Alternative Use of the Insecticide Diofenolan on Musca domestica (Diptera: Muscidae): A Morphological and Ultrastructural Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. House Fly Strain
2.2. Fecundity Bioassays
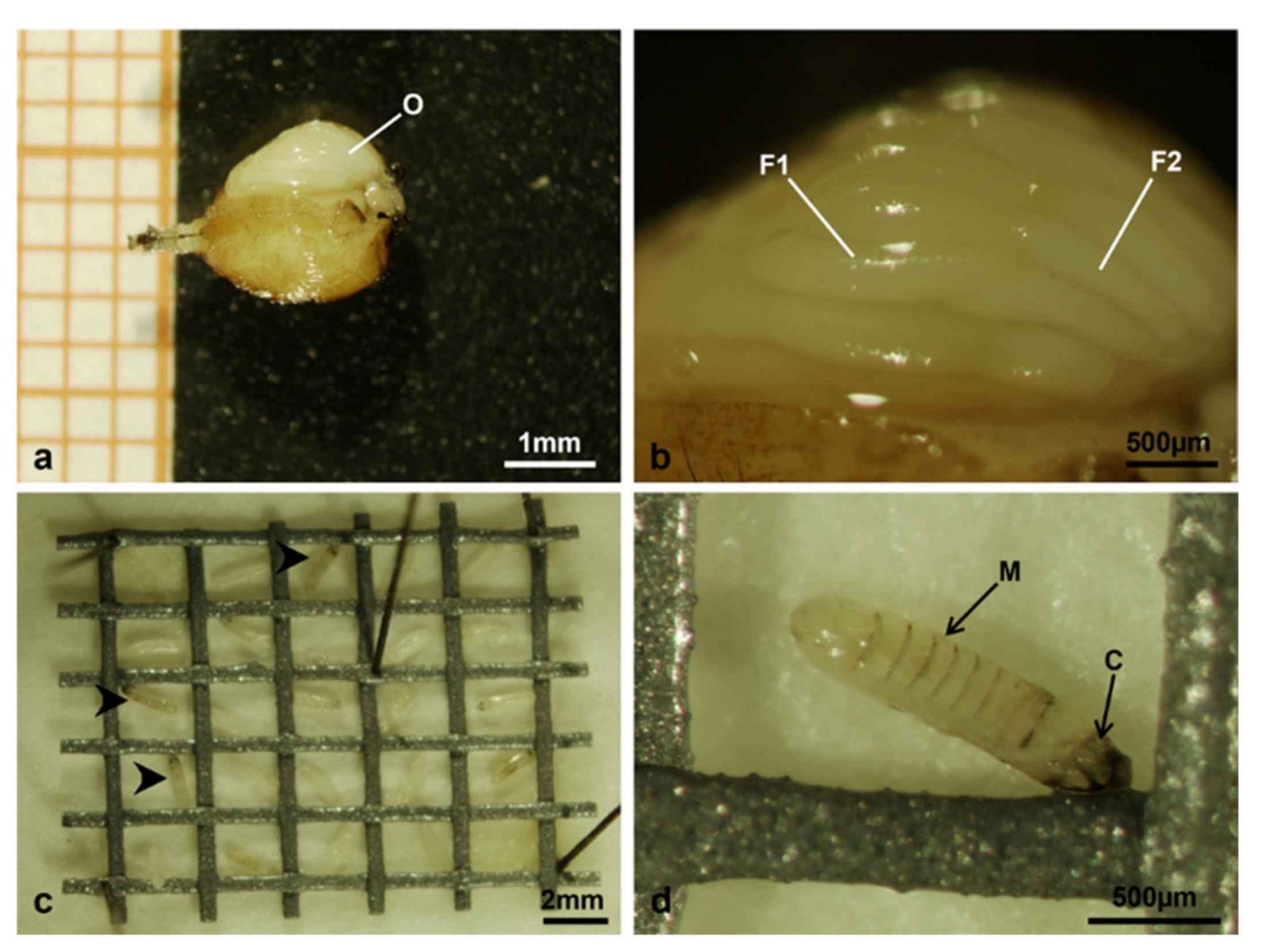
2.3. Stereomicroscope Observations
2.4. Fecundity and Hatching Tests in Egg Deposition Cycles
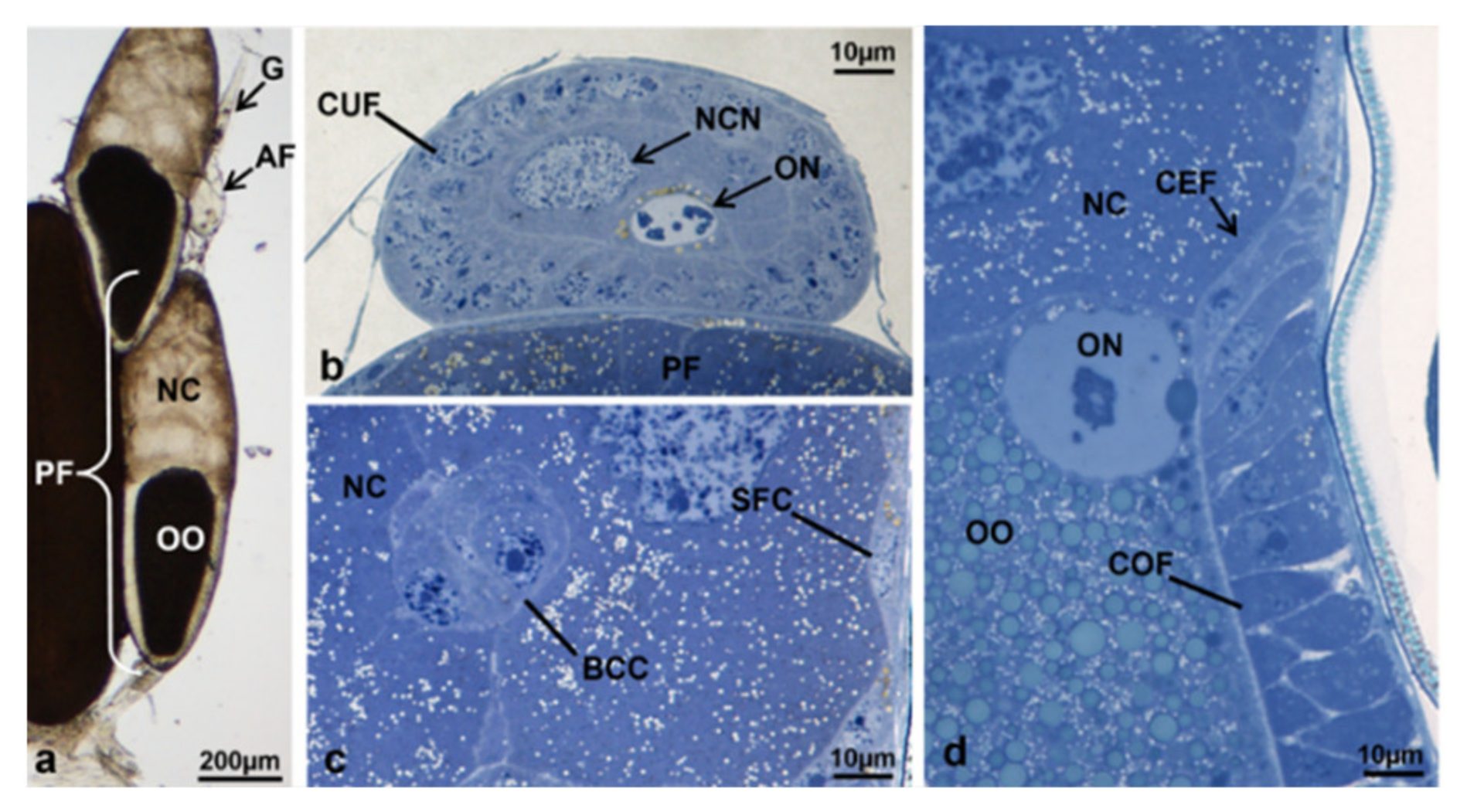
2.5. Analysis of Ovarioles by Optical Microscopy
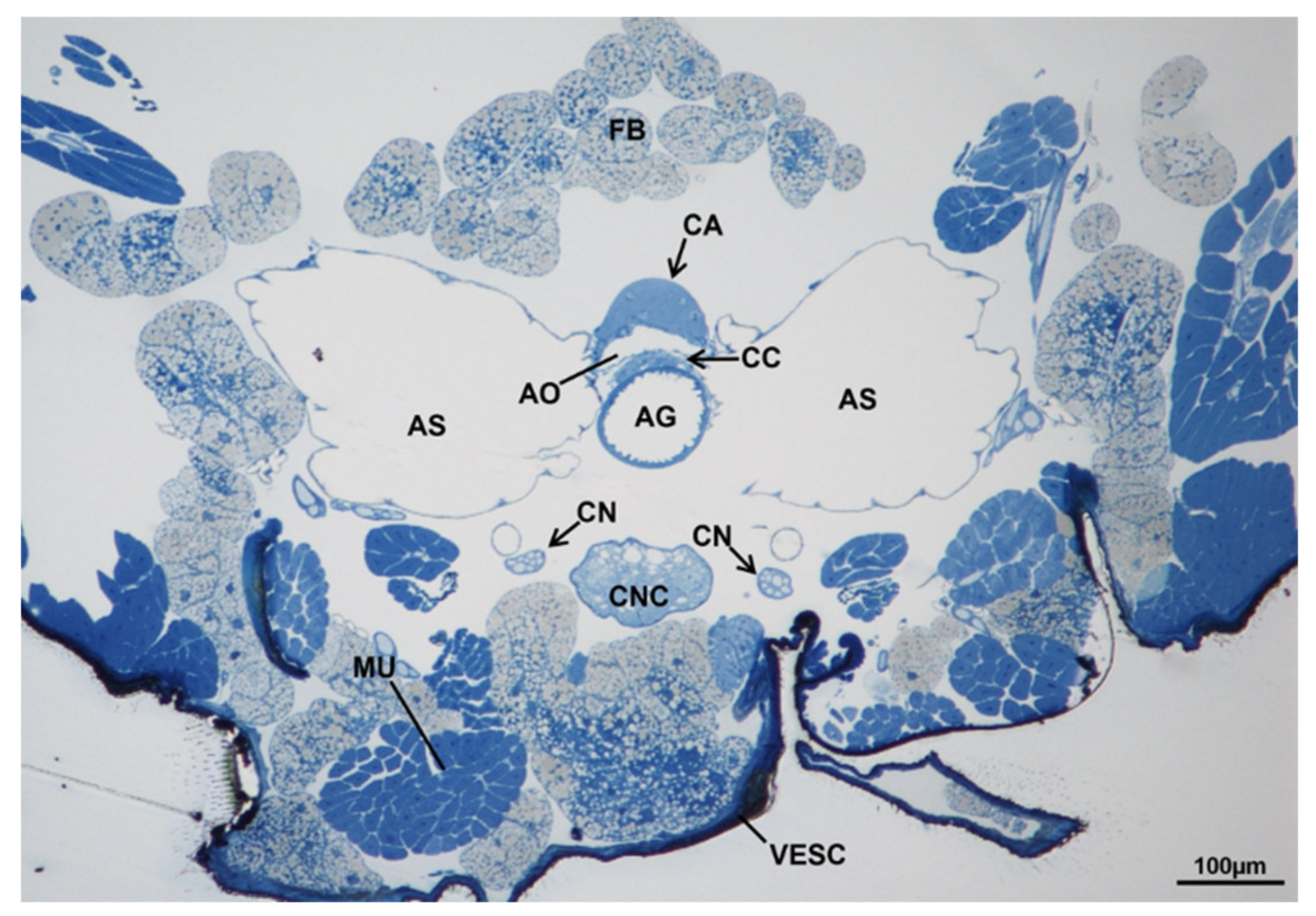
2.6. Mapping of Ovaries
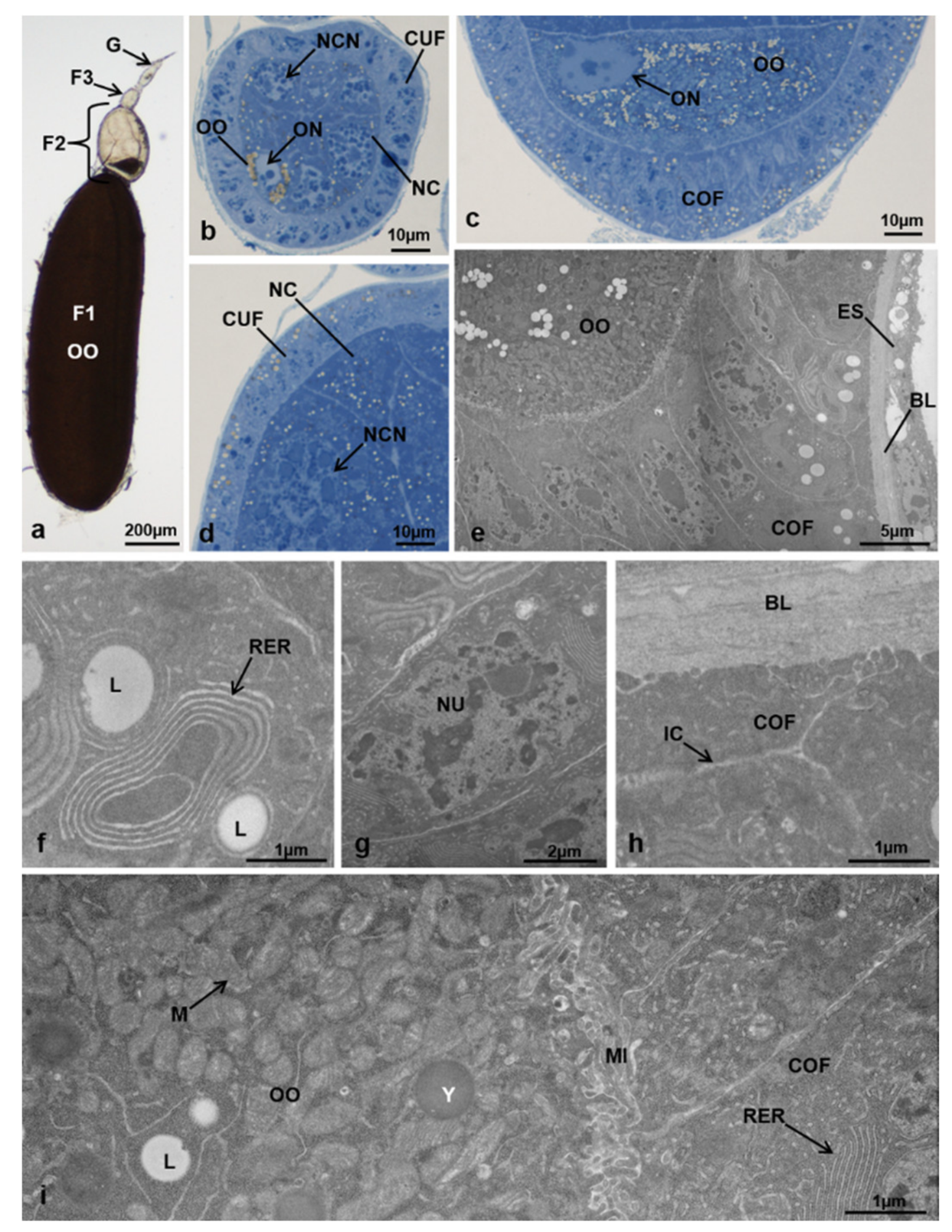
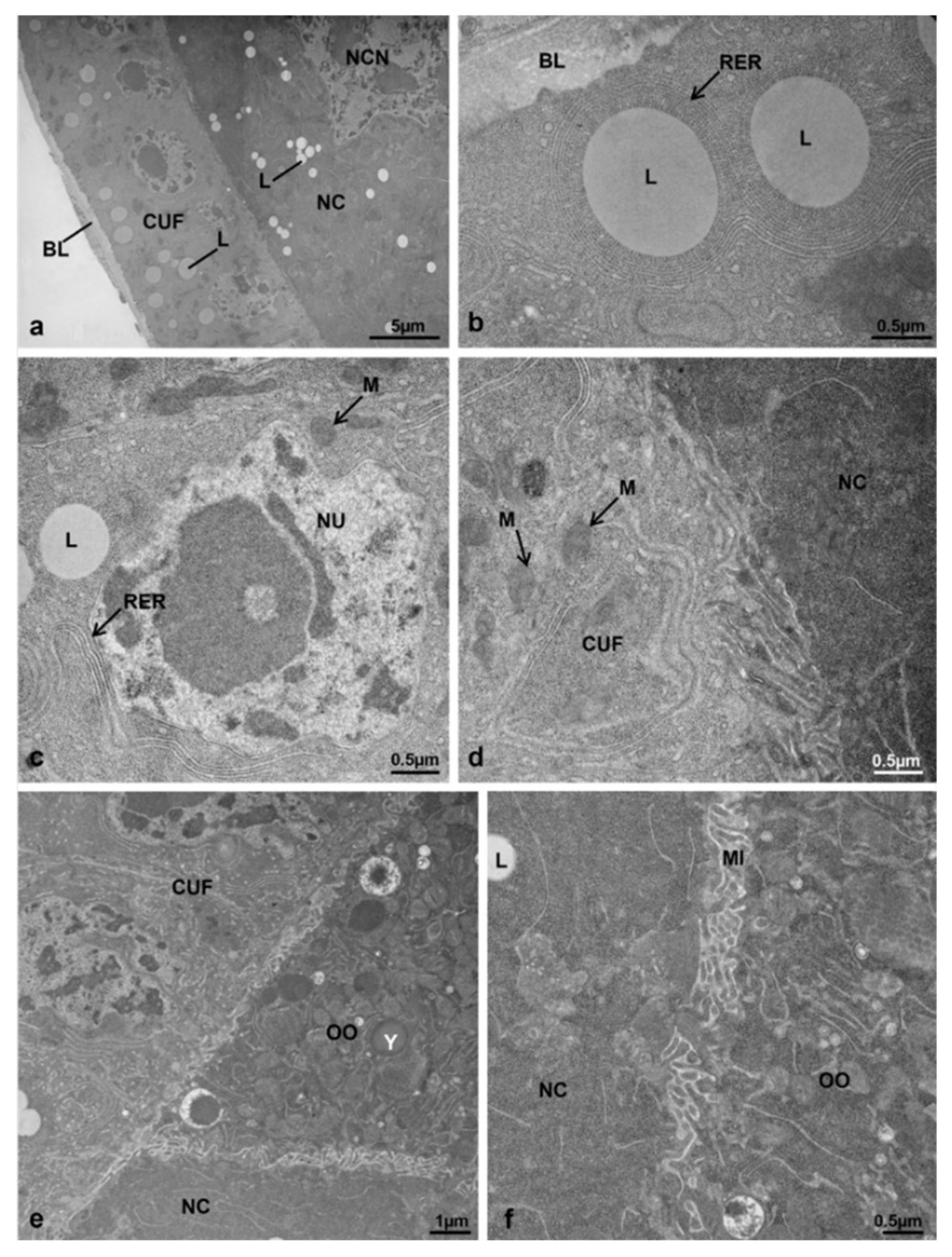
2.7. Analyses on Ovarioles
2.8. Analyses of Corpora Allata
3. Results
3.1. Fecundity Bioassays
3.2. Stereomicroscope Observations
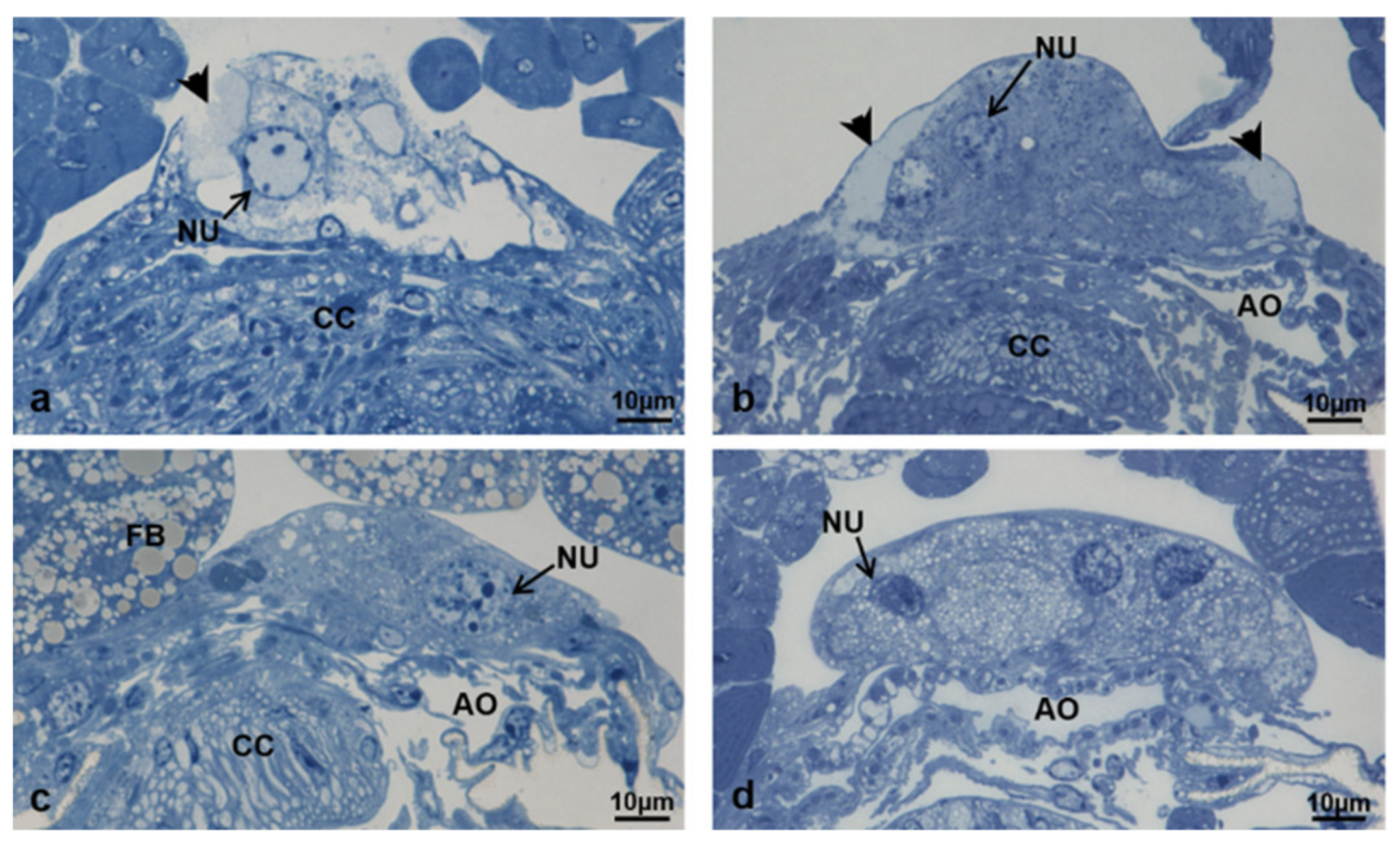
3.3. Ovariole Morphology at Different Exposure Times
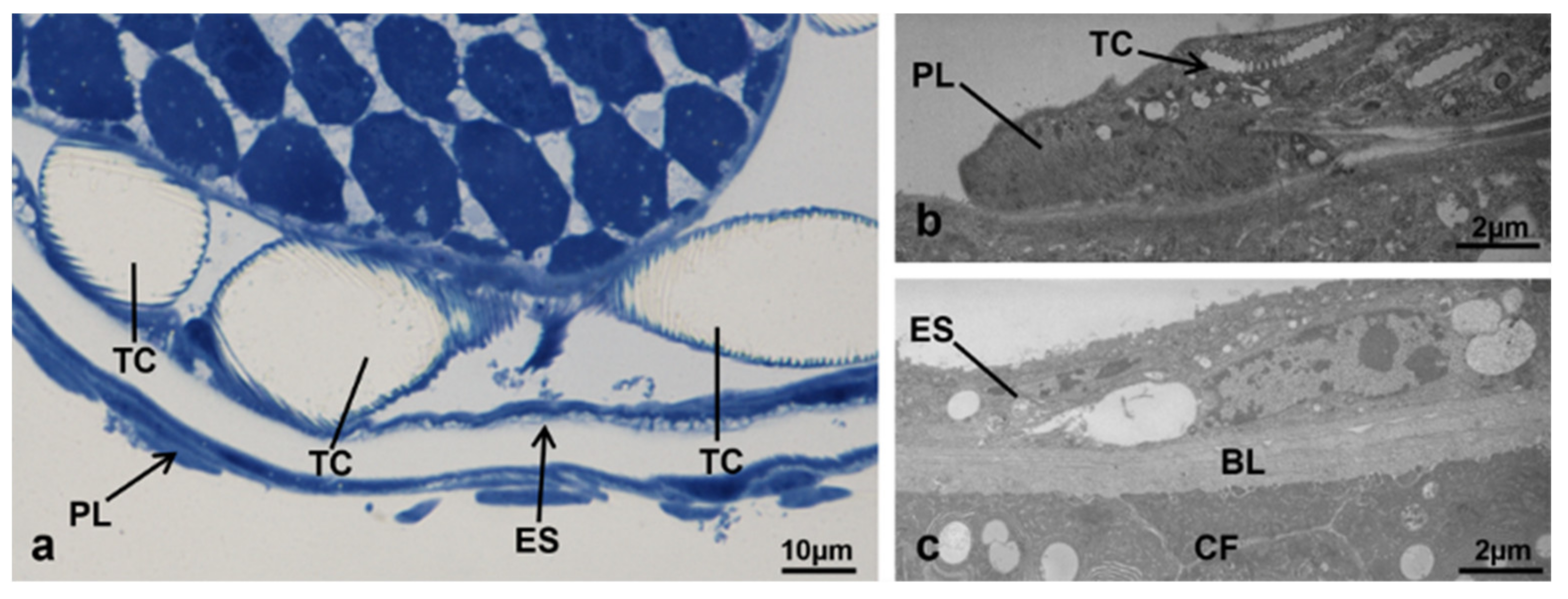
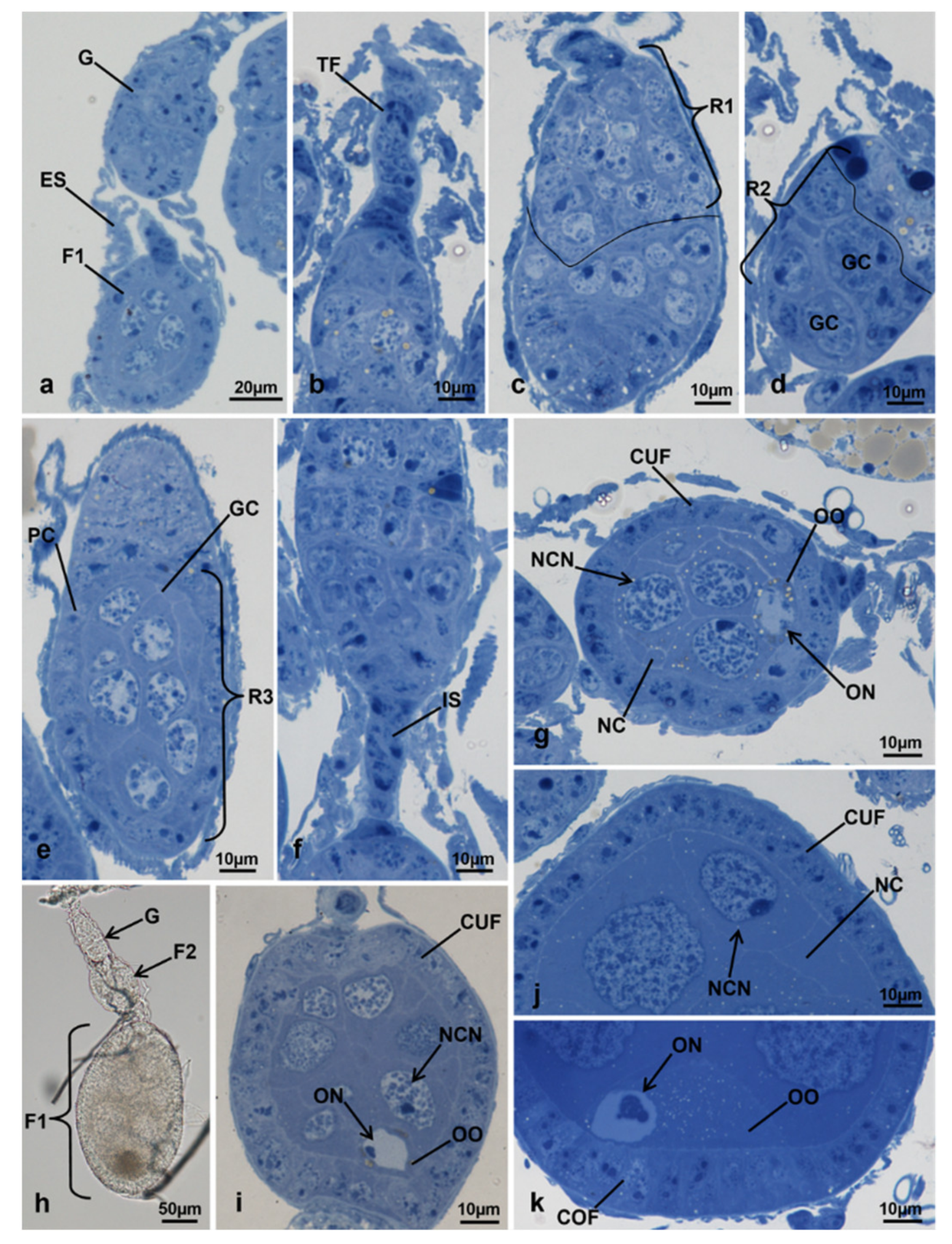
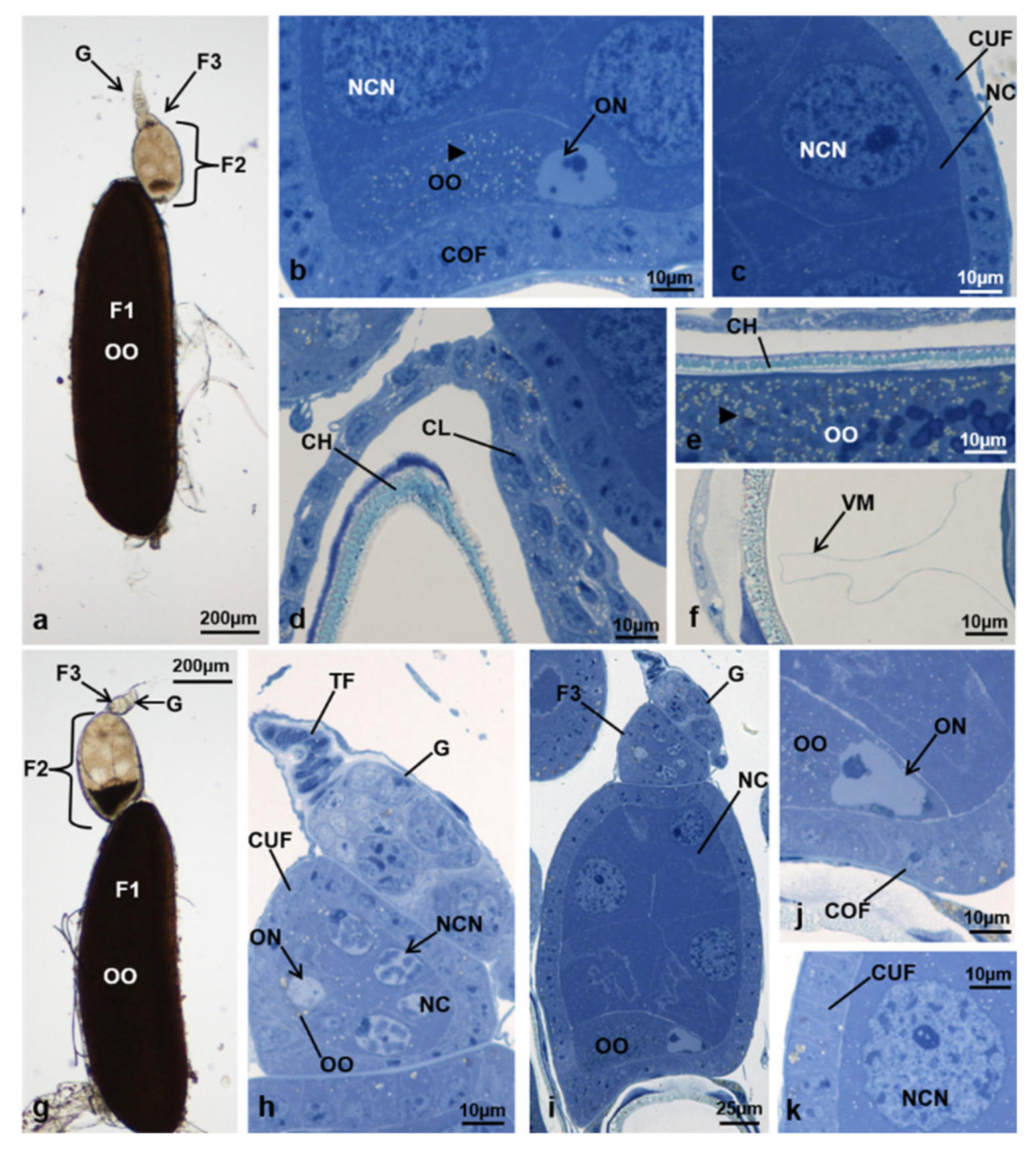
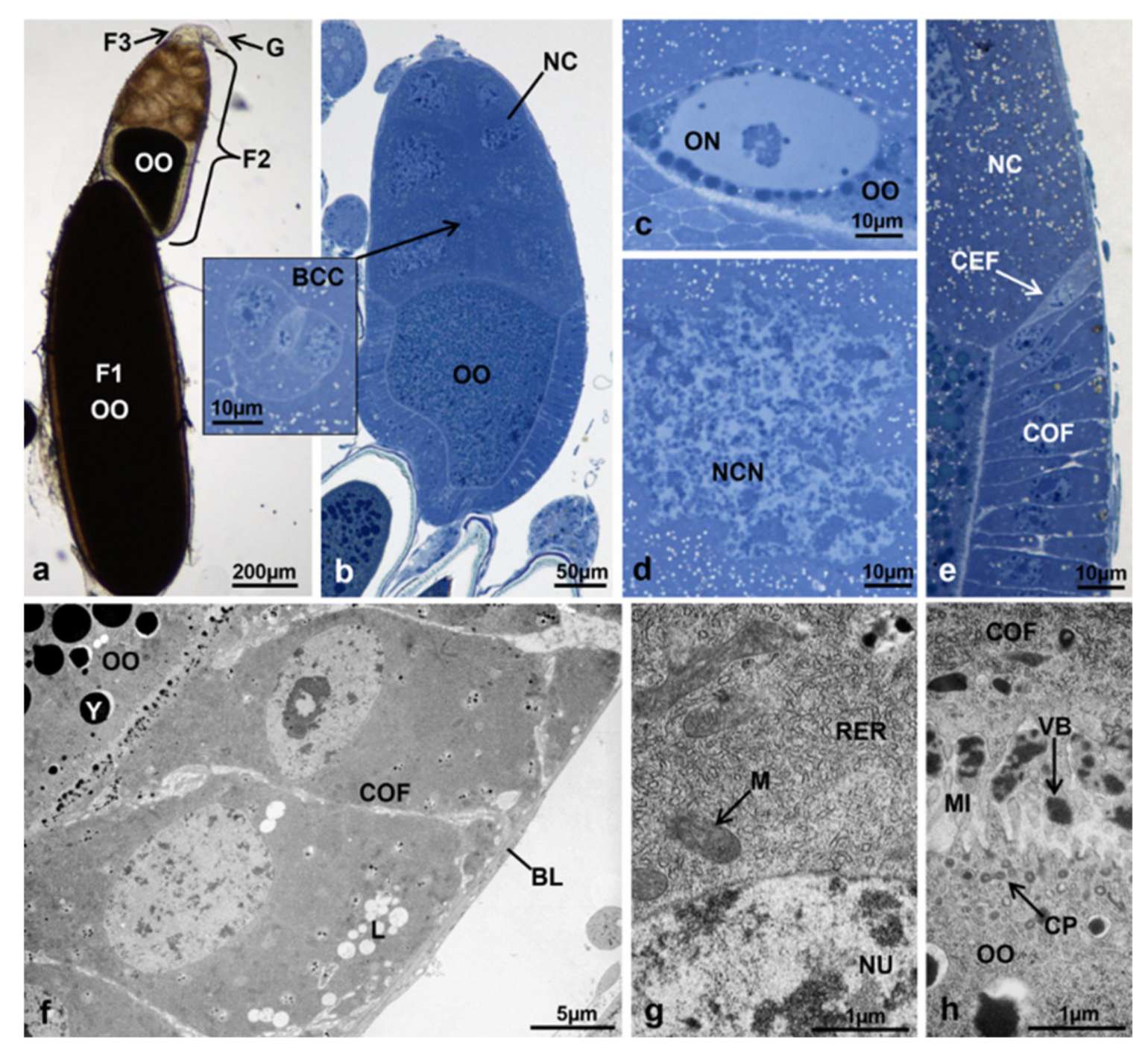
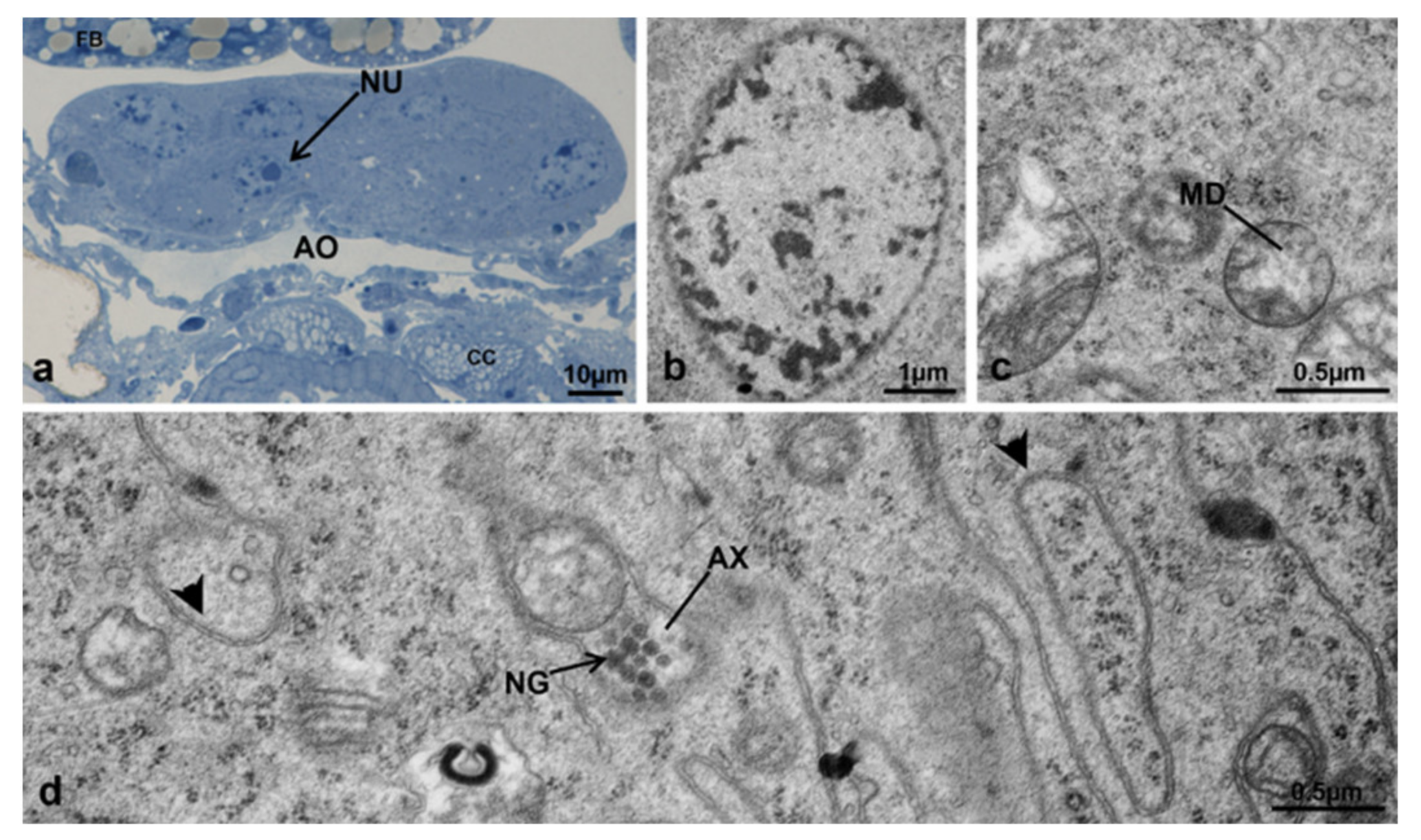
3.3.1. T0 (Emerging from Puparium)
3.3.2. T0t (15 h from Emerging, Topical Treatment)
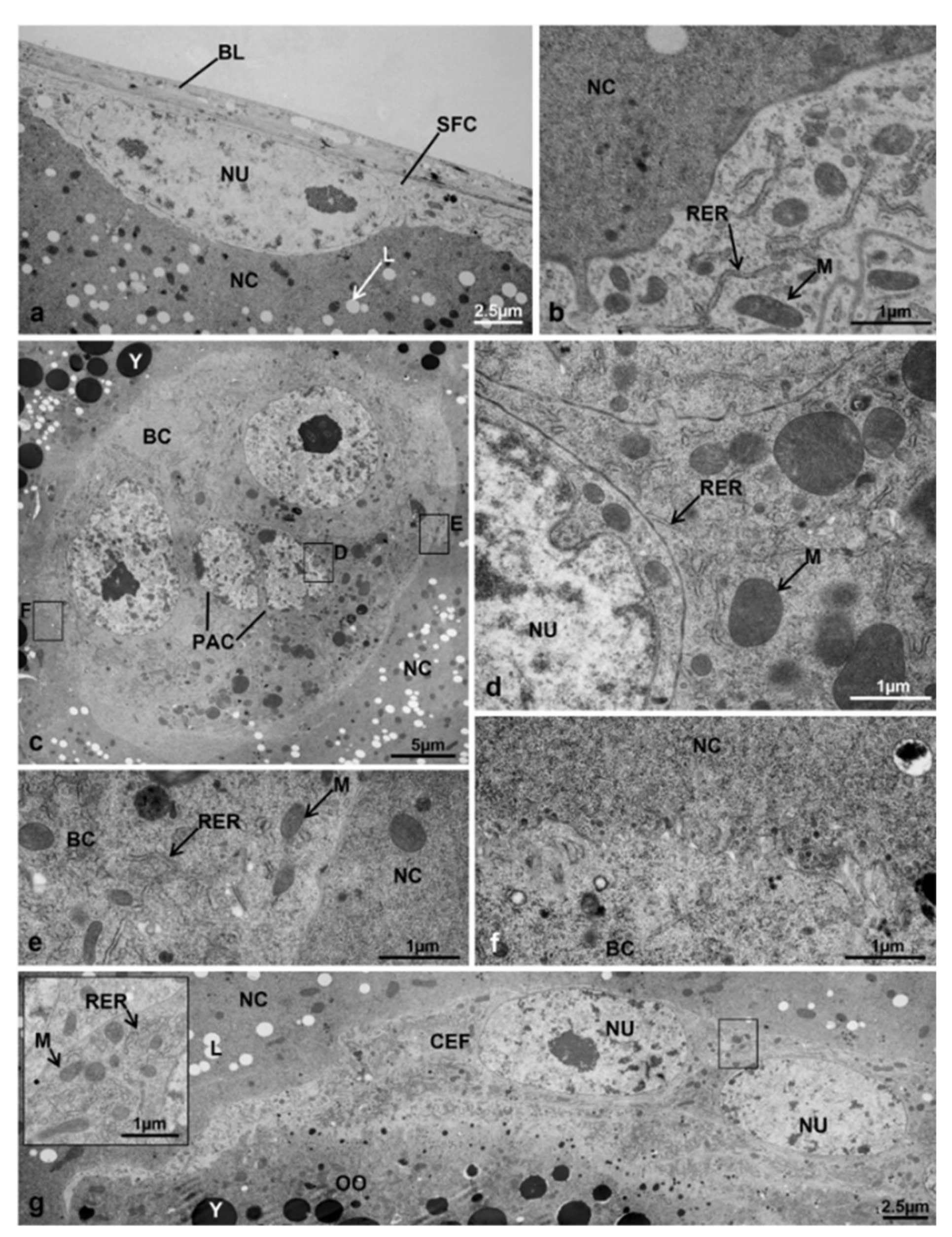
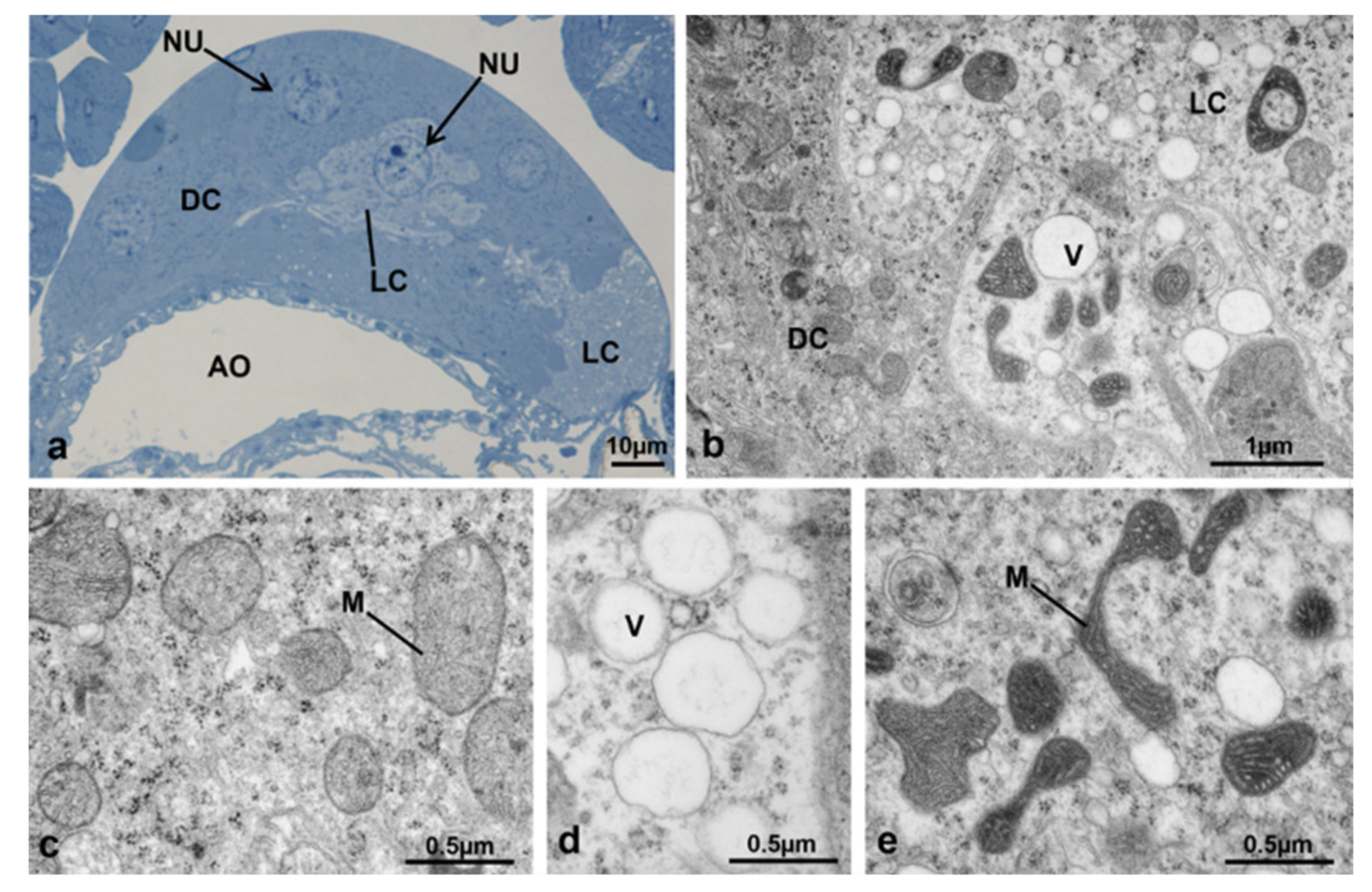
3.3.3. T1 Controls (45 h from Emerging, 30 h after Treatment)
3.3.4. T1 Treated (45 h from Emerging, 30 h after Treatment)
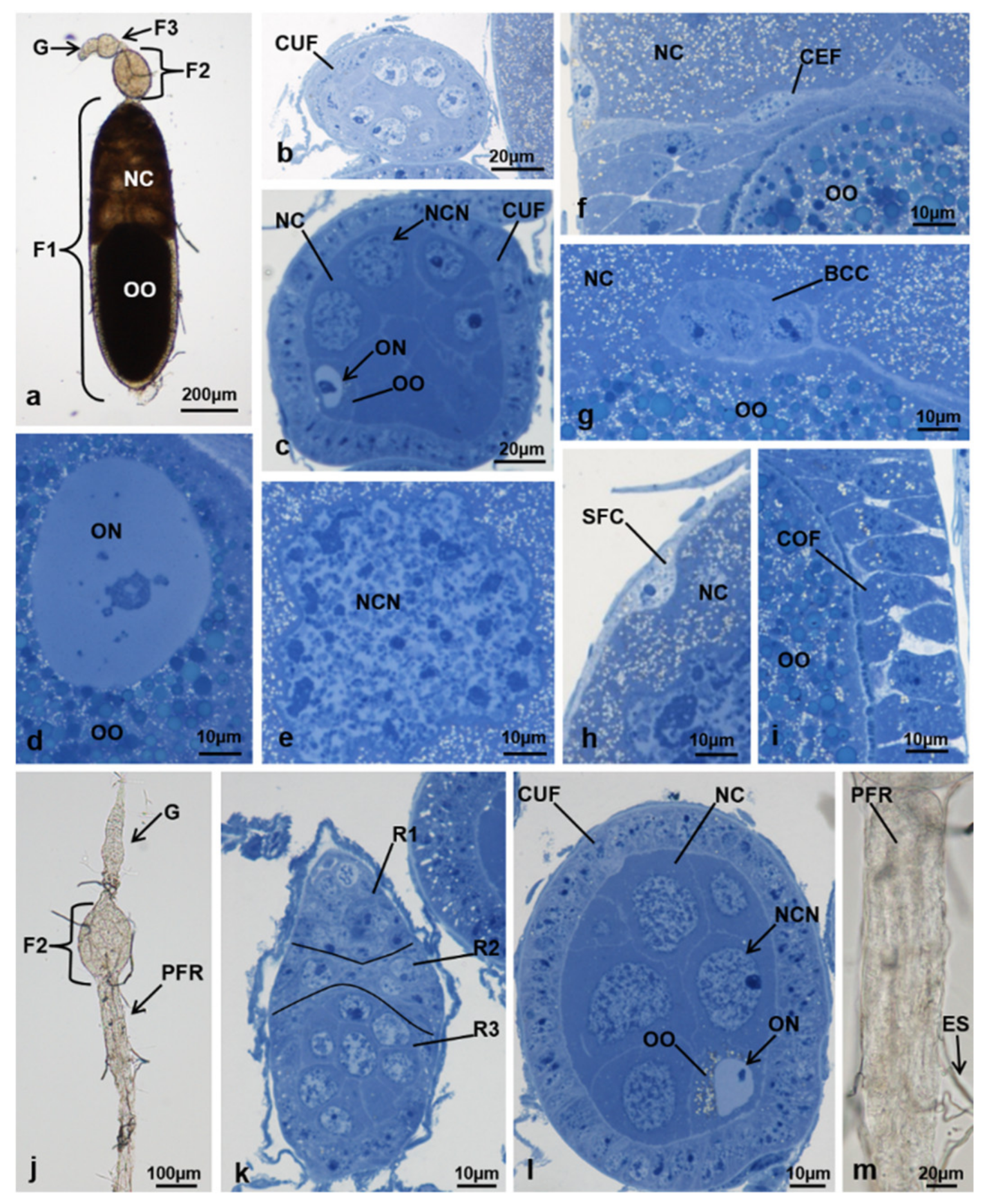
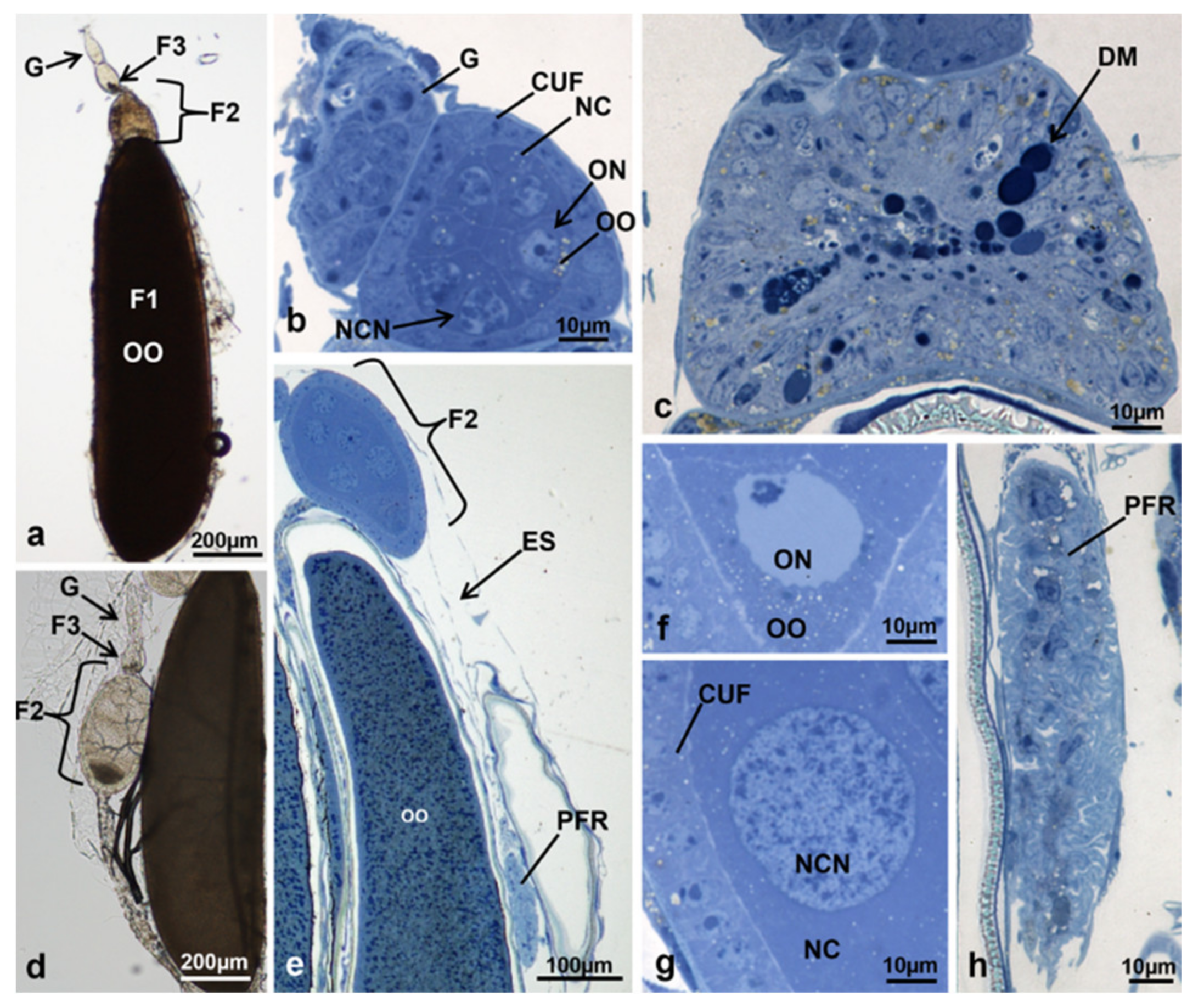
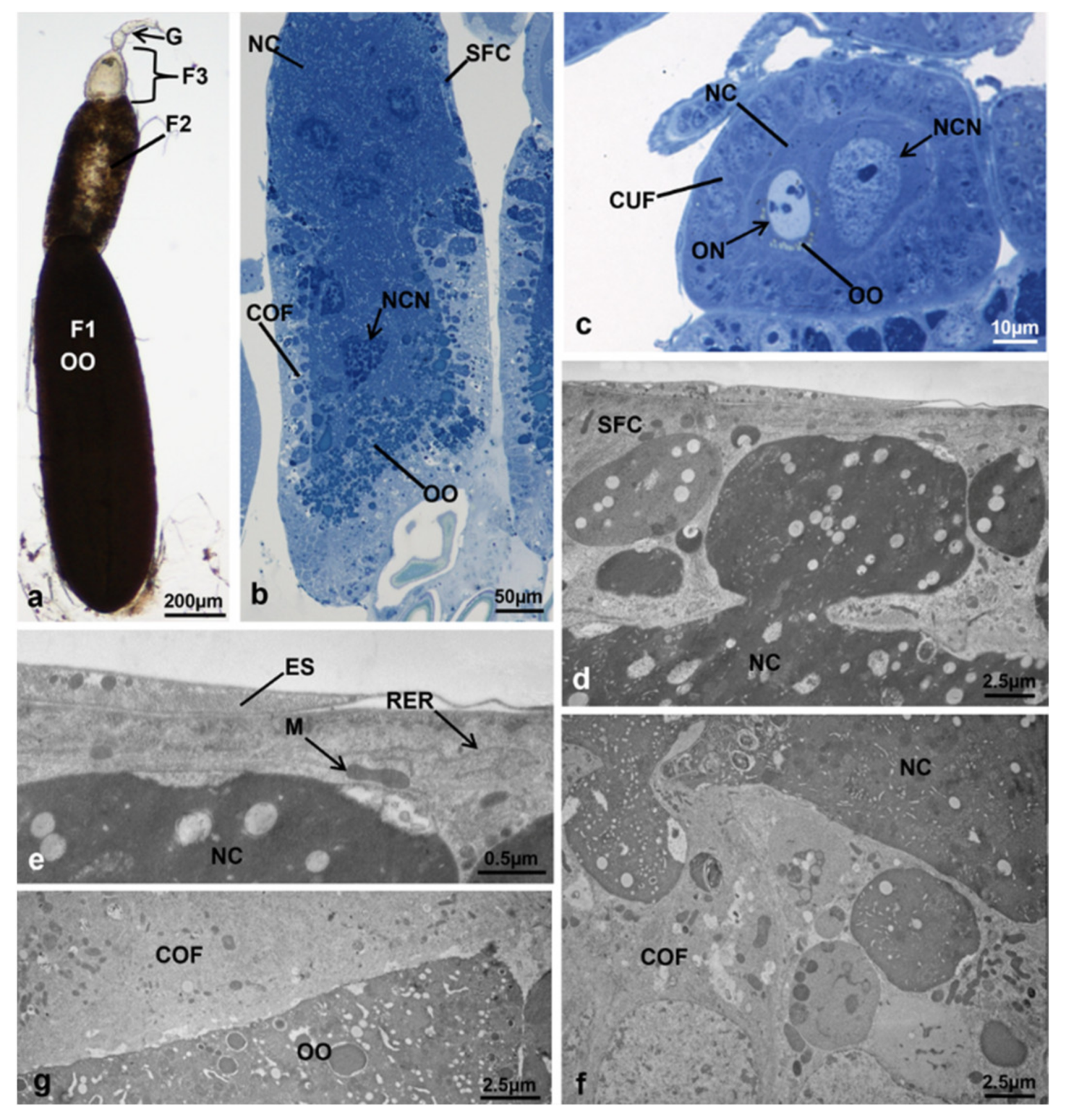
3.3.5. T2 Controls (70 h from Emerging, 55 h after Treatment)
3.3.6. T2 Treated (70 h from Emerging, 55 h after Treatment)
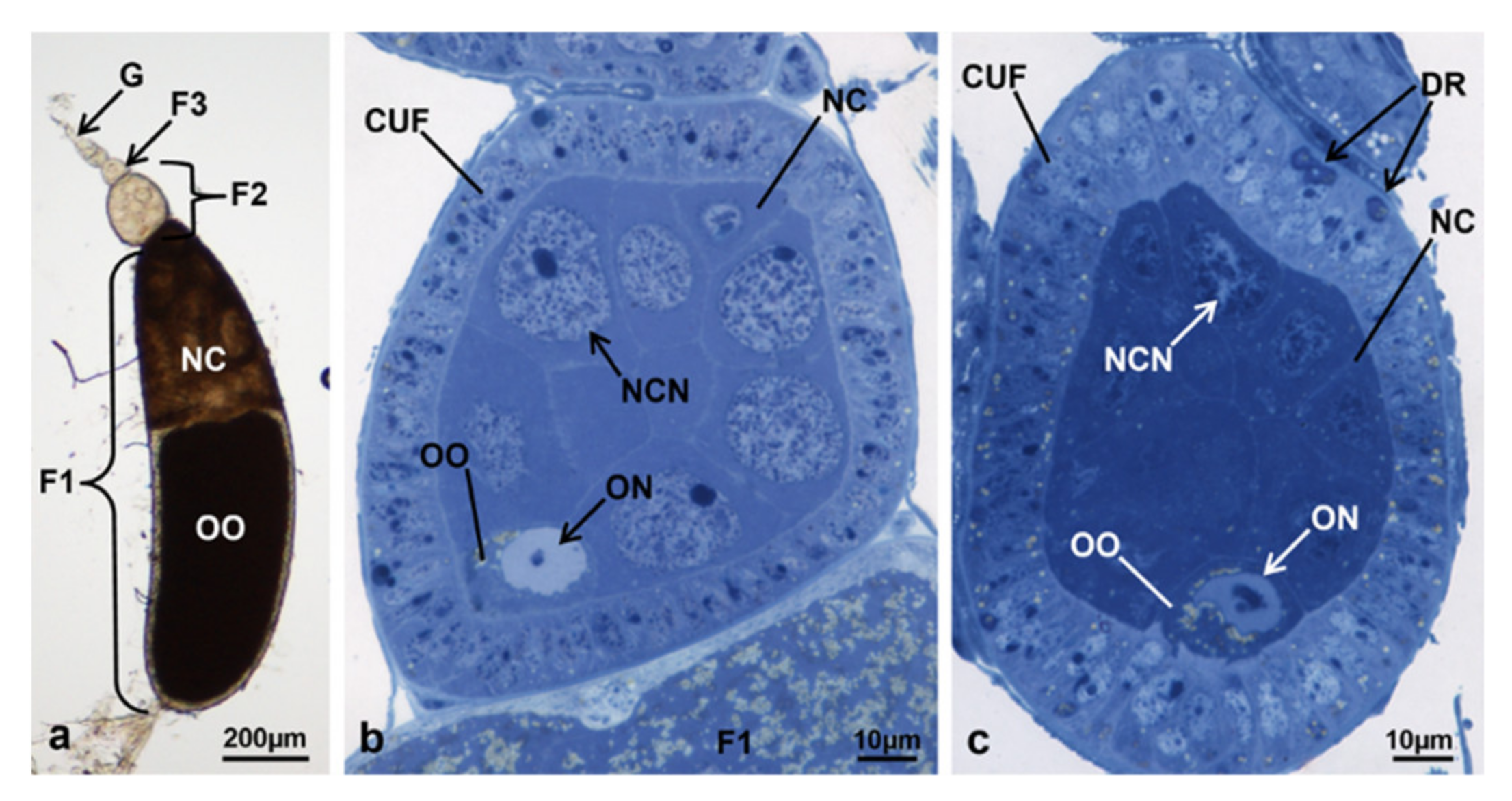
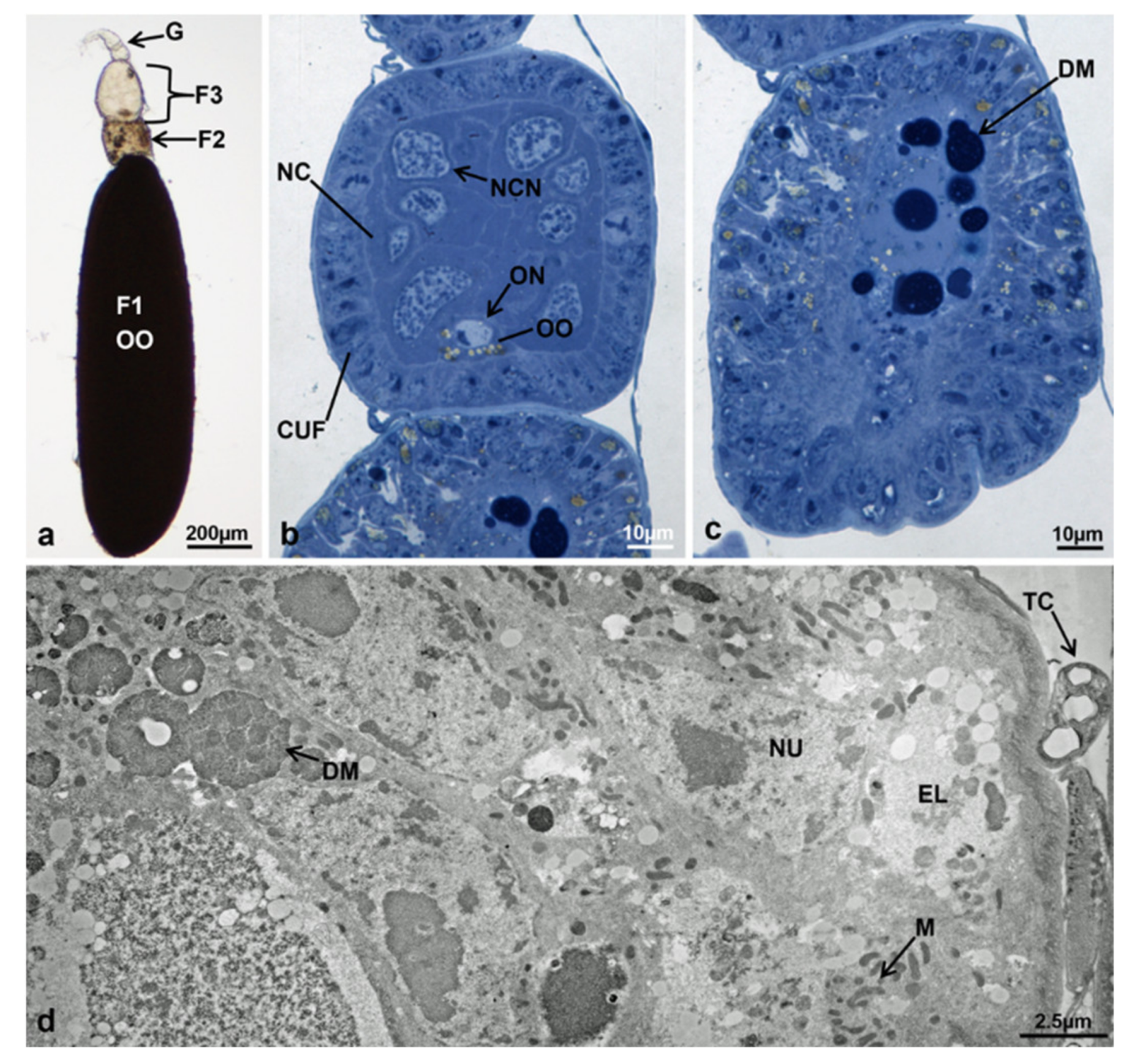
3.3.7. T3 Controls (90 h from Emerging, 75 h after Treatment)
