Plant Species Composition and the Perception of the Afforestation in Urban Public Green Spaces in a Municipality in Eastern Brazilian Amazon
Abstract
:1. Introduction
2. Materials and Methods
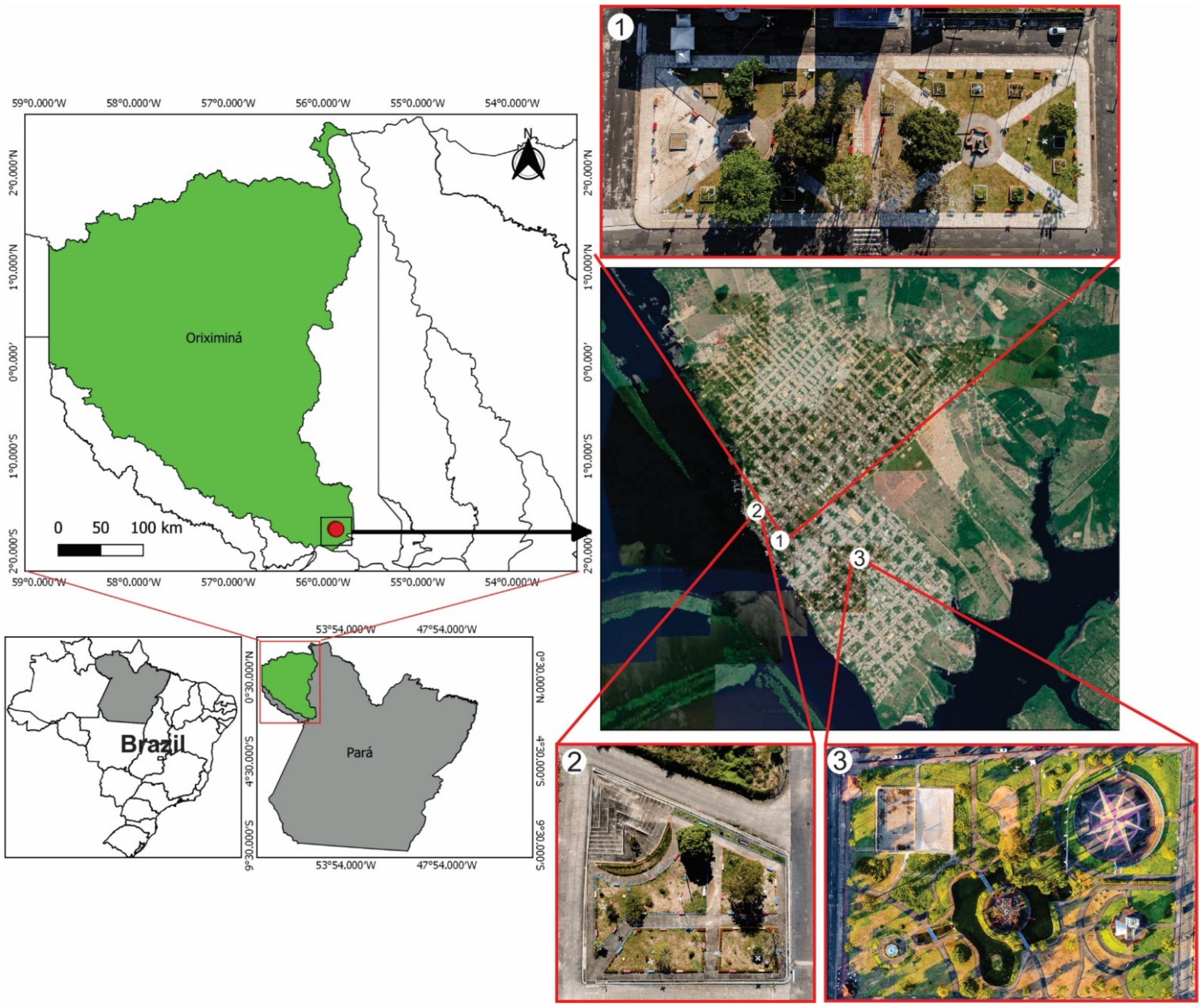
2.1. Characterization of the Study Area
2.2. Collection and Identification of Botanical Material
2.3. Perception of Squares’ Visitors
3. Results
3.1. Floristic Composition in Urban Public Areas
3.2. Analysis of the Questionnaires
4. Discussion
Perception of Regular Visitors
5. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, X.; Wang, Y.-C. Spatial–temporal dynamics of urban green space in response to rapid urbanization and greening policies. Landsc. Urban Plan. 2011, 100, 268–277. [Google Scholar] [CrossRef]
- Secretariat of the Convention on Biological Diversity. Cities and Biodiversity Outlook—Executive Summary. Montreal. 2012. 16 pages. Available online: https://www.cbd.int/authorities/doc/cbo-1/cbd-cbo1-summary-en-f-web.pdf (accessed on 6 September 2021).
