Performance of Anisole and Isobutanol as Gasoline Bio-Blendstocks for Spark Ignition Engines
Abstract
:1. Introduction
2. Research Background
2.1. Biocomponents in Gasoline
2.2. Anisole
2.3. Isobutanol
2.4. Merits of FFV
3. Materials and Methods
3.1. Tested Fuels
3.2. Experimental Engine Set-Up and Measurements
3.3. Modeling Performance of Ternary Blends in FFV
4. Results and Discussion
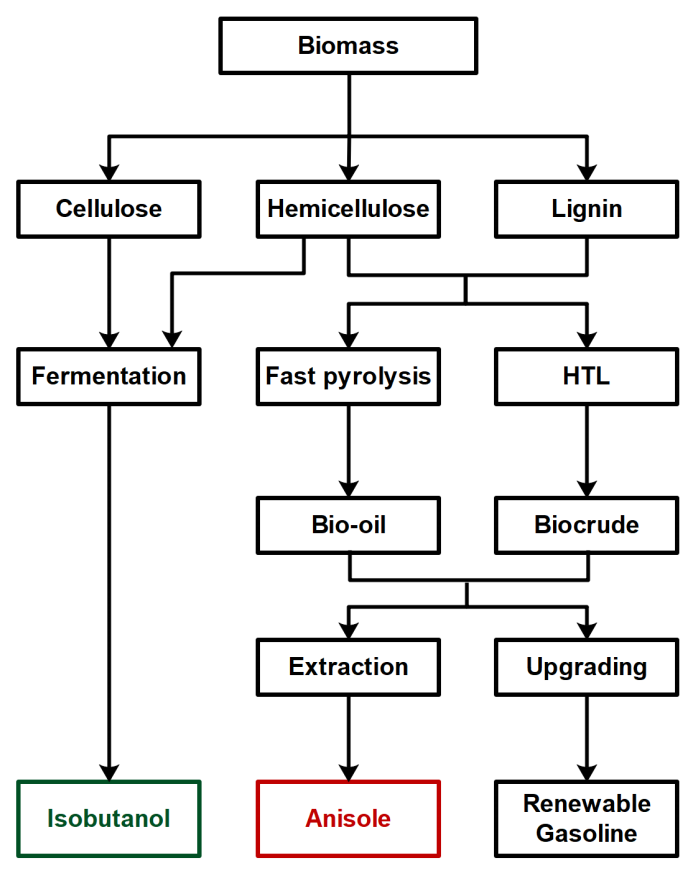
4.1. Lignocellulosic Biomass as a Precursor of SI Fuel Component
4.2. Experimental Results
4.3. Modeling Results for the FFV Fleet
4.4. GHG Emission Reductions and Cost Estimation
4.4.1. Anisole, HTL Biocrude, and Pyrolysis Bio-Oil
4.4.2. Isobutanol
4.4.3. Experimentally Tested Fuel Blends
5. Conclusions
- All selected blends performed well during experimental tests on the SI engine. No compatibility issues were detected, confirming the drop-in characteristic of all considered fuels. Fuels were stable and no phase separation was observed.
- In contrast to expectations, CO and HC emissions increased for oxygenated fuels, especially for alcohol blends. Nevertheless, the emissions of HC and CO and exhaust temperature were concluded to be in the range that can be accepted by TWC operation.
- Significantly higher BTE was observed for formulated blends when compared with base gasoline—on average, by 1.4%—which is in conformity with other studies.
- The potential of the anisole binary blend to decrease BSFC was reported. The estimated volumetric fuel consumption change (−1.8% for anisole binary, −1.4% for ternary and 4.6% in the case of the isobutanol binary blend) was better than expected based on LHV considerations.
- Tailpipe CO emissions from engine tests were reduced on average by 5.1% for anisole binary and ternary blends and 7.9% for the isobutanol binary blend when compared to base gasoline.
- From a fleet perspective, modeled FC and CO results were in line with experiments. An FFV with an optimized engine could benefit from the superior properties of anisole and isobutanol (high RON, oxygen content, heat of evaporation).
- The formulated blends can bring a decrease in GHG emissions, especially when looking at the WTW assessment. Significant reductions in GHG emissions were reported, ranging from 9.9 g CO-eq/MJ for the anisole binary blend to 20.2 g CO-eq/MJ for the isobutanol binary blend. These savings require sustainable lignocellulosic feedstock as well as advanced conversion processes such as hydrothermal liquefaction, fast pyrolysis, or fermentation.
