Multifunctional and Durable Coatings for Stone Protection Based on Gd-Doped Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. NPs Synthesis
2.3. Characterisation of Gd-TiO2 Nanoparticles
2.4. Preparation of Nanocomposites and Application Procedures
2.5. Testing Methods
- -
- L*, a*, b*(MB) and L*, a*, b*(t): the mean values after the application of methylene blue over surfaces and after t hours of UV-A light exposure, respectively.
- -
- L*(0), a*(0), and b*(0): three different chromatic coordinates of treated LS before staining with MB dye (the difference in between treated and untreated LS).
3. Results and Discussion
3.1. Characterisation of As-Prepared Pure and Doped TiO2 Nanoparticles
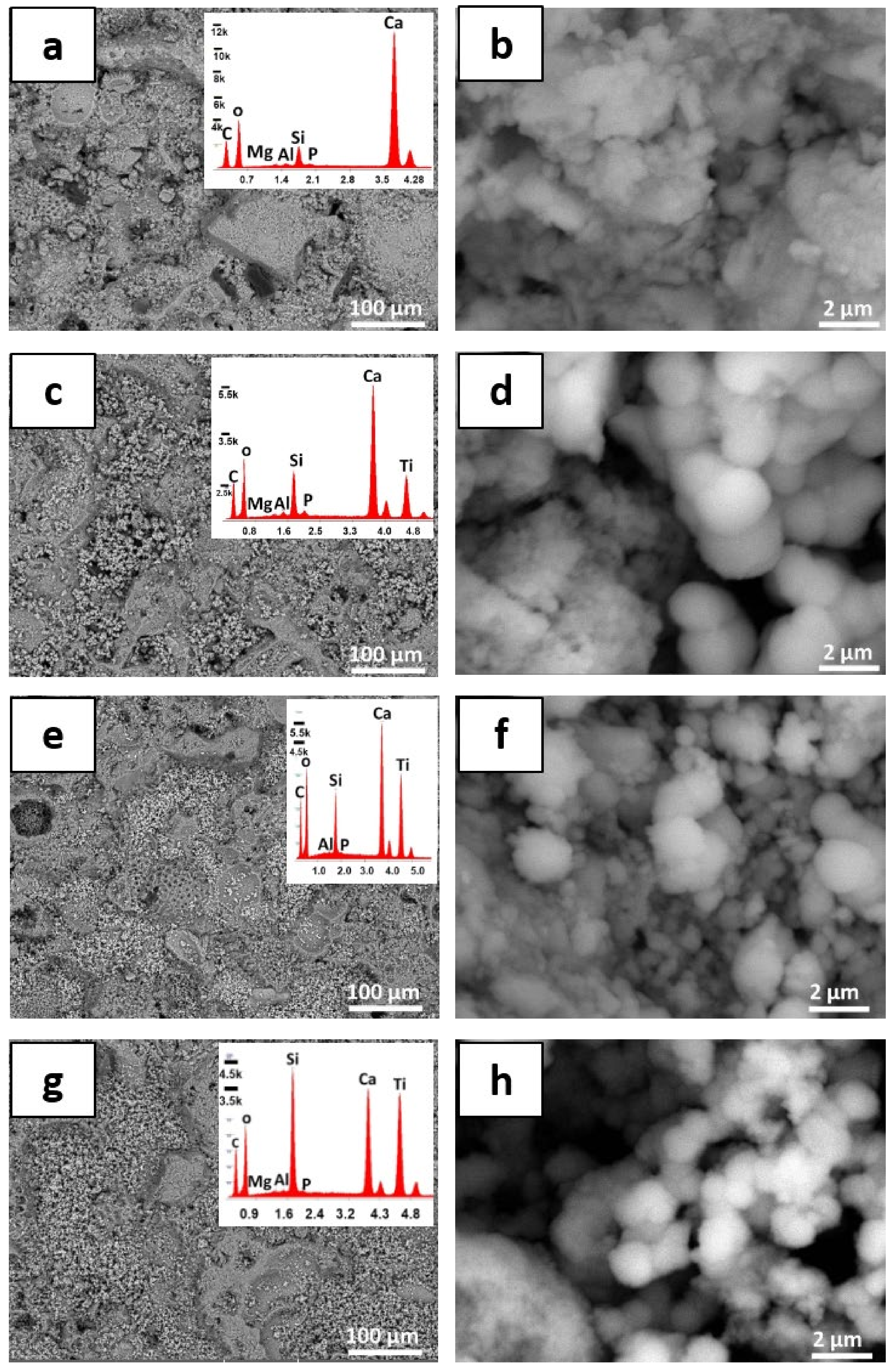
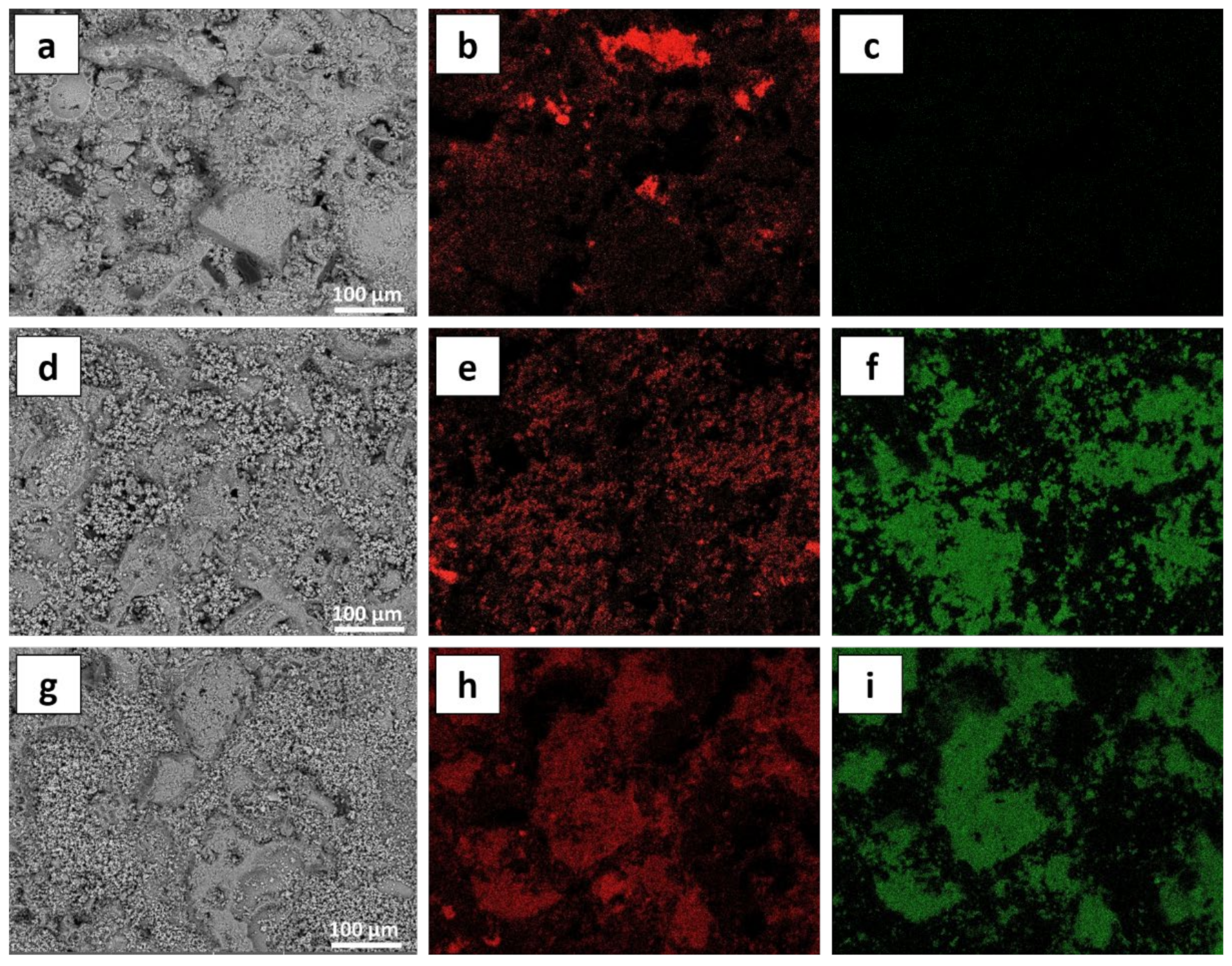
3.2. Modifications of the Stone Surface after Coatings Application
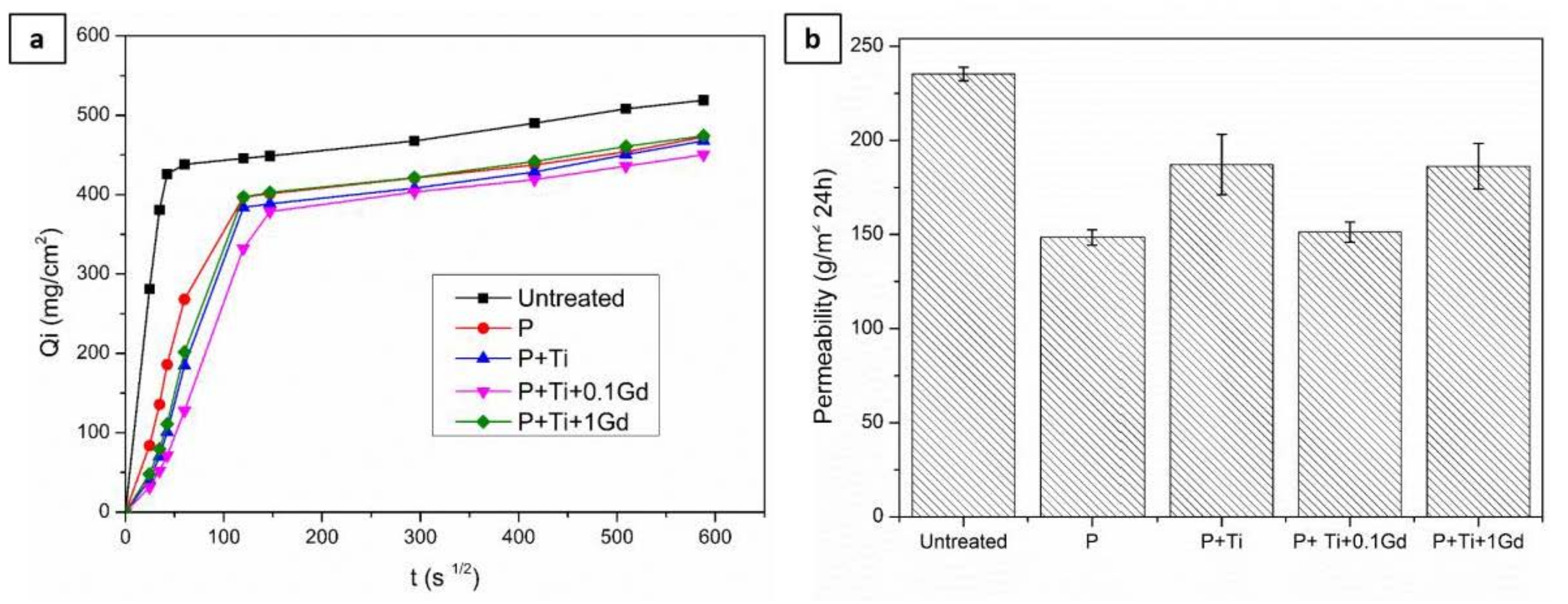
3.3. Evaluation of Hydrophobic Features of Different Treatments
3.4. Self-Cleaning Test
3.5. Antimicrobial Activity
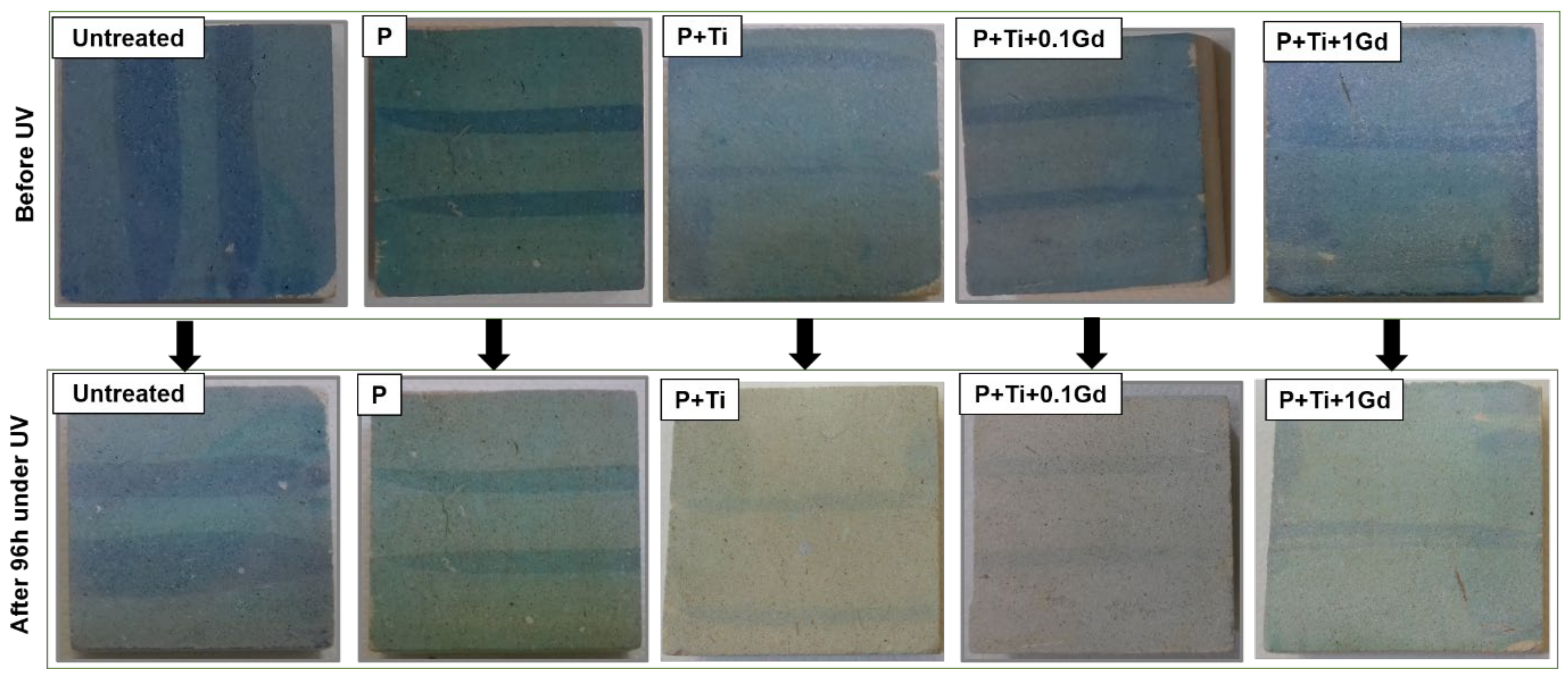
3.6. Durability of Nanocomposite Protective Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urzì, C. Microbial Deterioration of Rocks and Marble Monuments of the Mediterranean Basin: A Review, Corrosion Reviews. Corros. Rev. 2004, 22, 441–458. [Google Scholar] [CrossRef]
- Licchelli, M.; Malagodi, M.; Weththimuni, M.; Zanchi, C. Anti-graffiti nanocomposite materials for surface protection of a very porous stone. Appl. Phys. A 2014, 116, 1525–1539. [Google Scholar] [CrossRef]
- Belfiore, C.M.; Fichera, G.V.; la Russa, M.F.; Pezzino, A.; Ruffolo, S.A. The Baroque architecture of Scicli (south-eastern Sicily): Characterization of degradation materials and testing of protective products. Period. Mineral. 2012, 81, 19–33. [Google Scholar] [CrossRef]
- Ricca, M.; le Pera, E.; Licchelli, M.; Macchia, A.; Malagodi, M.; Randazzo, L.; Rovella, N.; Ruffolo, S.A.; Weththimuni, M.L.; la Russa, M.F. The CRATI Project: New Insights on the Consolidation of Salt Weathered Stone and the Case Study of San Domenico Church in Cosenza (South Calabria, Italy). Coatings 2019, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Licchelli, M.; Malagodi, M.; Weththimuni, M.L.; Zanchi, C. Water-repellent properties of fluoroelastomers on a very porous stone: Effect of the application procedure. Prog. Org. Coat. 2013, 76, 495–503. [Google Scholar] [CrossRef]
- Roig, P.B.; Ros, J.L.R.; Estellés, R.M. Biocleaning of nitrate alterations on wall paintings by Pseudomonas stutzeri. Int. Biodeterior. Biodegrad. 2013, 84, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Sanmartín, P.; DeAraujo, A.; Vasanthakumar, A.; Mitchell, R. Feasibility study involving the search for natural strains of microorganisms capable of degrading graffiti from heritage materials. Int. Biodeterior. Biodegrad. 2015, 103, 186–190. [Google Scholar] [CrossRef]
