The Direct Reduction of Iron Ore with Hydrogen
Abstract
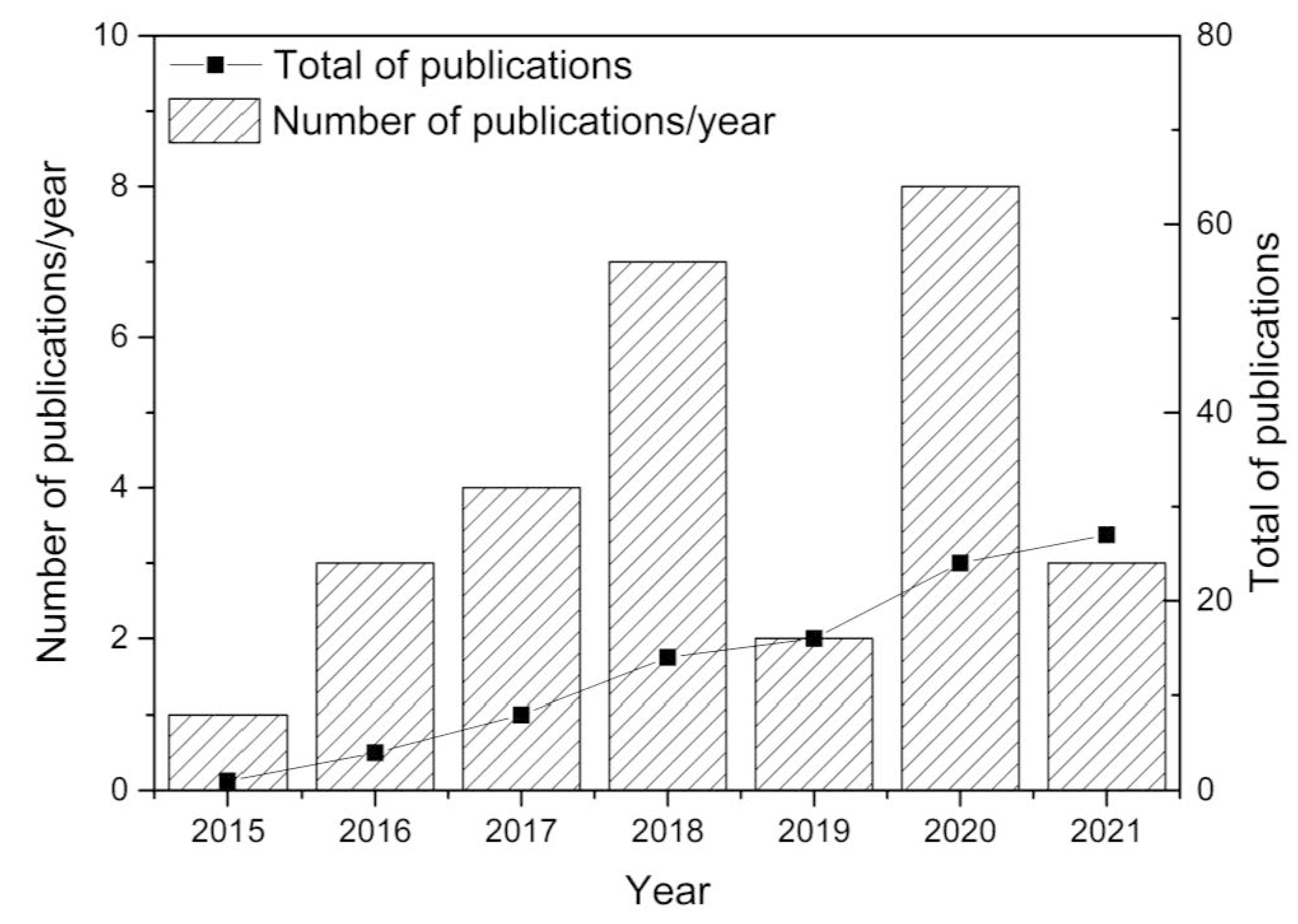
:1. Introduction
1.1. Background Information
1.2. Objectives, Structure and Contribution of the Reported Research
2. Materials and Methods
2.1. Materials
2.2. Methods and Procedures
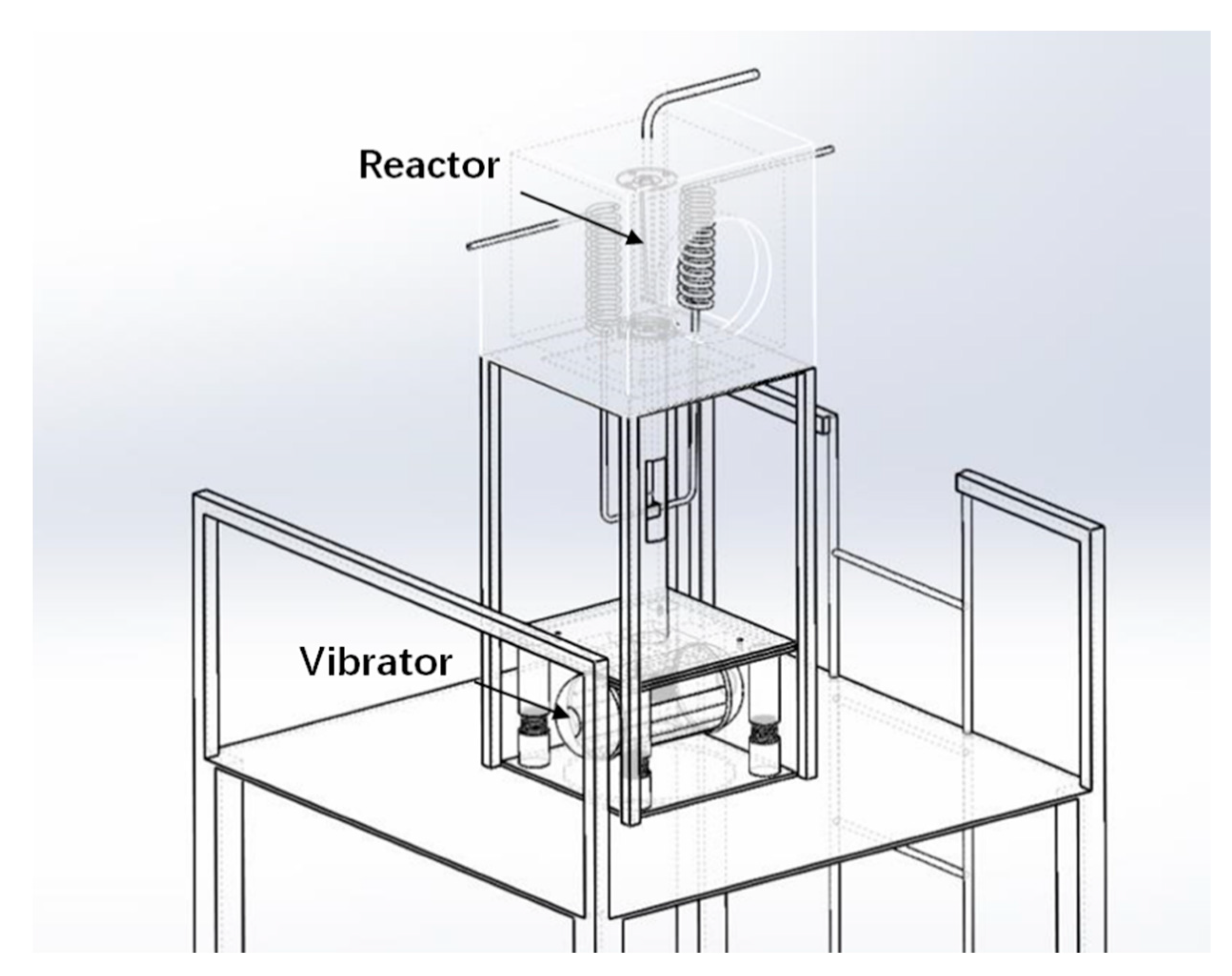
2.2.1. Experimental Set-Ups
2.2.2. Data Treatment