- Sirakaya, A.; Cliquet, A.; Harris, J. Ecosystem services in cities: Towards the international legal protection of ecosystem services in urban environments. Ecosyst. Serv. 2018, 29, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Alberti, M.; Correa, C.; Marzluff, J.M.; Hendry, A.P.; Palkovacs, E.P.; Gotanda, K.M.; Hunt, V.M.; Apgar, T.M.; Zhou, Y. Global urban signatures of phenotypic change in animal and plant populations. Proc. Natl. Acad. Sci. USA 2017, 114, 8951–8956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United Nations Department of Economic and Social Affairs. United Nations World Urbanization Prospects. The 2018 Revision. Available online: https://www.un.org/development/desa/publications/2018-revision-of-world-urbanization-prospects.html (accessed on 1 July 2020).
- Nowak, D.J.; Crane, D.E. Carbon storage and sequestration by urban trees in the USA. Environ. Pollut. 2002, 116, 381–389. [Google Scholar] [CrossRef]
- Liu, C.; Li, X. Carbon storage and sequestration by urban forests in Shenyang, China. Urban For. Urban Green. 2012, 11, 121–128. [Google Scholar] [CrossRef]
- Nowak, D.J.; Hirabayashi, S.; Bodine, A.; Hoehn, R. Modeled PM2.5 removal by trees in ten U.S. cities and associated health effects. Environ. Pollut. 2013, 178, 395–402. [Google Scholar] [CrossRef]
- Arcanjo, F.A.; Taglianetti, E.; Torezan, J.M.D. Big trees, big fall: Large-diameter trees and the fate of carbon stocks in Atlantic Forest remnants. Oecologia Aust. 2020, 24, 438–447. [Google Scholar] [CrossRef]
- Shimamoto, C.Y.; Botosso, P.C.; Marques, M.C.M. How much carbon is sequestered during the restoration of tropical forests? Estimates from tree species in the Brazilian Atlantic forest. For. Ecol. Manag. 2014, 329, 1–9. [Google Scholar] [CrossRef]
- Abreu, L.V.; Labaki, L.C. Conforto térmico propiciado por algumas espécies arbóreas: Avaliação do raio de influência através de diferentes índices de conforto. Ambient. Constr. 2010, 10, 103–117. [Google Scholar] [CrossRef]
- Kong, L.; Lau, K.K.-L.; Yuan, C.; Chen, Y.; Xu, Y.; Ren, C.; Ng, E. Regulation of outdoor thermal comfort by trees in Hong Kong. Sustain. Cities Soc. 2017, 31, 12–25. [Google Scholar] [CrossRef]
- Li, D.; Liao, W.; Rigden, A.J.; Liu, X.; Wang, D.; Malyshev, S.; Shevliakova, E. Urban heat island: Aerodynamics or imperviousness? Sci. Adv. 2019, 5, eaau4299. [Google Scholar] [CrossRef] [Green Version]
- Aronson, M.F.J.; Lepczyk, C.A.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S.; Nilon, C.H.; Vargo, T. Biodiversity in the city: Key challenges for urban green space management. Front. Ecol. Environ. 2017, 15, 189–196. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Urban green spaces: A brief for action. Reg. Off. Eur. 2017, 24. [Google Scholar] [CrossRef] [Green Version]
- Dearborn, D.C.; Kark, S. Motivations for conserving urban biodiversity. Conserv. Biol. 2010, 24, 432–440. [Google Scholar] [CrossRef]
- Nielsen, A.B.; van den Bosch, M.; Maruthaveeran, S.; van den Bosch, C.K. Species richness in urban parks and its drivers: A review of empirical evidence. Urban Ecosyst. 2014, 17, 305–327. [Google Scholar] [CrossRef]
- FAO. Forests and Sustainable Cities; FAO: Rome, Italy, 2018; Volume 69, ISBN 9789251303832. [Google Scholar]
