Effect of Ether Mono Amine Collector on the Cationic Flotation of Micaceous Minerals—A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Micro-Flotation
2.3. Contact Angle Measurements
2.4. Residual Surface Tension Measurements
2.5. Zeta Potential
3. Results
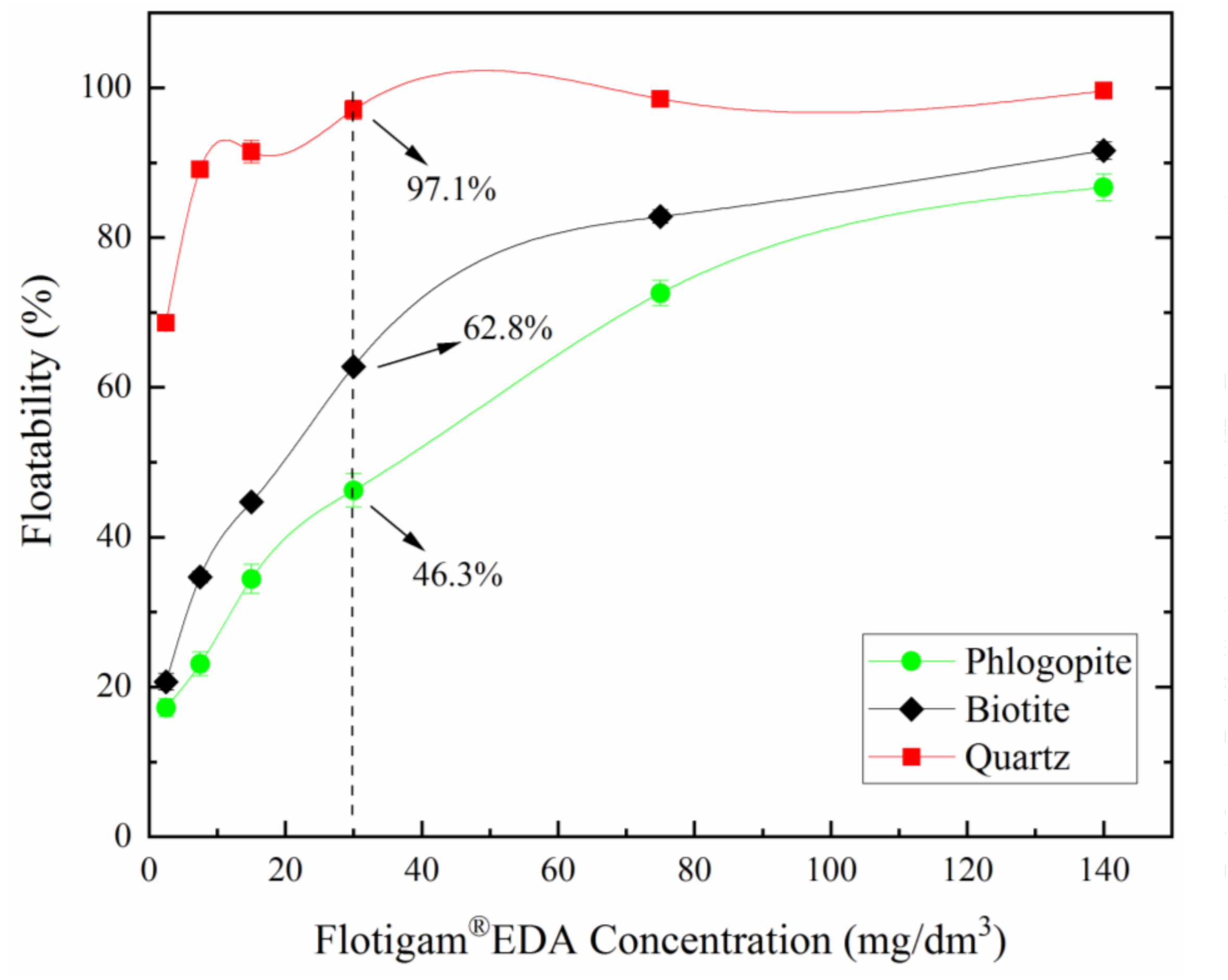
3.1. Micro-Flotation of Single Minerals
3.2. Contact Angle Measurements
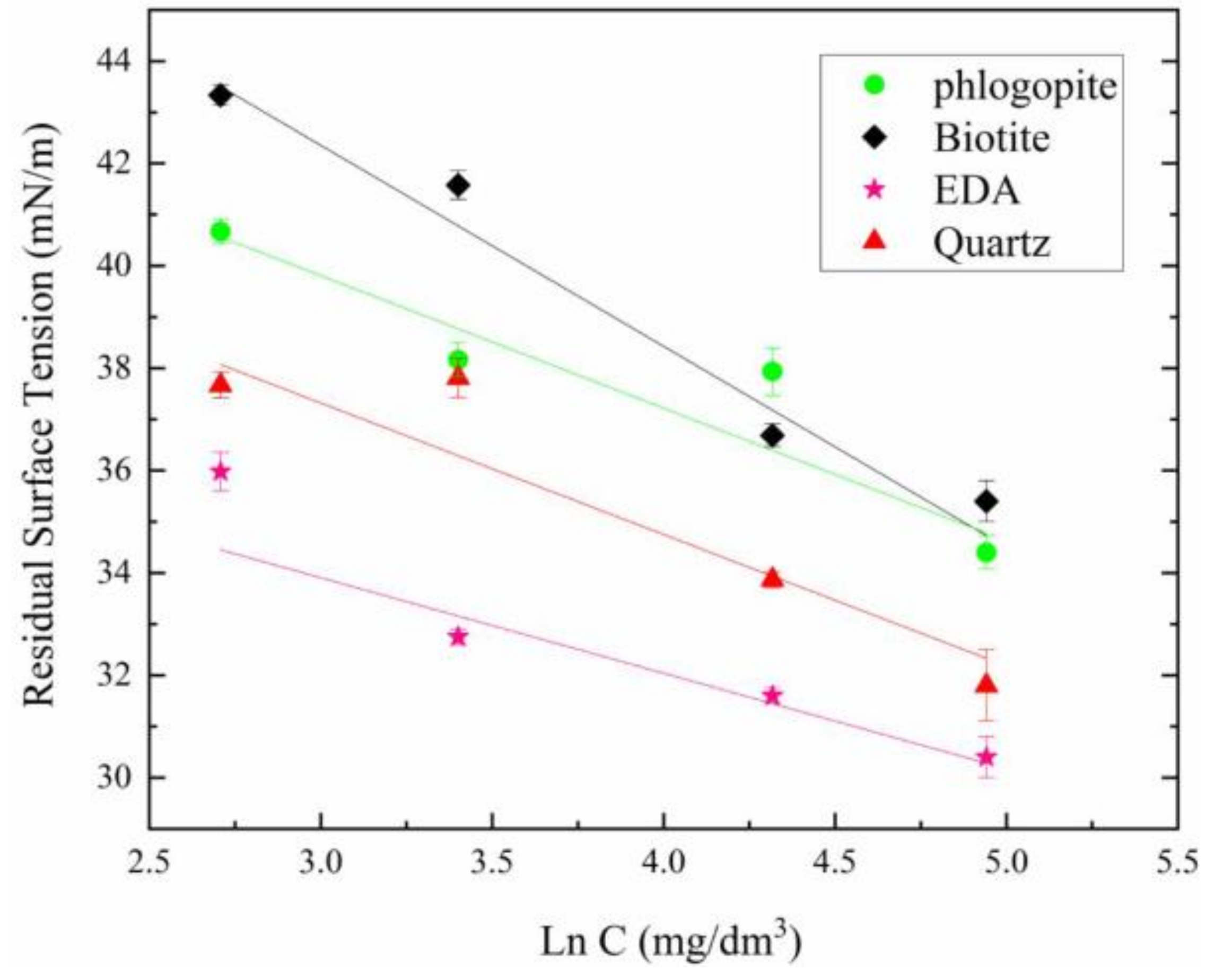
3.3. Residual Surface Tension
3.4. Zeta Potential
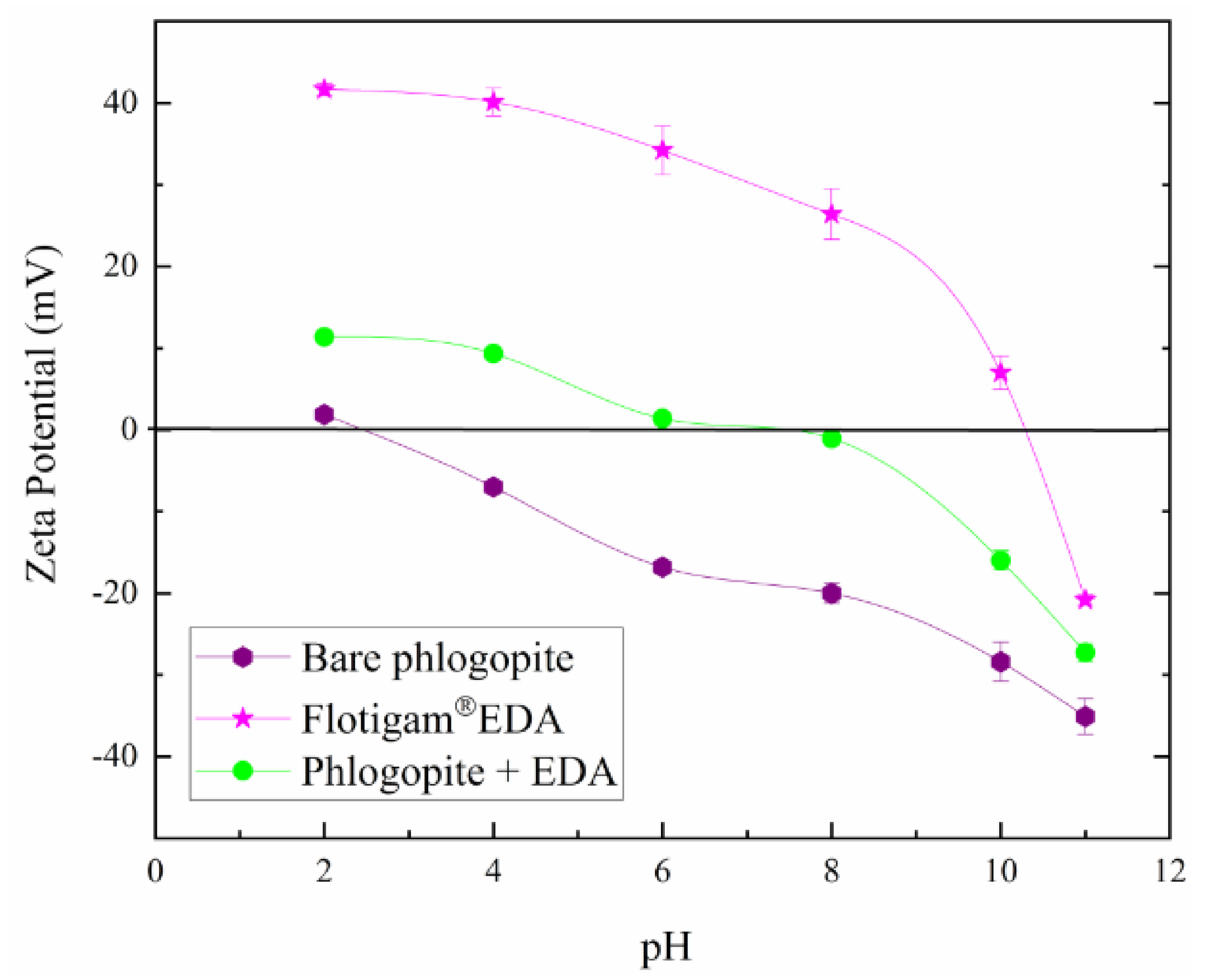
3.4.1. pH
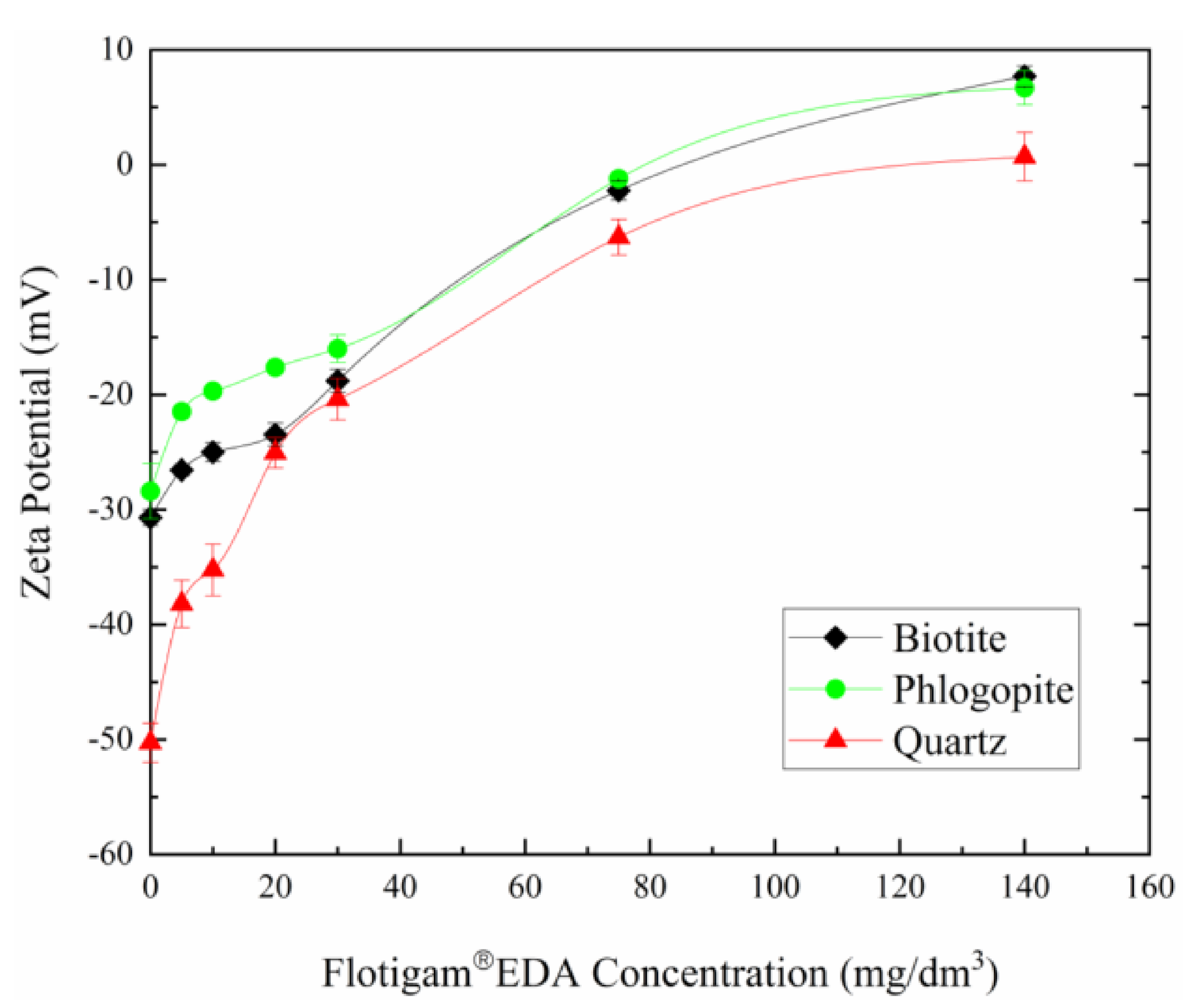
3.4.2. EDA Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leja, J. Surface Chemistry of Froth Flotation; Springer: Vancouver, BC, Canada, 1981. [Google Scholar] [CrossRef]
- Wills, B.A.; Napier–Munn, T. Wills’ Mineral Processing Technology; Elsevier BV: Cornwall, UK, 2005. [Google Scholar] [CrossRef]
- Han, G.; Wen, S.; Wang, H.; Feng, Q. Surface Sulfidization Mechanism of Cuprite and Its Response to Xanthate Adsorption and Flotation Performance. Miner. Eng. 2021, 169, 106982. [Google Scholar] [CrossRef]