3. Results and Discussion
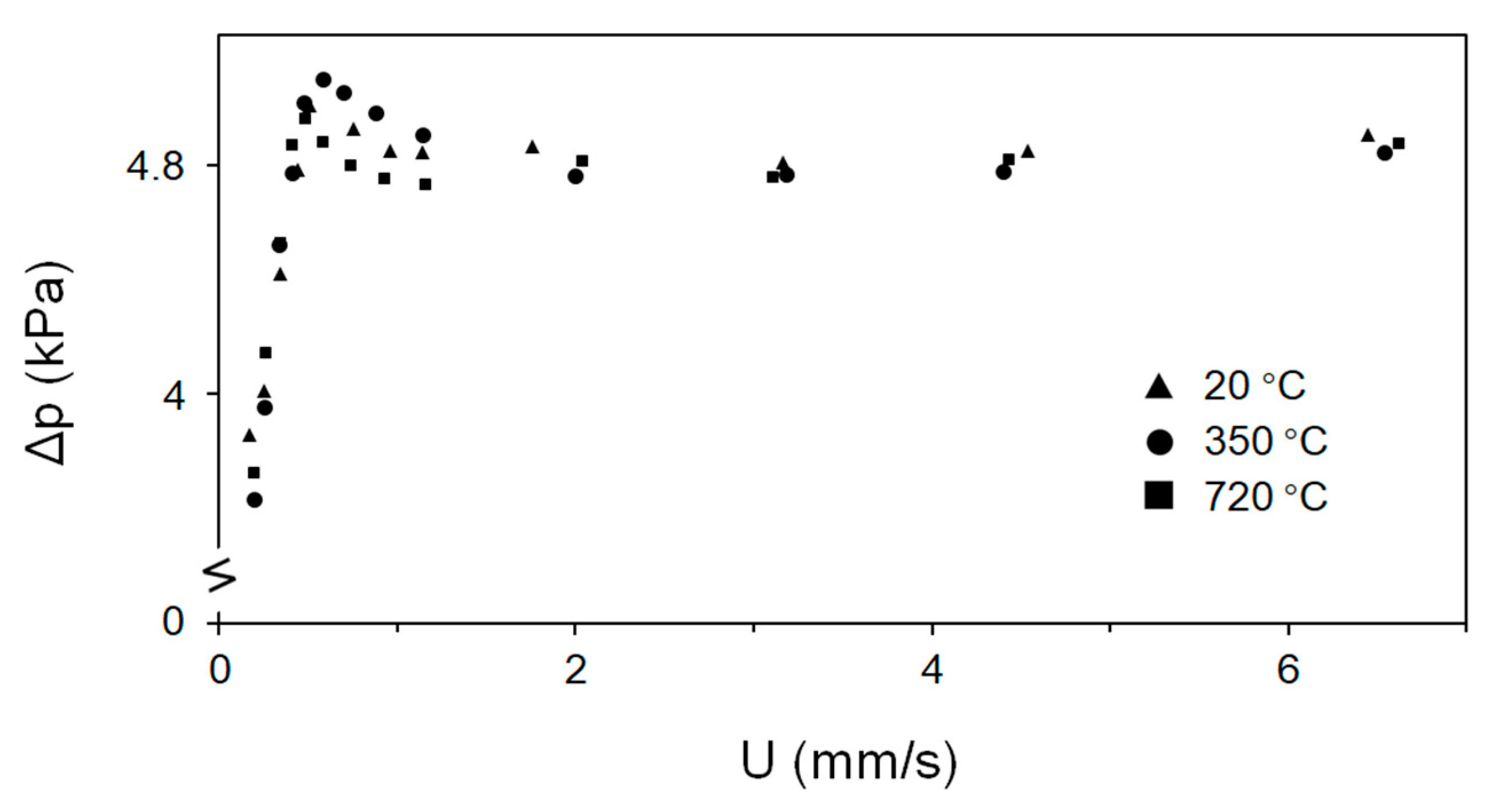
3.1. The Fluidization Characteristics of the Fine Fe2O3 Particles
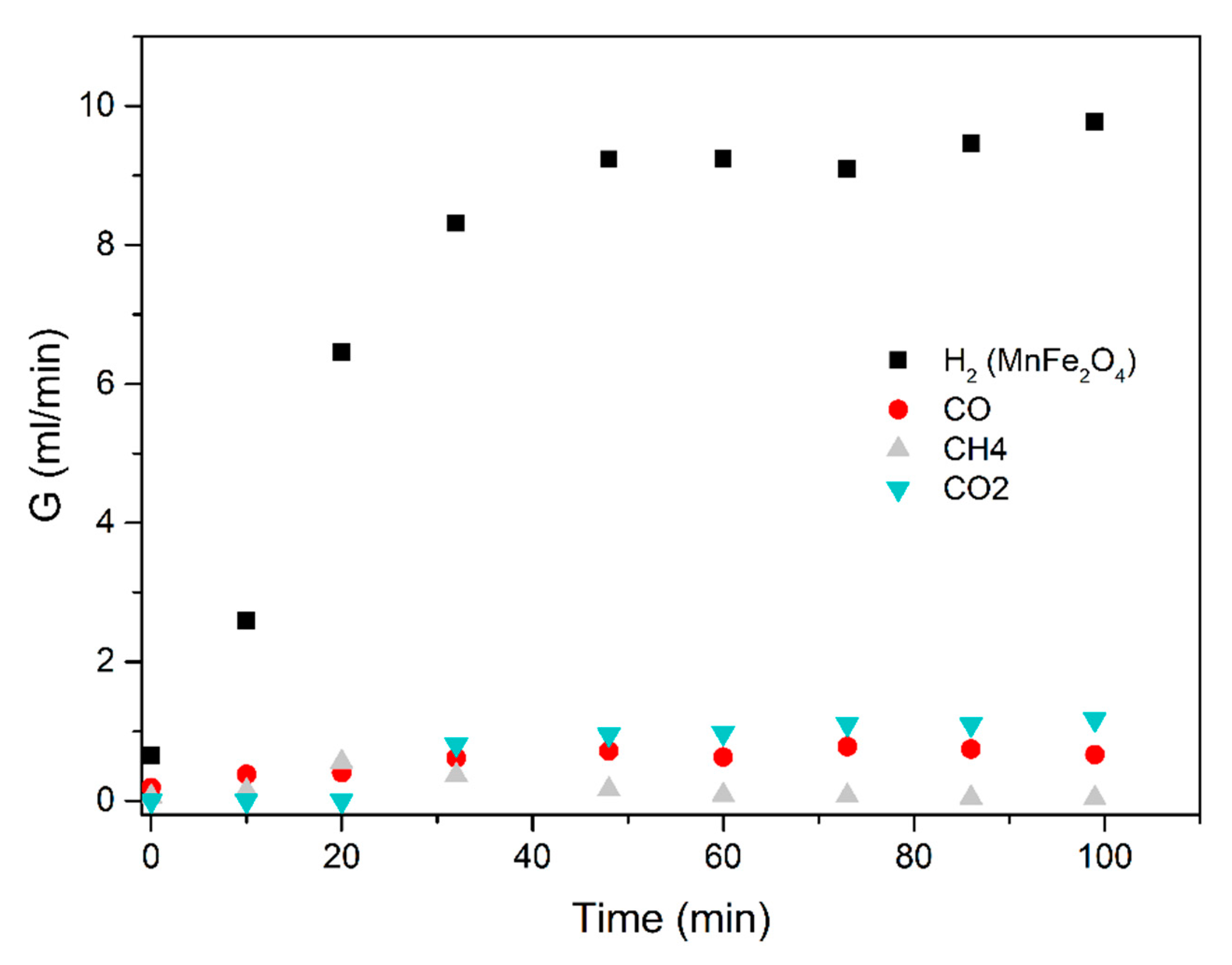
3.2. The Fe2O3 Reduction Rate
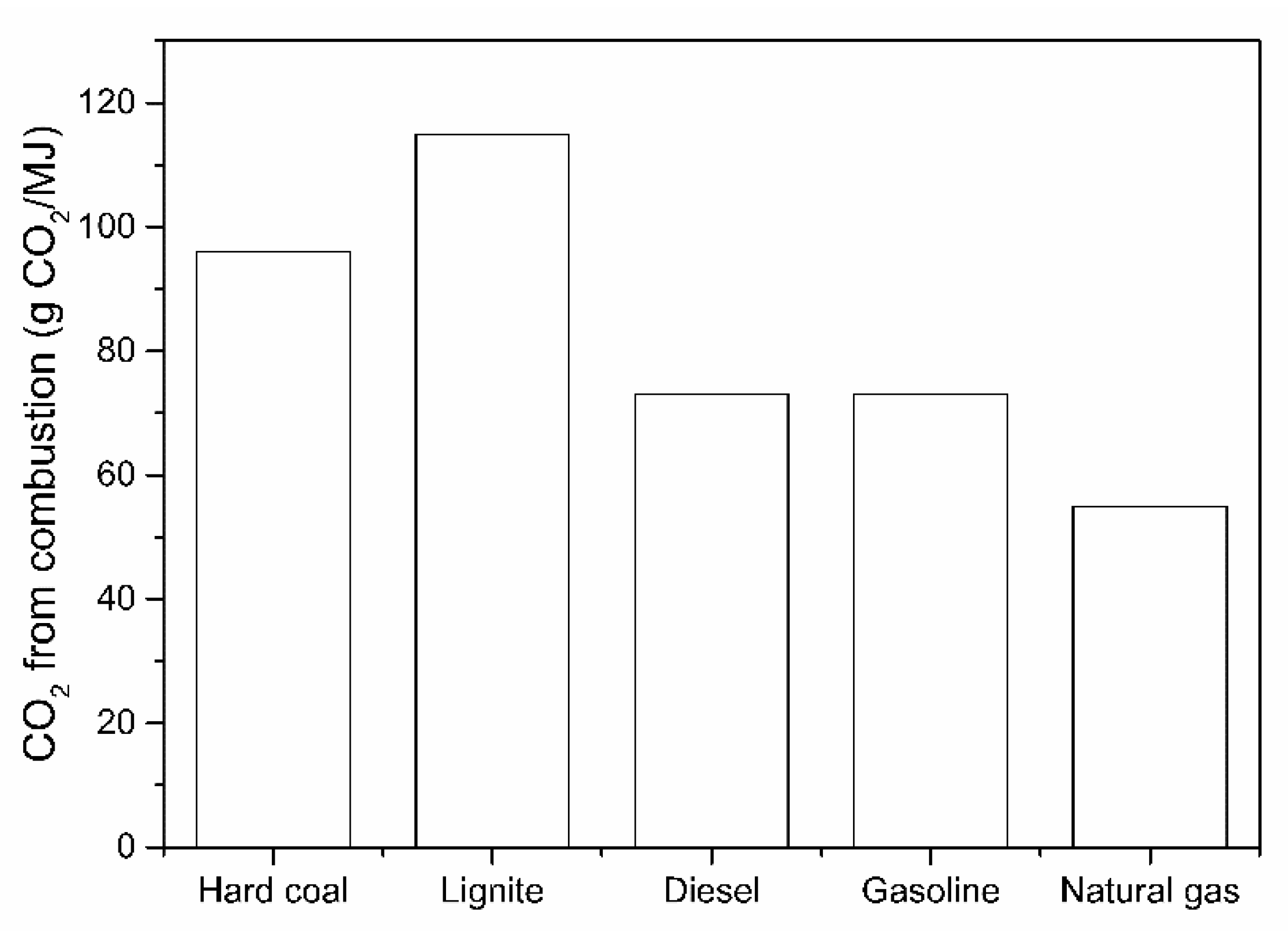
4. Potential “Green” H2 Production Methods
4.1. NH3 Decomposition
4.2. C2H5OH and CH3OH Catalytic Decomposition
4.3. Tentative Techno-Economic Comparison of Different H2 Production Technologies
5. Conclusions
- (i)
- Fe2O3 ore particles are of small size and cannot readily be processed by a H2 stream in a fixed or fluidized bed. A novel vibrated fluidized bed was developed and shown capable of fluidizing μm-particles at very low superficial fluidization velocities. The small-scale experiments need to be confirmed on a pilot-scale. Vibrated fluidization of the fine Fe2O3 particles caused good fluidization and yielded a nearly constant pressure drop. An increase in external force due to vibration slightly increased the pressure drop. Despite an increase in the reaction temperature, vibration produces a nearly constant minimum fluidization velocity.
