Utilisation of CO2 from Sodium Bicarbonate to Produce Chlorella vulgaris Biomass in Tubular Photobioreactors for Biofuel Purposes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae
2.2. Experimental Setup
2.3. Statistical Analysis
3. Results and Discussion
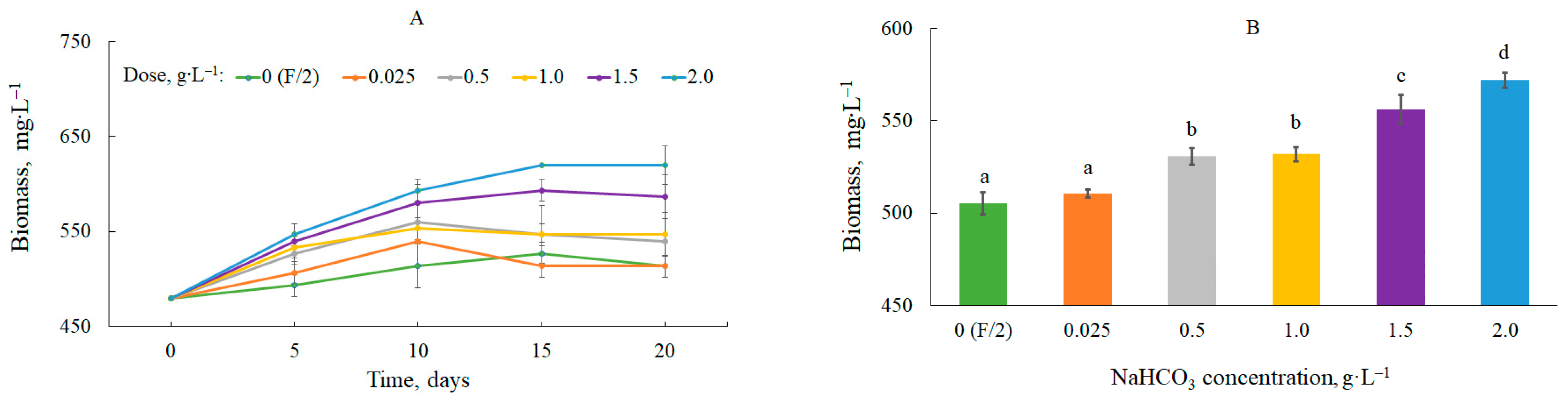
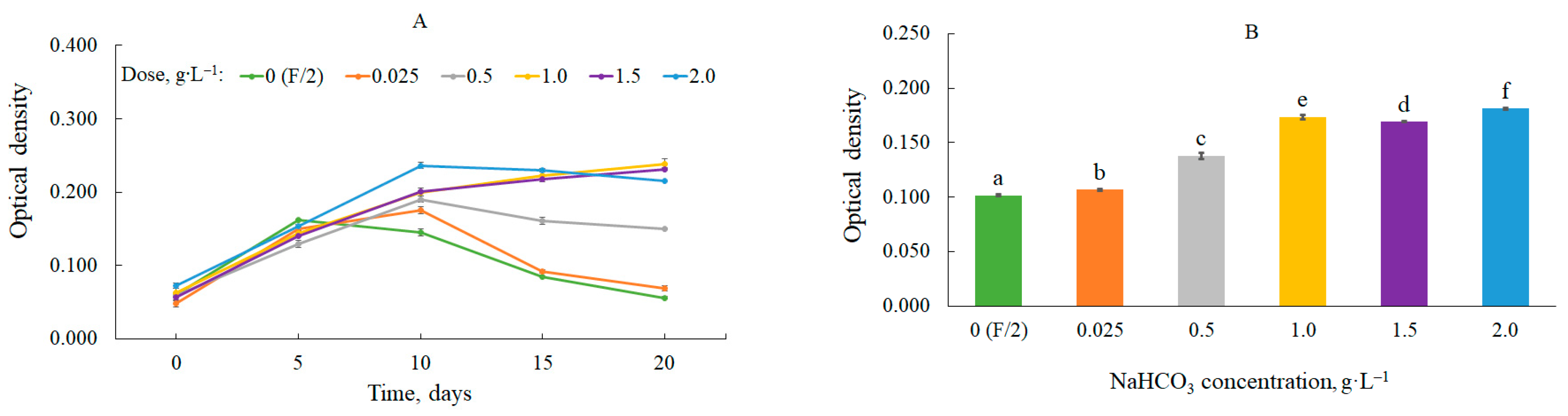
3.1. Biomass Production with CO2 from Sodium Bicarbonate
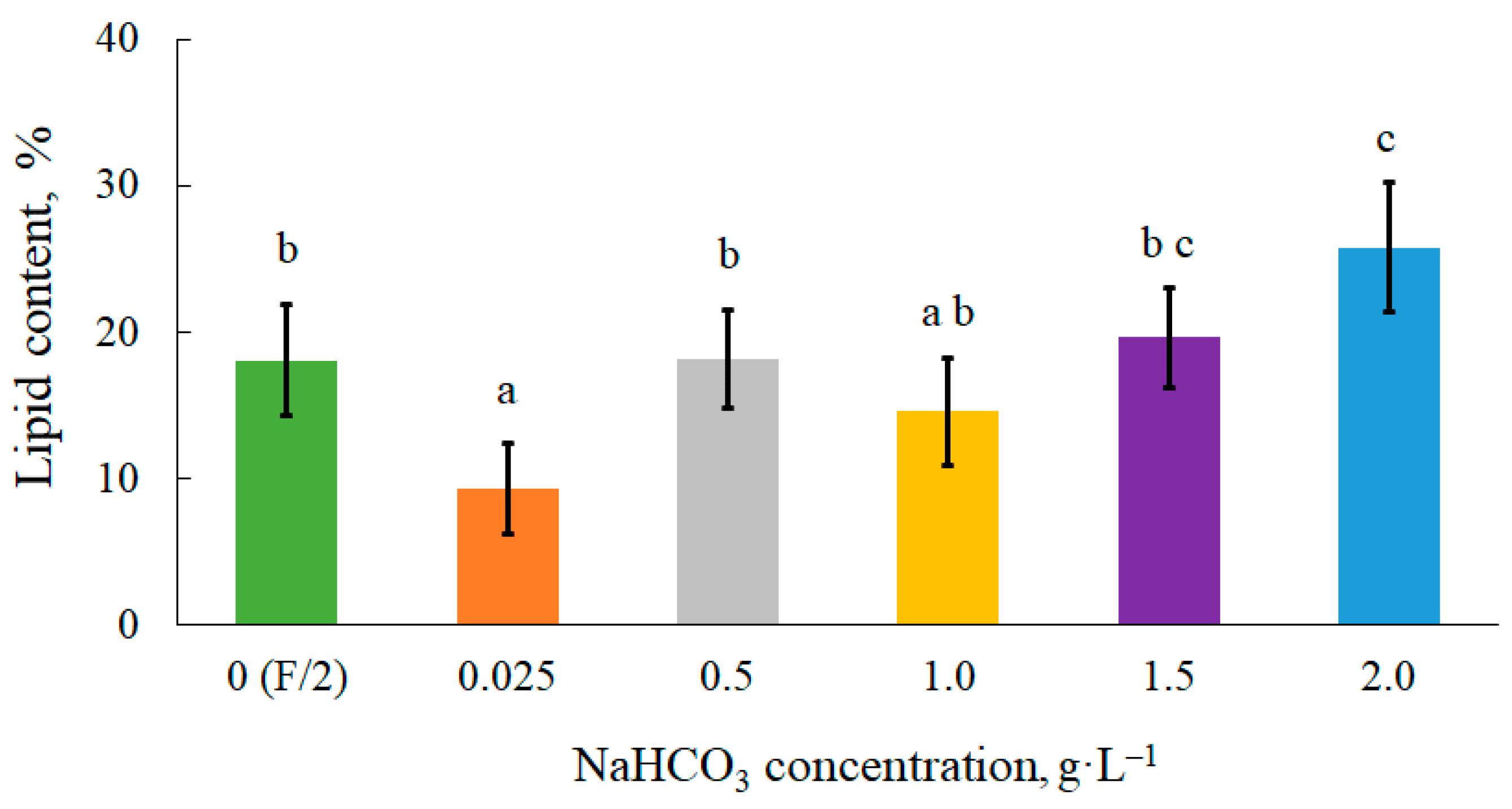
3.2. Effect of Sodium Bicarbonate on Lipid Accumulation in Microalgal Biomass
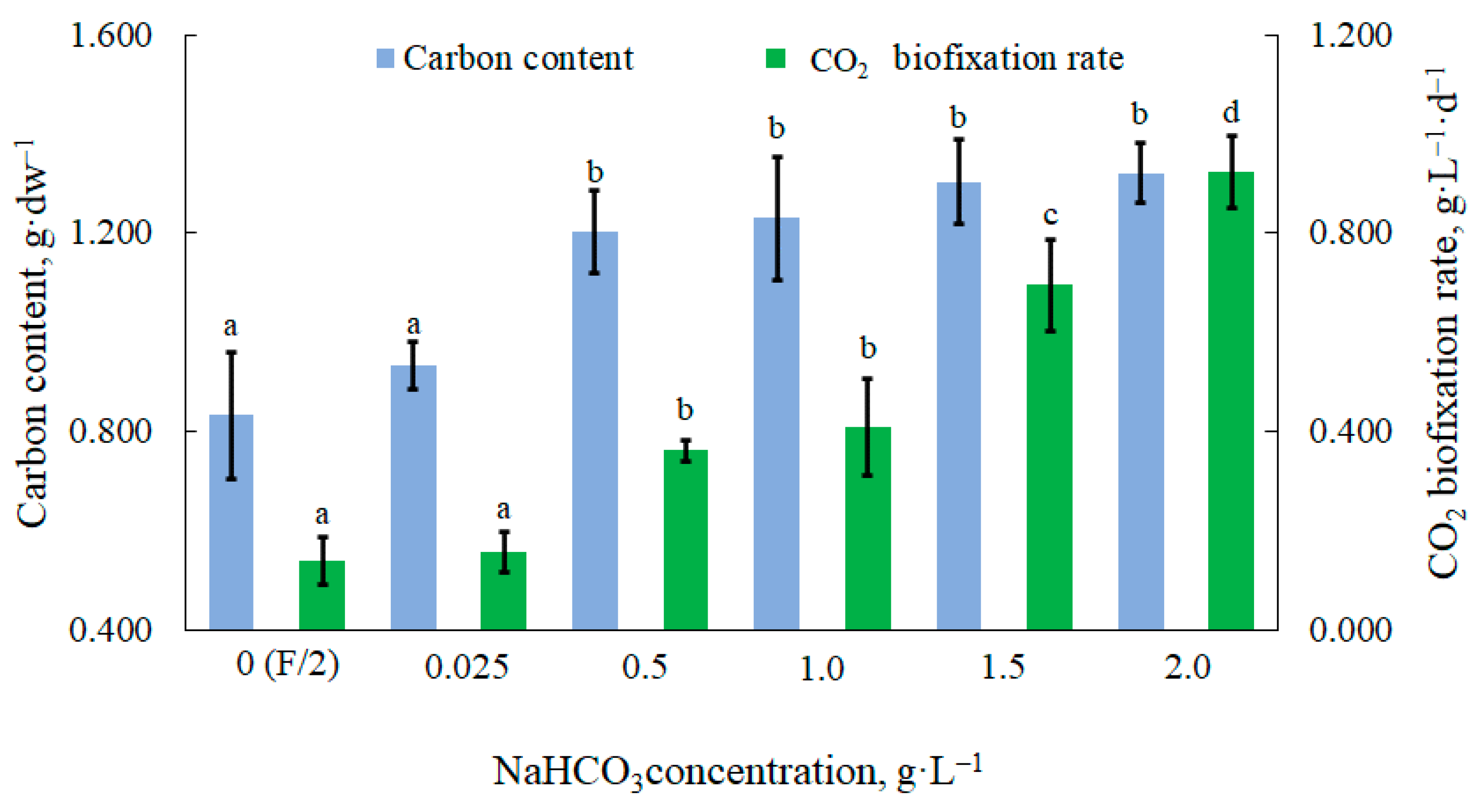
3.3. Carbon Content and CO2 Fixation Rate in Microalgal Biomass
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhatia, S.K.; Bhatia, R.K.; Jeon, J.M.; Pugazhendhi, A.; Awasthi, M.K.; Kumar, D.; Kumar, G.; Yoon, J.J.; Yang, Y.H. An overviewon advancements in biobased transesterification methods for biodiesel production: Oil resources, extraction, biocatalysts, andprocess intensification technologies. Fuel 2021, 285, 119117. [Google Scholar] [CrossRef]
- Stephens, E.; Ross, I.L.; Mussgnug, J.H.; Wagner, L.D.; Borowitzka, M.A.; Posten, C.; Kruse, O.; Hankamer, B. Future prospects of microalgal biofuel production systems. Trends Plant Sci. 2010, 15, 554–564. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Arumugam, M.; Agarwal, A.; Arya, M.C.; Ahmed, Z. Influence of nitrogen sources on biomass productivity of microalgae Scenedesmus bijugatus. Biores. Technol. 2013, 131, 246–249. [Google Scholar] [CrossRef]
- Ratledge, C.; Cohen, Z. Microbial and algal oils: Do they have a future for biodiesel or as commodity oils? Lipid Technol. 2008, 20, 155–160. [Google Scholar] [CrossRef]
- Beer, L.L.; Boyd, E.S.; Peters, J.W.; Posewitz, M.C. Engineering algae for biohydrogen and biofuel production. Curr. Opin. Biotechnol. 2009, 20, 264–271. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, S.; Cao, B.; Hu, Y.; Abomohra, A.E.-F.; Wang, Q.; Qian, L.; Liu, L.; Liu, X.; He, Z.; et al. Optimization of hydrothermal co-liquefaction of seaweeds with lignocellulosic biomass: Merging 2nd and 3rd generation feedstocks for enhanced bio-oil production. Energy 2019, 173, 413–422. [Google Scholar] [CrossRef]
