Co-Digestion of Extended Aeration Sewage Sludge with Whey, Grease and Septage: Experimental and Modeling Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. BMP Test
2.2. Bioreactor’s Test
2.3. Statistical Analysis
2.4. Modeling and Simulation
3. Results
3.1. BMP Test
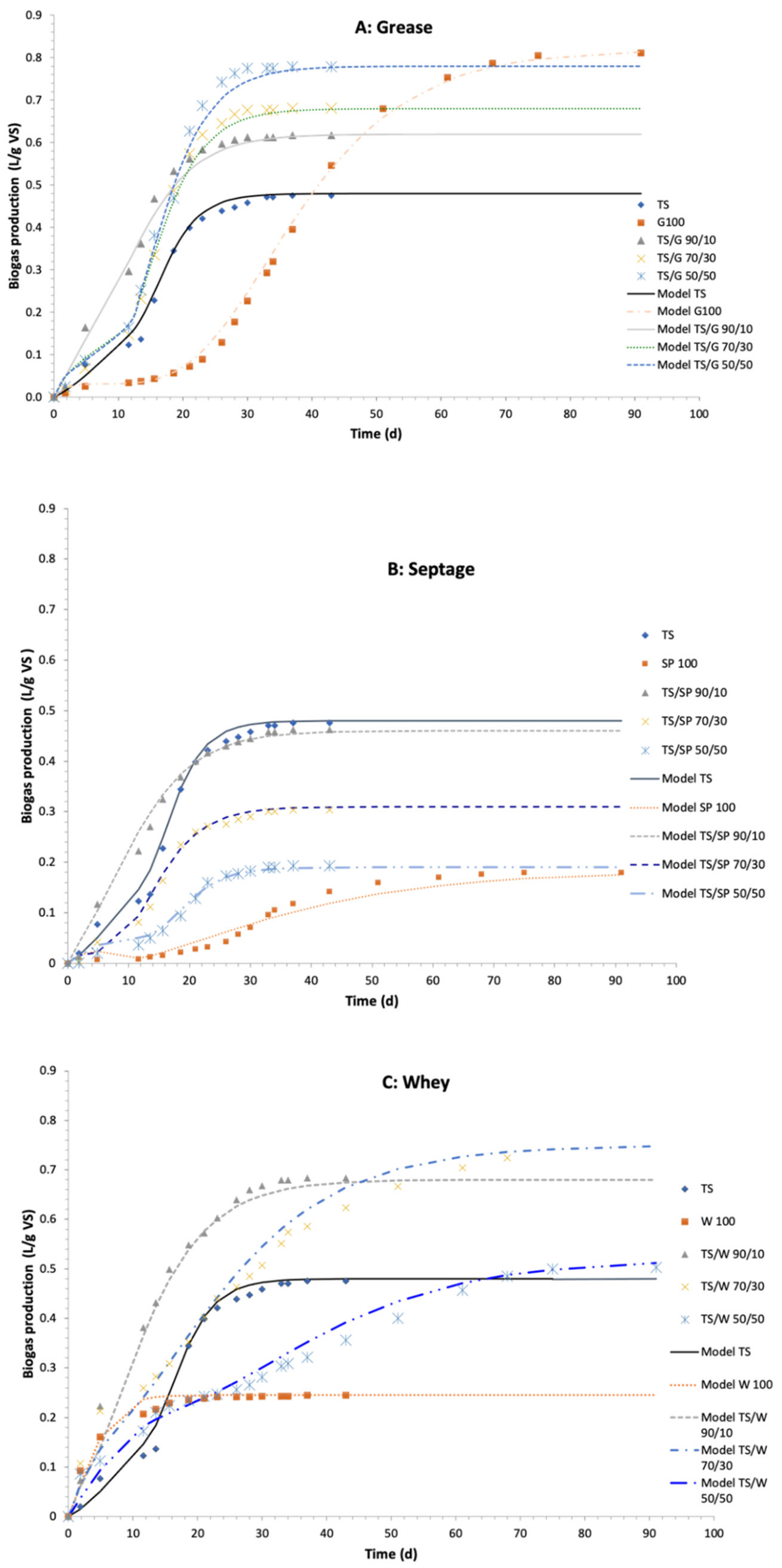
3.1.1. Thickened Sludge and Grease Mixtures
3.1.2. Thickened Sludge and Septage Mixtures
3.1.3. Thickened Sludge and Whey Mixtures
3.1.4. VS Removal Ratio
3.1.5. Methane Production
3.2. Bioreactor Test
3.2.1. Biogas Production
3.2.2. Qualitative Analysis of Biogas
3.2.3. VS and COD Removal
3.2.4. VFA Measurements
3.3. Modeling with ADM1
3.3.1. Biogas Production Per Reactor
3.3.2. Biogas Composition
3.3.3. pH
3.3.4. Microbial Degraders and Population Distribution Profiles Obtained from the Simulation with ADM1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GREENBIRDIE; CRIGEN. Evaluation du Potentiel de Production de Biométhane à Partir des Boues Issues des Stations D’épuration des Eaux Usées Urbaines. 2015, p. 93. Available online: https://www.ademe.fr/sites/default/files/assets/documents/potentiel-production-biomethane-boues-steu-201409-rapport-final.pdf (accessed on 1 June 2021).
- Hreiz, R.; Latifi, M.A.; Roche, N. Optimal design and operation of activated sludge processes: State-of-the-art. Chem. Eng. J. 2015, 281, 900–920. [Google Scholar] [CrossRef] [Green Version]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Falipou, E.; Gillot, S.; Canler, J.P.; Perret, J.M. Anaerobic digestion of sewage sludge: State of the art for France. WRRF TSM 2020, 9, 57–69. [Google Scholar] [CrossRef]
