The Effect of Nitrogen Fertilization on the Yield, Quality and Fatty Acid Composition of Opuntia ficus-indica Seed Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Collection
2.2. Extraction of the Seed
2.3. Lipid Extraction
2.4. Fatty Acids Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition
3.1.1. Oil Content
3.1.2. Fat Free Dry Matter (FFDM) Content
3.1.3. Moisture Content
3.2. The Effect of LAN, Urea and AmSul and Their Different Levels on the Fatty Acid Profile of Cactus Pear Seed Oil
3.3. Fatty Acid Ratios of Cactus Pear Seed Oil
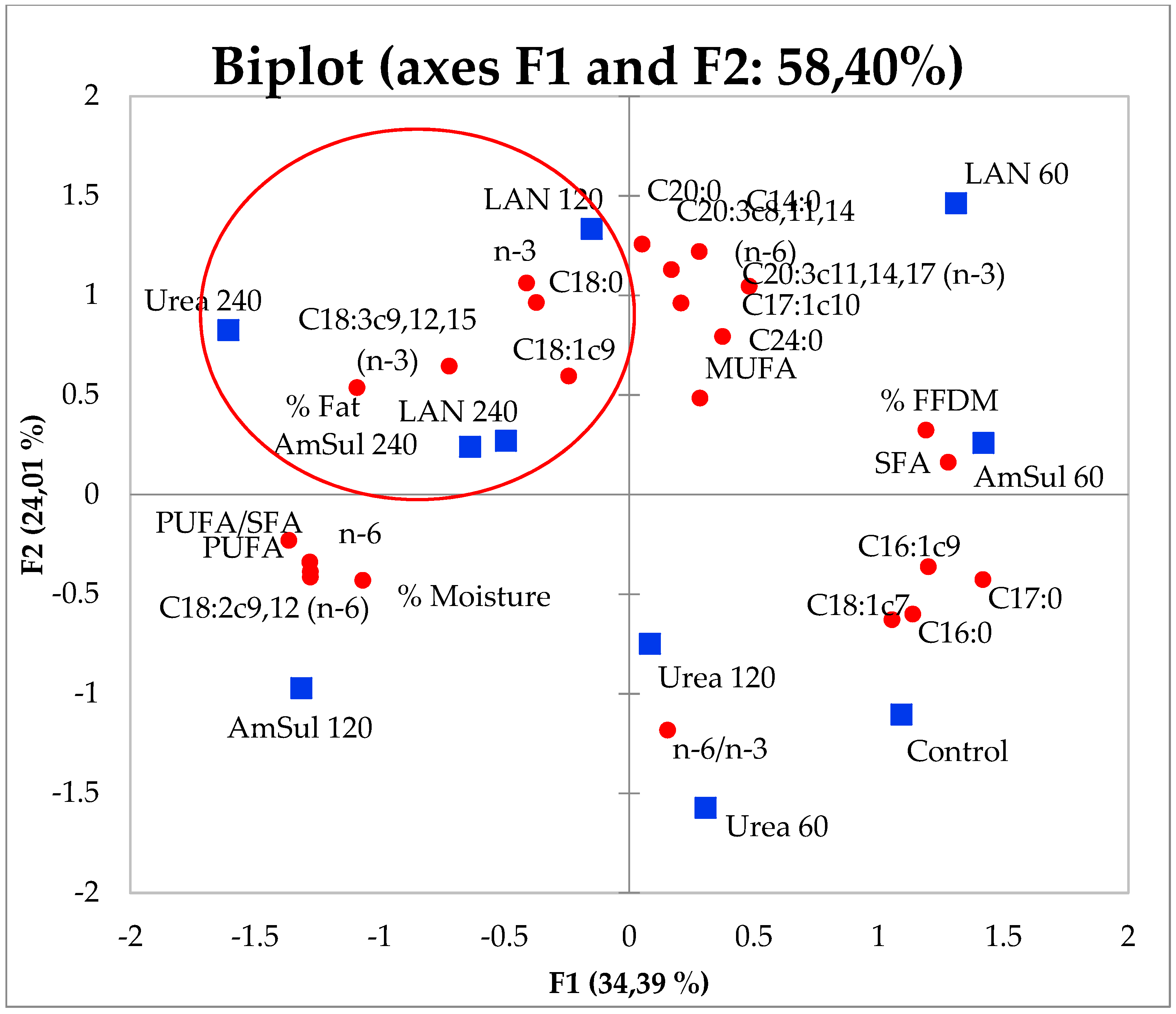
3.4. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wessels, A.B. Spine-Less Prickly Pear; Perskor Publishers: Johannesburg, South Africa, 1988; pp. 21–24. [Google Scholar]
- Ramakatane, M.E. Root dynamics and water studies on Opuntia ficus-indica and O. robusta. Master′s Thesis, University of the Free State, Bloemfontein, South Africa, 2003. [Google Scholar]
- Potgieter, J.P.; Mashope, B.K. Cactus pear (Opuntia spp.) germplasm conservation in South Africa. Acta Hortic. 2009, 811, 47–54. [Google Scholar] [CrossRef]
- Feugang, J.M.; Konarski, P.; Zou, D.; Stintzing, F.C.; Zou, C. Nutritional and medicinal use of cactus pear (Opuntia spp.) cladodes and fruits. Front Biosci. 2006, 11, 2574–2589. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Heux, L.; Mahrouz, M.; Vignon, M.R. Morphological and structural study of seed pericarp of Opuntia ficus-indica prickly pear fruits. Carbohydr. Polym. 2008, 72, 102–112. [Google Scholar] [CrossRef]
- Ledwaba, C.R.; Mmbi, N.E.; Maphahla, V.V.; Ngwepe, M.R.; Dikgwatlhe, S.B. Comparison of Fruit Quality Characteristics of 24 Cactus Pear Cultivars with Different Pulp Colours at Mara ADC, Limpopo; Limpopo Department of Agriculture: Limpopo, South Africa, 2012.
- Ramadan, M.F.; Mörsel, J.T. Oil cactus pear (Opuntia ficus-indica L.). Food Chem. 2003, 82, 339–345. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Mörsel, J.T. Lipid profile of prickly pear pulp fractions. JFAE 2003, 1, 66–70. [Google Scholar]
- Al-Juhaimi, F.; Özcan, M.M. Determination of some mineral contents of prickly pear (Opuntia ficus-indica L.) seed flours. Environ. Monit. Assess. 2013, 185, 3659–3663. [Google Scholar] [CrossRef]
- Du Toit, A.; de Wit, M.; Osthoff, G.; Hugo, A. Antioxidant content and capacity of fruit from different colour cactus pear (O. ficus-indica and O. robusta) cultivars. Acta Hortic. 2015, 1067, 187–192. [Google Scholar] [CrossRef]