- Chen, M.; Tang, H.; Ma, H.; Holland, T.C.; Ng, K.Y.S.; Salley, S.O. Effects of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour. Technol. 2011, 102, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Mohsenpour, S.F.; Willoughby, N. Luminescent photobioreactor design for improved algal growth and photosynthetic pigment production through spectral conversion of light. Bioresour. Technol. 2013, 142, 147–153. [Google Scholar] [CrossRef]
- Raheem, A.; Prinsen, P.; Vuppaladadiyam, A.K.; Zhao, M.; Luque, R. A review on sustainable microalgae based biofuel and bioenergy production: Recent developments. J. Clean. Prod. 2018, 181, 42–59. [Google Scholar] [CrossRef]
- Tomei, J.; Helliwell, R. Food Versus Fuel? Going Beyond Biofuels. Land Use Policy 2016, 56, 320–326. [Google Scholar] [CrossRef]
- Kazamia, E.; Smith, A.G. Assessing the environmental sustainability of biofuels. Trends Plant Sci. 2014, 19, 615–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collet, P.; Lardon, L.; Hélias, A.; Bricout, S.; Lombaert, I.; Perrier, V.B.; Lépine, O.; Steyer, J.P.; Bernard, O. Biodiesel from microalgae—Life cycle assessment and recommendations for potential improvements. Renew. Energy 2014, 71, 525–533. [Google Scholar] [CrossRef]
- Rawat, I.; Kumar, R.; Mutanda, T.; Bux, F. Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Appl. Energy. 2012, 103, 444–467. [Google Scholar] [CrossRef]
- Sahoo, N.K.; Gupta, S.K.; Rawat, I.; Ansari, F.A.; Singh, P.; Naik, S.N.; Bux, F. Sustainable dewatering and drying of self-flocculating microalgae and study of cake properties. J. Clean. Prod. 2017, 159, 248–256. [Google Scholar] [CrossRef]
- Mohd Udaiyappan, A.F.; Abu Hasan, H.; Takriff, M.S.; Sheikh Abdullah, S.R. A review of the potentials, challenges and currentstatus of microalgae biomass applications in industrial wastewater treatment. J. Water Process Eng. 2017, 20, 8–21. [Google Scholar] [CrossRef]
- Shahid, A.; Malik, S.; Zhu, H.; Xu, J.; Nawaz, M.Z.; Nawaz, S.; Asraful Alam, M.; Mehmood, M.A. Cultivating microalgae inwastewater for biomass production, pollutant removal, and atmospheric carbon mitigation: A review. Sci. Total Environ. 2020, 704, 135303. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Koniuszy, A.; Gałczyńska, M. Sustainable Production of Monoraphidium Microalgae Biomass as a Source of Bioenergy. Energies 2020, 13, 5975. [Google Scholar] [CrossRef]