- Agence de L’eau. Rhône Méditerranée Corse Méthanisation des Boues de Station D’épuration: Règle de L’art et Etat des Lieux sur les Bassins Rhône-Méditerranée et Corse; Decembre 2012; p. 56, report. Available online: https://www.eaurmc.fr/jcms/dma_40468/fr/methanisation-des-boues-de-stations-regle-de-l-art-et-etat-des-lieux-sur-les-bassins-rhone-mediterranee-et-corse (accessed on 1 May 2021).
- Sosnowski, P.; Wieczorek, A.; Ledakowicz, S. Anaerobic co-digestion of sewage sludge and organic fraction of municipal solid wastes. Adv. Environ. Res. 2003, 7, 609–616. [Google Scholar] [CrossRef]
- Edelmann, W.; Engeli, H.; Gradenecker, M. Co-digestion of organic solid waste and sludge from sewage treatment. Water Sci. Technol. 2000, 41, 213–221. [Google Scholar] [CrossRef]
- Grosser, A.; Neczaj, E.; Singh, B.R.; Almås, Å.R.; Brattebø, H.; Kacprzak, M. Anaerobic digestion of sewage sludge with grease trap sludge and municipal solid waste as co-substrates. Environ. Res. 2017, 155, 249–260. [Google Scholar] [CrossRef]
- Soni, M.N. Investigating the Inhibitory Effects of Fats, Oils and Grease Addition on Fatty Acids Degradation during Anaerobic Co-Digestion. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2018. Available online: https://tigerprints.clemson.edu/all_theses/2881 (accessed on 1 May 2021).