- Zhang, Q.; Wen, S.; Feng, Q.; Liu, Y. Activation Mechanism of Lead Ions in the Flotation of Sulfidized Azurite with Xanthate As Collector. Miner. Eng. 2021, 163, 106809. [Google Scholar] [CrossRef]
- Kohad, V.P. Flotation of Sulphide Ores–HZL Experience. Proceedings of Workshop on Froth Flotation: Recent Trends, Bhubaneswar, India, 22–24 September 1998. [Google Scholar]
- Lynch, A.J.; Johnson, N.W.; McKee, D.J.; Thorne, G.C. The Behaviour of Minerals in Sulphide Flotation Processes, With Reference to Simulation and Control. J. Sou. Afr. Inst. Min. And. Metal. 1974, 74, 349–361. [Google Scholar]
- Tohry, A.; Dehghani, A. Effect of Sodium Silicate on the Reverse Anionic Flotation of a siliceous–phosphorus Iron Ore. Sep. Purif. Technol. 2016, 164, 28–33. [Google Scholar] [CrossRef]
- Tohry, A.; Dehghani, A.; Mojtahedzadeh, A. Investigation of Silica Removal from Hematite Concentrate of Chador–Malu Flotation Circuit. Ir. J. Min. Eng. 2015, 10, 1–10. [Google Scholar]