- Wolch, J.R.; Byrne, J.; Newell, J.P. Urban green space, public health, and environmental justice: The challenge of making cities ‘just green enough’. Landsc. Urban Plan. 2014, 125, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Baycan-Levent, T.; Nijkamp, P. Planning and Management of Urban Green Spaces in Europe: Comparative Analysis. J. Urban Plan. Dev. 2009, 135, 1–12. [Google Scholar] [CrossRef]
- Govindarajulu, D. Urban green space planning for climate adaptation in Indian cities. Urban Clim. 2014, 10, 35–41. [Google Scholar] [CrossRef]
- Antonelli, A.; Sanmartín, I. Why are there so many plant species in the Neotropics? Taxon 2011, 60, 403–414. [Google Scholar] [CrossRef]
- Antonelli, A.; Zizka, A.; Carvalho, F.A.; Scharn, R.; Bacon, C.D.; Silvestro, D.; Condamine, F.L. Amazonia is the primary source of Neotropical biodiversity. Proc. Natl. Acad. Sci. USA 2018, 115, 6034–6039. [Google Scholar] [CrossRef] [Green Version]
- Gaertner, M.; Wilson, J.R.U.; Cadotte, M.W.; MacIvor, J.S.; Zenni, R.D.; Richardson, D.M. Non-native species in urban environments: Patterns, processes, impacts and challenges. Biol. Invasions 2017, 19, 3461–3469. [Google Scholar] [CrossRef] [Green Version]
- Moro, M.F.; Castro, A.S.F. A check list of plant species in the urban forestry of Fortaleza, Brazil: Where are the native species in the country of megadiversity? Urban Ecosyst. 2015, 18, 47–71. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Banerjee, A.K.; Dewanji, A. Native exotic relationships in plant communities: The role of exotic dominance in framing community composition. Ecol. Res. 2017, 32, 653–665. [Google Scholar] [CrossRef]
- Vieira, T.A.; Panagopoulos, T. Urban Forestry in Brazilian Amazonia. Sustainability 2020, 12, 3235. [Google Scholar] [CrossRef] [Green Version]
- Fitria, R.; Kim, D.; Baik, J.; Choi, M. Impact of biophysical mechanisms on Urban Heat Island associated with climate variation and urban morphology. Sci. Rep. 2019, 9, 19503. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatísticas (IBGE). Available online: https://cidades.ibge.gov.br/brasil/pa/oriximina/panorama (accessed on 22 April 2020).
- Instituto Socioambiental (ISA). Available online: https://www.socioambiental.org/pt-br/mapas/amazonia-brasileira-2014 (accessed on 1 July 2020).
- Peixoto, A.L.; Maia, E.C. Manual de Procedimentos Para Herbários; Editora Universitária UFPE: Recife, Brazil, 2013. [Google Scholar]
- Barroso, G.M. Sistemática de Angiospermas do Brasil; Imprensa Universitária: Viçosa, Brazil, 1991. [Google Scholar]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil; Instituto Plantarum: Nova Odessa, Brazil, 2009; Volume 3. [Google Scholar]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil; Instituto Plantarum: Nova Odessa, Brazil, 1998; Volume 2. [Google Scholar]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil; Instituto Plantarum: Nova Odessa, Brazil, 1992; Volume 1. [Google Scholar]
- Saueressig, D. Plantas do Brasil—Árvores Nativas; Editora Plantas do Brasil: Irati, Brazil, 2017. [Google Scholar]
- Souza, V.C.; Lorenzi, H. Botânica Sistemática: Guia Ilustrado Para Identificação das Famílias de Angiospermas da Flora Brasileira, Baseado em APG II; Instituto Plantarum: Nova Odessa, Brazil, 2005. [Google Scholar]
- The Angiosperm Phylogeny Group. APG IV An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef] [Green Version]
- JABOT Jardim Botânico do Rio de Janeiro. Available online: http://jabot.jbrj.gov.br (accessed on 1 July 2020).