- Chow, W.L.; Chong, S.; Lim, J.W.; Chan, Y.J.; Chong, M.F.; Tiong, T.J.; Chin, J.K.; Pan, G.-T. Anaerobic Co-Digestion of Wastewater Sludge: A Review of Potential Co-Substrates and Operating Factors for Improved Methane Yield. Processes 2020, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. Anaerobic co-digestion of food waste and FOG with sewage sludge—Realising its potential in Ireland. Int. J. Environ. Stud. 2018, 75, 496–517. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, B.; Yao, F. Biogas production from anaerobic co-digestion of waste activated sludge: Co-substrates and influencing parameters. Rev. Environ. Sci. Biotechnol. 2019, 18, 771–793. [Google Scholar] [CrossRef]
- Dar, R.A.; Parmar, M.; Dar, E.A.; Sani, R.K.; Phutela, U.G. Biomethanation of agricultural residues: Potential, limitations and possible solutions. Renew. Sustain. Energy Rev. 2021, 135, 110217. [Google Scholar] [CrossRef]
- Batstone, J.; Keller, J.; Angledaki, I.; Kalyuzhnyi, V.S.; Pavlostathis, G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.; Vavilin, V.A. The IWA Anaerobic Digestion Model No 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Rosén, C.; Jeppsson, U. Aspects on ADM1 Implementation within the BSM2 Framework; Department of Industrial Electrical Engineering and Automation, Lund Institute of Technology: Lund, Sweden, 2005; (TEIE.; Volume 7224); Available online: http://www.iea.lth.se/publications/Reports/LTH-IEA-7224.pdf (accessed on 1 March 2021).
- Hidaka, T.; Wang, F.; Tsumori, J. Comparative evaluation of anaerobic digestion for sewage sludge and various organic wastes with simple modeling. Waste Manag. 2015, 43, 144–151. [Google Scholar] [CrossRef]
- Wade, M.J. Not Just Numbers: Mathematical Modelling and Its Contribution to Anaerobic Digestion Processes. Processes 2020, 8, 888. [Google Scholar] [CrossRef]
- Holliger, V.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Envir. Sci. Biotech. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Waste Water; American Public Health Association: Washington, DC, USA, 1998; 874p. [Google Scholar]
- Filer, J.; Ding, H.H.; Chang, S. Biochemical Methane Potential (BMP) Assay Method for Anaerobic Digestion Research. Water 2019, 11, 921. [Google Scholar] [CrossRef] [Green Version]
- Grady, C.L., Jr.; Daigger, G.T.; Love, N.G.; Filipe, C.D. Biological Wastewater Treatment, 3rd ed.; CRC: Boca Raton, FL, USA, 2011. [Google Scholar]
- Metcalf & Eddy; Abu-Orf, M.; Bowden, G.; Burton, F.L.; Pfrang, W.; Stensel, H.D.; Tchobanoglous, G.; Tsuchihashi, R.; AECOM (Firm). Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Reichert, P.; Schulthess, R.V.; Wild, D. The use of AQUASIM for estimating parameters of activated sludge models. Water Sci. Technol. 1995, 31, 135–147. [Google Scholar] [CrossRef]
- Reichert, P. AQUASIM 2.0 User Manual; EAWAG Report; Swiss Federal Institute for Environmental Science and Technology: Dübendorf, Switzerland, 1998. [Google Scholar]