- Further emission studies are needed to monitor particulates in exhaust gases from turbocharged direct injection SI engines. Additionally, extended compatibility studies are recommended, especially focusing on tests with elastomers.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMEP | Brake mean effective pressure |
| BSFC | Brake specific fuel consumption |
| BTE | Brake thermal effciency |
| CO | Carbon monoxide |
| CO | Carbon dioxide |
| CO-eq | Amount of carbon dioxide equivalent emissions |
| CR | Compression ratio |
| DI | Direct injection |
| E10 | EN228 compliant gasoline with up to 10% ethanol vol. content |
| E85 | High ethanol content gasoline for FFV |
| ECU | Engine control unit |
| EGR | Exhaust Gas Recirculation |
| EU | European Union |
| FC | Fuel consumption |
| FFV | Flexi-fuel vehicle |
| GHG | Greenhouse gases |
| HC | Unburned hydrocarbon emission |
| HTL | Hydro-thermal liquefaction |
| ICE | Internal combustion engine |
| LDV | Light-duty vehicle |
| LHV | Lower heating value |
| MFSP | Minimum fuel selling price |
| MON | Motor octane number |
| NECP | National Energy and Climate Plans |
| PFI | Port fuel injection |
| RED | Renewable Energy Directive |
| RON | Research octane number |
| rpm | Revolutions per minute |
| SI | Spark ignition |
| T50 | Temperature at which 50% of the sample is evaporated |
| TWC | Three-way catalyst |
| wt. | Weight |
| WTW | Well-to-wheel |
| Greek letters | |
| Volumetric fuel consumption | |
| CO emissions | |
| Fuel density | |
| Subscripts | |
| On mass basis | |
| On volumetric basis | |
| Symbols | |
| A | RON |
| B | Density |
| C | Carbon content |
| D | Volume-based LHV |
| H | Hydrogen content |
| O | Oxygen content |
| S | Sulfur content |
References
- International Energy Agency. Energy Technology Perspectives 2020; International Energy Agency: Paris, France, 2020. [Google Scholar]
- European Environment Agency. Monitoring CO2 Emissions from New Passenger Cars and Vans in 2018; European Environment Agency: København, Denmark, 2020. [Google Scholar]
- European Automobile Manufacturers Association. Vehicles in Use—Europe 2019; European Automobile Manufacturers Association: Brussels, Belgium, 2019. [Google Scholar]
- European Environment Agency. Transport: Increasing Oil Consumption and Greenhouse Gas Emissions Hamper EU Progress towards Environment and Climate Objectives; European Environment Agency: København, Denmark, 2019. [Google Scholar]
- Technology Collaboration Programme for International Energy Agency. Advanced Motor Fuels Annual Report 2019; Technology Collaboration Programme for International Energy Agency: Paris, France, 2019. [Google Scholar]
- Eurostat. Energy, Transport and Environment Statistics, 2019th ed.; Eurostat: Luxembourg, 2019. [Google Scholar]
- European Union. Regulation (EU) 2018/1999 of the European Parliament and of the Council of 11 December 2018 on the Governance of the Energy Union and Climate Action, amending Regulations (EC) No 663/2009 and (EC) No 715/2009 of the European Parliament and of the Council; Technical Report, Directives 94/22/EC, 98/70/EC, 2009/31/EC, 2009/73/EC, 2010/31/EU, 2012/27; European Union: Brussels, Belgium, 2018. [Google Scholar]
- Ministry of Economic Affairs and Employment. Finland’s Integrated Energy and Climate Plan; Ministry of Economic Affairs and Employment: Helsinki, Finland, 2019. [Google Scholar]
- Finnish Ministry of Justice. Act on the Promotion of the Use of Biofuels in Transport, Amendment 419/2019; Finnish Ministry of Justice: Helsinki, Finland, 2019. [Google Scholar]
- Red, I. Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Source; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- International Energy Agency. Global EV Outlook 2020: Entering the Decade of Electric Drive? International Energy Agency: Paris, France, 2020. [Google Scholar]
- European Automobile Manufacturers Association. New Passenger Car Registrations by Fuel Type in the EU, Quarter 2; European Automobile Manufacturers Association: Brussels, Belgium, 2020. [Google Scholar]
- Berger, R. Integrated Fuels and Vehicles Roadmap to 2030 and Beyond; Roland Berger GmbH: Munich, Germany, 2016. [Google Scholar]
- Van Dyk, S.; Su, J.; Mcmillan, J.D.; Saddler, J. Potential synergies of drop-in biofuel production with further co-processing at oil refineries. Biofuels Bioprod. Biorefining 2019, 13, 760–775. [Google Scholar] [CrossRef] [Green Version]
- Engman, A.; Hartikka, T.; Honkanen, M.; Kiiski, U.; Kuronen, L.; Lehto, K.; Mikkonen, S.; Nortio, J.; Nuottimäki, J.; Saikkonen, P. Neste Renewable Diesel Handbook; Neste Proprietary Publication: Espoo, Finland, 2016. [Google Scholar]
- Gaspar, D.J.; West, B.H.; Ruddy, D.; Wilke, T.J.; Polikarpov, E.; Alleman, T.L.; George, A.; Monroe, E.; Davis, R.W.; Vardon, D.; et al. Top Ten Blendstocks Derived From Biomass For Turbocharged Spark Ignition Engines: Bio-Blendstocks with Potential for Highest Engine Efficiency; Technical Report; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2019. [Google Scholar]
- Farrell, J.; Holladay, J.; Wagner, R. Fuel Blendstocks with the Potential to Optimize Future Gasoline Engine Performance: Identification of Five Chemical Families for Detailed Evaluation; US Department of Energy: Washington, DC, USA, 2018.