- (ii)
- Although very interesting reduction reaction yields for fine Fe2O3 particles were obtained on a small-scale, it is important to investigate the production of such fine Fe2O3 feedstock on an industrial scale.
- (iii)
- The remaining issue relates to the “green” production of H2, currently based primarily on fossil fuel feedstock with a high degree of GHG emissions. Although steam reforming of ethanol and methanol offers an important potential, the methane process is preferred since its GHG emission potential is the most attractive (except for electrolysis and biomass gasification) while producing H2 at a very interesting cost. Further pilot-scale experiments are required to confirm these initial lab-scale results.
- (iv)
- To confirm the solar production potential, additional experiments will be conducted in the solar furnace as soon as the Direct Normal Irradiance values are higher (from July onwards).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, P.; Ryberg, M.; Yang, Y.; Feng, K.; Kara, S.; Hauschild, M.; Chen, W.-Q. Efficiency stagnation in global steel production urges joint supply- and demand-side mitigation efforts. Nat. Commun. 2021, 12, 2066. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Fan, D.-Q.; Abdelghany, A. Design of Novel Flash Ironmaking Reactors for Greatly Reduced Energy Consumption and CO2 Emissions. Metals 2021, 11, 332. [Google Scholar] [CrossRef]
- Abolpour, B.; Afsahi, M.M.; Azizkarimi, M. Hydrogen reduction of magnetite concentrate particles. Miner. Process. Extr. Metall. 2021, 130, 59–72. [Google Scholar] [CrossRef]
- Murakami, T.; Wakabayashi, H.; Maruoka, D.; Kasai, E. Effect of Hydrogen Concentration in Reducing Gas on the Changes in Mineral Phases during Reduction of Iron Ore Sinter. ISIJ Int. 2020, 60, 2678–2685. [Google Scholar] [CrossRef]
- Patisson, F.; Mirgaux, O. Hydrogen Ironmaking: How It Works. Metals 2020, 10, 922. [Google Scholar] [CrossRef]
- Zhang, A.; Monaghan, B.J.; Longbottom, R.J.; Nusheh, M.; Bumby, C.W. Reduction Kinetics of Oxidized New Zealand Ironsand Pellets in H2 at Temperatures up to 1443 K. Metall. Mater. Trans. B 2020, 51, 492–504. [Google Scholar] [CrossRef]
- Bai, M.; Long, H.; Li, L.; Liu, D.; Ren, S.-B.; Zhao, C.-F.; Cheng, J. Kinetics of iron ore pellets reduced by H2N2 under non-isothermal condition. Int. J. Hydrogen Energy 2018, 43, 15586–15592. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, J.; Qin, B.; Dong, Y.; Lu, Y.; Li, Y.; Hao, W.; Zhang, Y. Reduction kinetics of hematite ore fines with H2 in a rotary drum reactor. Powder Technol. 2018, 332, 18–26. [Google Scholar] [CrossRef]
- Bai, M.-H.; Long, H.; Ren, S.-B.; Liu, D.; Zhao, C.-F. Reduction Behavior and Kinetics of Iron Ore Pellets under H2–N2 Atmosphere. ISIJ Int. 2018, 58, 1034–1041. [Google Scholar] [CrossRef] [Green Version]
- Lyu, Q.; Qie, Y.; Liu, X.; Lan, C.; Li, J.; Liu, S. Effect of hydrogen addition on reduction behavior of iron oxides in gas-injection blast furnace. Thermochim. Acta 2017, 648, 79–90. [Google Scholar] [CrossRef]
- Elzohiery, M.; Sohn, H.Y.; Mohassab, Y. Kinetics of Hydrogen Reduction of Magnetite Concentrate Particles in Solid State Relevant to Flash Ironmaking. Steel Res. Int. 2017, 88, 1600133. [Google Scholar] [CrossRef]
- Du, W.; Yang, S.; Pan, F.; Shangguan, J.; Lu, J.; Liu, S.; Fan, H. Hydrogen Reduction of Hematite Ore Fines to Magnetite Ore Fines at Low Temperatures. J. Chem. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Mohassab, Y.; Elzohiery, M.; Fan, D.-Q.; Abdelghany, A. Status of the Development of Flash Ironmaking Technology. In Applications of Process Engineering Principles in Materials Processing, Energy and Environmental Technologies; The Minerals, Metals & Materials Series; Wang, S., Free, M., Alam, S., Zhang, M., Taylor, P., Eds.; Springer: Cham, Switzerland, 2017; pp. 15–23. [Google Scholar] [CrossRef]
- Htet, T.T.; Yan, Z.; Spooner, S.; Degirmenci, V.; Meijer, K.; Li, Z. Gasification and physical-chemical characteristics of carbonaceous materials in relation to HIsarna ironmaking process. Fuel 2021, 289, 119890. [Google Scholar] [CrossRef]
- Bhaskar, A.; Assadi, M.; Nikpey Somehsaraei, H. Decarbonization of the Iron and Steel Industry with Direct Reduction of Iron Ore with Green Hydrogen. Energies 2020, 13, 758. [Google Scholar] [CrossRef] [Green Version]