- Derbal, K.; Bencheikh-Lehocine, M.; Cecchi, F.; Meniai, A.-H.; Pavan, P. Application of the IWA ADM1 model to simulate anaerobic co-digestion of organic waste with waste activated sludge in mesophilic condition. Bioresour. Technol. 2009, 100, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Arnell, M.; Astals, S.; Åmand, L.; Batstone, D.J.; Jensen, P.D.; Jeppsson, U. Modelling anaerobic co-digestion in Benchmark Simulation Model No. 2: Parameter estimation, substrate characterisation and plant-wide integration. Water Res. 2016, 98, 138–146, ISSN 0043-1354. [Google Scholar] [CrossRef] [Green Version]
- Keucken, A.; Habagil, M.; Batstone, D.; Jeppsson, U.; Arnell, M. Anaerobic Co-Digestion of Sludge and Organic Food Waste—Performance, Inhibition, and Impact on the Microbial Community. Energies 2018, 11, 2325. [Google Scholar] [CrossRef] [Green Version]
- Fisgativa, H.; Zennaro, B.; Charnier, C.; Richard, C.; Accarion, G.; Béline, F. Comprehensive determination of input state variables dataset required for anaerobic digestion modelling (ADM1) based on characterisation of organic substrates. Data Brief 2020, 29, 105212, ISSN 2352-3409. [Google Scholar] [CrossRef] [PubMed]
- Champagne, C.; Li, P.; Anderson, B.C. Enhanced biogas production from anaerobic co-digestion of municipal wastewater treatment sludge and fat, oil and grease (FOG) by a modified two-stage thermophilic digester system with selected thermo-chemical pre-treatment. Renew. Energy 2015, 83, 474–482, ISSN 0960-1481. [Google Scholar]
- Luostarinen, S.; Luste, S.; Sillanpää, M. Increased biogas production at wastewater treatment plants through co-digestion of sewage sludge with grease trap sludge from a meat processing plant. Bioresour. Technol. 2009, 100, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Moletta, R. La Methanisation, 3rd ed.; Editions Lavoisier: Paris, France, 2015; 513p. [Google Scholar]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. A Review of the Role of Critical Parameters in the Design and Operation of Biogas Production Plants. Appl. Sci. 2019, 9, 1915. [Google Scholar] [CrossRef] [Green Version]
- Long, J.H.; Aziz, T.N.; Francis, L., III; Ducoste, J.J. Anaerobic co-digestion of fat, oil, and grease (FOG): A review of gas production and process limitations. Process. Saf. Environ. Prot. 2012, 90, 231–245. [Google Scholar] [CrossRef]
- Poulsen, T.G.; Adelard, L. Improving biogas quality and methane yield via co-digestion of agricultural and urban biomass wastes. Waste Manag. 2016, 54, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Solon, K.; Flores-Alsina, X.; Gernaey, K.V.; Jeppsson, U. Effects of influent fractionation, kinetics, stoichiometry and mass transfer on CH4, H2 and CO2 production for (plant-wide) modeling of anaerobic digesters. Water Sci. Technol. 2015, 71, 870–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galí, A.; Benabdallah, T.; Astals, S.; Mata-Alvarez, J. Modified version of ADM1 model for agro-waste application. Bioresour. Technol. 2009, 100, 2783–2790. [Google Scholar] [CrossRef]