3.3.8. T3 Treated (90 h from Emerging, 75 h after Treatment)
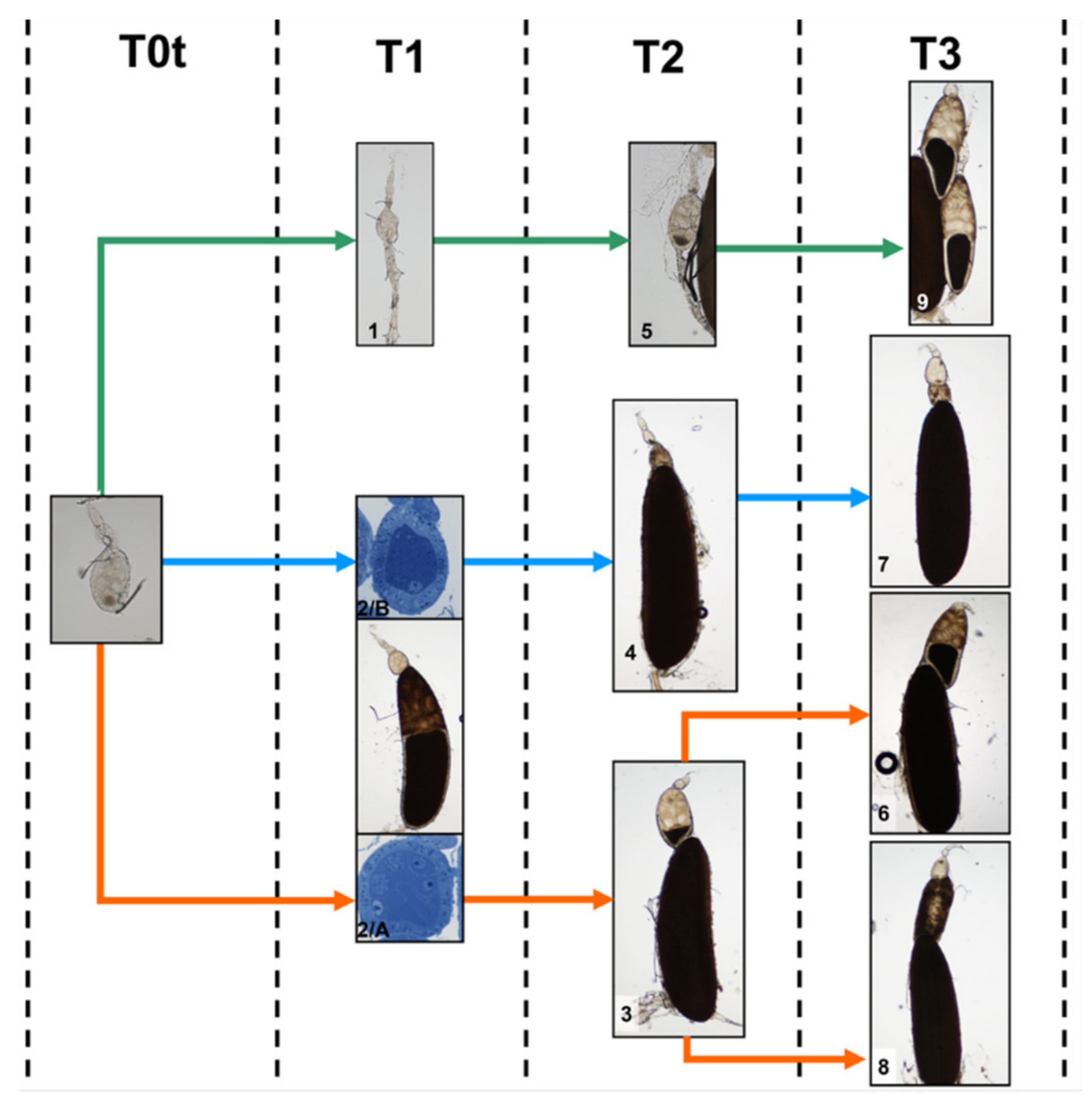
3.4. Paths of Ovariole Transformation
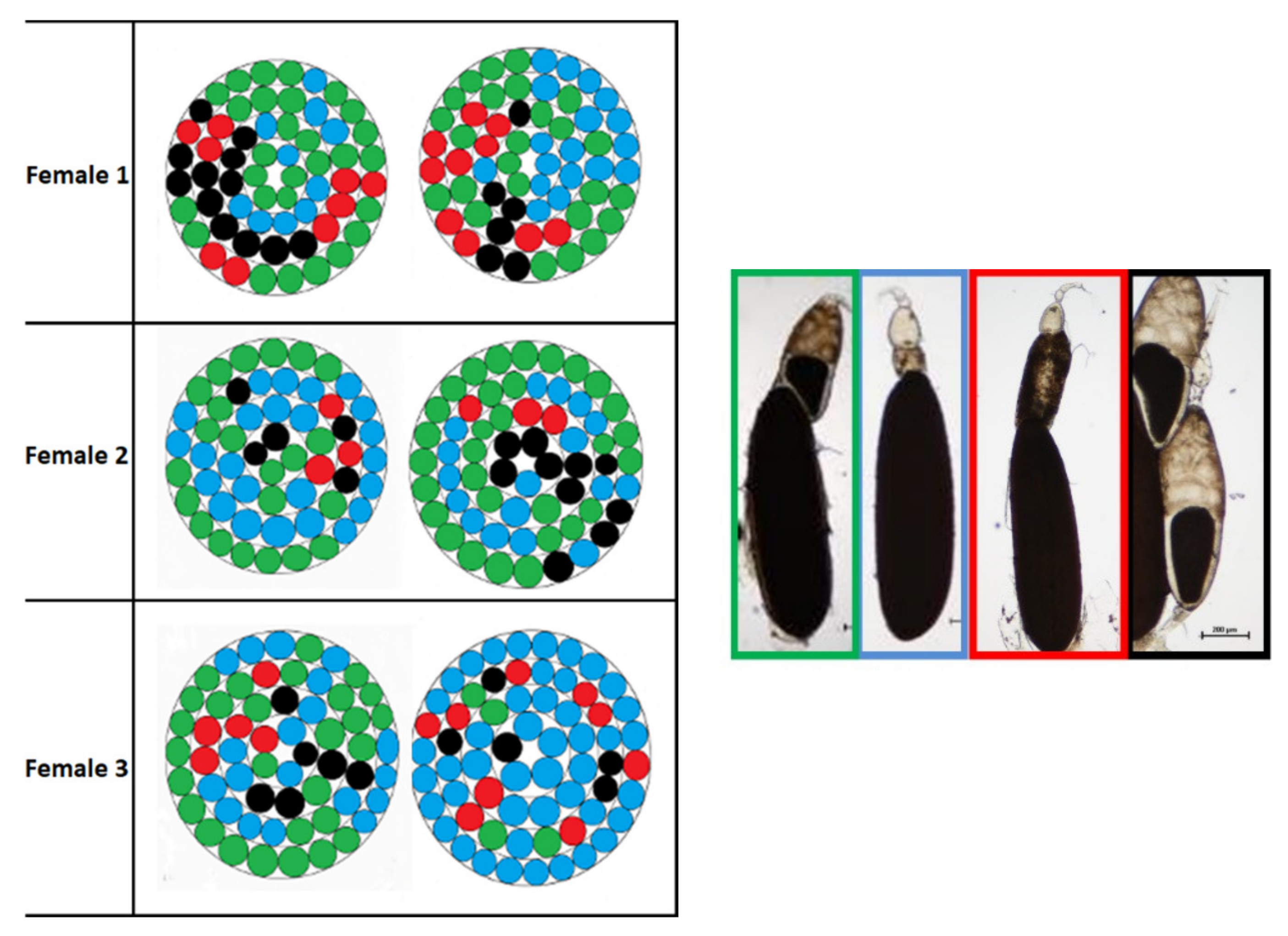
3.5. Ovariole Mapping and Counting
3.6. Fecundity and Hatching at Each Deposition Cycle
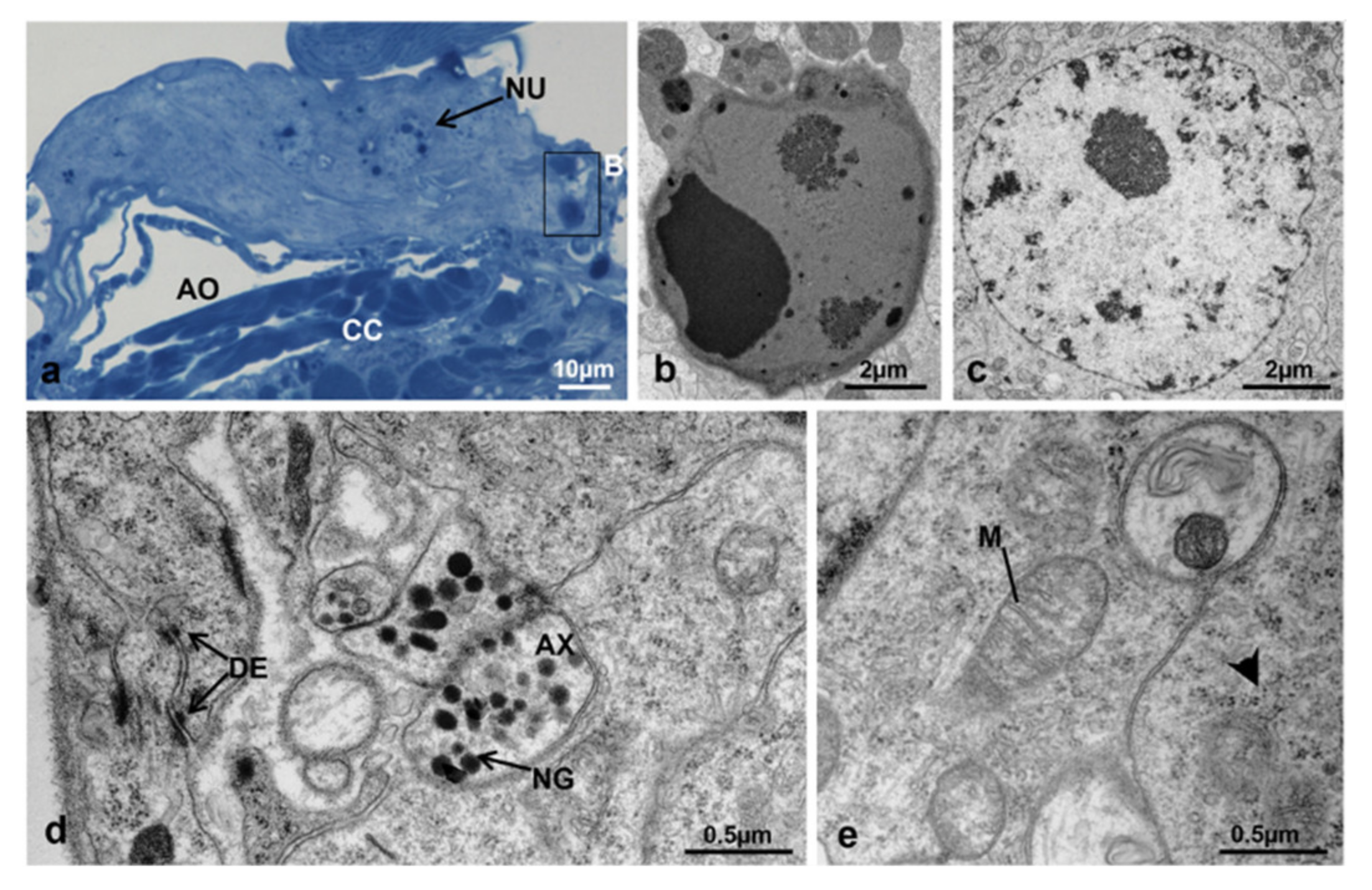
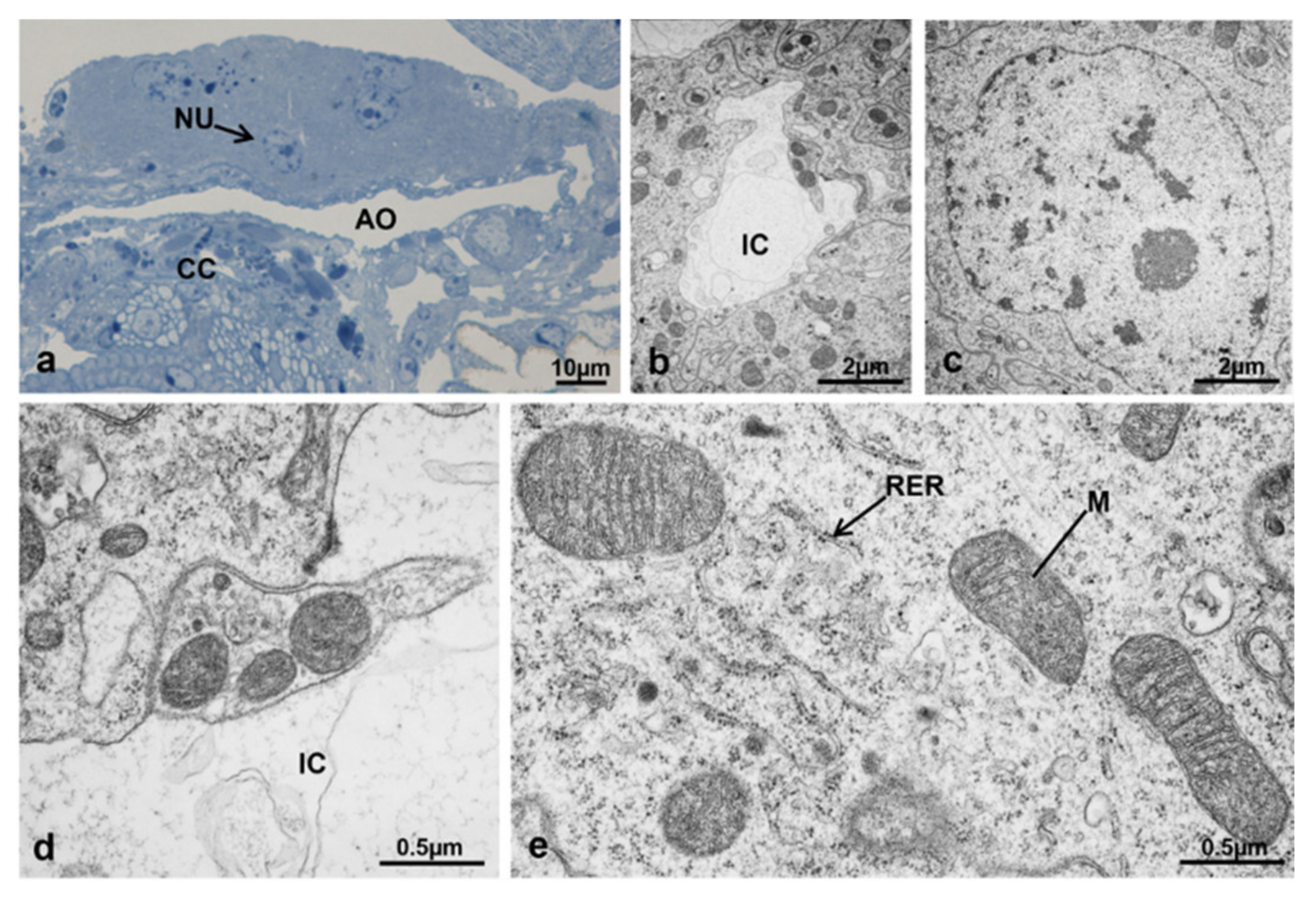
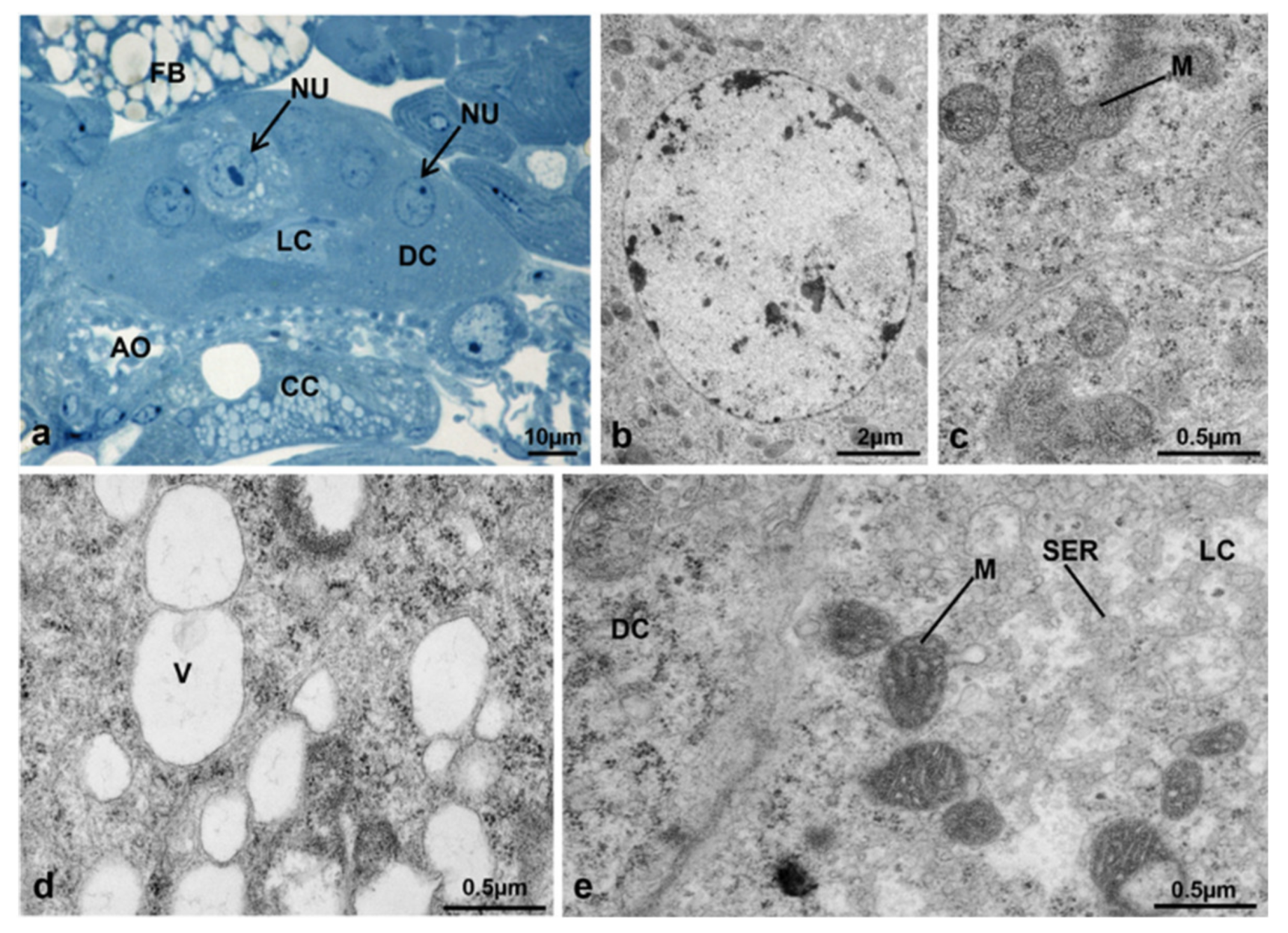
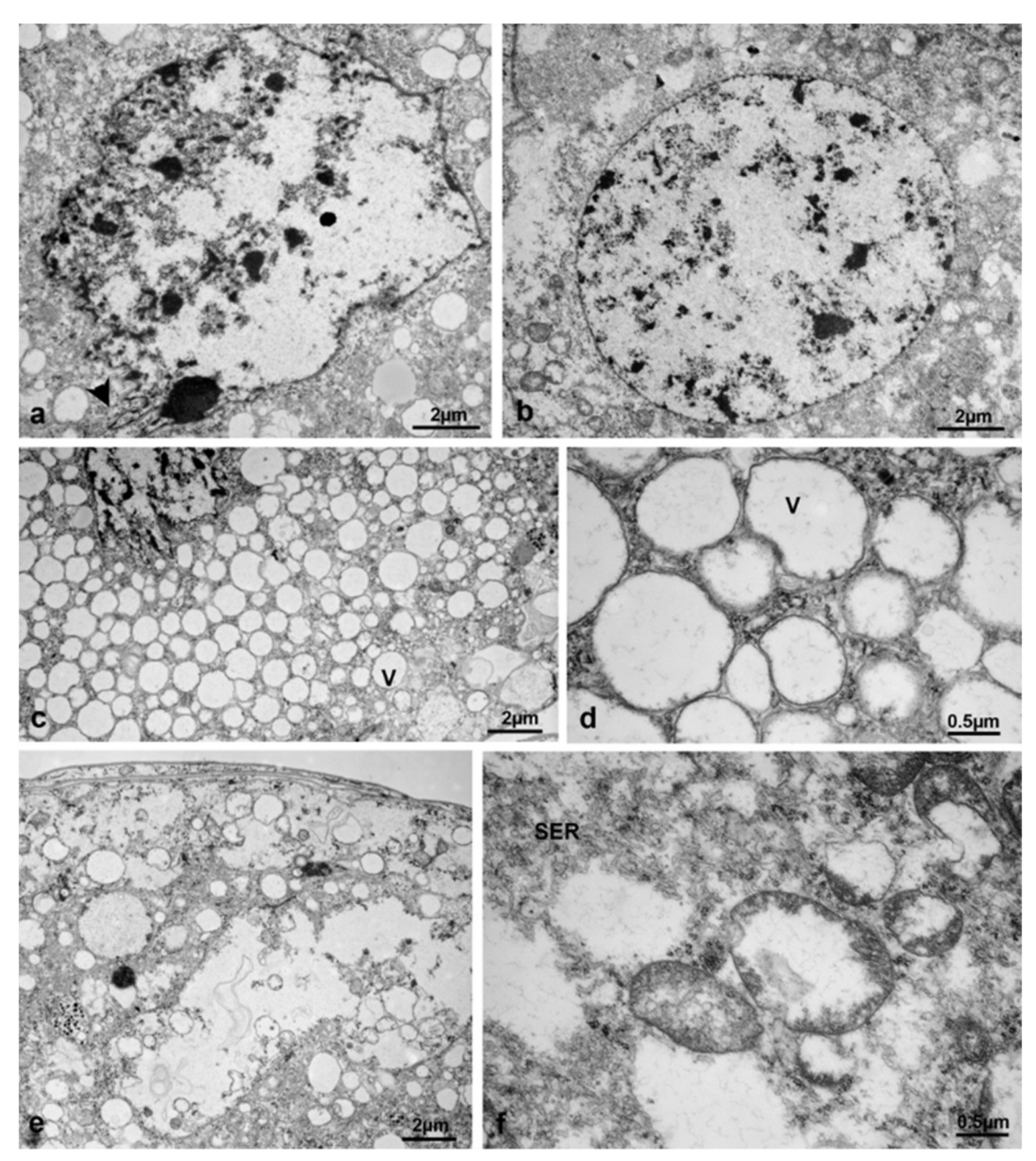
3.7. Corpora Allata
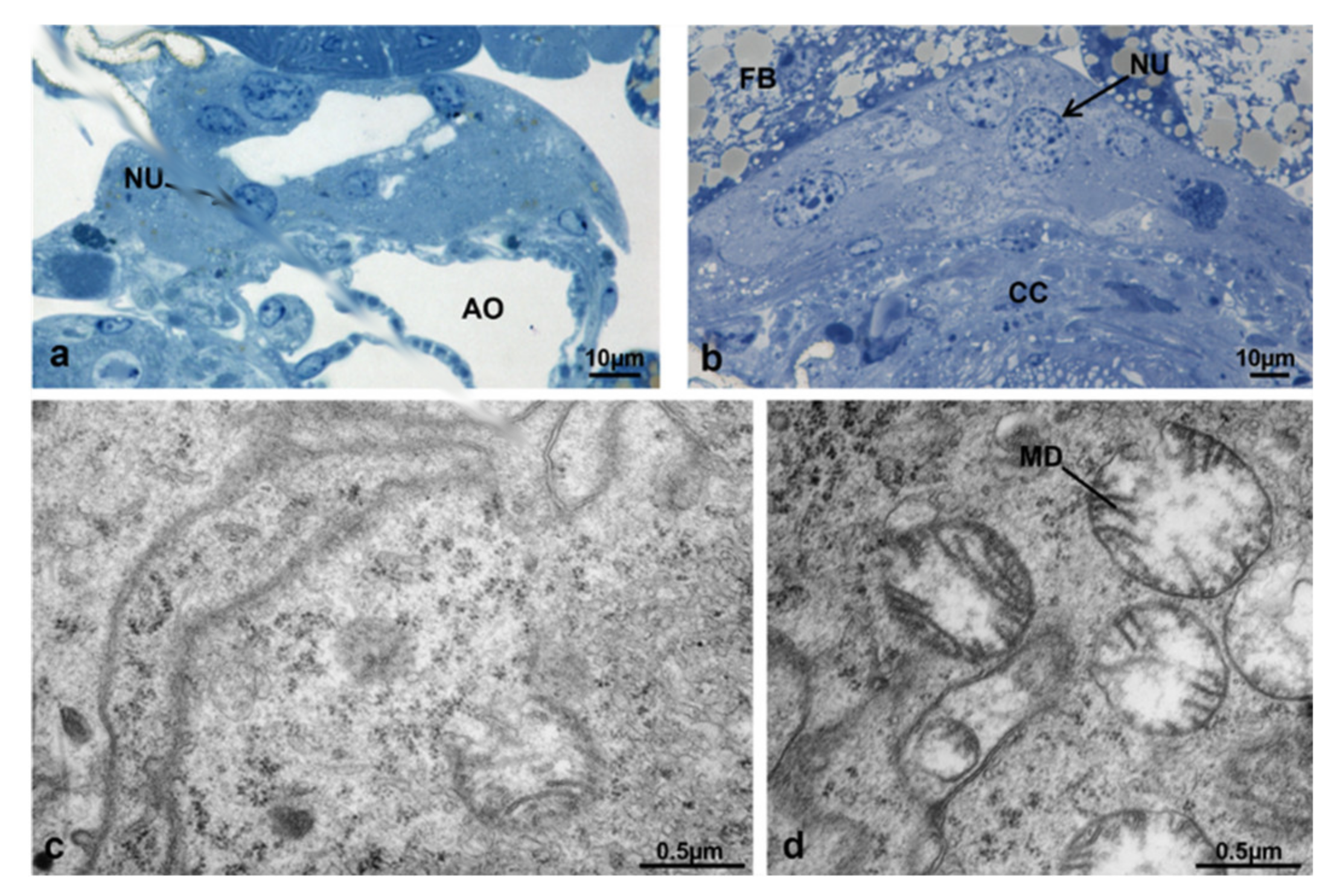
3.7.1. T0 (Emerging from Puparium)
3.7.2. T0t (15 h from Emerging, Topical Treatment)
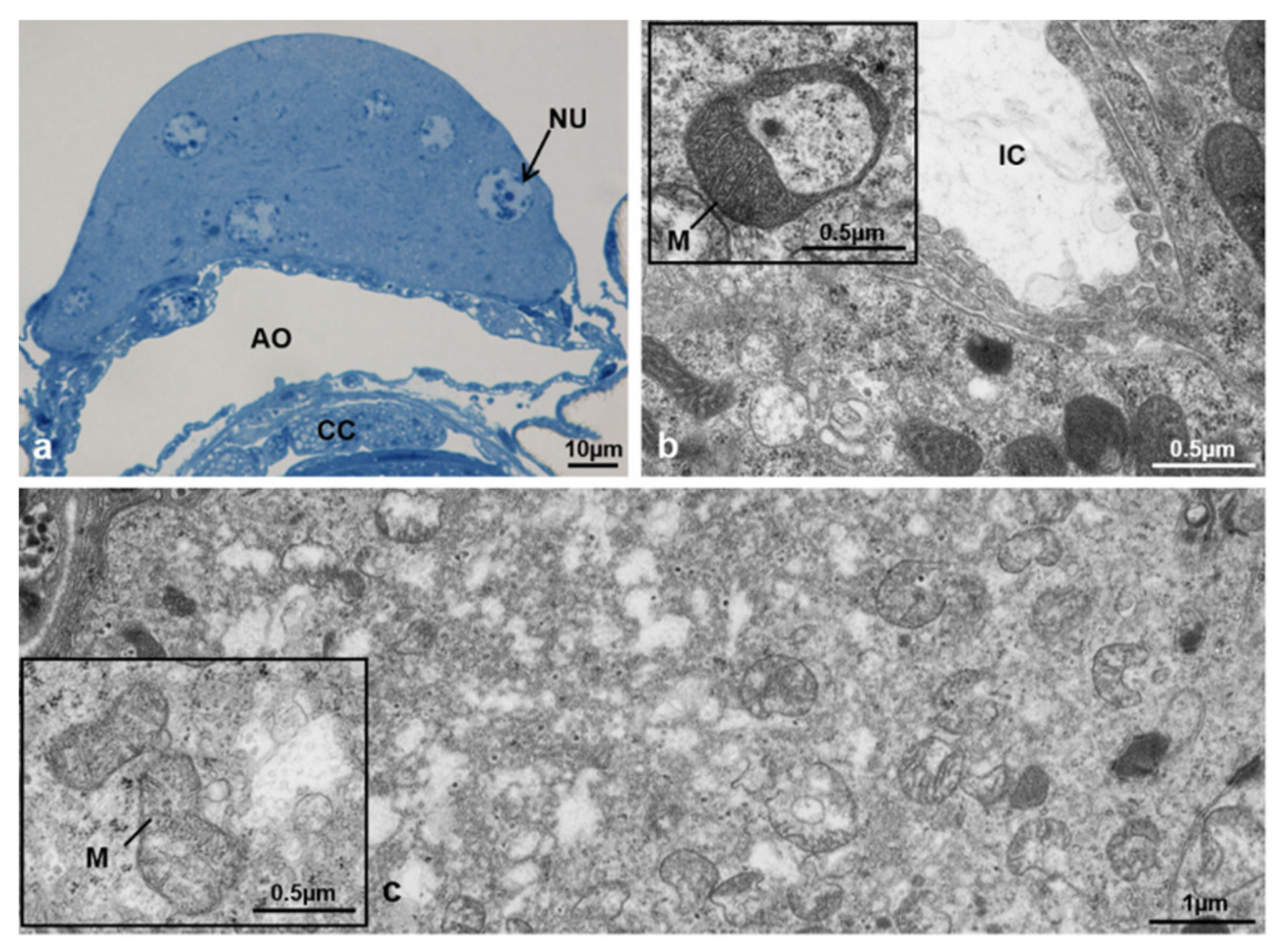
3.7.3. T1 Controls (45 h from Emerging, 30 h after Treatment)
3.7.4. T1 Treated (45 h from Emerging, 30 h after Treatment)
3.7.5. T2 Controls (70 h from Emerging, 55 h after Treatment)
3.7.6. T2 Treated (70 h from Emerging, 55 h after Treatment)
3.7.7. T3 Controls (90 h from Emerging, 75 h after Treatment)
3.7.8. T3 Treated (90 h from Emerging, 75 h after Treatment)
3.8. Cell Number in Corpora Allata
3.9. Volume of Corpora Allata
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, W. Urban Insects and Arachnids: A Handbook of Urban Entomology; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Giangaspero, A. Le Mosche di Interesse Veterinario. I Muscidae. Guida alla Conoscenza ed al Riconoscimento; Edizioni Agricole Calderini s.r.l.: Bologna, Italy, 1997. [Google Scholar]
- Tremblay, E. Entomologia Applicata. Vol. III: Ditteri Brachiceri (Caliptrati), Sifonatteri e Strepsitteri; Liguori Editore: Napoli, Italy, 1997. [Google Scholar]
- Dogra, V.; Aggarwal, A.K. Association of poultry farms with housefly and morbidity: A comparative study from Raipur Rani, Haryana. Indian J. Community Med. 2010, 35, 473–477. [Google Scholar] [CrossRef]