- Vogl, V.; Åhman, M.; Nilsson, L.J. Assessment of hydrogen direct reduction for fossil-free steelmaking. J. Clean. Prod. 2018, 203, 736–745. [Google Scholar] [CrossRef]
- He, K.; Zheng, Z.; Chen, H.; Hao, W. Reduction Behaviors of Hematite to Metallic Iron by Hydrogen at Low Temperatures. In Energy Technology 2021: Carbon Dioxide Management and Other Technologies; Springer: Cham, Switzerland, 2021; pp. 111–122. [Google Scholar]
- Spreitzer, D.; Schenk, J. Fluidization behavior and reducibility of iron ore fines during hydrogen-induced fluidized bed reduction. Particuology 2020, 52, 36–46. [Google Scholar] [CrossRef]
- Knop, K.; Kubiak, H. Process engineering and cost efficiency of steelmaking by means of hydrogen-based direct reduction. Stahl und Eisen 1996, 116, 55–64. [Google Scholar]
- Hamadeh, H.; Mirgaux, O.; Patisson, F. Detailed Modeling of the Direct Reduction of Iron Ore in a Shaft Furnace. Materials 2018, 11, 1865. [Google Scholar] [CrossRef] [Green Version]
- Ranzani da Costa, A.; Wagner, D.; Patisson, F. Modelling a new, low CO2 emissions, hydrogen steelmaking process. J. Clean. Prod. 2013, 46, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Lazou, A.; van der Eijk, C.; Balomenos, E.; Kolbeinsen, L.; Safarian, J. On the Direct Reduction Phenomena of Bauxite Ore Using H2 Gas in a Fixed Bed Reactor. J. Sustain. Metall. 2020, 6, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Moldenhauer, P.; Linderholm, C.; Rydén, M.; Lyngfelt, A. Avoiding CO2 capture effort and cost for negative CO2 emissions using industrial waste in chemical-looping combustion/gasification of biomass. Mitig. Adapt. Strateg. Glob. Chang. 2020, 25, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Hoseinzadeh, S.; Stephan Heyns, P. Advanced Energy, Exergy, and Environmental (3E) Analyses and Optimization of a Coal-Fired 400 MW Thermal Power Plant. J. Energy Resour. Technol. 2021, 143, 082106. [Google Scholar] [CrossRef]
- Tjahjono, T.; Ehyaei, M.A.; Ahmadi, A.; Hoseinzadeh, S.; Memon, S. Thermo-Economic Analysis on Integrated CO2, Organic Rankine Cycles, and NaClO Plant Using Liquefied Natural Gas. Energies 2021, 14, 2849. [Google Scholar] [CrossRef]
- Mahmoudan, A.; Samadof, P.; Hosseinzadeh, S.; Garcia, D.A. A multigeneration cascade system using ground-source energy with cold recovery: 3E analyses and multi-objective optimization. Energy 2021, 233, 121185. [Google Scholar] [CrossRef]
- Torres, E.; Rodriguez-Ortiz, L.A.; Zalazar, D.; Echegaray, M.; Rodriguez, R.; Zhang, H.; Mazza, G. 4-E (environmental, economic, energetic and exergetic) analysis of slow pyrolysis of lignocellulosic waste. Renew. Energy 2020, 162, 296–307. [Google Scholar] [CrossRef]
- Li, S.; Baeyens, J.; Dewil, R.; Appels, L.; Zhang, H.; Deng, Y. Advances in rigid porous high temperature filters. Renew. Sustain. Energy Rev. 2021, 139, 110713. [Google Scholar] [CrossRef]
- Kong, W.; Tan, T.; Baeyens, J.; Flamant, G.; Zhang, H. Bubbling and Slugging of Geldart Group A Powders in Small Diameter Columns. Ind. Eng. Chem. Res. 2017, 56, 4136–4144. [Google Scholar] [CrossRef]
- Fernandez, A.; Soria, J.; Rodriguez, R.; Baeyens, J.; Mazza, G. Macro-TGA steam-assisted gasification of lignocellulosic wastes. J. Environ. Manag. 2019, 233, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Geldart, D.; Baeyens, J. The design of distributors for gas-fluidized beds. Powder Technol. 1985, 42, 67–78. [Google Scholar] [CrossRef]
- Haitao, W.; Sohn, H.Y. Reduction of Magnetite Concentrate Particles by H2+CO at 1673 K. ISIJ Int. 2015, 55, 706–708. [Google Scholar]
- Zhang, B.; Wang, Z.; Gong, X.; Guo, Z. Characterization of Precipitated Carbon by XPS and Its Prevention Mechanism of Sticking during Reduction of Fe2O3 Particles in the Fluidized Bed. ISIJ Int. 2013, 53, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Hsu, W.-Y.; Huang, A.-N.; Kuo, H.-P. Analysis of interparticle forces and particle-wall interactions by powder bed pressure drops at incipient fluidization. Powder Technol. 2018, 325, 64–68. [Google Scholar] [CrossRef]
- Wank, J.R.; George, S.M.; Weimer, A.W. Vibro-fluidization of fine boron nitride powder at low pressure. Powder Technol. 2001, 121, 195–204. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Busoul, M.; Al-Hassan, M. Hydro-thermal behavior of magnetically stabilized fluidized beds. Powder Technol. 2001, 115, 58–67. [Google Scholar] [CrossRef]