- Flora do Brasil 2020 Jardim Botânico do Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/ (accessed on 1 July 2020).
- IPNI. International Plant Names Index. 2021. Available online: http://www.ipni.org (accessed on 27 July 2021).
- Instituto Hórus 2020 Base de Dados Nacional de Espécies Exóticas Invasoras em I3N-Brasil. Available online: http://www.institutohorus.org.br/ (accessed on 13 February 2020).
- SINITOX 2020 Dados de Intoxicações. Dados Nacionais. Available online: https://sinitox.icict.fiocruz.br (accessed on 18 February 2020).
- da Silva Rios, M.N.; Pastore, F., Jr. Plantas da Amazônia: 450 Espécies de Uso Geral; Universidade de Brasília: Brasília, Brazil, 2011. [Google Scholar]
- João, R.A.L.; João, P.d.C.L.; Stoney, D.N.P.; Ary, V.d.P. Germinação de sementes e crescimento inicial de plântulas de ingá-mirim—Inga laurina (S W.) Willd—Utilizada na arborização urbana de Rio Branco, Acre. Rev. Soc. Bras. Arborização Urbana 2019, 7, 11. [Google Scholar] [CrossRef]
- Souza, O.P.S.; Souza, P.T.S.; Freitas, A.D.D.; Paraense, V.C.; Silva, S.A.S. Diagnóstico quali-quantitativo da arborização das praças do município de Altamira, Pará. Encicl. Biosfera 2013, 9, 1080. [Google Scholar]
- Gomes, A.C.; de Figueredo, L.F.; Souza, J.T.A.; Souto, J.S.; de Lacerda, A.V. Flora of Public Squares in the City of Areia, Paraíba, Brazil. Floresta Ambiente 2019, 26. [Google Scholar] [CrossRef]
- Lorenzi, H.; Bacher, L.B.; Torres, M.A. Árvores e Arvoretas Exóticas no Brasil: Madeireiras, Ornamentais e Aromáticas, 1st ed.; Instituto Plantarum de Estudos da Flora: Nova Odessa, Brazil, 2018; ISBN 978-85-86714-56-6. [Google Scholar]
- Santamour, F.S. Trees for urban planting: Diversity, Uniformity, and Common Sense. In Proceedings of the Seventh Conference of The Metropolitan Tree Improvement Alliance, Lisle, IL, USA, 11–12 June 1990; Volume 7, pp. 57–65. [Google Scholar]
- De Freitas, W.K.; Magalhães, L.M.S.; de Santana, C.A.A.; Pereira Junior, E.R.; de Castro Machado de Souza, L.; Toledo, R.A.B.; Garção, B.R. Tree composition of urban public squares located in the Atlantic Forest of Brazil: A systematic review. Urban For. Urban Green. 2020, 48, 126555. [Google Scholar] [CrossRef]
- Asgarzadeh, M.; Vahdati, K.; Lotfi, M.; Arab, M.; Babaei, A.; Naderi, F.; Pir Soufi, M.; Rouhani, G. Plant selection method for urban landscapes of semi-arid cities (a case study of Tehran). Urban For. Urban Green. 2014, 13, 450–458. [Google Scholar] [CrossRef]
- de Souza e Silva, J.L.; de Oliveira, M.T.P.; Oliveira, W.; Borges, L.A.; Cruz-Neto, O.; Lopes, A.V. High richness of exotic trees in tropical urban green spaces: Reproductive systems, fruiting and associated risks to native species. Urban For. Urban Green. 2020, 50, 126659. [Google Scholar] [CrossRef]