- Nobel, P.S. Cacti: Biology and Uses, 1st ed.; University of California Press, Ltd.: Berkeley, CA, USA, 2002; pp. 1–291. [Google Scholar]
- Cantwell, M. Agricultural ecology, cultivation and uses of cactus pears. Plant Production and Protection Paper 132. In Agro-Ecology, Cultivation and Uses of Cactus Pear; Barbera, G., Inglese, P., Pimiento-Barrios, E., Eds.; FAO: Rome, Italy, 1995; pp. 120–136. [Google Scholar]
- Sawaya, W.N.; Khalil, J.K.; Al-Mohammad, M.M. Nutritive value of prickly pear seeds, Opuntia ficus-indica. Plant Foods Hum. Nutr. 1983, 33, 91–97. [Google Scholar] [CrossRef]
- Labuschagne, M.T.; Hugo, A. Oil content and fatty acid composition of cactus pear seed compared with cotton and grape seed. J. Food Biochem. 2010, 34, 93–100. [Google Scholar] [CrossRef]
- Matthäus, B.; Özcan, M.M. Habitat effects on yield, fatty acid composition and tocopherol contents of prickly pear (Opuntia ficus-indica L.) seed oils. Sci. Hortic. 2011, 131, 95–98. [Google Scholar] [CrossRef]
- Sawaya, W.N.; Khan, P. Chemical characterization of prickly pear seed oil, Opuntia ficus-indica. J. Food Sci. 1982, 47, 2060–2061. [Google Scholar] [CrossRef]
- Salvo, F.; Galati, E.M.; Lo Curto, S.; Tripodo, M.M. Chemical characterization of Opuntia ficus-indica seed oil. Acta Hortic. 2002, 581, 283–289. [Google Scholar] [CrossRef]
- Coşkuner, Y.N.; Tekin, A. Monitoring of seed composition of prickly pear (Opuntia ficus-indica) fruits during maturation period. J. Sci. Food Agric. 2003, 83, 846–849. [Google Scholar] [CrossRef]
- Ennouri, M.; Evelyn, B.; Laurence, M.; Hamadi, A. Fatty acid composition and rheological behavior of prickly pear seed oils. Food Chem. 2005, 93, 431–437. [Google Scholar] [CrossRef]
- Lee, D. Essential Fatty Acids; Woodlands Publishing Inc.: Pleasant Grove, UT, USA, 1997; pp. 6–32. [Google Scholar]
- García Pantaleón, G.D.; Flores Ortiz, M.; Moreno Álvarez, M.; Belén Camacho, D.; Medina Martínez, C.; Ojeda Escalona, C.E.; Padrón Pereira, C.A. Chemical, biochemical, and fatty acids composition of seeds of Opuntia boldinghii Britton et Rose. J. Prof. Assoc. Cactus Dev. 2009, 11, 45–52. [Google Scholar]
- Knothe, G.; Dunn, R.O. Dependence of oil stability index of fatty compounds on their structure and concentration and presence of metals. J. Am. Oil Chem. Soc. 2003, 80, 1021–1026. [Google Scholar] [CrossRef]
- Pimienta-Barrios, E. El Nopal Tunero; Universidad de Guadalajara: Guadalajara, Mexico, 1990. [Google Scholar]