- Birkemoe, T.; Sverdrup-Thygeson, A. Stable fly (Stomoxys calcitrans) and house fly (Musca domestica) densities: A comparison of three monitoring methods on pig farms. J. Pest Sci. 2011, 84, 273–280. [Google Scholar] [CrossRef]
- Axtell, R.C. Fly management in poultry production: Cultural, biological, and chemical. Poultry Sci. 1986, 65, 657–667. [Google Scholar] [CrossRef]
- Gerry, A.C.; Higginbotham, G.E.; Periera, L.N.; Lam, A.; Shelton, C.R. Evaluation of surveillance methods for monitoring house fly abundance and activity on large commercial dairy operations. J. Econom. Entomol. 2011, 104, 1093–1102. [Google Scholar] [CrossRef]
- Moon, R.D. Muscid flies (Muscidae). In Medical and Veterinary Entomology, 2nd ed.; Mullen, G.R., Durden, L.A., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 275–295. [Google Scholar]
- Singh, A.; Singh, Z. Incidence of myiasis among humans—A review. Parasitol. Res. 2015, 114, 3183–3199. [Google Scholar] [CrossRef]
- Crosskey, R.W. Introduction to the Diptera. In Medical Insects and Arachnids; Lane, R.P., Crosskey, R.W., Eds.; British Museum (Natural History), Chapman and Hall: London, UK, 1993; pp. 51–77. [Google Scholar]
- Graczyk, T.K.; Knight, R.; Gilman, R.H.; Cranfield, M.R. The role of non-biting flies in the epidemiology of human infectious diseases. Microbes Infect. 2001, 3, 231–235. [Google Scholar] [CrossRef]
- Kubrakiewicz, J.; Biliński, S.M.; Mazurkiewicz, M. Diptera—Ovary structure and oogenesis in midges and flies. Folia Histochem. Cytobiol. 1998, 36, 197–203. [Google Scholar]
- Kleine-Schonnefeld, H.; Engels, W. Symmetrical pattern of follicle arrangement in the ovary of Musca domestica (Insecta, Diptera). Zoomorphology 1981, 98, 185–190. [Google Scholar] [CrossRef]