- Oliveira, M.T.P.; Silva, J.L.S.; Cruz-Neto, O.; Borges, L.A.; Girão, L.C.; Tabarelli, M.; Lopes, A.V. Urban green areas retain just a small fraction of tree reproductive diversity of the Atlantic forest. Urban For. Urban Green. 2020, 54, 126779. [Google Scholar] [CrossRef]
- Terra, C. OS Jardins do Brasil do Século XIX: Glaziou Revisitado; EBA/UFRJ: Rio de Janeiro, Brazil, 1993. [Google Scholar]
- dos Santos, A.R.; da Rocha, C.F.D.; Bergallo, H.G. Native and exotic species in the urban landscape of the city of Rio de Janeiro, Brazil: Density, richness, and arboreal deficit. Urban Ecosyst. 2010, 13, 209–222. [Google Scholar] [CrossRef]
- Barros, V.D.S.; Martins, C.M.; Dos Santos, M.A.S.; Rebello, F.K.; Monteiro, C.W.B.; Mesquita, I.S.B. Avaliação da organização árborea e a percepção dos usuários das praças do município de Mocajuba, estado do Pará, Brasil. Rev. Soc. Bras. Arborização Urbana 2019, 13, 1. [Google Scholar] [CrossRef] [Green Version]
- Silva, I.D.R.; Oliveira, À.T.D.S.; Da Silva, L.B.O.; Baia, R.S.; Correa, T.B.C.; Martins, W.B.R. Diagnóstico visual e fitossociologia na arborização de praças em Paragominas, Pará. Rev. Soc. Bras. Arborização Urbana 2018, 13, 1. [Google Scholar] [CrossRef] [Green Version]
- Rufino, M.R.; Silvino, A.S.; Moro, M.F. Exóticas, exóticas, exóticas: Reflexões sobre a monótona arborização de uma cidade brasileira. Rodriguésia 2019, 70. [Google Scholar] [CrossRef] [Green Version]
- ICMBIO. Guia de Orientação Para o Manejo de Espécies Exóticas Invasoras em Unidades Conservação Federais; Instituto Chico Mendes: Brasilia, Brazil, 2019. [Google Scholar]
- Zenni, R.D.; Ziller, S.R. An overview of invasive plants in Brazil. Rev. Bras. Botânica 2011, 34, 431–446. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.L.; Malheiros, T.F.; Fernandes, V.; Dagostin Darós, T. A percepção ambiental como instrumento de apoio na gestão e na formulação de políticas públicas ambientais. Saúde Soc. 2012, 21, 96–110. [Google Scholar] [CrossRef] [Green Version]
- Bochner, R.; Lemos, E. Plantas tóxicas em espaços escolares infantis: Do risco à informação. J. Health NPEPS 2017, 2, 102–112. [Google Scholar]
- Buckeridge, M. Árvores urbanas em São Paulo: Planejamento, economia e água. Estudos Avançados 2015, 29, 85–101. [Google Scholar] [CrossRef] [Green Version]
- Oldfield, E.E.; Warren, R.J.; Felson, A.J.; Bradford, M.A. FORUM: Challenges and future directions in urban afforestation. J. Appl. Ecol. 2013, 50, 1169–1177. [Google Scholar] [CrossRef]
- Carrus, G.; Scopelliti, M.; Lafortezza, R.; Colangelo, G.; Ferrini, F.; Salbitano, F.; Agrimi, M.; Portoghesi, L.; Semenzato, P.; Sanesi, G. Go greener, feel better? The positive effects of biodiversity on the well-being of individuals visiting urban and peri-urban green areas. Landsc. Urban Plan. 2015, 134, 221–228. [Google Scholar] [CrossRef]
- Fongar, C.; Aamodt, G.; Randrup, T.B.; Solfjeld, I. Does perceive green space quality matter? Linking Norwegian adult perspectives on perceived quality to motivation and frequency of visits. Int. J. Environ. Res. Public Health 2019, 16, 2327. [Google Scholar] [CrossRef] [Green Version]