- Inglese, P. Orchard planting and management. Plant Production and Protection Paper 132. In Agro-Ecology, Cultivation and Uses of Cactus Pear; Barbera, G., Inglese, P., Pimienta-Barrios, E., Eds.; FAO: Rome, Italy, 1995; pp. 78–90. [Google Scholar]
- Hopkins, W.G.; Hüner, N.P.A. Nitrogen assimilation. In Introduction to Plant Physiology, 4th ed.; Witt, K., Foxman, J., Muriello, L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 195–211. [Google Scholar]
- Nobel, P.S. Nutrient levels in cacti: Relation to nocturnal acid accumulation and growth. Am. J. Bot. 1983, 70, 1244–1253. [Google Scholar] [CrossRef]
- Gathaara, G.N.; Felker, P.; Land, M. Influence of nitrogen and phosphorus application on Opuntia engelmanii tissue N and P concentrations, biomass production and fruit yields. J. Arid Environ. 1989, 16, 337–346. [Google Scholar] [CrossRef]
- Claassens, A.S.; Wessels, A.B. The fertilizer requirements of cactus pear (Opuntia ficus-indica) under summer rainfall conditions in South Africa. Acta Hortic. 1997, 438, 83–95. [Google Scholar] [CrossRef]
- Nerd, A.; Karady, A.; Mizrahi, Y. Irrigation, fertilization, and polyethylene covers influence bud development in prickly pear. Hort. Sci. 1989, 24, 773–775. [Google Scholar]
- Nerd, A.; Karady, A.; Mizrahi, Y. Out-of-season prickly pear: Fruit characteristics and effect of fertilization and short drought on productivity. Hort. Sci. 1991, 26, 527–529. [Google Scholar] [CrossRef] [Green Version]
- Nerd, A.; Mizrahi, Y. Effect of nitrogen fertilization and organ removal on rebudding in Opuntia ficus-indica (L.) Miller. Scientia Hortic. 1994, 59, 115–122. [Google Scholar] [CrossRef]
- Inglese, P.; Barbera, G.; La Mantia, T. Seasonal reproductive and vegetative growth patterns and resource allocation during cactus pear fruit growth. Hort. Sci. 1999, 34, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Zegbe, J.A.; Mena-Covarrubias, J. Retraso de la cosecha en nopal tunero cv. Cristalina. Rev. Chapingo Ser. Hortic. 2008, 13, 85–90. [Google Scholar] [CrossRef]
- Zegbe, J.A.; Serna-Pérez, A.; Mena-Covarrubias, J. Soil applications of NPK affect fruit quality and shelf-life of ‘Cristalina’ cactus pear. Fruits 2015, 70, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Galizzi, F.A.; Felker, P.; González, C.; Gardiner, D. Correlation between soil and cladode nutrient concentrations and fruit yield and quality in cactus pear, Opuntia ficus-indica, in a traditional farm setting in Argentina. J. Arid Environ. 2004, 59, 115–132. [Google Scholar] [CrossRef]