- McCormick, R.L.; Fioroni, G.; Fouts, L.; Christensen, E.; Yanowitz, J.; Polikarpov, E.; Albrecht, K.; Gaspar, D.J.; Gladden, J.; George, A. Selection criteria and screening of potential biomass-derived streams as fuel blendstocks for advanced spark-ignition engines. SAE Int. J. Fuels Lubr. 2017, 10, 442–460. [Google Scholar] [CrossRef]
- Miles, P. Efficiency Merit Function for Spark-Ignition Engines: Revision and Improvements Based on FY16-17 Research; US Department of Energy, Office of Energy Efficiency & Renewable Energy: Washington, DC, USA, 2018.
- Szybist, J.P.; Busch, S.; McCormick, R.L.; Pihl, J.A.; Splitter, D.A.; Ratcliff, M.A.; Kolodziej, C.P.; Storey, J.M.; Moses-DeBusk, M.; Vuilleumier, D.; et al. What fuel properties enable higher thermal efficiency in spark-ignited engines? Prog. Energy Combust. Sci. 2021, 82, 100876. [Google Scholar] [CrossRef]
- Ulonska, K.; König, A.; Klatt, M.; Mitsos, A.; Viell, J. Optimization of multiproduct biorefinery processes under consideration of biomass supply chain management and market developments. Ind. Eng. Chem. Res. 2018, 57, 6980–6991. [Google Scholar] [CrossRef]
- Dahmen, M.; Marquardt, W. Model-based formulation of biofuel blends by simultaneous product and pathway design. Energy Fuels 2017, 31, 4096–4121. [Google Scholar] [CrossRef]
- König, A.; Ulonska, K.; Mitsos, A.; Viell, J. Optimal applications and combinations of renewable fuel production from biomass and electricity. Energy Fuels 2019, 33, 1659–1672. [Google Scholar] [CrossRef]
- König, A.; Neidhardt, L.; Viell, J.; Mitsos, A.; Dahmen, M. Integrated design of processes and products: Optimal renewable fuels. Comput. Chem. Eng. 2020, 134, 106712. [Google Scholar] [CrossRef]
- Tews, I.J.; Zhu, Y.; Drennan, C.; Elliott, D.C.; Snowden-Swan, L.J.; Onarheim, K.; Solantausta, Y.; Beckman, D. Biomass Direct Liquefaction Options. In TechnoEconomic and Life Cycle Assessment; Technical Report; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2014. [Google Scholar]
- Rahzani, B.; Saidi, M.; Rahimpour, H.R.; Gates, B.C.; Rahimpour, M.R. Experimental investigation of upgrading of lignin-derived bio-oil component anisole catalyzed by carbon nanotube-supported molybdenum. RSC Adv. 2017, 7, 10545–10556. [Google Scholar] [CrossRef] [Green Version]
- Roussos, A.; Misailidis, N.; Koulouris, A.; Zimbardi, F.; Petrides, D. A Feasibility Study of Cellulosic Isobutanol Production—Process Simulation and Economic Analysis. Processes 2019, 7, 667. [Google Scholar] [CrossRef] [Green Version]
- Dunn, J.B.; Biddy, M.; Jones, S.; Cai, H.; Benavides, P.T.; Markham, J.; Tao, L.; Tan, E.; Kinchin, C.; Davis, R.; et al. Environmental, economic, and scalability considerations and trends of selected fuel economy-enhancing biomass-derived blendstocks. ACS Sustain. Chem. Eng. 2018, 6, 561–569. [Google Scholar] [CrossRef]
- Tao, L.; Tan, E.C.; McCormick, R.; Zhang, M.; Aden, A.; He, X.; Zigler, B.T. Techno-economic analysis and life-cycle assessment of cellulosic isobutanol and comparison with cellulosic ethanol and n-butanol. Biofuels Bioprod. Biorefining 2014, 8, 30–48. [Google Scholar] [CrossRef]
- Cai, H.; Markham, J.; Jones, S.; Benavides, P.T.; Dunn, J.B.; Biddy, M.; Tao, L.; Lamers, P.; Phillips, S. Techno-economic analysis and life-cycle analysis of two light-duty bioblendstocks: Isobutanol and aromatic-rich hydrocarbons. ACS Sustain. Chem. Eng. 2018, 6, 8790–8800. [Google Scholar] [CrossRef]
- Saidi, M.; Samimi, F.; Karimipourfard, D.; Nimmanwudipong, T.; Gates, B.C.; Rahimpour, M.R. Upgrading of lignin-derived bio-oils by catalytic hydrodeoxygenation. Energy Environ. Sci. 2014, 7, 103–129. [Google Scholar] [CrossRef]