- Amha, Y.M.; Sinha, P.; Lagman, J.; Gregori, M.; Smith, A.L. Elucidating microbial community adaptation to anaerobic co-digestion of fats, oils, and grease and food waste. Water Res. 2017, 123, 277–289. [Google Scholar] [CrossRef]
- Tandukar, M.; Pavlostathis, S.G. Co-digestion of municipal sludge and external wastes for enhanced biogas production under realistic plant constraints. Water Res. 2015, 87, 432–445. [Google Scholar] [CrossRef]
- Wang, L.; Aziz, T.N.; de los Reyes, F.L. Determining the limits of anaerobic co-digestion of thickened waste activated sludge with grease interceptor waste. Water Res. 2013, 47, 3835–3844. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Zha, L.; Abomohra, A.E.; Li, X.; Zhang, C.; Salama, E.S. Evaluation of animal- and plant-based lipidic waste in anaerobic digestion: Kinetics of long-chain fatty acids degradation. Crit. Rev. Biotechnol. 2020, 40, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Merlin, G.; Boileau, H. Anaerobic digestion of agricultural waste: State of the art and future trends. In Anaerobic Digestion: Types, Processes and Environmental Impact; Torres, A., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2013. [Google Scholar]
- Merlin, G.; Kohler, F.; Bouvier, M.; Lissolo, T.; Boileau, H. Importance of heat transfer in an anaerobic digestion plant in a continental climate context. Bioresour. Technol. 2012, 124, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Elalami, D.; Carrere, H.; Monlau, F.; Abdelouahdi, K.; Oukarroum, A.; Barakat, A. Pretreatment and co-digestion of wastewater sludge for biogas production: Recent research advances and trends. Renew. Sustain. Energy Rev. 2019, 114, 109287. [Google Scholar] [CrossRef]
- Fang, C.; Boe, K.; Angelidaki, I. Biogas production from potato-juice, a byproduct from potato-starch processing, in upflow anaerobic sludge blanket (UASB) and expanded granular sludge bed (EGSB) reactors. Bioresour. Technol. 2011, 102, 5734–5741. [Google Scholar] [CrossRef] [PubMed]
- Traversi, D.; Bonetta, S.; Degan, R.; Villa, S.; Porfido, A.; Bellero, M.; Carraro, E.; Gilli, G. Environmental advances due to the integration of food industries and anaerobic digestion for biogas production: Perspectives of the italian milk and dairy product Sector. Bioenergy Res. 2013, 6, 851–863. [Google Scholar] [CrossRef]
- Hagen, L.H.; Vivekanand, V.; Linjordet, R.; Pope, P.B.; Eijsink, V.G.; Horn, S.J. Microbial community structure and dynamics during co-digestion of whey permeate and cow manure in continuous stirred tank reactor systems. Bioresour. Technol. 2014, 171, 350–359. [Google Scholar] [CrossRef]
- Siciliano, A.; Limonti, C.; Curcio, G.M.; Calabrò, V. Biogas Generation through Anaerobic Digestion of Compost Leachate in Semi-Continuous Completely Stirred Tank Reactors. Processes 2019, 7, 635. [Google Scholar] [CrossRef] [Green Version]
- Hublin, A.; Zelić, B. Modelling of the whey and cow manure co-digestion process. Waste Manag. Res. 2013, 31, 353–360. [Google Scholar] [CrossRef]