- Staicu, A.; Apostol, I.; Pascu, A.; Urzica, I.; Pascu, M.L.; Damian, V. Minimal invasive control of paintings cleaning by LIBS. Opt. Laser Technol. 2016, 77, 187–192. [Google Scholar] [CrossRef]
- Balliana, E.; Ricci, G.; Pesce, C.; Zendri, E. Assessing the value of green conservation for cultural heritage: Positive and critical aspects of already available methodologies. Int. J. Conserv. Sci. 2016, 7, 185–202. [Google Scholar]
- Sierra-Fernandez, A.; Gomez-Villalba, L.S.; Rabanal, M.E.; Fort, R. New nanomaterials for applications in conservation and restoration of stony materials: A review. Mater. Construcción 2017, 67, 107. [Google Scholar] [CrossRef]
- D’Orazio, L.; Grippo, A. A water dispersed Titanium dioxide/poly(carbonate urethane) nanocomposite for protecting cultural heritage: Preparation and properties. Prog. Org. Coat. 2015, 79, 1–7. [Google Scholar] [CrossRef]
- la Russa, M.F.; Rovella, N.; de Buergo, M.A.; Belfiore, C.M.; Pezzino, A.; Crisci, G.M.; Ruffolo, S.A. Nano-TiO2 coatings for cultural heritage protection: The role of the binder on hydrophobic and self-cleaning efficacy. Prog. Org. Coat. 2016, 91, 1–8. [Google Scholar] [CrossRef]
- Quagliarini, E.; Bondioli, F.; Goffredo, G.B.; Cordoni, C.; Munafò, P. Self-cleaning and de-polluting stone surfaces: TiO2 nanoparticles for limestone. Constr. Build. Mater. 2012, 37, 51–57. [Google Scholar] [CrossRef]
- Munafò, P.; Goffredo, G.B.; Quagliarini, E. TiO2-based nanocoatings for preserving architectural stone surfaces: An overview. Constr. Build. Mater. 2015, 84, 201–218. [Google Scholar] [CrossRef]
- Graziani, L.; Quagliarini, E.; Bondioli, F.; D’Orazio, M. Durability of self-cleaning TiO2 coatings on fired clay brick façades: Effects of UV exposure and wet & dry cycles. Build. Environ. 2014, 71, 193–203. [Google Scholar] [CrossRef]
- Crupi, V.; Fazio, B.; Gessini, A.; Kis, Z.; la Russa, M.F.; Majolino, D.; Masciovecchio, C.; Ricca, M.; Rossi, B.; Ruffolo, S.A.; et al. TiO2–SiO2–PDMS nanocomposite coating with self-cleaning effect for stone material: Finding the optimal amount of TiO2. Constr. Build. Mater. 2018, 166, 464–471. [Google Scholar] [CrossRef]
- Xu, A.W.; Gao, Y.; Liu, H.Q. The preparation, characterization, and their photocatalytic activities of rare-earth-doped TiO2 nanoparticles. J. Catal. 2002, 207, 151–157. [Google Scholar] [CrossRef]
- Yadav, S.; Jaiswar, G. Review on Undoped/Doped TiO2 Nanomaterial; Synthesis and Photocatalytic and Antimicrobial Activity: Review on Undoped/Doped TiO2 Nanomaterial. J. Chin. Chem. Soc. 2017, 64, 103–116. [Google Scholar] [CrossRef]
- Chobba, M.B.; Weththimuni, M.L.; Messaoud, M.; Urzi, C.; Bouaziz, J.; de Leo, F.; Licchelli, M. Ag-TiO2/PDMS nanocomposite protective coatings: Synthesis, characterization, and use as a self-cleaning and antimicrobial agent. Prog. Org. Coat. 2021, 158, 106342. [Google Scholar] [CrossRef]