- Sakurai, H. Studies on the ovarian development in the housefly, Musca domestica vicina Macquart. I. Stages of oogenesis and the function of the follicle. Jpn J. Med. Sci. Biol. 1973, 26, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Ogienko, A.A.; Fedorova, S.A.; Baricheva, E.M. Basic aspects of ovarian development in Drosophila melanogaster. Russ. J. Genet. 2007, 43, 1120–1134. [Google Scholar] [CrossRef]
- Trepte, H.-H. Rate of follicle growth, change in follicle volume and stages of macromolecular synthesis during ovarian development in Musca domestica. J. Insect Physiol. 1979, 25, 199–203. [Google Scholar] [CrossRef]
- Chaiwong, T.; Sukontason, K.; Chaisri, U.; Kuntalue, B.; Vogtsberger, R.C.; Sukontason, K.L. Ovarian ultrastructure and development of the blow fly, Chrysomya megacephala (Diptera: Calliphoridae). Int. J. Parasitol. Res. 2012, 4, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Cassier, P. The corpora allata of insects. Int. Rev. Cytol. 1979, 57, 1–73. [Google Scholar] [CrossRef]
- King, R.C. Ovarian Development in Drosophila melanogaster; Academic Press: New York, NY, USA, 1970. [Google Scholar]
- Wyatt, G.R. Juvenile hormone in insect reproduction—A paradox? Eur. J. Entomol. 1997, 94, 323–333. [Google Scholar]
- Nijhout, H.F. Insect Hormones; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Gäde, G.; Hoffmann, K.H.; Spring, J.H. Hormonal regulation in insects: Facts, gaps, and future directions. Physiol. Rev. 1997, 77, 963–1032. [Google Scholar] [CrossRef]
- Adams, T.S.; Li, Q.J. Ecdysteroidostatin from the house fly, Musca domestica. Arch. Insect Biochem. Physiol. 1998, 38, 166–176. [Google Scholar] [CrossRef]