- Sartori, R.A.; Martins, G.A.C.; Zaú, A.S.; Brasil, L.S.C. Urban afforestation and favela: A study in a community of Rio de Janeiro, Brazil. Urban For. Urban Green. 2019, 40, 84–92. [Google Scholar] [CrossRef]
- Fuller, R.A.; Irvine, K.N.; Devine-Wright, P.; Warren, P.H.; Gaston, K.J. Psychological benefits of greenspace increase with biodiversity. Biol. Lett. 2007, 3, 390–394. [Google Scholar] [CrossRef]
- Alves, F.R.N.; de Aquino, M.G.C.; Maestri, M.P.; Tenório, R.S.; das Silva, J.J.N.; Carneiro, F.D.S.; dos Santos, J.L.; de Figueira, E.P.O. Percepção da arborização urbana pelos moradores de duas zonas do município de Santarém (PA). Nat. Conserv. 2019, 12, 60–76. [Google Scholar] [CrossRef]


| Public Green Space | Area (m2) | Coordinates |
|---|---|---|
| Centenário | 25,381.83 m2 | 1°46′10.5″ S 55°51′32.9″ W |
| Santo Antônio | 3841.14 m2 | 1°46′03.5″ S 55°52′05.2″ W |
| Saudade | 1822.99 m2 | 1°45′51.9″ S 55°52′15.0″ W |
| Families/Species | Common Name | Voucher HSTM | N | NI | FR% | Origin | IUCN | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Life Form | C | SA | S | |||||||
| Anacardiaceae | ||||||||||
| Anacardium occidentale L. | Cajueiro/Cashew | Tree | 14480 | 01 | 00 | 00 | 01 | 0.68 | N | NE |
| Apocynaceae | ||||||||||
| Allamanda blanchetii A.DC. | Alamanda roxa/Purple Allamanda | Flowering shrub | 14488 | 00 | 00 | 01 | 01 | 0.68 | NEA | NE |
| Allamanda cathartica L. | Alamanda/Golden trumpet | Flowering shrub | 14483 | 00 | 18 | 00 | 18 | 12.33 | N | NE |
| Plumeria pudica Jacq. | Buquê-de-noiva/Golden Arrow | Flowering shrub | 14485 | 00 | 00 | 08 | 08 | 5.48 | E | LC |
| Arecaceae | ||||||||||
| Caryota urens L. | Palmeira-rabo-de-peixe/Solitary fishtail palm | Palm | - | 00 | 01 | 00 | 01 | 0.68 | E | NE |
| Dypsis lutescens (H. Wendl.) Beentje & J. Dransf. | Palmeira areca-bambu/Areca palm | Palm | - | 00 | 06 | 00 | 06 | 4.1 | E | NT |
| Euterpe oleracea M art. | Açaí-do-pará/Açai berry | Palm | - | 12 | 00 | 00 | 12 | 8.22 | N | NE |
| Mauritia flexuosa L. f. | Buriti/Moriche palm | Palm | - | 13 | 00 | 00 | 13 | 8.90 | N | NE |
| Pritchardia pacifica Seem. & H. Wendl. | Palmeira-de-leque/Fiji fan palm | Palm | - | 00 | 01 | 01 | 02 | 1.37 | E | NE |
| Roystonea oleracea (Jacq.) O.F. Cook. | Palmeira-imperial/Imperial Palm | Palm | - | 00 | 03 | 00 | 03 | 2.05 | E | NE |
| Asparagaceae | ||||||||||
| Dracaena fragans (L.) Ker Gawl | Dracaena | Flowering shrub | - | 04 | 00 | 00 | 04 | 2.74 | E | NE |
| Bignoniaceae | ||||||||||
| Handroanthus barbatus (E.Mey.) Mattos | Ipê-roxo/Purple ipe | Tree | 14486 | 01 | 00 | 00 | 01 | 0.68 | N | NE |
| Handroanthus serratifolius (Vahl) S.Grose | Ipê-amarelo/Yellow ipe | Tree | 14492 | 13 | 05 | 01 | 19 | 13.01 | N | NE |