- Merenda, A.; Rana, A.; Guirguis, A.; Zhu, D.M.; Kong, L.; Dumée, L.F. Enhanced Visible Light Sensitization of N-Doped TiO2 Nanotubes Containing Ti-Oxynitride Species Fabricated via Electrochemical Anodization of Titanium Nitride. J. Phys. Chem. C 2019, 123, 2189–2201. [Google Scholar] [CrossRef]
- Langlet, M.; Coutier, C.; Fick, J.; Audier, M.; Meffre, W.; Jacquier, B.; Rimet, R. Sol–gel thin film deposition and characterization of a new optically active compound: Er2Ti2O7. Opt. Mater. 2001, 16, 463–473. [Google Scholar] [CrossRef]
- Fonseca, A.J.; Pina, F.; Macedo, M.F.; Leal, N.; Romanowska-Deskins, A.; Laiz, L.; Gómez-Bolea, A.; Saiz-Jimenez, C. Anatase as an alternative application for preventing biodeterioration of mortars: Evaluation and comparison with other biocides. Int. Biodeterior. Biodegrad. 2010, 64, 388–396. [Google Scholar] [CrossRef] [Green Version]
- Ruffolo, S.A.; Macchia, A.; la Russa, M.F.; Mazza, L.; Urzì, C.; de Leo, F.; Barberio, M.; Crisci, G.M. Marine Antifouling for Underwater Archaeological Sites: TiO2 and Ag-Doped TiO2. Int. J. Photoenergy 2013, 2013, 251647. [Google Scholar] [CrossRef] [Green Version]
- la Russa, M.F.; Macchia, A.; Ruffolo, S.A.; de Leo, F.; Barberio, M.; Barone, P.; Crisci, G.M.; Urzì, C. Testing the antibacterial activity of doped TiO2 for preventing biodeterioration of cultural heritage building materials. Int. Biodeterior. Biodegrad. 2014, 96, 87–96. [Google Scholar] [CrossRef]
- Luo, W.; Hou, J.; Zou, D.; Cui, L.; Zhu, Q.; Dai, J. Lanthanide-titanium-oxalate clusters and their degradation products, photocurrent response and photocatalytic behaviours. New J. Chem. 2018, 42, 11629–11634. [Google Scholar] [CrossRef]
- Saqib, N.U.; Adnan, R.; Shah, I. A mini-review on rare earth metal-doped TiO2 for photocatalytic remediation of wastewater. Environ. Sci. Pollut. Res. 2016, 23, 15941–15951. [Google Scholar] [CrossRef] [PubMed]
- Mazierski, P.; Mikolajczyk, A.; Bajorowicz, B.; Malankowska, A.; Zaleska-Medynska, A.; Nadolna, J. The role of lanthanides in TiO2-based photocatalysis: A review. Appl. Catal. B Environ. 2018, 233, 301–317. [Google Scholar] [CrossRef]
- Xu, J.; Ao, Y.; Fu, D.; Yuan, C. Synthesis of Gd-doped TiO2 nanoparticles under mild condition and their photocatalytic activity. Colloids Surf. A Physicochem. Eng. Asp. 2009, 334, 107–111. [Google Scholar] [CrossRef]
- Paul, S.; Chetri, P.; Choudhury, B.; Ahmed, G.A.; Choudhury, A. Enhanced visible light photocatalytic activity of Gadolinium doped nanocrystalline titania: An experimental and theoretical study. J. Colloid Interface Sci. 2015, 439, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.; El-Molla, S.A.; Aboul-Fotouh, S.M.K.; Ibrahim, M.M.; Ismail, L.F.M.; Dahn, D.C. Nanostructured Gd3+-TiO2 surfaces for self-cleaning application. J. Mol. Struct. 2014, 1067, 120–126. [Google Scholar] [CrossRef]