- Bi, Z.; Zhang, J.; Peterson, E.; Zhu, Z.; Xia, C.; Liang, Y.; Wiltowski, T. Biocrude from pretreated sorghum bagasse through catalytic hydrothermal liquefaction. Fuel 2017, 188, 112–120. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Laskar, D.D.; Yang, B.; Wang, H.; Lee, J. Pathways for biomass-derived lignin to hydrocarbon fuels. Biofuels Bioprod. Biorefining 2013, 7, 602–626. [Google Scholar] [CrossRef]
- Ramirez, J.A.; Brown, R.J.; Rainey, T.J. A review of hydrothermal liquefaction bio-crude properties and prospects for upgrading to transportation fuels. Energies 2015, 8, 6765–6794. [Google Scholar] [CrossRef] [Green Version]
- Christensen, E.; Fioroni, G.M.; Kim, S.; Fouts, L.; Gjersing, E.; Paton, R.S.; McCormick, R.L. Experimental and theoretical study of oxidative stability of alkylated furans used as gasoline blend components. Fuel 2018, 212, 576–585. [Google Scholar] [CrossRef]
- Gschwend, D.; Soltic, P.; Wokaun, A.; Vogel, F. Review and performance evaluation of fifty alternative liquid fuels for spark-ignition engines. Energy Fuels 2019, 33, 2186–2196. [Google Scholar] [CrossRef]
- Singerman, G.M. Novel Anisole Mixture and Gasoline Containing the Same. U.S. Patent 4,312,636, 26 January 1982. [Google Scholar]
- Tian, M.; McCormick, R.L.; Ratcliff, M.A.; Luecke, J.; Yanowitz, J.; Glaude, P.A.; Cuijpers, M.; Boot, M.D. Performance of lignin derived compounds as octane boosters. Fuel 2017, 189, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Büttgen, R.; Tian, M.; Fenard, Y.; Minwegen, H.; Boot, M.; Heufer, K. An experimental, theoretical and kinetic modelling study on the reactivity of a lignin model compound anisole under engine-relevant conditions. Fuel 2020, 269, 117190. [Google Scholar] [CrossRef]
- Wu, Y.; Rossow, B.; Modica, V.; Yu, X.; Wu, L.; Grisch, F. Laminar flame speed of lignocellulosic biomass-derived oxygenates and blends of gasoline/oxygenates. Fuel 2017, 202, 572–582. [Google Scholar] [CrossRef] [Green Version]
- Szybist, J.P.; Splitter, D.A. Understanding chemistry-specific fuel differences at a constant RON in a boosted SI engine. Fuel 2018, 217, 370–381. [Google Scholar] [CrossRef]
- Ratcliff, M.A.; Burton, J.; Sindler, P.; Christensen, E.; Fouts, L.; Chupka, G.M.; McCormick, R.L. Knock resistance and fine particle emissions for several biomass-derived oxygenates in a direct-injection spark-ignition engine. SAE Int. J. Fuels Lubr. 2016, 9, 59–70. [Google Scholar] [CrossRef] [Green Version]
- da Silva Trindade, W.R.; dos Santos, R.G. Review on the characteristics of butanol, its production and use as fuel in internal combustion engines. Renew. Sustain. Energy Rev. 2017, 69, 642–651. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Mathimani, T.; Varjani, S.; Rene, E.R.; Kumar, G.; Kim, S.H.; Ponnusamy, V.K.; Yoon, J.J. Biobutanol as a promising liquid fuel for the future-recent updates and perspectives. Fuel 2019, 253, 637–646. [Google Scholar] [CrossRef]
- Al-Shorgani, N.K.N.; Shukor, H.; Abdeshahian, P.; Kalil, M.S.; Yusoff, W.M.W.; Hamid, A.A. Enhanced butanol production by optimization of medium parameters using Clostridium acetobutylicum YM1. Saudi J. Biol. Sci. 2018, 25, 1308–1321. [Google Scholar] [CrossRef] [Green Version]
- Baez, A.; Cho, K.M.; Liao, J.C. High-flux isobutanol production using engineered Escherichia coli: A bioreactor study with in situ product removal. Appl. Microbiol. Biotechnol. 2011, 90, 1681–1690. [Google Scholar] [CrossRef] [Green Version]
- Ryan, C. An Overview Of Gevo’s Biobased Isobutanol Production Process. 2021. Available online: https://gevo.com/wp-content/uploads/2021/05/Gevo-Whitepaper-%E2%80%93-Isobutanol-Production-Process.pdf (accessed on 8 May 2021).