- Karim, R.; Felker, P.; Bingam, R.L. Correlations between cactus pear (Opuntia spp.) cladode nutrient concentrations and fruit yield and quality. Ann. Arid Zone 1998, 37, 159–171. [Google Scholar]
- FERTASA. Fertilizer Handbook, 7th ed.; FERTASA: Pretoria, South Africa, 2016; pp. 112–127. [Google Scholar]
- Grain SA Fertilizer Report 11. Available online: https://www.grainsa.co.za/upload/report_files/Kunsmisverslag-Volledig.pdf (accessed on 21 July 2021).
- Potgieter, J.P. Guidelines for the Cultivation of Spine-Less Cactus Pears for Fruit Production, 2nd ed.; Group 7 Trust Printers: Sinoville, South Africa, 1997. [Google Scholar]
- Kruger, A.C. Climate of South Africa: Climatic Regions; South African Weather Service: Pretoria, South Africa, 2004; pp. 24–25. [Google Scholar]
- Coetzer, G.; Fouche, H. Fruit yield and quality of cactus pear (Opuntia spp.) cultivars in the Central Free State, South Africa. Acta Hortic. 2015, 1067, 89–95. [Google Scholar] [CrossRef]
- Soil Classification Working Group. Memoirs on the Agricultural Natural Resources of South Africa No 15. In Soil Classification: A Taxonomic System for South Africa; Department of Agricultural Development: Pretoria, South Africa, 1991; p. 257. [Google Scholar]
- De Wit, M.; Hugo, A.; Shongwe, N.; van der Merwe, R. Effect of cultivar, season and locality on lipid content and fatty acid composition of cactus pear seed oil. S. Afr. J. Plant Soil. 2016, 33, 279–288. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissue. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Slover, H.T.; Lanza, E. Quantitative analysis of food fatty acids by capillary gas chromatography. J. Am. Oil Chem. Soc. 1979, 56, 933–943. [Google Scholar] [CrossRef]
- Diaz, M.T.; Alvarez, I.; De La Fuente, J.; Sanudo, C.; Campo, M.M.; Oliver, M.A.; Font i Furnols, M.; Montossi, F.; San Julian, R.; Nute, G.R.; et al. Fatty acid composition of meat from typical lamb production systems of Spain, United Kingdom, Germany and Uruguay. Meat Sci. 2005, 71, 256–263. [Google Scholar] [CrossRef]
- Hur, S.J.; Ye, B.W.; Lee, J.L.; Ha, Y.L.; Park, G.B.; Joo, S.T. Effects of conjugated linoleic acid on color and lipid oxidation of beef patties during cold storage. Meat Sci. 2004, 66, 771–775. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, D.; Wang, Z.; Chen, Y.; Yang, Z.; Wu, C.; Yang, H.; Chen, L. Nitrogen application modifies the seed and oil yields and fatty acid composition of Nicotiana tabacum. Hort. Sci. 2020, 55, 1898–1902. Available online: https://journals.ashs.org/hortsci/view/journals/hortsci/55/12/article-p1898.xml (accessed on 20 August 2021). [CrossRef]