- Xiaoning, L.; Guangyao, C.; Yi, T.; Jun, W. Application of effluent from WWTP in cultivation of four microalgae for nutrients removal and lipid production under the supply of CO2. Renew. Energy 2020, 149, 708–715. [Google Scholar]
- Umetani, I.; Janka, E.; Sposób, M.; Hulatt, C.J.; Kleiven, S.; Bakke, R. Bicarbonate for microalgae cultivation: A case study in a chlorophyte, Tetradesmus wisconsinensis isolated from a Norwegian lake. J. Appl. Phycol. 2021, 33, 1341–1352. [Google Scholar] [CrossRef]
- Zhang, X. Microalgae removal of CO2 from flue gas; IEA Clean Coal Centre: London, UK, 2015. [Google Scholar]
- Klinthong, W.; Yang, Y.-H.; Huang, C.-H.; Tan, C.-S. A review: Microalgae and their applications in CO2 capture and renewable energy. Aerosol Air Qual. Res. 2015, 15, 712–742. [Google Scholar] [CrossRef]
- Ho, S.H.; Chen, C.Y.; Lee, D.J.; Chang, J.S. Perspectives on microalgal CO2-emission mitigation systems—A review. Biotechnol Adv. 2011, 29, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Rodolfi, L.; Zittelli, G.C.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotech. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef]
- Hussain, F.; Zahir, S.Z.; Zhou, W.; Iqbal, M. Microalgae screening under CO2 stress: Growth and micro-nutrients removal efficiency. J. Photochem. Photobiol. B Biol. 2017, 170, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.P.; Liu, X.P.; Li, X.; Chen, Y.M. Quantifying the relationship between urban forms and carbon emissions using panel data analysis. Landsc. Ecol. 2013, 28, 1889–1907. [Google Scholar] [CrossRef]
- Bezyk, Y.; Sówka, I.; Górka, M.; Blachowski, J. GIS-Based Approach to Spatio-Temporal Interpolation of Atmospheric CO2 Concentrations in Limited Monitoring Dataset. Atmosphere 2021, 12, 384. [Google Scholar] [CrossRef]
- Markou, G.; Vandamme, D.; Muylaert, K. Microalgal and cyanobacterial cultivation: The supply of nutrients. Water Res. 2014, 65, 186–202. [Google Scholar] [CrossRef] [Green Version]