- Mahalakshmi, M.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Preparation, Characterization, and Photocatalytic Activity of Gd3+ Doped TiO2 Nanoparticles. J. Nanosci. Nanotechnol. 2007, 7, 3277–3285. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.Q.; Ma, C.Y.; Yi, X.Y.; Yuan, F.; Xie, Y.; Hu, J.M.; Hu, B.C.; Zhang, Q.Y. Structural, morphological, optical and photocatalytic properties of Gd-doped TiO2 films. Thin Solid Films 2016, 615, 13–18. [Google Scholar] [CrossRef]
- Thomas, P.J.; Carpenter, D.; Boutin, C.; Allison, J.E. Rare earth elements (REEs): Effects on germination and growth of selected crop and native plant species. Chemosphere 2014, 96, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Pagano, G.; Guida, M.; Tommasi, F.; Oral, R. Health effects and toxicity mechanisms of rare earth elements—Knowledge gaps and research prospects. Ecotoxicol. Environ. Saf. 2015, 115, 40–48. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, C.; Tai, P.; Sun, L.; Chen, Z. The exposure of gadolinium at environmental relevant levels induced genotoxic effects in Arabidopsis thaliana (L.). Ecotoxicol. Environ. Saf. 2021, 215, 112138. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Capsoni, D.; Malagodi, M.; Licchelli, M. Improving Wood Resistance to Decay by Nanostructured ZnO-Based Treatments. J. Nanomater. 2019, 2019, 6715756. [Google Scholar] [CrossRef]
- Alexandru, M.; Cazacu, M.; Doroftei, F.; Ignat, M.; Timpu, D.; Grigoras, C.V.; Simionescu, B.C. On the morphology and potential application of polydimethylsiloxane-silica-titania composites. Express Polym. Lett. 2011, 5, 188–196. [Google Scholar] [CrossRef]
- Tavares, M.T.S.; Santos, A.S.F.; Santos, I.M.G.; Silva, M.R.S.; Bomio, M.R.D.; Longo, E.; Paskocimas, C.A.; Motta, F.V. TiO2/PDMS nanocomposites for use on self-cleaning surfaces. Surf. Coat. Technol. 2014, 239, 16–19. [Google Scholar] [CrossRef] [Green Version]
- Kapridaki, C.; Pinho, L.; Mosquera, M.J.; Maravelaki-kalaitzaki, P. Producing photoactive, transparent and hydrophobic SiO2-crystalline TiO2 nanocomposites at ambient conditions with application as self-cleaning coatings. Appl. Catal. B Environ. 2014, 156–157, 416–427. [Google Scholar] [CrossRef]
- Kapridaki, C.; Maravelaki-Kalaitzaki, P. TiO2-SiO2-PDMS nano-composite hydrophobic coating with self-cleaning properties for marble protection. Prog. Org. Coat. 2013, 76, 400–410. [Google Scholar] [CrossRef]
- Chobba, M.B.; Messaoud, M.; Weththimuni, M.L.; Bouaziz, J.; Licchelli, M.; de Leo, F.; Urzì, C. Preparation and characterization of photocatalytic Gd-doped TiO2 nanoparticles for water treatment. Environ. Sci. Pollut. Res. 2019, 26, 32734–32745. [Google Scholar] [CrossRef]
- Licchelli, M.; Malagodi, M.; Weththimuni, M.; Zanchi, C. Nanoparticles for conservation of bio-calcarenite stone. Appl. Phys. A 2014, 114, 673–683. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Licchelli, M.; Malagodi, M.; Rovella, N.; Russa, M.L. Consolidation of bio-calcarenite stone by treatment based on diammonium hydrogenphosphate and calcium hydroxide nanoparticles. Measurement 2018, 127, 396–405. [Google Scholar] [CrossRef]