- Adams, T.S.; Filipi, P.A. Interaction between juvenile hormone, 20-hydroxyecdysone, the corpus cardiacum-allatum complex, and the ovaries in regulating vitellogenin levels in the house fly, Musca domestica. J. Insect Physiol. 1988, 34, 11–19. [Google Scholar] [CrossRef]
- Williams, C.M. The juvenile hormone of insects. Nature 1956, 178, 212–213. [Google Scholar] [CrossRef]
- Tunaz, H.; Uygun, N. Insect growth regulators for insect pest control. Turk J. Agric. For. 2004, 28, 377–387. [Google Scholar]
- Singh, S.; Kumar, K. Diofenolan: A novel insect growth regulator in common citrus butterfly, Papilio demoleus. Phytoparasitica 2011, 39, 205–213. [Google Scholar] [CrossRef]
- Di Domenico, D.; Ruggeri, L.; Pampiglione, G. Primo caso italiano di resistenza al larvicida ciromazina da parte di Musca domestica. Avicoltura 2005, 4, 34–37. [Google Scholar]
- Pezzi, M.; Lanfredi, M.; Chicca, M.; Tedeschi, P.; Brandolini, V.; Leis, M. Preliminary evaluation of insecticide resistance in a strain of Musca domestica (Diptera: Muscidae) from an intensive chicken farm of Northern Italy. J. Environ. Sci. Health. B 2011, 46, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, K.S.; Amer, M.S.; Bream, A.S.; Al-Dali, A.G.; Hamadah, K.S. Effectiveness of Margosan-O and Jojoba on some reproductive aspects of the house fly, Musca domestica (Diptera: Muscidae). Int. J. Agri. Biol. 2007, 9, 338–341. [Google Scholar]
- Ghoneim, K.S.; Amer, M.S.; Bream, A.S.; Al-Dali, A.G.; Hamadah, K.S. Developmental and morphogenetic response of the house fly Musca domestica to the CSIs: Lufefuron and diofenolan. Al-Azhar Bull. Sci. 2004, 15, 25–42. [Google Scholar]