- Salih, F. Microalgae Tolerance to High Concentrations of Carbon Dioxide: A Review. J. Environ. Prot. 2011, 2, 648–654. [Google Scholar] [CrossRef] [Green Version]
- Pourjamshidian, R.; Abolghasemi, H.; Esmaili, M.; Amrei, H.D.; Parsa, M.; Rezaei, S. Carbon dioxide biofixation by Chlorella sp in a bubble column reactor at different flow rates and CO2 concentrations. Brazilian J. Chem. Eng. 2019, 36, 639–645. [Google Scholar] [CrossRef] [Green Version]
- Solovchenko, A.; Khozin-Goldberg, I. High-CO2 tolerance in microalgae: Possible mechanisms and implications for biotechnology and bioremediation. Biotechnol. Lett. 2013, 35, 1745–1752. [Google Scholar] [CrossRef]
- Fulke, A.B.; Chambhare, K.; Giripunje, M.D.; Sangolkar, L.; Krishnamurthi, K.; Juwarkar, A.A.; Chakrabarti, T. Potential of wastewater grown algae for biodiesel production and CO2 sequestration. Afr. J. Biotechnol. 2013, 12, 2939–2948. [Google Scholar]
- Chi, Z.; O’Fallon, J.V.; Chen, S. Bicarbonate produced from carbon capture for algae culture. Trends Biotechnol. 2011, 29, 537–541. [Google Scholar] [CrossRef]
- De Farias Silva, C.E.; Gris, B.; Sforza, E.; La Rocca, N.; Bertucco, A. Effects of sodium bicarbonate on biomass and carbohydrate production in Synechococcus pcc 7002. Chem. Eng. Trans. 2016, 49, 241–246. [Google Scholar] [CrossRef]
- Chisti, Y. Constraints to commercialization of algal fuels. J. Biotechnol. 2013, 167, 201–214. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, S.; Ji, Y.; Schwaneberg, U.; Chi, Z. Progress toward a bicarbonate-based microalgae production system. Trends Biotechnol. 2021. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, W.; Li, L.; Wang, Z.; Wang, S.; Ding, H.; Zhang, Z.; Sun, L.; Wang, W. Effective Capture of Carbon Dioxide Using Hydrated Sodium Carbonate Powders. Materials 2018, 11, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillard, R.R.L.; Ryther, J.J. Studies of marine planktonic diatoms in Cyclotella nana Hustedt and Detonula confervacea Cleve. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Ratomski, P.; Hawrot-Paw, M. Production of Chlorella vulgaris biomass in tubular photobioreactors during different culture conditions. Appl. Sci. 2021, 11, 3106. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Walkley, A.; Black, I.A. An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–37. [Google Scholar] [CrossRef]