- UNI 10921:2001, Beni Culturali—Materiali Lapidei Naturali ed Artificiali—Prodotti Idrorepellenti—Applicazione su Provini e Determinazione in Laboratorio delle loro Caratteristiche; Milan, Italy, 2001; Available online: https://www.biblio.units.it/SebinaOpac/resource/beni-culturali-materiali-lapidei-naturali-ed-artificiali-prodotti-idrorepellenti-applicazione-su-pro/TSA1388731 (accessed on 30 September 2021).
- Chobba, M.B.; Messaoud, M.; Bouaziz, J.; de Leo, F.; Urzì, C. The Effect of Heat Treatment on Photocatalytic Performance and Antibacterial Activity of TiO2 Nanoparticles Prepared by Sol-Gel Method. In Advances in Materials, Mechanics and Manufacturing; Chaari, F., Barkallah, M., Bouguecha, A., Zouari, B., Khabou, M.T., Kchaou, M., Haddar, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 71–79. [Google Scholar] [CrossRef]
- Kapridaki, C.; Verganelaki, A.; Dimitriadou, P.; Maravelaki-Kalaitzaki, P. Conservation of Monuments by a Three-Layered Compatible Treatment of TEOS-Nano-Calcium Oxalate Consolidant and TEOS-PDMS-TiO2 Hydrophobic/Photoactive Hybrid Nanomaterials. Materials 2018, 11, 684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weththimuni, M.; Crivelli, F.; Galimberti, C.; Malagodi, M.; Licchelli, M. Evaluation of commercial consolidating agents on very porous biocalcarenite. Int. J. Conserv. Sci. 2020, 11, 251–260. [Google Scholar]
- Koschwanez, J.H.; Carlson, R.H.; Meldrum, D.R. Thin PDMS films using long spin times or tert-butyl alcohol as a solvent. PLoS ONE 2009, 4, e4572. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Capsoni, D.; Malagodi, M.; Milanese, C.; Licchelli, M. Shellac/nanoparticles dispersions as protective materials for wood. Appl. Phys. A 2016, 122, 1058. [Google Scholar] [CrossRef]
- UNI EN 15886:2010, Conservazione dei Beni Culturali, Metodi di prova, Misura del Colore Delle Superfici; UNI Ente Italiano di Normazione: Milan, Italy, 2010.
- UNI EN 15802:2010, Conservazione dei Beni Culturali—Metodi di Prova—Determinazione Dell’angolo di Contatto Statico; UNI: Milan, Italy, 2010.
- UNI EN 15801:2010, Conservazione dei Beni Culturali, Metodi di Prova, Determinazione Dell’assorbimento Dell’acqua per Capillarità; UNI: Milan, Italy, 2010.
- UNI EN 15803:2010, Conservazione dei Beni Culturali, Metodi di Prova, Determinazione Della Permeabilità al Vapore D’acqua; UNI: Milan, Italy, 2010.
- ISO 15184:1998, Paints and Varnishes—Determination of Film Hardness by Pencil Test; International Organization for Standardization: Genève, Switzerland, 1998.
- ASTM C1327-03, Standard Test Method for Vickers Indentation Hardness of Advanced Ceramics; ASTM International: West Conshohocken, PA, USA, 2003.