- Lucentini, I.; García Colli, G.; Luzi, C.D.; Serrano, I.; Martínez, O.M.; Llorca, J. Catalytic ammonia decomposition over Ni-Ru supported on CeO2 for hydrogen production: Effect of metal loading and kinetic analysis. Appl. Catal. B Environ. 2021, 286, 119896. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, X.; Zhao, S.; Zhang, Y. FeCe nanocomposite with high iron content as efficient catalyst for generation of COx-free hydrogen via ammonia decomposition. J. Rare Earths 2020, 38, 1053–1059. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Cha, J.; Kwak, Y.; Park, Y.; Jo, Y.S.; Jeong, H.; Sohn, H.; Yoon, C.W.; Kim, Y.; Kim, K.-B.; et al. Top-Down Syntheses of Nickel-Based Structured Catalysts for Hydrogen Production from Ammonia. ACS Appl. Mater. Interfaces 2021, 13, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.A.; Carter, J.H.; Dummer, N.F.; Richards, N.; Morgan, D.J.; Golunski, S.E.; Hutchings, G.J. Ammonia Decomposition Enhancement by Cs-Promoted Fe/Al2O3 Catalysts. Catal. Lett. 2020, 150, 3369–3376. [Google Scholar] [CrossRef]
- Ye, T.-N.; Lu, Y.; Kobayashi, Y.; Li, J.; Park, S.-W.; Sasase, M.; Kitano, M.; Hosono, H. Efficient Ammonia Synthesis over Phase-Separated Nickel-Based Intermetallic Catalysts. J. Phys. Chem. C 2020, 124, 28589–28595. [Google Scholar] [CrossRef]
- Yu, Y.; Gan, Y.-M.; Huang, C.; Lu, Z.-H.; Wang, X.; Zhang, R.; Feng, G. Ni/La2O3 and Ni/MgO–La2O3 catalysts for the decomposition of NH3 into hydrogen. Int. J. Hydrogen Energy 2020, 45, 16528–16539. [Google Scholar] [CrossRef]







| References | Major Findings |
|---|---|
| Hydrogen reduction of iron ores | |
| Sohn et al. [2] | Developed a novel iron-making technology based on fine iron ore concentrate in a flash reactor. Optimization towards using multiple burners, preheating the feed gas, or both is examined. |
| Abolpour et al. [3] | Studied the reaction kinetics of H2 reduction of iron ore concentrate particles between 700 and 900 °C. |
| Murakami et al. [4] | Studied the effect of H2 concentration in the reducing gas on the changes in mineral phases during reduction of iron ore sinter. The mineral composition of 10 types of sinter samples was analyzed. |
| Patisson & Mirgauxb [5] | Analyzed a new route for making steel from iron ore based on the use of H2, with a 90% reduction in CO2 emissions compared to those of the blast-furnace route. |
| Zhang et al. [6] | Described the reduction kinetics by a pellet-scale single-interface shrinking core model at temperatures below 1143 K. At higher temperatures, the rate-limiting step is the interfacial chemical reaction process. |
| Bai et al. [7] | Investigated the thermal reduction of pellets by H2. An un-reacted core and interfacial chemical reaction are reaction rate controlling steps in function of the operating temperature. |
| Wei et al. [8] | Conducted reduction experiments with a H2-N2 mixture in a rotary drum reactor. A dense, increasingly thick layer of iron was formed during the reduction reaction. The reduction process was controlled by a first-order reaction model. |
| Bai et al. [9] | Studied the direct reduction behavior and dynamic characteristics of oxidized pellets under the 75% H2-25% N2 atmosphere at 760, 900, 1000 °C. Holes and cracks were observed at higher temperature, hampering the reduction degradation behavior of the pellets. |
| Lyu et al. [10] | Investigated the effect of hydrogen addition on gas utilization in a blast furnace. The H2 content should be controlled in the range of 5%–10%. |
| Elzohiery et al. [11] | Demonstrated that the reduction is of a first-order dependence with the hydrogen partial pressure. |
| Du et al. [12] | Proposed a new process that could simultaneously enrich CH4 from coke oven gas and produce separated magnetite from low-grade hematite. |
| Sohn et al. [13] | Reduced the concentrate by H2 and CO gas mixtures formed from the partial oxidation of natural gas in a flash reactor. The rate equations were applied to experimental results from a laboratory flash reactor using CFD simulation. |