| Handronthus sp.1 | Ipê/Ipe | Tree | 14476 | 01 | 00 | 00 | 01 | 0.68 | NID | - |
| Handronthus sp.2 | Ipê/Ipe | Tree | 14475 | 00 | 00 | 02 | 02 | 1.37 | NID | - |
| Chrysobalanaceae | ||||||||||
| Licania tomentosa (Benth.) Fritsch | Oiti | Tree | 14491 | 00 | 02 | 00 | 02 | 1.37 | NEA | NE |
| Combretaceae | ||||||||||
| Terminalia catappa L. | Castanholeira/Country almond | Tree | 14487 | 00 | 02 | 00 | 02 | 1.37 | E | NE |
| Cycadaceae | ||||||||||
| Cycas circinalis L. | Cica/Queen sago | Palm | - | 09 | 02 | 03 | 14 | 9.59 | E | NE |
| Fabaceae | ||||||||||
| Andira cf. surinamensis (Bondt) Splitg. ex Amshoff | Andira-da-várzea | Tree | 14484 | 02 | 00 | 00 | 02 | 1.37 | N | NE |
| Clitoria fairchildiana R.A.Howard | Sombreiro | Tree | 14489 | 00 | 00 | 01 | 01 | 0.68 | N | NE |
| Delonix regia (Bojer ex Hook.) Raf. | Flamboyant/Royal poinciana | Tree | 14482 | 00 | 00 | 01 | 01 | 0.68 | E | NE |
| Malpighiaceae | ||||||||||
| Lophanthera lactescens Ducke | Lofântera-da-amazônia | Tree | 14478 | 11 | 00 | 00 | 11 | 7.53 | N | NE |
| Malvaceae | ||||||||||
| Pseudobombax munguba (Mart.) Dugand | Munguba | Tree | 14490 | 00 | 00 | 01 | 01 | 0.68 | N | NE |
| Meliaceae | ||||||||||
| Azadirachta indica A. Juss. | Nim/Neem | Tree | 14479 | 14 | 01 | 00 | 15 | 10.27 | E | NE |
| Moraceae | ||||||||||
| Ficus benjamina L. | Ficus/Weeping fig | Tree | 14493 | 00 | 00 | 02 | 02 | 1.37 | E | NE |
| Myrtaceae | ||||||||||
| Syzygium malaccense (L.) Merr. & L.M. Perry | Jambeiro/Malay apple | Tree | 14477 | 00 | 00 | 01 | 01 | 0.68 | E | LC |
| Nyctaginaceae | ||||||||||
| Bougainvillea sp. | Buganvílea | Vine | 14474 | 00 | 00 | 00 | 01 | 0.68 | NID | - |
| Rubiaceae | ||||||||||
| Ixora coccinea L. | Ixora vermelha/Jungle geranium | Flowering shrub | 14481 | 764 | 386 | 320 | 1.47 | * | E | NE |
| Total | 847 | 427 | 342 | 1.616 | 100 | |||||
| Species | Centenário | Santo Antônio | Saudade |
|---|---|---|---|
| RF% | RF% | RF% | |
| Allamanda blanchetii | - | - | 4.55 |
| Allamanda cathartica | - | 43.90 | - |
| Anacardium occidentale | 1.22 | - | - |
| Andira cf. surinamensis | 2.44 | - | - |
| Azadirachta indica | 17.07 | 2.44 | - |
| Bougainvillea sp. | 1.22 | - | - |
| Caryota urens | - | 2.44 | - |
| Clitoria fairchildiana | - | - | 4.55 |
| Cycas circinalis | 10.98 | 4.88 | 13.64 |
| Delonix regia | - | - | 4.55 |
| Dracaena fragans | 4.88 | - | - |
| Dypsis lutescens | - | 14.63 | - |
| Euterpe oleracea | 14.63 | - | - |
| Ficus benjamina | - | - | 9.09 |
| Handroanthus barbatus | 1.22 | - | - |
| Handroanthus serratifolius | 15.85 | 12.2 | 4.55 |
| Handronthus sp.1 | 1.22 | - | - |
| Handronthus sp.2 | - | - | 9.09 |
| Licania tomentosa | - | 4.88 | - |
| Lophanthera lactescens | 13.41 | - | - |
| Mauritia flexuosa | 15.85 | - | - |
| Plumeria pudica | - | - | 36.36 |
| Pritchardia pacifica | - | 2.44 | 4.55 |
| Pseudobombax munguba | - | - | 4.55 |