- Aakko-Saksa, P.; Koponen, P.; Kihlman, J.; Reinikainen, M.; Skyttä, E.; Rantanen-Kolehmainen, L.; Engman, A. Biogasoline Options for Conventional Spark-Ignition Cars; VTT Technical Research Centre of Finland: Espoo, Finland, 2011. [Google Scholar]
- Irimescu, A. Fuel conversion efficiency of a port injection engine fueled with gasoline—Isobutanol blends. Energy 2011, 36, 3030–3035. [Google Scholar] [CrossRef]
- Rodríguez-Antón, L.; Gutiérrez-Martín, F.; Hernández-Campos, M. Physical properties of gasoline-ETBE-isobutanol (in comparison with ethanol) ternary blends and their impact on regulatory compliance. Energy 2019, 185, 68–76. [Google Scholar] [CrossRef]
- Stansfield, P.A.; Bisordi, A.; OudeNijeweme, D.; Williams, J.; Gold, M.; Ali, R. The Performance of a Modern Vehicle on a Variety of Alcohol-Gasoline Fuel Blends. SAE Int. J. Fuels Lubr. 2012, 5, 813–822. [Google Scholar] [CrossRef]
- Karavalakis, G.; Short, D.; Vu, D.; Russell, R.; Asa-Awuku, A.; Durbin, T. A complete assessment of the emissions performance of ethanol blends and iso-butanol blends from a fleet of nine PFI and GDI vehicles. SAE Int. J. Fuels Lubr. 2015, 8, 374–395. [Google Scholar] [CrossRef] [Green Version]
- Leone, T.G.; Anderson, J.E.; Davis, R.S.; Iqbal, A.; Reese, R.A.; Shelby, M.H.; Studzinski, W.M. The effect of compression ratio, fuel octane rating, and ethanol content on spark-ignition engine efficiency. Environ. Sci. Technol. 2015, 49, 10778–10789. [Google Scholar] [CrossRef]
- Balki, M.K.; Sayin, C. The effect of compression ratio on the performance, emissions and combustion of an SI (spark ignition) engine fueled with pure ethanol, methanol and unleaded gasoline. Energy 2014, 71, 194–201. [Google Scholar] [CrossRef]
- Akihisa, D.; Daisaku, S. Research on Improving Thermal Efficiency Through Variable Super-High Expansion Ratio Cycle; SAE Technical Report; SAE: Warrendale, PA, USA, 2010. [Google Scholar]
- Curran, J.M. Air/Fuel Control System for Flexible Fuel Vehicles. U.S. Patent 5,253,631, 19 October 1993. [Google Scholar]
- Deng, B.; Fu, J.; Zhang, D.; Yang, J.; Feng, R.; Liu, J.; Li, K.; Liu, X. The heat release analysis of bio-butanol/gasoline blends on a high speed SI (spark ignition) engine. Energy 2013, 60, 230–241. [Google Scholar] [CrossRef]
- Edwards, R.; Padella, M.; Giuntoli, J.; Koeble, R.; O’Connell, A.; Bulgheroni, C.; Marelli, L. Definition of Input Data to Assess GHG Default Emissions from Biofuels in EU Legislation; Version 1c–July; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar]
- Robert Bosch GmbH. Electronic Automotive Handbook; Robert Bosch GmbH: Stuttgart, Germany, 2002; Volume 1. [Google Scholar]
- Bielaczyc, P.; Woodburn, J.; Szczotka, A. Particulate emissions from European vehicles featuring direct injection spark ignition engines tested under laboratory conditions. SAE Int. J. Fuels Lubr. 2014, 7, 580–590. [Google Scholar] [CrossRef]
- Kroyan, Y.; Wojcieszyk, M.; Kaario, O.; Larmi, M.; Zenger, K. Modeling the end-use performance of alternative fuels in light-duty vehicles. Energy 2020, 205, 117854. [Google Scholar] [CrossRef]
- Anderson, J.; Kramer, U.; Mueller, S.; Wallington, T. Octane numbers of ethanol- and methanol- gasoline blends estimated from molar concentrations. Energy Fuels 2010, 24, 6576–6585. [Google Scholar] [CrossRef]
- Wojcieszyk, M.; Kroyan, Y.; Larmi, M.; Kaario, O.; Bani, A. End-use performance of alternative fuels in various modes of transportation. Adv. Proj. Deliv. D 2020, 5, 5. [Google Scholar]
- Zabed, H.; Sahu, J.; Boyce, A.N.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Bagnato, G.; Sanna, A.; Paone, E.; Catizzone, E. Recent Catalytic Advances in Hydrotreatment Processes of Pyrolysis Bio-Oil. Catalysts 2021, 11, 157. [Google Scholar] [CrossRef]