- Vivekanand, D.; Mulat, G.; Eijsink, V.G.H.; Horn, S.J. Synergistic effects of anaerobic co-digestion of whey, manure and fish ensilage. Bioresour. Technol. 2018, 249, 35–41. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496, ISSN 1364-0321. [Google Scholar] [CrossRef]
- García-Gen, S.; Lema, J.M.; Rodríguez, J. Generalised modelling approach for anaerobic co-digestion of fermentable substrates. Bioresour. Technol. 2013, 147, 525–533. [Google Scholar] [CrossRef]
- Yang, S.T.; Tang, I.C. Methanogenesis from lactate by a co-culture of Clostridium formicoaceticum and Methanosarcina mazei. Appl. Microbiol. Biotechnol. 1991, 35, 119–123. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Ortiga, V.; Lesty, Y.; Vanden Bossche, H.; Olivares, D. Addressing the synergy determination in anaerobic co-digestion and the inoculum activity impact on BMP test. Water Sci. Technol. 2019, 80, 387–396. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Ortega, V.; Lesty, Y.; Vanden Bossche, H.; Olivares, D. Assessing the stability of anaerobic co-digestion in the context of a WWTP with thermal pre-treatment of sewage sludge. Case study in Chile. Waste Manag. 2019, 100, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Siatou, A.; Manali, A.; Gikas, P. Energy Consumption and Internal Distribution in Activated Sludge Wastewater Treatment Plants of Greece. Water 2020, 12, 1204. [Google Scholar] [CrossRef]





| Inoculum | Thickened Sludge | Grease | Septage | Whey | |
|---|---|---|---|---|---|
| DM (g·L−1) | 36.5 ± 0.5 | 36.8 ± 3.2 | 44.8 ± 0.5 | 42.3 ± 3.3 | 55.8 ± 3.8 |
| VS (g·L−1) | 22.2 ± 0.3 | 25.6 ± 0.3 | 37.2 ± 0.3 | 26.3 ± 0.4 | 48 ± 0.5 |
| COD (g·L−1) | 42.4 ± 2 | 65.9 ± 2.8 | 72.5 ± 12.9 | 23.2 ± 1.12 | 69.4 ± 4 |
| COD/VS | 1.9 | 2.57 | 1.95 | 0.88 | 1.45 |
| * BMP Reactor | λ1 (d) | λ2 (d) | k1 (LgVS−1d−1) | k2 (LgVS−1d−1) | K1 (LgVS−1) | K2 (LgVS−1) | R2 |
|---|---|---|---|---|---|---|---|
| TS 100 | 1.5 | 13 | 0.015 | 0.051 | 0.2 | 0.48 | 0.993 |
| G 100 | 0 | 21 | 0.012 | 0.025 | 0.03 | 0.82 | 0.998 |
| TS/G 90/10 | 0 | 6.5 | 0.027 | 0.04 | 0.18 | 0.62 | 0.993 |
| TS/G 70/30 | 0 | 10.4 | 0.027 | 0.054 | 0.1 | 0.68 | 0.997 |
| TS/G 50/50 | 0 | 10.4 | 0.028 | 0.058 | 0.09 | 0.78 | 0.996 |
| SP 100 | 1 | 8 | 0.004 | 0.017 | 0.22 | 0.05 | 0.977 |
| TS/SP 90/10 | 0 | 6.5 | 0.02 | 0.026 | 0.24 | 0.46 | 0.993 |
| TS/SP 70/30 | 0 | 8.2 | 0.01 | 0.023 | 0.02 | 0.31 | 0.993 |
| TS/SP 50/50 | 0 | 14.1 | 0.008 | 0.017 | 0.05 | 0.19 | 0.995 |
| W 100 | 0 | NaN | 0.034 | NaN | 0.245 | NaN | 0.975 |
| TS/W 90/10 | 1 | NaN | 0.035 | NaN | 0.68 | NaN | 0.993 |
| TS/W 70/30 | 0 | 4 | 0.021 | 0.021 | 0.1 | 0.75 | 0.991 |
| TS/W 50/50 | 0 | 16.3 | 0.019 | 0.012 | 0.2 | 0.52 | 0.985 |
| * BMP Reactor | λ1 | λ2 | k1 | k2 | K1 | K2 | Score |
|---|---|---|---|---|---|---|---|
| TS 100 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| G 100 | −1.00 | 0.62 | −0.20 | −0.51 | −0.85 | 0.71 | −0.12 |
| TS/G 90/10 | −1.00 | −0.50 | 0.80 | −0.22 | −0.10 | 0.29 | 0.39 |
