, J. Targeting HPC with the help of nanoparticles: An overview. Constr. Build. Mater. 2013, 38, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Heikal, M.; El Aleem, S.; Morsi, W.M. Characteristics of blended cements containing nano-silica. HBRC J. 2013, 9, 243–255. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Jiang, G. Experimental study on properties of pervious concrete pavement materials. Cem. Concr. Res. 2003, 33, 381–386. [Google Scholar] [CrossRef]
- Senff, L.; Labrincha, J.A.; Ferreira, V.M.; Hotza, D.; Repette, W.L. Effect of nano-silica on rheology and fresh properties of cement pastes and mortars. Constr. Build. Mater. 2009, 23, 2487–2491. [Google Scholar] [CrossRef]
- Al-Qahtani, K.M. Cadmium removal from aqueous solution by green synthesis zero valent silver nanoparticles with Benjamina leaves extract. Egypt. J. Aquat. Res. 2017, 43, 269–274. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Yang, L.; Han, M.; Zhao, J.; Cheng, X. Nanomaterials as Sorbents to Remove Heavy Metal Ions in Wastewater Treatment. J. Environ. Anal. Toxicol. 2012, 2, 154. [Google Scholar] [CrossRef]
- Suman; Kardam, A.; Gera, M.; Jain, V. A novel reusable nanocomposite for complete removal of dyes, heavy metals and microbial load from water based on nanocellulose and silver nano-embedded pebbles. Environ. Technol. 2014, 36, 706–714. [Google Scholar] [CrossRef]
- Rengga, W.D.P.; Chafidz, A.; Sudibandriyo, M.; Nasikin, M.; Abasaeed, A.E. Silver nano-particles deposited on bamboo-based activated carbon for removal of formaldehyde. J. Environ. Chem. Eng. 2017, 5, 1657–1665. [Google Scholar] [CrossRef]
- Ramsey, A.J. The Evaluation of Permeable Cementitious Media for Nutrient Removal. Master’s Thesis, University of Missouri-Kansas City, Kansas City, MI, USA, 2017. [Google Scholar]
- Doudrick, K.; Monzón, O.; Mangonon, A.; Hristovski, K.; Westerhoff, P. Nitrate Reduction in Water Using Commercial Titanium Dioxide Photocatalysts (P25, P90, and Hombikat UV100). J. Environ. Eng. 2012, 138, 852–861. [Google Scholar] [CrossRef]
- Dylla, H.L. Quantification of The Environmental Impact of Titanium Dioxide Photocatalytic Pavements For Air Pollution Remediation. Ph.D. Thesis, Louisiana State University, Baton Rouge, LA, USA, 2013. [Google Scholar]
- Okano, K.; Uemoto, M.; Kagami, J.; Miura, K.; Aketo, T.; Toda, M.; Honda, K.; Ohtake, H. Novel technique for phosphorus recovery from aqueous solutions using amorphous calcium silicate hydrates (A-CSHs). Water Res. 2013, 47, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lin, J.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesized iron nanoparticles by green tea and eucalyptus leaves extracts used for removal of nitrate in aqueous solution. J. Clean. Prod. 2014, 83, 413–419. [Google Scholar] [CrossRef]
- An, Y.-J.; Kampbell, D.H.; Breidenbach, G.P. Escherichia coli and total coliforms in water and sediments at lake marinas. Environ. Pollut. 2002, 120, 771–778. [Google Scholar] [CrossRef]





| Aggregates | Bulk Density (kg/m3) | Dry Density (kg/m3) | Saturated Density (kg/m3) | Water Absorption (%) | Ref. |
|---|---|---|---|---|---|
| Scoria | 2150 | 1710 | 1910 | 11.90 | [133] |
| Limestone | 1460, 2510 | 1530 | 2550 | 2.58, 1.8 | [141,142,143] |
| Basalt | 1500 | 2230 | 2670 | 1.20 | [139,144,145] |
| Size Number (Nominal Size) | Amounts Finer than Each Laboratory Sieve, Mass Percent | |||||||
|---|---|---|---|---|---|---|---|---|