- Quagliarini, E.; Bondioli, F.; Goffredo, G.B.; Licciulli, A.; Munafò, P. Self-cleaning materials on Architectural Heritage: Compatibility of photo-induced hydrophilicity of TiO2 coatings on stone surfaces. J. Cult. Herit. 2013, 14, 1–7. [Google Scholar] [CrossRef]
- de Leo, F.; Marchetta, A.; Capillo, G.; Germanà, A.; Primerano, P.; Schiavo, S.L.; Urzì, C. Surface Active Ionic Liquids Based Coatings as Subaerial Anti-Biofilms for Stone Built Cultural Heritage. Coatings 2021, 11, 26. [Google Scholar] [CrossRef]
- Urzì, C.; de Leo, F. Sampling with adhesive tape strips: An easy and rapid method to monitor microbial colonization on monument surfaces. J. Microbiol. Methods 2001, 44, 1–11. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Yu, X.; Xu, L.; Kang, W.; Yan, W.; Gao, H.; Liu, Z.; Guo, Y. Rare earth oxide-doped titania nanocomposites with enhanced photocatalytic activity towards the degradation of partially hydrolysis polyacrylamide. Appl. Surf. Sci. 2009, 255, 3731–3738. [Google Scholar] [CrossRef]
- Agorku, E.S.; Mamba, B.B.; Pandey, A.C.; Mishra, A.K. Sulfur/Gadolinium-Codoped TiO2 Nanoparticles for Enhanced Visible-Light Photocatalytic Performance. J. Nanomater. 2014, 2014, 289150. [Google Scholar] [CrossRef] [Green Version]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Wagner, P.; Fürstner, R.; Barthlott, W.; Neinhuis, C. Quantitative assessment to the structural basis of water repellency in natural and technical surfaces. J. Exp. Bot. 2003, 54, 1295–1303. [Google Scholar] [CrossRef] [Green Version]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Kolinkeová, B.; Panayiotou, C. Superhydrophobic films for the protection of outdoor cultural heritage assets. Appl. Phys. A 2009, 97, 351–360. [Google Scholar] [CrossRef]
- Cappelletti, G.; Fermo, P.; Camiloni, M. Smart hybrid coatings for natural stones conservation. Prog. Org. Coat. 2015, 78, 511–516. [Google Scholar] [CrossRef]