- Ahamed, T.S.; Anto, S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Upgrading of bio-oil from thermochemical conversion of various biomass—Mechanism, challenges and opportunities. Fuel 2020, 287, 119329. [Google Scholar] [CrossRef]
- de Rezende Pinho, A.; de Almeida, M.B.; Mendes, F.L.; Casavechia, L.C.; Talmadge, M.S.; Kinchin, C.M.; Chum, H.L. Fast pyrolysis oil from pinewood chips co-processing with vacuum gas oil in an FCC unit for second generation fuel production. Fuel 2017, 188, 462–473. [Google Scholar] [CrossRef] [Green Version]
- Castello, D.; Pedersen, T.H.; Rosendahl, L.A. Continuous hydrothermal liquefaction of biomass: A critical review. Energies 2018, 11, 3165. [Google Scholar] [CrossRef] [Green Version]
- Funkenbusch, L.T.; Mullins, M.E.; Vamling, L.; Belkhieri, T.; Srettiwat, N.; Winjobi, O.; Shonnard, D.R.; Rogers, T.N. Technoeconomic assessment of hydrothermal liquefaction oil from lignin with catalytic upgrading for renewable fuel and chemical production. Wiley Interdiscip. Rev. Energy Environ. 2019, 8, e319. [Google Scholar] [CrossRef] [Green Version]
- Funkenbusch, L.T.; Mullins, M.E.; Salam, M.A.; Creaser, D.; Olsson, L. Catalytic hydrotreatment of pyrolysis oil phenolic compounds over Pt/Al2O3 and Pd/C. Fuel 2019, 243, 441–448. [Google Scholar] [CrossRef]
- Wagnon, S.W.; Thion, S.; Nilsson, E.J.; Mehl, M.; Serinyel, Z.; Zhang, K.; Dagaut, P.; Konnov, A.A.; Dayma, G.; Pitz, W.J. Experimental and modeling studies of a biofuel surrogate compound: Laminar burning velocities and jet-stirred reactor measurements of anisole. Combust. Flame 2018, 189, 325–336. [Google Scholar] [CrossRef]
- Yakovlev, V.; Khromova, S.; Sherstyuk, O.; Dundich, V.; Ermakov, D.Y.; Novopashina, V.; Lebedev, M.Y.; Bulavchenko, O.; Parmon, V. Development of new catalytic systems for upgraded bio-fuels production from bio-crude-oil and biodiesel. Catal. Today 2009, 144, 362–366. [Google Scholar] [CrossRef]
- Si, Z.; Zhang, X.; Wang, C.; Ma, L.; Dong, R. An overview on catalytic hydrodeoxygenation of pyrolysis oil and its model compounds. Catalysts 2017, 7, 169. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, X.; Xu, Y.; Gao, X.; Dai, Y.; Tang, Y. Palladium-Incorporated α-MoC Mesoporous Composites for Enhanced Direct Hydrodeoxygenation of Anisole. Catalysts 2021, 11, 370. [Google Scholar] [CrossRef]
- Taghvaei, H.; Kheirollahivash, M.; Ghasemi, M.; Rostami, P.; Gates, B.C.; Rahimpour, M.R. Upgrading of anisole in a dielectric barrier discharge plasma reactor. Energy Fuels 2014, 28, 4545–4553. [Google Scholar] [CrossRef]
- Wang, H.; Feng, M.; Yang, B. Catalytic hydrodeoxygenation of anisole: An insight into the role of metals in transalkylation reactions in bio-oil upgrading. Green Chem. 2017, 19, 1668–1673. [Google Scholar] [CrossRef]
- Talvenmaa, P. Introduction to chromic materials. In Intelligent Textiles and Clothing; Woodhed Puhlishing in Textiles: Cambridge, UK, 2006; pp. 193–205. [Google Scholar]
- Durbin, T.D.; Karavalakis, G.; Norbeck, J.M.; Park, C.S.; Castillo, J.; Rheem, Y.; Bumiller, K.; Yang, J.; Van, V.; Hunter, K. Material compatibility evaluation for elastomers, plastics, and metals exposed to ethanol and butanol blends. Fuel 2016, 163, 248–259. [Google Scholar] [CrossRef] [Green Version]
- McCormick, R.L.; Ratcliff, M.A.; Christensen, E.; Fouts, L.; Luecke, J.; Chupka, G.M.; Yanowitz, J.; Tian, M.; Boot, M. Properties of oxygenates found in upgraded biomass pyrolysis oil as components of spark and compression ignition engine fuels. Energy Fuels 2015, 29, 2453–2461. [Google Scholar] [CrossRef] [Green Version]