| Roystonea oleracea | - | 7.32 | - |
| Syzygium malaccense | - | - | 4.55 |
| Terminalia catappa | - | 4.88 | - |
| Total | 100 | 100 | 100 |
| Species | Common Name | Life Form | Família |
|---|---|---|---|
| Alibertia edulis (Rich.) A. Rich. | Puru | Tree | Rubiaceae |
| Andira inermis (W.Wright) DC. | Morcegueira/Cabbage tree | Tree | Fabaceae |
| Andira parviflora Ducke | Sucupira-vermelha | Tree | Fabaceae |
| Apeiba tibourbou Aubl. | Pente-de-macaco | Tree | Malvaceae |
| Annona montana Macfad. | Araticum/Mountain soursop | Tree | Annonaceae |
| Bauhinia ungulata L. | Pata-de-vaca | Shrub/Tree | Fabaceae |
| Cenostigma tocantinum Ducke | Pau-pretinho | Shrub/Tree | Fabaceae |
| Costus spiralis (Jacq.) Roscoe | Cana-do-brejo/Spiral ginger | Herb | Costaceae |
| Dipteryx odorata (Aubl.) Willd. | Cumaru/Tonka beans | Tree | Fabaceae |
| Heliconia acuminata L.C.Rich. | Helicônia | Herb | Heliconiaceae |
| Heliconia hirsuta L.f. | Helicônia | Herb | Heliconiaceae |
| Heliconia psittacorum L.f. | Helicônia-papagaio/Parrot’s beak | Herb | Heliconiaceae |
| Hevea brasiliensis (Willd. ex A.Juss.) Müll.Arg. | Seringueira/Pará rubber tree | Tree | Euphorbiaceae |
| Himatanthus drasticus (Mart.) Plumel | Janaúba, sucuuba | Tree | Apocynaceae |
| Humiria balsamifera (Aubl.) A.St.-Hil. | Umiri | Shrub/Tree | Humiriaceae |
| Inga laurina (Sw.) Wild | Ingá-chichica, ingá-de-macaco | Tree | Fabaceae |
| Inga capitata Desv. | Ingá-ferro | Tree | Fabaceae |
| Inga edulis Mart. | Ingá-de-metro/Ice-cream-bean | Tree | Fabaceae |
| Jacaranda copaia (Aubl.) D.Don | Caroba | Tree | Bignoniaceae |
| Oenocarpus bacaba Mart. | Bacaba | Palm | Arecaceae |
| Pachira aquatica Aubl. | Monguba, mamorana/Guiana chestut | Tree | Malvaceae |
| Pouteria ramiflora (Mart.) Radlk. | Abiu-do-campo | Shrub/Tree | Sapotaceae |
| Tabebuia aurea (Silva Manso) Benth. & Hook.f. ex S.Moore | Carobeira, ipê amarelo/Silver trumpet tree | Tree | Bignoniaceae |
| Talisia esculenta (Cambess.) Radlk. | Pitomba | Tree | Sapindaceae |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, E.B.; Nogueira, F.M.; Talgatti, D.M. Plant Species Composition and the Perception of the Afforestation in Urban Public Green Spaces in a Municipality in Eastern Brazilian Amazon. Sustainability 2021, 13, 10332. https://doi.org/10.3390/su131810332
dos Santos EB, Nogueira FM, Talgatti DM. Plant Species Composition and the Perception of the Afforestation in Urban Public Green Spaces in a Municipality in Eastern Brazilian Amazon. Sustainability. 2021; 13(18):10332. https://doi.org/10.3390/su131810332
Chicago/Turabian Styledos Santos, Ediane Bó, Fernanda Mayara Nogueira, and Dávia Marciana Talgatti. 2021. "Plant Species Composition and the Perception of the Afforestation in Urban Public Green Spaces in a Municipality in Eastern Brazilian Amazon" Sustainability 13, no. 18: 10332. https://doi.org/10.3390/su131810332