- Anjos, M.; Fernandes, B.D.; Vicente, A.A.; Teixeira, J.A.; Dragone, G. Optimization of CO2 bio-mitigation by Chlorella Vulgaris. Bioresour. Technol. 2013, 139, 149–154. [Google Scholar]
- Yeh, K.L.; Chang, J.S.; Chen, W.M. Effect of light supply and carbon source on cell growth and cellular composition of a newly isolated microalga Chlorella vulgaris ESP-31. Eng. Life Sci. 2010, 10, 201–208. [Google Scholar] [CrossRef]
- Mokashi, K.; Shetty, V.; George, S.; Sibi, G. Sodium Bicarbonate as Inorganic Carbon Source for Higher Biomass and Lipid Production Integrated Carbon Capture in Chlorella vulgaris. Achiev. Life Sci. 2016, 10, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Molazadeh, M.; Danesh, S.; Ahmadzadeh, H.; Pourianfar, H.R. Influence of CO2 concentration and N:P ratio on Chlorella vulgaris-assisted nutrient bioremediation, CO2 biofixation and biomass production in a lagoon treatment plant. J Taiwan Inst Chem Eng. 2019, 96, 114–120. [Google Scholar] [CrossRef]
- Rodas-Gaitán, H.A.; Rodríguez-Fuentes, H.; Luna-Maldonad, A.I.; Alcalá Jáuregui, J.; Vidales-Contreras, J.A.; Flores-Breceda, H. Biomass production and quality estimation of chlorella vulgaris(clv2) under large scale production conditions. JEBAS 2016, 4, 493–498. [Google Scholar] [CrossRef]
- Aishvarya, V.; Pradhan, N.; Nayak, R.R.; Sukla, L.B.; Mishra, B.K. Enhanced inorganic carbon uptake by Chlorella sp. IMMTCC-2 under autotrophic conditions for lipid production and CO2 sequestration. J. Appl. Phycol. 2012, 24, 1455–1463. [Google Scholar] [CrossRef]
- Salbitani, G.; Bolinesi, F.; Affuso, M.; Carraturo, F.; Mangoni, O.; Carfagna, S. Rapid and Positive Effect of Bicarbonate Addition on Growth and Photosynthetic Efficiency of the Green Microalgae Chlorella Sorokiniana (Chlorophyta, Trebouxiophyceae). Appl. Sci. 2020, 10, 4515. [Google Scholar] [CrossRef]