| 25 mm | 19.0 mm | 12.50 mm | 9.50 mm | 4.75 mm | 2.36 mm | 1.18 mm | 300 µm | |
| #67 (19.0 to 4.75 mm) | 100 | 90–100 | - | 20–55 | 0–10 | 0–5 | - | - |
| #7 (12.5 to 4.75 mm) | - | 100 | 90–100 | 40–70 | 0–15 | 0–5 | - | - |
| #8 (9.5 to 2.36 mm) | - | - | 100 | 85–100 | 10–30 | 0–10 | 0–5 | - |
| #89 (9.5 to 1.18 mm) | - | - | 100 | 90–100 | 20–55 | 5–30 | 0–10 | 0–5 |
| Details | Proportions in kg/m3 |
|---|---|
| Cementitious materials | 270 to 415 |
| Aggregate | 1190 to 1480 |
| w/c ratio, by mass | 0.27 to 0.34 |
| Aggregate: cement, by mass | 4 to 4.50:1 |
| Fine: coarse aggregate, by mass | 0 to 1:1 |
| Sample Number | nTiO2 Application Methods | TiO2 Solution (g/L) | W/C | Material Contents (%) | ||
|---|---|---|---|---|---|---|
| Cement | Water | Aggregate | ||||
| 1 | Dipping | 0.50 | ||||
| 2 | 1.00 | |||||
| 3 | 1.50 | |||||
| 4 | Painting | 0.50 | ||||
| 5 | 1.00 | 0.31 | 15.80 | 4.90 | 69.30 | |
| 6 | 1.50 | |||||
| 7 | Soaking | 0.50 | ||||
| 8 | 1.00 | |||||
| 9 | 1.50 | |||||
| Nanoparticle | Dose | Permeability (CM) + (mm/s) | MCS ++ (CM) + (MPa) | Ref. |
|---|---|---|---|---|
| TiO2 | 0.5–1.5 (g/L) | 18.65–22.04 (18.16) | [11] | |
| 1.99–2.19 (2.21) | ||||
| Fe2O3 | 3 and 5% | −(8.39) | −(6) | [32] |
| Fe2O3 | 6% (NI/B *) | 5.60 (26.70) | 22.80 (13.90) | [55] |
| 0.5% (NI/B) | 7.60 (26.70) | 21.40 (13.90) | ||
| Fe | 30 (kg/m3) (6.30%) | 9.40 (15) | 16.20 (9.60) | [23] |
| SiO2 | 2.28 (kg/m3) (0.50%) | 8.80 (15) | 17.20 (9.60) | |
| ENPFe-surf | 5% (ENP/B) | 18.80 (11.5) | 10.41 (7.93) | [186] |
| Nanoparticle | Dose | Contaminant | Removal Rate (%) | Ref. |
|---|---|---|---|---|
| TiO2 | 1 (g) | NO3 | 100% | [237] |
| TiO2 | 0.5–1.5 (g/L) | MB | 60–90 | [11] |
| TP | 60–90 | |||
| AN | 60–90 | |||
| Fe2O3 | 3% | Fe | 84 | [32] |
| 5% | Fe | 87.7 | ||
| 3% | Mn | 46 | ||
| 5% | Mn | 58.79 | ||
| 3% | Pb | 76.8 | ||
| 5% | Pb | 78.4 | ||
| - | NH-3N | NE * | ||
| - | Phenol | NE | ||
| Fe2O3 | 6% (NI/B **) | FC | 72.4 | [55] |
| 6% (NI/B) | P | 49.8 | ||
| 0.5% (NI/B) | FC | 77.9 | ||
| 0.5% (NI/B) | P | 40.5 | ||
| Fe | 30 (kg/m3) (6.30%) | Turbidity | NE | |
| >80% | ||||
| FC | >80% | [23] | ||
| SiO2 | 2.28 (kg/m3) (0.50%) | Turbidity | NI *** | |
| >80% | ||||
| FC | >80% | |||
| ENPFe-surf | 5% (ENP/B) | Phosphate | Increased (7%) | [186] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alimohammadi, V.; Maghfouri, M.; Nourmohammadi, D.; Azarsa, P.; Gupta, R.; Saberian, M. Stormwater Runoff Treatment Using Pervious Concrete Modified with Various Nanomaterials: A Comprehensive Review. Sustainability 2021, 13, 8552. https://doi.org/10.3390/su13158552
Alimohammadi V, Maghfouri M, Nourmohammadi D, Azarsa P, Gupta R, Saberian M. Stormwater Runoff Treatment Using Pervious Concrete Modified with Various Nanomaterials: A Comprehensive Review. Sustainability. 2021; 13(15):8552. https://doi.org/10.3390/su13158552
Chicago/Turabian StyleAlimohammadi, Vahid, Mehdi Maghfouri, Delaram Nourmohammadi, Pejman Azarsa, Rishi Gupta, and Mohammad Saberian. 2021. "Stormwater Runoff Treatment Using Pervious Concrete Modified with Various Nanomaterials: A Comprehensive Review" Sustainability 13, no. 15: 8552. https://doi.org/10.3390/su13158552
APA StyleAlimohammadi, V., Maghfouri, M., Nourmohammadi, D., Azarsa, P., Gupta, R., & Saberian, M. (2021). Stormwater Runoff Treatment Using Pervious Concrete Modified with Various Nanomaterials: A Comprehensive Review. Sustainability, 13(15), 8552. https://doi.org/10.3390/su13158552