- Facio, D.S.; Mosquera, M.J. Simple Strategy for Producing Superhydrophobic Nanocomposite Coatings In Situ on a Building Substrate. ACS Appl. Mater. Interfaces 2013, 5, 7517–7526. [Google Scholar] [CrossRef]
- Toniolo, L.; Poli, T.; Castelvetro, V.; Manariti, A.; Chiantore, O.; Lazzari, M. Tailoring new fluorinated acrylic copolymers as protective coatings for marble. J. Cult. Herit. 2002, 3, 309–316. [Google Scholar] [CrossRef]
- Mills, A.; Wang, J. Photobleaching of methylene blue sensitised by TiO2: An ambiguous system? J. Photochem. Photobiol. A Chem. 1999, 127, 123–134. [Google Scholar] [CrossRef]
- Ruot, B.; Plassais, A.; Olive, F.; Guillot, L.; Bonafous, L. TiO2-containing cement pastes and mortars: Measurements of the photocatalytic efficiency using a rhodamine B-based colourimetric test. Sol. Energy 2009, 83, 1794–1801. [Google Scholar] [CrossRef]
- NORMAL 20/85, Interventi Conservativi: Progettazione Esecuzione e Valutazione Preventiva; Milan, Italy, 1996; Available online: https://scoprirete.bibliotecheromagna.it/SebinaOpac/resource/normal-2085-interventi-conservativi-progettazione-esecuzione-e-valutazione-preventiva/RAV1021964 (accessed on 30 September 2021).
- Smaoui, S.; Elleuch, L.; Bejar, W.; Karray-Rebai, I.; Ayadi, I.; Jaouadi, B.; Mathieu, F.; Chouayekh, H.; Bejar, S.; Mellouli, L. Inhibition of Fungi and Gram-Negative Bacteria by Bacteriocin BacTN635 Produced by Lactobacillus plantarum sp. TN635. Appl. Biochem. Biotechnol. 2010, 162, 1132–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
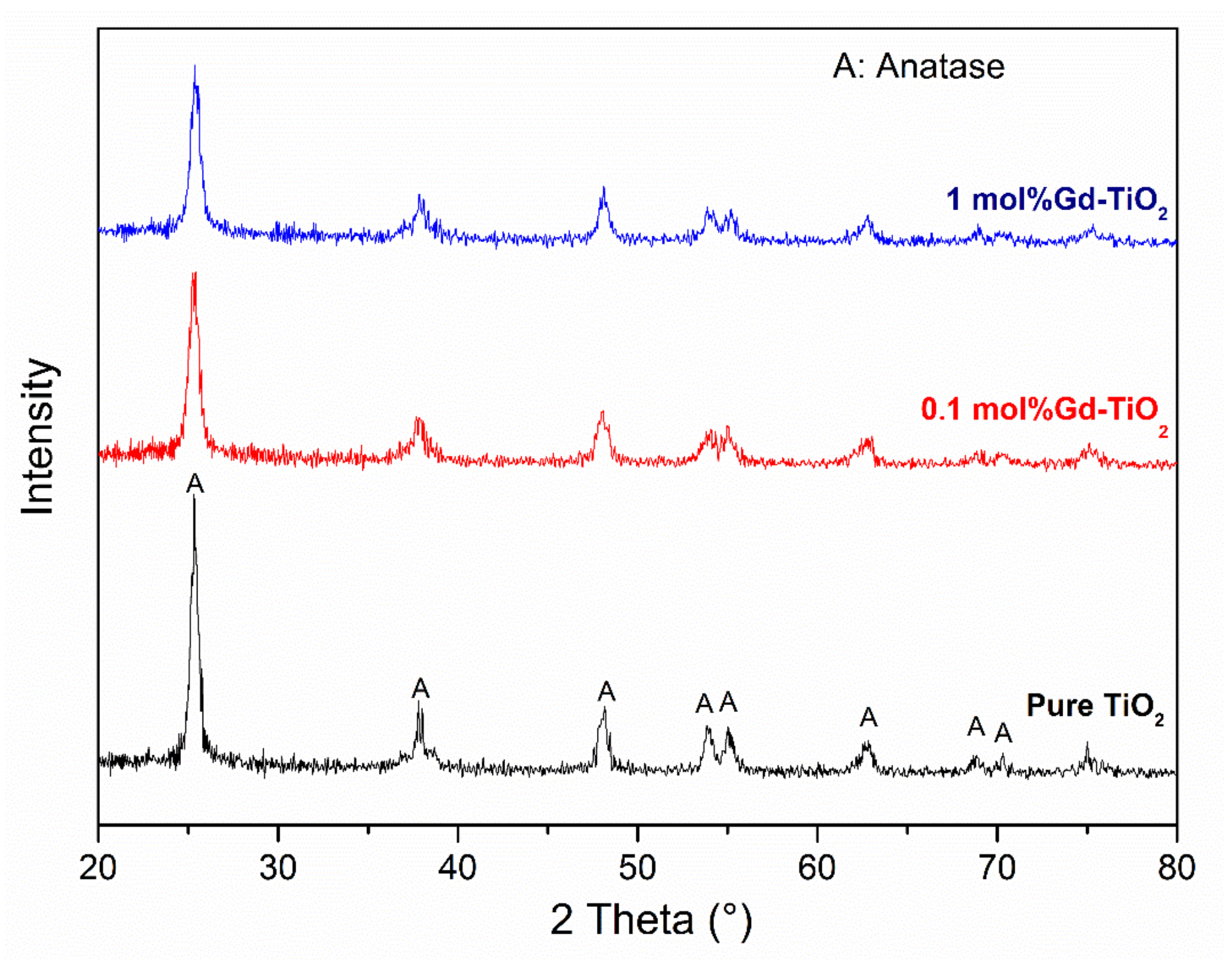
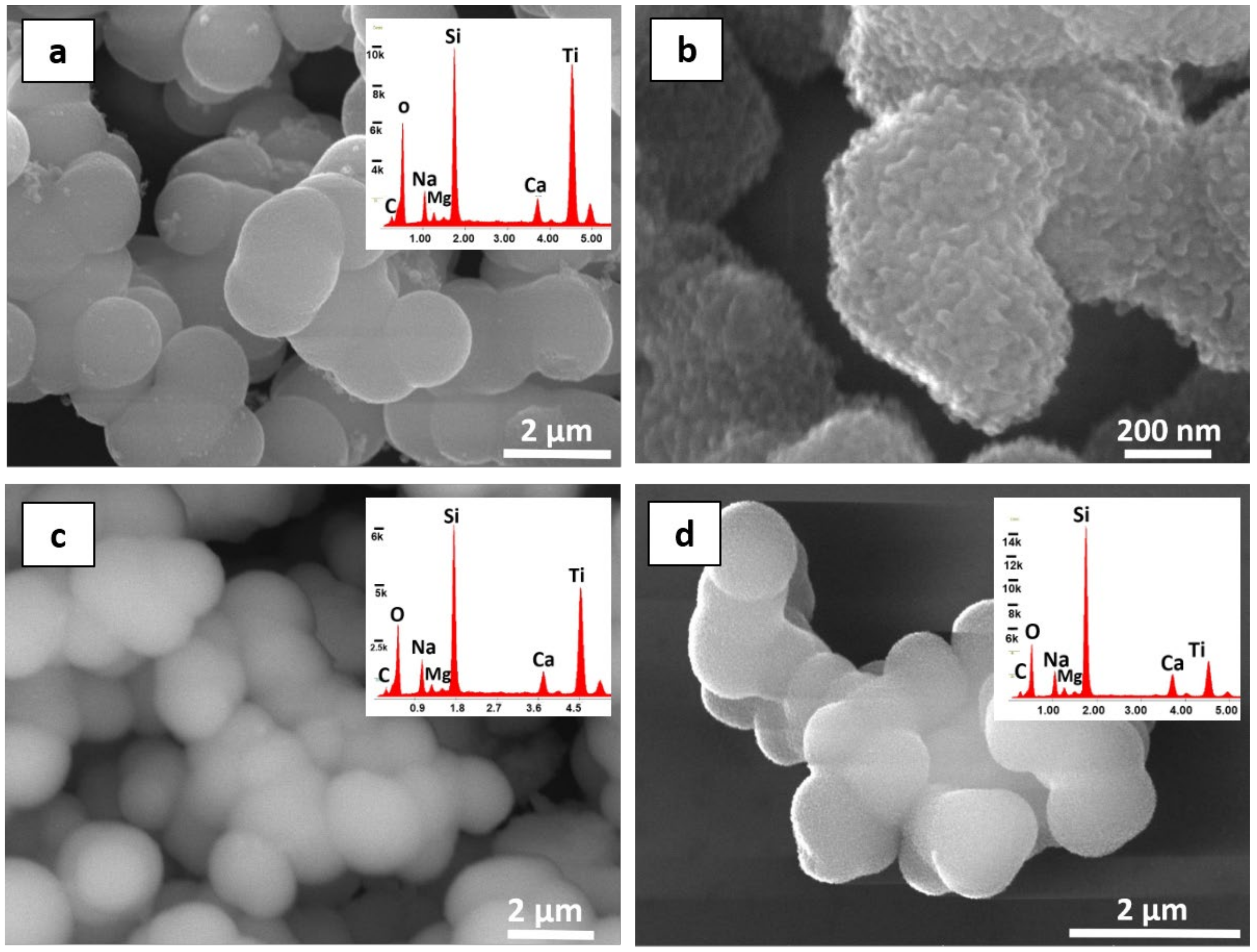
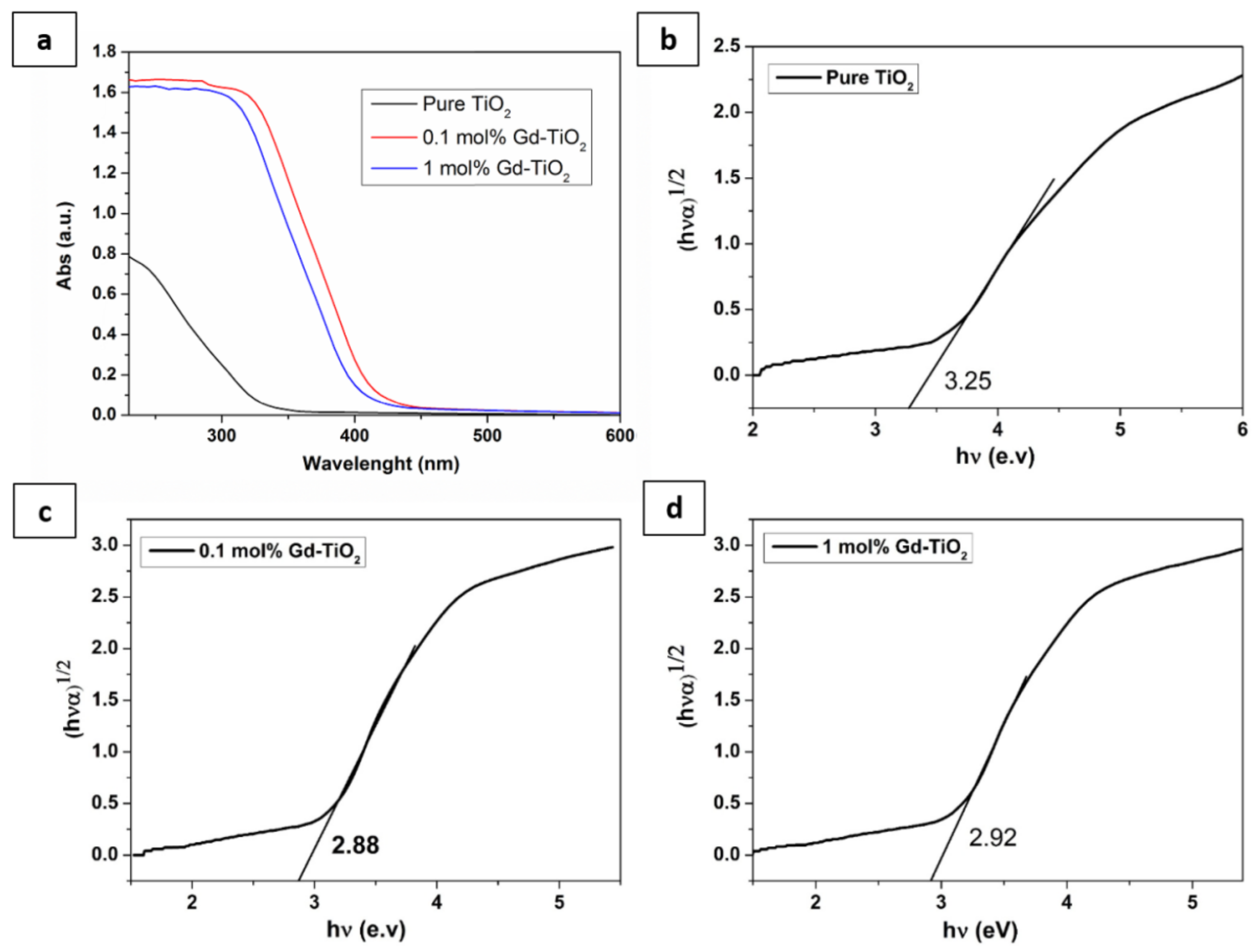




| Samples | D (nm) | a = b(Å) | c(Å) | c/a | V(Å3) |
|---|---|---|---|---|---|
| Pure TiO2 | 20 ± 2 | 3.793 | 9.518 | 2.509 | 136.93 |
| 0.1 mol% Gd-TiO2 | 16 ± 3 | 3.79 | 9523 | 2.501 | 136.8 |
| 1 mol% Gd-TiO2 | 15 ± 2 | 3.797 | 9.475 | 2.495 | 136.6 |
| Samples | Before Ageing Test | After Ageing Test | ||||
|---|---|---|---|---|---|---|
| ΔL* | Δa* | Δb* | ΔL* | Δa* | Δb* | |
| P | −2.22 ± 1.18 | 0.31 ± 0.15 | 1.33 ± 0.08 | 5.33 ± 0.39 | −1.24 ± 0.14 | −4.08 ± 0.18 |