- Tohry, A.; Dehghan, R.; Hatefi, P.; Chelgani, S.C. A Comparative Study Between the Adsorption Mechanisms of Sodium Co–Silicate and Conventional Depressants for the Reverse Anionic Hematite Flotation. Sep. Sci. Technol. 2021, 1–18. [Google Scholar] [CrossRef]
- Tohry, A.; Dehghan, R.; Filho, L.D.S.L.; Chelgani, S.C. Tannin: An Eco–Friendly Depressant for the Green Flotation Separation of Hematite from Quartz. Miner. Eng. 2021, 168, 106917. [Google Scholar] [CrossRef]
- Tohry, A.; Dehghan, R.; Chelgani, S.C.; Rosenkranz, J.; Rahmani, O.A. Selective Separation of Hematite by a Synthesized Depressant in Various Scales of Anionic Reverse Flotation. Miner. 2019, 9, 124. [Google Scholar] [CrossRef] [Green Version]
- Tohry, A.; Dehghan, R.; Zarei, M.; Chelgani, S.C. Mechanism of Humic Acid Adsorption As a Flotation Separation Depressant on the Complex Silicates and Hematite. Miner. Eng. 2021, 162, 106736. [Google Scholar] [CrossRef]
- Leal Filho, L.S.; Assist, S.M.; Barros, L.A.F.; Peres, A.E.C. Activation and Adepression of Silicates During Anionic Flotation of Igneous Apatite. In Benefication of Phosphate (Fundamentals and Technology); Society for Mining, Metallurgy and Exploration Inc. (SME): Littleton, CO, USA, 2002. [Google Scholar]