| TS/G 70/30 | −1.00 | −0.20 | 0.80 | 0.06 | −0.50 | 0.42 | 0.30 |
| TS/G 50/50 | −1.00 | −0.20 | 0.87 | 0.14 | −0.55 | 0.63 | 0.35 |
| SP 100 | −0.33 | −0.38 | −0.73 | −0.67 | 0.10 | −0.90 | −0.26 |
| TS/SP 90/10 | −1.00 | −0.50 | 0.33 | −0.49 | 0.20 | −0.04 | 0.25 |
| TS/SP 70/30 | −1.00 | −0.37 | −0.33 | −0.55 | −0.90 | −0.35 | −0.21 |
| TS/SP 50/50 | −1.00 | 0.08 | −0.47 | −0.67 | −0.75 | −0.60 | −0.35 |
| W 100 | −1.00 | NaN | 1.27 | NaN | 0.23 | NaN | 0.58 |
| TS/W 90/10 | −0.33 | NaN | 1.33 | NaN | 2.40 | NaN | 1.39 |
| TS/W 70/30 | −1.00 | −0.69 | 0.40 | −0.59 | −0.50 | 0.56 | 0.29 |
| TS/W 50/50 | −1.00 | 0.25 | 0.27 | −0.76 | 0.00 | 0.08 | 0.04 |
| % CH4 | normoL CH4 gVSr−1 | |||
|---|---|---|---|---|
| BMP Reactor | Average | SD | Average | SD |
| TS 100 | 62.13 | 9.3 | 0.416 | 0.085 |
| G 100 | 34.40 | 14.9 | 0.327 | 0.171 |
| TS/G 90/10 | 69.85 | 1.6 | 0.669 | 0.082 |
| TS/G 70/30 | 67.86 | 4.6 | 0.657 | 0.041 |
| TS/G 50/50 | 62.34 | 17.3 | 0.699 | 0.109 |
| SP 100 | 41.25 | 14.4 | 0.140 | 0.053 |
| TS/SP 90/10 | 69.16 | 2.2 | 0.453 | 0.114 |
| TS/SP 70/30 | 70.02 | 0.6 | 0.247 | 0.023 |
| TS/SP 50/50 | 65.38 | 12.2 | 0.160 | 0.038 |
| W 100 | 4.26 | 1.6 | 0.016 | 0.003 |
| TS/W 90/10 | 62.06 | 5.9 | 0.719 | 0.258 |
| TS/W 70/30 | 55.05 | 13 | 0.658 | 0.030 |
| TS/W 50/50 | 43.95 | 2.1 | 0.296 | 0.028 |
| Volumetric Loading Rates | TS | TS/W | TS/G |
|---|---|---|---|
| COD | 2.5 ± 0.6 | 2.5 ± 0.6 | 2.6 ± 0.6 |
| VS | 1.1 ± 0.3 | 1.3 ± 0.5 | 1.3 ± 0.2 |
| DM | 1.8 ± 0.4 | 1.8 ± 0.4 | 1.7 ± 0.2 |
| Biogas Composition (%) | TS | TS/W | TS/G |
|---|---|---|---|
| CH4 | 71.3 ± 1.3 | 69.7 ± 5.4 | 68.7 ± 1.5 |
| CO2 | 28.7 ± 1.3 | 30.3 ± 5.4 | 31.3 ± 1.5 |
| Experimental Results (L d−1) | Modeling Results (L d−1) | ||||||
|---|---|---|---|---|---|---|---|
| TS | TS_W | TS_G | TS | TS_W | TS_G | ||
| Before co-substrate addition | Average | 7.32 | 8.81 * | 7.02 | 5.74 | 6.75 | 4.71 |
| ±SD | 1.32 | 0.98 | 0.50 | 2.01 | 2.64 | 1.61 | |
| Median | 6.50 | 9.00 | 6.90 | 5.10 | 5.58 | 3.91 | |
| After co-substrate addition | Average | 11.61 | 11.94 | 9.82 * | 10.01 | 10.18 | 9.11 * |
| ±SD | 1.52 | 2.70 | 2.05 | 1.74 | 1.96 | 1.47 | |
| Median | 11.80 | 12.30 | 9.90 | 10.79 | 10.43 | 9.17 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merlin, G.; Outin, J.; Boileau, H. Co-Digestion of Extended Aeration Sewage Sludge with Whey, Grease and Septage: Experimental and Modeling Determination. Sustainability 2021, 13, 9199. https://doi.org/10.3390/su13169199
Merlin G, Outin J, Boileau H. Co-Digestion of Extended Aeration Sewage Sludge with Whey, Grease and Septage: Experimental and Modeling Determination. Sustainability. 2021; 13(16):9199. https://doi.org/10.3390/su13169199
Chicago/Turabian StyleMerlin, Gérard, Jonathan Outin, and Hervé Boileau. 2021. "Co-Digestion of Extended Aeration Sewage Sludge with Whey, Grease and Septage: Experimental and Modeling Determination" Sustainability 13, no. 16: 9199. https://doi.org/10.3390/su13169199