- Basiouny, A.; Ghoneim, K.; Tanani, M.; Hamadah, K.; Waheeb, H. Disturbed protein content in Egyptian cotton leafworm Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae) by some novel chitin synthesis inhibitors. Int. J. Adv. Res. Biol. Sci. 2016, 3, 1–12. [Google Scholar]
- Tanani, M.; Ghoneim, K.; Hamadah, K.; Basiouny, A. Waheeb, H. Disruptive effects of some novel chitin synthesis inhibitors on transaminase activity in larval tissues of Spodoptera littoralis (Lepidoptera: Noctuidae). Int. J. Res. Stud. Zool. 2016, 2, 1–12. [Google Scholar]
- Singh, S.; Kumar, K. Effect of juvenoids pyriproxyfen and diofenolan on embryogenesis and postembryonic development of blow fly Chrysomya megacephala (Diptera: Calliphoridae) following egg treatment. Parasitol. Res. 2015, 114, 3213–3222. [Google Scholar] [CrossRef] [PubMed]
- Tanani, M.A.; Ghoneim, K.; Hassan, H.A.; Bakr, N.A. Perturbation of main body metabolites in the pink bollworm Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae) by the chitin synthesis inhibitors novaluron and diofenolan. Bio Bulletin 2017, 3, 8–21. [Google Scholar]
- Tanani, M.A.; Bakr, N.A. Effectiveness of the chitin synthesis inhibitor, diofenolan, on survival and development of the pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae). J. Entomol. Zool. Stud. 2018, 6, 1209–1219. [Google Scholar]
- Ghoneim, K.S.; Bream, A.S.; Tanani, M.A.; Nassar, M.M. Effectiveness of IGRs (CGA-184699) and (CGA-259205) on the respiratory metabolism of the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Med. Fac. Landbouww. Univ. Gent 2001, 66/2a, 413–424. [Google Scholar]
- Ghoneim, K.S.; Al-Dali, A.G.; Abdel-Ghaffar, A.A. Effectiveness of lufenuron (CGA-184699) and diofenolan (CGA-59205) on the general body metabolism of the red palm weevil, Rhynchophorus ferrugineus (Curculionidae: Coleoptera). Pak. J. Biol. Sci. 2003, 6, 1125–1129. [Google Scholar] [CrossRef] [Green Version]