- Derhy, M.; Taha, Y.; Hakkou, R.; Benzaazoua, M. Review of the Main Factors Affecting the Flotation of Phosphate Ores. Minerals 2020, 10, 1109. [Google Scholar] [CrossRef]
- Arol, A.; Aydogan, A. Recovery Enhancement of Magnetite Fines in Magnetic Separation. Colloids Surfaces A Physicochem. Eng. Asp. 2004, 232, 151–154. [Google Scholar] [CrossRef]
- Filippov, L.; Severov, V.; Filippova, I. An Overview of the Beneficiation of Iron Ores via Reverse Cationic Flotation. Int. J. Miner. Process. 2014, 127, 62–69. [Google Scholar] [CrossRef]
- Filippov, L.; Filippova, I.; Severov, V. The Use of Collectors Mixture in the Reverse Cationic Flotation of Magnetite Ore: The Role of Fe–Bearing Silicates. Miner. Eng. 2010, 23, 91–98. [Google Scholar] [CrossRef]
- Tohry, A.; Dehghani, A.; Hosseini–Nasab, M. Removal of Fine Gangue Minerals from Chador–Malu Iron Concentrate Using Hydroseparator. Physicochem. Prob. Mine. Proc 2017, 53, 250–263. [Google Scholar] [CrossRef]