| P + Ti | −0.90 ± 1.34 | −0.32 ± 0.15 | −1.80 ± 1.50 | 4.68 ± 0.54 | −0.45 ± 0.42 | −1.65± 0.08 |
| P + Ti + 0.1Gd | −1.22 ± 2.41 | 0.24 ± 0.69 | −0.88 ± 0.50 | 2.14 ± 0.68 | −0.63 ± 0.33 | −2.02 ± 0.37 |
| P + Ti + 1Gd | −0.03 ± 0.46 | 0.05 ± 0.05 | −2.44 ± 0.73 | 0.95 ± 0.88 | −0.27 ± 0.01 | −0.08 ± 0.90 |
| Samples | Before Ageing | After Ageing |
|---|---|---|
| P | H | F |
| P + Ti | 2H | H |
| P + Ti + 0.1Gd | 2H | 2H |
| P + Ti + 1Gd | 3H | 3H |
| Samples | α (°) Before Ageing | α (°) After Ageing |
|---|---|---|
| P | 122 ± 2 | 114 ± 8 |
| P + Ti | 133 ± 5 | 113 ± 7 |
| P + Ti + 0.1Gd | 135 ± 5 | 113 ± 4 |
| P + Ti + 1Gd | 132 ± 3 | 110 ± 1 |
| Samples | D*(%) | |||
|---|---|---|---|---|
| Before Ageing | After Ageing | |||
| 48 h | 96 h | 48 h | 96 h | |
| Untreated | 13.74 ± 3.14 | 20.47 ± 3.95 | - | - |
| P | 33.74 ± 4.75 | 38.85 ± 3.16 | 27.7 ± 1.1 | 33.6 ± 3 |
| P + Ti | 38.4 ± 3.6 | 48.27 ± 3.3 | 31.9 ± 1.12 | 40.46 ± 0.7 |
| P + Ti + 0.1Gd | 54.90 ± 2.96 | 70.54 ± 2.2 | 44.4 ± 2.1 | 63.42 ± 1 |
| P + Ti + 1Gd | 42.02 ± 1.48 | 53.01 ± 3.14 | 36.4 ± 1.3 | 49.79 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chobba, M.B.; Weththimuni, M.L.; Messaoud, M.; Sacchi, D.; Bouaziz, J.; De Leo, F.; Urzi, C.; Licchelli, M. Multifunctional and Durable Coatings for Stone Protection Based on Gd-Doped Nanocomposites. Sustainability 2021, 13, 11033. https://doi.org/10.3390/su131911033
Chobba MB, Weththimuni ML, Messaoud M, Sacchi D, Bouaziz J, De Leo F, Urzi C, Licchelli M. Multifunctional and Durable Coatings for Stone Protection Based on Gd-Doped Nanocomposites. Sustainability. 2021; 13(19):11033. https://doi.org/10.3390/su131911033
Chicago/Turabian StyleChobba, Marwa Ben, Maduka Lankani Weththimuni, Mouna Messaoud, Donatella Sacchi, Jamel Bouaziz, Filomena De Leo, Clara Urzi, and Maurizio Licchelli. 2021. "Multifunctional and Durable Coatings for Stone Protection Based on Gd-Doped Nanocomposites" Sustainability 13, no. 19: 11033. https://doi.org/10.3390/su131911033