- Trepte, H.-H.; Trepte-Feuerborn, C. Development and physiology of follicular atresia during ovarian growth in the house fly, Musca domestica. J. Insect Physiol. 1980, 26, 329–338. [Google Scholar] [CrossRef]
- Chiang, A.-S.; Burns, E.L.; Schal, C. Ovarian regulation of cyclic changes in size and activity of corpus allatum cells in Blattella germanica. J. Insect Physiol. 1991, 37, 907–917. [Google Scholar] [CrossRef]
- Sakurai, H. Endocrine control of oögenesis in the housefly, Musca domestica vicina. J. Insect Physiol. 1977, 23, 1295–1302. [Google Scholar] [CrossRef]
- Scharrer, B. Histophysiological studies on the corpus allatum of Leucophaea maderae. IV. Ultrastructure during normal activity cycle. Z. Zellforsch. Mikrosk. Anat. 1964, 62, 125–148. [Google Scholar] [CrossRef] [PubMed]

| DC (µg/µL) | F ± SD | FI (%) |
|---|---|---|
| Control (0) | 2461.3 ± 330.8 | --- |
| 5 | 1668.6 ± 82.1 | 32 |
| 25 | 837.3 ± 76.5 | 66 |
| 50 | 736.0 ± 52.5 | 70 |
| 75 | 690.0 ± 86.9 | 72 |
| ON ± SD | ||||
|---|---|---|---|---|
| I° Circle | II° Circle | III° Circle | IV° Circle | |
| Control | 24.2 ± 1.2 | 18.5 ± 1.0 | 11.5 ± 1.0 | 5.2 ± 0.8 |
| Treated | 24.2 ± 1.0 | 18.2 ± 1.5 | 11.5 ± 0.8 | 5.2 ± 0.8 |
| Time Intervals | ON ± SD | |
|---|---|---|
| T0t | -- | 60.1 ± 2.0 |
| T1 | Control | 58.7 ± 2.9 |
| Treated | 58.8 ± 1.8 | |
| T2 | Control | 59.8 ± 2.4 |
| Treated | 58.3 ± 3.4 | |
| T3 | Control | 59.7 ± 2.1 |
| Treated | 59.0 ± 2.5 | |
| EC | FC ± SD | FI (%) | HC ± SD | H (%) | SI (%) | |
|---|---|---|---|---|---|---|
| Control (n = 10) | 1° | 90.2 ± 19.0 | --- | 86.9 ± 19.4 | 96 | --- |