- Elbadawy, N.; Hegazi, E.; Yehia, T.; Abourayya, M.; Mahmoud, T. Effect of nitrogen fertilizer on yield, fruit quality and oil content in manzanillo olive trees. J. Arid Land Stud. 2016, 26, 175–177. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Vick, B.A.; Baldwin, B.S.; Buehring, N.; Astatkie, T.; Johnson, B. Oil content and saturated fatty acids in sunflower as a function of planting date, nitrogen, and hybrid. Agron. J. 2009, 101, 1003–1011. [Google Scholar] [CrossRef] [Green Version]
- Aminpanah, H. Effect of nitrogen rate on seed yield, protein and oil content of two canola (Brassica napus L.) cultivars. ACAS 2013, 101, 183–190. [Google Scholar]
- De Wit, M.; Hugo, A.; Shongwe, N. South African cactus pear seed oil: A comprehensive study on 42 spineless burbank Opuntia ficus-indica and Opuntia robusta cultivars. Eur. J. Lipid Sci. Technol. 2018, 120, 1700343. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated fatty acids and risk of coronary heart disease: Modulation by replacement nutrients. Curr. Atheroscler. Rep. 2010, 12, 384–390. [Google Scholar] [CrossRef] [Green Version]
- De Wit, M.; Motsamai, V.K.; Hugo, A. Cold-pressed cactus pear seed oil: Quality and stability. Grasa. Aceite. 2021, 72, e415. [Google Scholar] [CrossRef]
- Kheir, N.F.; Harb, E.Z.; Moursi, H.A.; El-Gayar, S.H. Effect of salinity and fertilization on flax plants (Linum usitatissimum L.). II. chemical composition. Bull. Fac. Agric. 1991, 42, 57–70. [Google Scholar]
- Crossa, J. Statistical analyses of multi-locational trials. Adv. Agron. 1990, 44, 55–85. [Google Scholar] [CrossRef]
- Tsige, G.K. Genetic Diversity Analysis and Genotype X Environment Interaction in Ethopia Mustard. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2002. [Google Scholar]

| Property * | |
|---|---|
| Clay (%) | 27 |
| pH (KCl) | 7.1 |
| Nutrients (mg kg−1) | |
| P (NaHCO3) | 7.8 |
| Ca (NH4OAc) | 4126 |
| Mg (NH4OAc) | 727.0 |
| K (NH4OAc) | 164.1 |
| Na (NH4OAc) | 81.2 |
| Treatment | Control (0) | LAN 60 | LAN 120 | LAN 240 | Urea 60 | Urea 120 | Urea 240 | AmSul 60 | AmSul 120 | AmSul 240 | Sign. Level |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % Fat | 8.15 a ± 0.60 | 8.64 ab ± 0.44 | 8.73 ab ± 0.61 | 8.89 ab ± 0.88 | 8.38 ab ± 0.75 | 8.65 ab ± 0.82 | 9.54 b ± 0.84 | 7.96 a ± 0.67 | 8.75 ab ± 0.88 | 8.21 ab ± 1.05 | p = 0.035 |
| % FFDM | 89.23 b ± 2.36 | 90.58 b ± 2.67 | 88.30 ab ± 1.63 | 88.07 ab ± 1.71 | 88.35 ab ± 2.31 | 86.91 ab ± 0.68 | 85.79 a ± 1.27 | 91.12 b ± 0.77 | 88.55 ab ± 1.26 | 88.85 ab ± 3.18 | p = 0.003 |