- Abinandan, S.; Shanthakumar, S. Evaluation of photosynthetic efficacy and CO2 removal of microalgae grown in an enriched bicarbonate medium. 3 Biotech 2016, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Patyna, A.; Biłos, Ł.; Płaczek, M.; Witczak, S. Productivity of microalgae Chlorella vulgaris in laboratory condition. Ecol. Eng. 2017, 18, 99–105. [Google Scholar]
- Tossavainen, M.; Lahti, K.; Edelmann, M.; Eskola, R.; Lampi, A.M.; Piironen, V.; Korvonen, P.; Ojala, A.; Romantschuk, M. Integrated utilization of microalgae cultured in aquaculture wastewater: Wastewater treatment and production of valuable fatty acids and tocopherols. J. Appl. Phycol. 2019, 31, 1753–1763. [Google Scholar] [CrossRef] [Green Version]
- Jegan, G.; Mukund, S.; Senthilkumarand, N.S.; Vallinayagam, R.R. Influence of different concentrations of sodium bicarbonate on growth rateand biochemical composition of microalgae. J. Algal Biomass Utln. 2013, 4, 81–87. [Google Scholar]
- Chi, Z.; Elloy, F.; Xie, Y.; Hu, Y.; Chen, S. Selection of microalgae and cyanobacteria strains for bicarbonate-based integrated carbon capture and algae production system. Appl. Biochem. Biotechnol. 2014, 172, 447–457. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.G. Osmotic responses of Dunaliella to the changes of salinity. J. Cell Physiol. 2009, 219, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Ishika, T.; Moheimani, N.R.; Bahri, P.A.; Laird, D.W.; Blair, S.; Parlevliet, D. Halo-adapted microalgae for fucoxanthin production: Effect of incremental increase in salinity. Algal Res. 2017, 28, 66–73. [Google Scholar] [CrossRef]
- White, D.A.; Pagarette, A.; Rooks, P.; Ali, S.T. The effect of sodium bicarbonate supplementation on growth and biochemical composition of marine microalgae cultures. J. Appl. Phycol. 2013, 25, 153–165. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Lan, C.G.; Liao, D. Effects of sodium bicarbonate on cell growth, lipid accumulation, and morphology of Chlorella vulgaris. Microb. Cell Factories 2018, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Bywaters, K.F.; Fritsen, C.H. Biomass and neutral lipid production in geothermal microalgal consortia. Front. Bioeng. Biotechnol. 2015, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sampathkumar, S.J.; Gothandam, K.M. Sodium bicarbonate augmentation enhances lutein biosynthesis in green microalgae Chlorella pyrenoidosa. Biocatal. Agric. Biotechnol. 2019, 22, 101406. [Google Scholar] [CrossRef]