- Vom Lehn, F.; Cai, L.; Tripathi, R.; Broda, R.; Pitsch, H. A property database of fuel compounds with emphasis on spark-ignition engine applications. Appl. Energy Combust. Sci. 2021, 5, 100018. [Google Scholar]
- Zervas, E.; Montagne, X.; Lahaye, J. Emissions of regulated pollutants from a spark ignition engine. Influence of fuel and air/fuel equivalence ratio. Environ. Sci. Technol. 2003, 37, 3232–3238. [Google Scholar] [CrossRef]
- Elfasakhany, A. State of art of using biofuels in spark ignition engines. Energies 2021, 14, 779. [Google Scholar] [CrossRef]
- Larsson, T.; Mahendar, S.K.; Christiansen-Erlandsson, A.; Olofsson, U. The Effect of Pure Oxygenated Biofuels on Efficiency and Emissions in a Gasoline Optimised DISI Engine. Energies 2021, 14, 3908. [Google Scholar] [CrossRef]
- Yusoff, M.; Zulkifli, N.; Masum, B.; Masjuki, H. Feasibility of bioethanol and biobutanol as transportation fuel in spark-ignition engine: A review. RSC Adv. 2015, 5, 100184–100211. [Google Scholar] [CrossRef]
- Göktaş, M.; Balki, M.K.; Sayin, C.; Canakci, M. An evaluation of the use of alcohol fuels in SI engines in terms of performance, emission and combustion characteristics: A review. Fuel 2021, 286, 119425. [Google Scholar] [CrossRef]
- Elfasakhany, A. Dual and Ternary Biofuel Blends for Desalination Process: Emissions and Heat Recovered Assessment. Energies 2021, 14, 61. [Google Scholar] [CrossRef]
- Bielaczyc, P.; Woodburn, J.; Klimkiewicz, D.; Pajdowski, P.; Szczotka, A. An examination of the effect of ethanol–gasoline blends’ physicochemical properties on emissions from a light-duty spark ignition engine. Fuel Process. Technol. 2013, 107, 50–63. [Google Scholar] [CrossRef]
- Regalbuto, C.; Pennisi, M.; Wigg, B.; Kyritsis, D. Experimental Investigation of Butanol Isomer Combustion in Spark Ignition Engines; SAE Technical Report; SAE: Warrendale, PA, USA, 2012. [Google Scholar]
- Nithyanandan, K.; Lee, C.F.F.; Wu, H.; Zhang, J. Performance and emissions of acetone-butanol-ethanol (ABE) and gasoline blends in a port fuel injected spark ignition engine. In Internal Combustion Engine Division Fall Technical Conference; American Society of Mechanical Engineers: New York, NY, USA, 2014; Volume 46162, p. V001T02A010. [Google Scholar]
- Dernotte, J.; Mounaïm-Rousselle, C.; Halter, F.; Seers, P. Evaluation of butanol–gasoline blends in a port fuel-injection, spark-ignition engine. Oil Gas Sci. Technol. Rev. L’Institut Français Pétrole 2010, 65, 345–351. [Google Scholar] [CrossRef]
- Masum, B.; Kalam, M.; Masjuki, H.; Palash, S.; Fattah, I.R. Performance and emission analysis of a multi cylinder gasoline engine operating at different alcohol—Gasoline blends. RSC Adv. 2014, 4, 27898–27904. [Google Scholar] [CrossRef]
- Sayin, C. The impact of varying spark timing at different octane numbers on the performance and emission characteristics in a gasoline engine. Fuel 2012, 97, 856–861. [Google Scholar] [CrossRef]
- Sayin, C.; Balki, M.K. Effect of compression ratio on the emission, performance and combustion characteristics of a gasoline engine fueled with iso-butanol/gasoline blends. Energy 2015, 82, 550–555. [Google Scholar] [CrossRef]
- Laurikko, J. On Exhaust Emissions from Petrol-Fuelled Passenger Cars at Low Ambient Temperatures; Technical Research Centre of Finland: Espoo, Finland, 1998. [Google Scholar]
- Meyer, P.A.; Snowden-Swan, L.J.; Jones, S.B.; Rappé, K.G.; Hartley, D.S. The effect of feedstock composition on fast pyrolysis and upgrading to transportation fuels: Techno-economic analysis and greenhouse gas life cycle analysis. Fuel 2020, 259, 116218. [Google Scholar] [CrossRef]