| Htet et al. [14] | Investigated the gasification reactivity of carbon black compared to the carbonaceous materials used in the HIsarna process by TGA method at 1250 °C, 1350 °C and 1450 °C under atmospheric pressure. Carbon black is less reactive than thermal coal and charcoal. The random pore model best describes the gasification reaction of the selected samples. Activation energies were determined. |
| Energetic and environmental importance of H2-based steel production | |
| Bhaskar et al. [15] | Developed a mass and energy flow model based upon Python to explore the feasibility of using H2- direct reduction of iron ore (HDRI) coupled with an electric arc furnace (EAF) for carbon-free steel production. This technology could reduce specific emissions from steel production in the EU by more than 35%, at present grid emission levels (295 kgCO2/MWh). |
| Vogl et al. [16] | Hydrogen direct reduction (H-DR) steelmaking needs 3.48 MWh of electricity per ton of liquid steel, mainly for the electrolytic hydrogen production. H-DR emits only 2.8% of blast furnace CO2. |
| Mixed/moving and fluidized bed direct reduction reactors | |
| He et al. [17] | Investigated the micro-fluidized bed reduction behavior of Brazilian hematite in 20% H2 and 80% Ar at 400–570 °C. The reaction rate was suggested to be determined by the phase boundary reaction. |
| Spreitzer & Schenk [18] | Investigated the fluidization behavior and reducibility of various iron ore grades. A stable fluidization of hematite- and limonite-based iron ores was observed, while magnetite-based iron ore could not be fluidized. |
| Knop and Kubiak [19] | Studies the comparison of a combined H2-based direct reduction and electric steelmaking, and the ore-fines reduction in a novel fluidized bed process. |
| Hamadeh et al. [20] | Studied the moving bed principle in de direct reduction shaft furnace, providing evidence of 8 different zones according to the predominant thermal and reaction characteristics. |
| da Costa et al. [21] | In a counter-current direct-reduction moving bed reactor, CO2 emissions were reduced by more than 80%. |
| Lazou et al. [22] | Studied the CO2 reduction in the conventional Pedersen Process by reducing iron or bauxite ore at temperatures below and above 560 °C. At temperatures above 860 °C, the formation of FeAl2O4 retards the reduction to metallic iron. |
| Moldenauer et al. [23] | A circulating fluidized bed reactor was applied in a chemical looping combustion. CO2 reductions of 75 to 92% were achieved. |
| Particle Property | Value |
|---|---|
| Mean particle size (µm) | 10–15 |
| Particle density (kg/m3) | 4800 |
| Shape | Non-spherical, sphericity ~0.85 |
| Chemical composition | Iron 68%, Oxygen 30% |
| Parameter | |
|---|---|
| Fluidizing carrier gas | N2 |
| Reduction gas | H2 |
| Superficial gas velocity (m/s) | <0.02 |
| Bed height (m) | 0.25 |
| Vibration frequency [Hz] | <50 |
| Vibration amplitude [mm] | 0.6 |
| Vibration direction | Vertical |
| Reaction temperature [K] | 773–873 |
| Temperature (°C) | NH3 Conversion (%) |
|---|---|
| 300 | 95.7 |
| 400 | 99.1 |
| 500 | 99.7 |
| 600 | 99.9 |
| Methane Steam Reforming | Coal Gasification | Membrane Electrolysis (Renewable Energy) | Biomass Gasification | Methanol Steam Reforming | Ethanol Steam Reforming | Methane Decomposition | |
|---|---|---|---|---|---|---|---|
| LCOH ($/kg H2) | 1.25 | 1.5 | 7.7 | 3 (for large scale) Otherwise > 5 | 1.8–2.2 | 1.5–2.0 (if acetone can be recovered and sold) | 1.0–1.5 (if carbon black can be sold at 250–1000 $/ton) |
| GHG emission (kg CO2-eq/kg H2) | 8.1–11 | 13–17 | 0 | +/−0 | >7 | 5–7 | 2.5 |
| H2 yield (%) | 70–85 | 50–60 | 70 | 20–40 | >90 | 60–80 | 75–80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhang, H.; Nie, J.; Dewil, R.; Baeyens, J.; Deng, Y. The Direct Reduction of Iron Ore with Hydrogen. Sustainability 2021, 13, 8866. https://doi.org/10.3390/su13168866
Li S, Zhang H, Nie J, Dewil R, Baeyens J, Deng Y. The Direct Reduction of Iron Ore with Hydrogen. Sustainability. 2021; 13(16):8866. https://doi.org/10.3390/su13168866
Chicago/Turabian StyleLi, Shuo, Huili Zhang, Jiapei Nie, Raf Dewil, Jan Baeyens, and Yimin Deng. 2021. "The Direct Reduction of Iron Ore with Hydrogen" Sustainability 13, no. 16: 8866. https://doi.org/10.3390/su13168866
APA StyleLi, S., Zhang, H., Nie, J., Dewil, R., Baeyens, J., & Deng, Y. (2021). The Direct Reduction of Iron Ore with Hydrogen. Sustainability, 13(16), 8866. https://doi.org/10.3390/su13168866