- Pimolrat, P.; Direkbusarakom, S.; Chinajariyawong, C.; Powtongsook, S. The effect of sodium bicarbonate concentrations on growth and biochemical composition of Chaetoceros gracilis Schutt. Kasetsart Univ. Fish. Res. Bull. 2010, 34, 40–47. [Google Scholar]
- Delgadillo-Mirquez, L.; Lopes, F.; Taidi, B.; Pareau, D. Nitrogen and phosphate removal from wastewater with a mixed microalgae and bacteria culture. Biotechnol. Rep. 2016, 11, 18–26. [Google Scholar] [CrossRef]
- Salbitani, G.; Barone, C.M.A.; Carfagna, S. Effect of bicarbonate on growth of the oleaginous microalga Botryococcus braunii. Int. J. Plant. Biol. 2019, 10, 8273. [Google Scholar] [CrossRef] [Green Version]
- Moheimani, N.R. Inorganic carbon and pH effect on growth and lipid productivity of Tetraselmis suecica and Chlorella sp (Chlorophyta) grown outdoors in bag photobioreactors. J. Appl. Phycol 2013, 25, 387–398. [Google Scholar] [CrossRef]
- Ryu, H.J.; Oh, K.K.; Kim, Y.S. Optimization of the influential factors for the improvement of CO2 utilization efficiency and CO2 mass transfer rate. J. Ind. Eng. Chem. 2009, 15, 471–475. [Google Scholar] [CrossRef]
- Prabakaran, P.; Ravindran, A.D. Lipid extraction and CO2 mitigation by microalgae. J. Biochem. Technol. 2012, 4, 469–472. [Google Scholar]
- Nayak, M.; Suh, W.I.; Lee, B.; Chang, K.C. Enhanced carbon utilization efficiency and FAME production of Chlorella sp. HS2 through combined supplementation of bicarbonate and carbon dioxide. Energy Convers. Manag. 2018, 156, 45–52. [Google Scholar] [CrossRef]
- Tang, D.; Han, W.; Li, P.; Miao, X.; Zhong, J. CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour. Technol. 2011, 102, 3071–3076. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Karatza, D.; Chianese, S.; Iovine, A.; Casella, P.; Marino, T.; Musmarra, D. Bench-Scale Cultivation of Microalgae Scenedesmus almeriensis for CO2 Capture and Lutein Production. Energies 2019, 12, 2806. [Google Scholar] [CrossRef] [Green Version]