| % Moisture | 2.69 ab ± 1.95 | 0.78 a ± 0.46 | 2.97 ab ± 1.67 | 3.03 ab ± 1.62 | 3.27 ab ± 1.82 | 4.44 b ± 0.41 | 4.67 b ± 1.38 | 0.92 a ± 0.31 | 2.70 ab ± 1.96 | 2.94 ab ± 2.19 | p = 0.002 |
| Treatment | Control | LAN 60 | LAN 120 | LAN 240 | Urea 60 | Urea 120 | Urea 240 | AmSul 60 | AmSul 120 | AmSul 240 | Sign. Level |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C14:0 | 0.07 ab ± 0.02 | 0.09 b ± 0.02 | 0.08 ab ± 0.02 | 0.06 ab ± 0.02 | 0.06 a ± 0.02 | 0.07 ab ± 0.01 | 0.08 ab ± 0.02 | 0.08 ab ± 0.02 | 0.06 ab ± 0.02 | 0.09 ab ± 0.01 | p = 0.044 |
| C16:0 | 13.95 b ± 0.17 | 13.82 ab ± 0.07 | 13.78 ab ± 0.10 | 13.71 a ± 0.07 | 13.85 ab ± 0.16 | 13.83 ab ± 0.06 | 13.71 a ± 0.09 | 13.79 ab ± 0.19 | 13.71 a ± 0.08 | 13.70 a ± 0.12 | p = 0.002 |
| C16:1c9 | 0.68 b ± 0.02 | 0.66 ab ± 0.02 | 0.65 a ± 0.01 | 0.64 a ± 0.02 | 0.65 a ± 0.01 | 0.65 a ± 0.01 | 0.64 a ± 0.01 | 0.66 ab ± 0.01 | 0.64 a ± 0.01 | 0.64 a ± 0.01 | p < 0.001 |
| C17:0 | 0.17 c ± 0.01 | 0.16 bc ± 0.01 | 0.14 ab ± 0.01 | 0.14 ab ± 0.02 | 0.16 bc ± 0.01 | 0.15 abc ± 0.02 | 0.13 a ± 0.01 | 0.16 bc ± 0.01 | 0.13 ab ± 0.01 | 0.14 abc ± 0.01 | p < 0.001 |
| C17:1c10 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 | p = 0.271 |
| C18:0 | 3.05 a ± 0.05 | 3.17 b ± 0.10 | 3.18 b ± 0.03 | 3.21 b ± 0.07 | 3.12 ab ± 0.04 | 3.19 b ± 0.08 | 3.21 b ± 0.01 | 3.20 b ± 0.07 | 3.13 ab ± 0.06 | 3.15 ab ± 0.02 | p < 0.001 |
| C18:1c9 | 12.88 a ± 0.09 | 13.19 b ± 0.12 | 13.16 ab ± 0.11 | 13.23 b ± 0.09 | 13.15 ab ± 0.24 | 13.20 b ± 0.28 | 13.17 ab ± 0.16 | 13.31 b ± 0.05 | 13.16 ab ± 0.36 | 13.25 b ± 0.25 | p < 0.001 |
| C18:1c7 | 6.11 c ± 0.05 | 6.06 abc ± 0.03 | 6.03 ab ± 0.06 | 6.01 ab ± 0.05 | 6.07 bc ± 0.04 | 6.02 ab ± 0.04 | 5.98 a ± 0.03 | 6.06 abc ± 0.03 | 6.05 abc ± 0.03 | 6.05 abc ± 0.05 | p ≤ 0.001 |
| C18:2c9,12 (n-6) | 61.96 ± 0.15 | 61.79 ± 0.32 | 61.91 ± 0.22 | 61.93 ± 0.18 | 61.91 ± 0.19 | 61.86 ± 0.36 | 62.06 ± 0.18 | 61.72 ± 0.15 | 62.09 ± 0.36 | 61.94 ± 0.25 | p = 0.396 |
| C20:0 | 0.22 ± 0.01 | 0.23 ± 0.01 | 0.23 ± 0.01 | 0.23 ± 0.01 | 0.22 ± 0.01 | 0.22 ± 0.01 | 0.22 ± 0.01 | 0.22 ± 0.01 | 0.22 ± 0.01 | 0.22 ± 0.01 | p = 0.121 |
| C18:3c9,12,15 (n-3) | 0.39 ± 0.01 | 0.39 ± 0.01 | 0.40 ± 0.01 | 0.39 ± 0.01 | 0.39 ± 0.01 | 0.39 ± 0.01 | 0.40 ± 0.01 | 0.39 ± 0.01 | 0.40 ± 0.01 | 0.40 ± 0.01 | p = 0.650 |
| C20:3c8,11,14 (n-6) | 0.13 ab ± 0.01 | 0.13 ab ± 0.01 | 0.13 ab ± 0.01 | 0.13 b ± 0.01 | 0.12 a ± 0.01 | 0.12 ab ± 0.01 | 0.13 ab ± 0.01 | 0.13 ab ± 0.01 | 0.13 ab ± 0.01 | 0.13 ab ± 0.01 | p = 0.024 |