| 2° | 87.4 ± 33.8 | --- | 85.3 ± 34.1 | 98 | --- | |
| 3° | 83.8 ± 33.0 | --- | 81.4 ± 32.2 | 97 | --- | |
| Treated (n = 10) | 1° | 70.8 ± 45.1 | 22 | 51.4 ± 36.71 | 73 | 41 |
| 2° | 29.2 ± 44.8 | 67 | 14.3 ± 33.17 | 49 | 83 | |
| 3° | 20.9 ± 38.8 | 75 | 5.0 ± 10.2 | 24 | 94 |
| Time Intervals | CN ± SD | V ± SD | |
|---|---|---|---|
| T0 | --- | 16.4± 1.1 | 110.8 ± 8.4 × 103 |
| T0t | --- | 19.4 ± 1.1 | 158.2 ± 12.3 × 103 |
| T1 | Control | 19.6 ± 1.1 | 205.6 ± 14.4 × 103 |
| Treated | 17.6 ± 1.5 | 160.1 ± 13.6 × 103 | |
| T2 | Control | 19.8 ± 1.3 | 323.4 ± 17.8 × 103 |
| Treated | 17.4 ± 0.9 | 184.4 ± 17.2 × 103 | |
| T3 | Control | 19.8 ± 1.3 | 214.1 ± 24.0 × 103 |
| Treated | 18.0 ± 1.0 | 153.8 ± 21.1 × 103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzi, M.; Scapoli, C.; Marchetti, M.G.; Chicca, M.; Krčmar, S.; Leis, M.; Bonacci, T. Alternative Use of the Insecticide Diofenolan on Musca domestica (Diptera: Muscidae): A Morphological and Ultrastructural Investigation. Sustainability 2021, 13, 10122. https://doi.org/10.3390/su131810122
Pezzi M, Scapoli C, Marchetti MG, Chicca M, Krčmar S, Leis M, Bonacci T. Alternative Use of the Insecticide Diofenolan on Musca domestica (Diptera: Muscidae): A Morphological and Ultrastructural Investigation. Sustainability. 2021; 13(18):10122. https://doi.org/10.3390/su131810122
Chicago/Turabian StylePezzi, Marco, Chiara Scapoli, Maria Gabriella Marchetti, Milvia Chicca, Stjepan Krčmar, Marilena Leis, and Teresa Bonacci. 2021. "Alternative Use of the Insecticide Diofenolan on Musca domestica (Diptera: Muscidae): A Morphological and Ultrastructural Investigation" Sustainability 13, no. 18: 10122. https://doi.org/10.3390/su131810122