- Aussant, J.; Guihéneuf, F.; Stengel, D.B. Impact of temperature on fatty acid composition and nutritional value in eight species of microalgae. Appl. Microbiol. Biotechnol. 2018, 102, 5279–5297. [Google Scholar] [CrossRef] [PubMed]

| Level of Sodium Bicarbonate (g∙L–1) | Biomass Productivity (mg·L–1·d–1) | |||
|---|---|---|---|---|
| Day 5 | Day 10 | Day 15 | Day 20 | |
| 0 (F/2) | 2.7 ± 2.3 | 3.3 ± 2.3 | 3.1 ± 0.8 | 1.7 ± 0.6 |
| 0.025 | 5.3 ± 2.3 | 6.0 ± 0.0 | 2.2 ± 0.8 | 1.7 ± 0.6 |
| 0.5 | 9.3 ± 2.3 | 8.0 ± 2.0 | 4.4 ± 0.8 | 3.0 ± 0.0 |
| 1.0 | 10.7 ± 2.3 | 7.3 ± 1.2 | 4.4 ± 2.0 | 3.3 ± 1.2 |
| 1.5 | 12.0 ± 0.0 | 10.0 ± 2.0 | 7.6 ± 0.8 | 5.3 ± 1.2 |
| 2.0 | 13.3 ± 2.3 | 11.3 ± 1.2 | 9.3 ± 0.0 | 7.0 ± 1.0 |
| Strain | Carbon Source and Dose | Experimental Scale | CO2 Fixation, g·L–1·d–1 | References |
|---|---|---|---|---|
| Chlorellavulgaris | NaHCO3, 2.0 g·L−1 | 100 L | 0.93 | This study |
| Chlorella vulgaris | NaHCO3, 1.0 g·L−1 | 100 mL | 0.69 | [44] |
| Chlorella sp. | NaHCO3, 7.5 g·L−1 | 500 mL | 0.21 | [66] |
| Scenedesmus abliquus | CO2, 10% | 1 L | 0.26 | [67] |
| Scenedesmus almeriensis | CO2, 3% | 28.5 L | 0.24 | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratomski, P.; Hawrot-Paw, M.; Koniuszy, A. Utilisation of CO2 from Sodium Bicarbonate to Produce Chlorella vulgaris Biomass in Tubular Photobioreactors for Biofuel Purposes. Sustainability 2021, 13, 9118. https://doi.org/10.3390/su13169118
Ratomski P, Hawrot-Paw M, Koniuszy A. Utilisation of CO2 from Sodium Bicarbonate to Produce Chlorella vulgaris Biomass in Tubular Photobioreactors for Biofuel Purposes. Sustainability. 2021; 13(16):9118. https://doi.org/10.3390/su13169118
Chicago/Turabian StyleRatomski, Patryk, Małgorzata Hawrot-Paw, and Adam Koniuszy. 2021. "Utilisation of CO2 from Sodium Bicarbonate to Produce Chlorella vulgaris Biomass in Tubular Photobioreactors for Biofuel Purposes" Sustainability 13, no. 16: 9118. https://doi.org/10.3390/su13169118
APA StyleRatomski, P., Hawrot-Paw, M., & Koniuszy, A. (2021). Utilisation of CO2 from Sodium Bicarbonate to Produce Chlorella vulgaris Biomass in Tubular Photobioreactors for Biofuel Purposes. Sustainability, 13(16), 9118. https://doi.org/10.3390/su13169118