- Nie, Y.; Bi, X. Life-cycle assessment of transportation biofuels from hydrothermal liquefaction of forest residues in British Columbia. Biotechnol. Biofuels 2018, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Biddy, M.J.; Jones, S.B.; Elliott, D.C.; Schmidt, A.J. Techno-economic analysis of liquid fuel production from woody biomass via hydrothermal liquefaction (HTL) and upgrading. Appl. Energy 2014, 129, 384–394. [Google Scholar] [CrossRef]







| Base Gasoline | Anisole | Isobutanol | Anisole Binary Blend | Isobutanol Binary Blend | Ternary Blend | ||
|---|---|---|---|---|---|---|---|
| Energy content [%] | Gasoline | 100 | 0 | 0 | 85 | 70 | 70 |
| Anisole | 0 | 100 | 0 | 15 | 0 | 15 | |
| Isobutanol | 0 | 0 | 100 | 0 | 30 | 15 | |
| RON | 95 | 103 | 105 | 96.4 | 99.2 | 98.5 | |
| LHV [MJ/kg] | 43.6 | 32.7 | 33.4 | 41.5 | 39.9 | 39.8 | |
| LHV [MJ/L] | 32.6 | 32.4 | 26.8 | 32.5 | 30.5 | 31.5 | |
| Density [kg/m] | 747 | 990 | 802 | 784 | 764 | 791 | |
| Molecular Weight [g/mol] | 95.9 | 108.1 | 74.1 | 98 | 86.8 | 92.9 | |
| Oxygen [wt. %] | 0.1 | 14.8 | 21.6 | 2.9 | 7.8 | 6.6 | |
| Engine | Volvo B4204 |
|---|---|
| Engine type | 4 cylinders, 4 valves per cylinder |
| Displacement volume | 1.95 dm |
| Bore × stroke | 83 × 90 mm |
| Compression ratio | 10.2:1 |
| Power | 120 kW |
| Injection system | Port fuel injection (PFI) |
| Charge cooling | Air-cooled |
| Boosting system | Turbocharger Garret Gt3071 |
| EGR | No |
| Steady State | Engine Speed [rpm] | Load [Nm] | Load [% of max] | BMEP [bar] | Corresponding Vehicle Speed and Gear Number |
|---|---|---|---|---|---|
| 1 | 1550 | 23 | 10 | 1.5 | 50 km/h, 4th |
| 2 | 1470 | 33 | 14 | 2.1 | 60 km/h, 5th |
| 3 | 1700 | 38 | 16 | 2.4 | 70 km/h, 5th |
| 4 | 2000 | 43 | 18 | 2.7 | 80 km/h, 5th |
| 5 | 2500 | 54 | 23 | 3.5 | 100 km/h, 5th |
| 6 | 3000 | 70 | 29 | 4.6 | 120 km/h, 5th |
| Fuel | RON | MON | S | LHVvol MJ/L | LHVmass MJ/kg | Density g/L | O % wt. | C % wt. | H % wt. | AMW g/mol |
|---|---|---|---|---|---|---|---|---|---|---|
| Gasoline | 95.0 | 84.8 | 10.2 | 32.6 | 43.6 | 747.3 | 0.1 | 86.4 | 13.6 | 95.9 |
| Isobutanol | 105.0 | 90.0 | 15.0 | 26.8 | 33.4 | 802.0 | 21.6 | 64.8 | 13.6 | 74.1 |
| Anisole | 103.0 | 91.0 | 12.0 | 32.4 | 32.7 | 990.0 | 14.8 | 77.7 | 7.5 | 108.1 |
| Conversion Process | Feedstock | GHG Emissions [g CO2-eq/MJ] | Reference |
|---|---|---|---|
| Fast pyrolysis with upgrading of bio-oil | Logging residues and forest thinnings | 33.8 | Tews et al. [25] |
| Strand board | 26.1 | Meyer et al. [97] | |
| Corn stover | 29.9 | ||
| Clean pine | 42.4 | ||
| Switchgrass | 36.9 | ||
| Hydrothermal liquefaction process with upgrading to HTL gasoline | Forest residues (small branches) | 27.2 | Tews et al. [25] |
| Forest residues | 17–20.5 | Nie et al. [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojcieszyk, M.; Knuutila, L.; Kroyan, Y.; de Pinto Balsemão, M.; Tripathi, R.; Keskivali, J.; Karvo, A.; Santasalo-Aarnio, A.; Blomstedt, O.; Larmi, M. Performance of Anisole and Isobutanol as Gasoline Bio-Blendstocks for Spark Ignition Engines. Sustainability 2021, 13, 8729. https://doi.org/10.3390/su13168729
Wojcieszyk M, Knuutila L, Kroyan Y, de Pinto Balsemão M, Tripathi R, Keskivali J, Karvo A, Santasalo-Aarnio A, Blomstedt O, Larmi M. Performance of Anisole and Isobutanol as Gasoline Bio-Blendstocks for Spark Ignition Engines. Sustainability. 2021; 13(16):8729. https://doi.org/10.3390/su13168729
Chicago/Turabian StyleWojcieszyk, Michał, Lotta Knuutila, Yuri Kroyan, Mário de Pinto Balsemão, Rupali Tripathi, Juha Keskivali, Anna Karvo, Annukka Santasalo-Aarnio, Otto Blomstedt, and Martti Larmi. 2021. "Performance of Anisole and Isobutanol as Gasoline Bio-Blendstocks for Spark Ignition Engines" Sustainability 13, no. 16: 8729. https://doi.org/10.3390/su13168729