| C20:3c11,14,17 (n-3) | 0.15 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.16 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.01 | p = 0.206 |
| C24:0 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | p = 0.105 |
| Treatment | Control | LAN 60 | LAN 120 | LAN 240 | Urea 60 | Urea 120 | Urea 240 | AmSul 60 | AmSul 120 | AmSul 240 | Sign. Level |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SFA | 17.56 ± 0.19 | 17.57 ± 0.19 | 17.51 ± 0.13 | 17.45 ± 0.11 | 17.51 ± 0.16 | 17.56 ± 0.12 | 17.45 ± 0.09 | 17.55 ± 0.18 | 17.35 ± 0.05 | 17.40 ± 0.11 | p = 0.232 |
| MUFA | 19.72 ± 0.12 | 19.96 ± 0.14 | 19.89 ± 0.16 | 19.92 ± 0.06 | 19.91 ± 0.27 | 19.91 ± 0.27 | 19.84 ± 0.18 | 20.07 ± 0.03 | 19.89 ± 0.35 | 19.99 ± 0.26 | p = 0.103 |
| PUFA | 62.63 ± 0.15 | 62.47 ± 0.31 | 62.60 ± 0.21 | 62.60 ± 0.18 | 62.57 ± 0.20 | 62.52 ± 0.36 | 62.75 ± 0.19 | 62.39 ± 0.16 | 62.77 ± 0.37 | 62.62 ± 0.26 | p = 0.393 |
| n-6 | 62.09 ± 0.15 | 61.92 ± 0.31 | 62.04 ± 0.22 | 62.06 ± 0.18 | 62.03 ± 0.20 | 61.98 ± 0.36 | 62.19 ± 0.19 | 61.85 ± 0.15 | 62.22 ± 0.37 | 62.07 ± 0.25 | p = 0.403 |
| n-3 | 0.54 ± 0.01 | 0.55 ± 0.01 | 0.56 ± 0.02 | 0.54 ± 0.01 | 0.54 ± 0.01 | 0.54 ± 0.01 | 0.56 ± 0.01 | 0.54 ± 0.01 | 0.55 ± 0.01 | 0.55 ± 0.02 | p = 0.559 |
| PUFA/SFA | 3.57 ± 0.04 | 3.56 ± 0.06 | 3.58 ± 0.03 | 3.59 ± 0.03 | 3.57 ± 0.03 | 3.56 ± 0.04 | 3.60 ± 0.02 | 3.55 ± 0.05 | 3.62 ± 0.03 | 3.60 ± 0.03 | p = 0.242 |
| n-6/n-3 | 114.98 ± 2.23 | 112.58 ± 3.13 | 110.79 ± 3.30 | 114.93 ± 2.10 | 114.87 ± 1.77 | 114.78 ± 2.89 | 111.05 ± 1.55 | 114.54 ± 1.75 | 113.13 ± 2.03 | 112.85 ± 2.92 | p = 0.616 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkoi, V.; Wit, M.d.; Fouche, H.; Coetzer, G.; Hugo, A. The Effect of Nitrogen Fertilization on the Yield, Quality and Fatty Acid Composition of Opuntia ficus-indica Seed Oil. Sustainability 2021, 13, 10123. https://doi.org/10.3390/su131810123
Nkoi V, Wit Md, Fouche H, Coetzer G, Hugo A. The Effect of Nitrogen Fertilization on the Yield, Quality and Fatty Acid Composition of Opuntia ficus-indica Seed Oil. Sustainability. 2021; 13(18):10123. https://doi.org/10.3390/su131810123
Chicago/Turabian StyleNkoi, Vuyelwa, Maryna de Wit, Herman Fouche, Gesine Coetzer, and Arno Hugo. 2021. "The Effect of Nitrogen Fertilization on the Yield, Quality and Fatty Acid Composition of Opuntia ficus-indica Seed Oil" Sustainability 13, no. 18: 10123. https://doi.org/10.3390/su131810123