- Manser, R.M. Handbook of Silicate Flotation; Warren Spring Laboratory: Stevenage, UK, 1975. [Google Scholar]
- Araujo, A.; Viana, P.; Peres, A. Reagents in Iron Ores Flotation. Miner. Eng. 2005, 18, 219–224. [Google Scholar] [CrossRef]
- Severov, V.; Filippov, L.; Filippova, I. Relationship Between Cation Distribution With Electrochemical and Flotation Properties of Calcic Amphiboles. Int. J. Miner. Process. 2016, 147, 18–27. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, X.; Xu, Z. Role of Crystal Structure in Flotation Separation of Diaspore from Kaolinite, Pyrophyllite and Illite. Miner. Eng. 2003, 16, 219–227. [Google Scholar] [CrossRef]
- Rao, K.H.; Antti, B.–M.; Forssberg, K.S.E. Flotation of Mica Minerals and Selectivity Between Muscovite and Biotite While Using Mixed anionic/Cationic Collectors. Mining Met. Explor. 1990, 7, 127–132. [Google Scholar] [CrossRef]
- Filippov, L.; Duverger, A.; Filippova, I.V.; Kasaini, H.; Thiry, J. Selective Flotation of Silicates and Ca–Bearing Minerals: The Role of Non–Ionic Reagent on Cationic Flotation. Miner. Eng. 2012, 36–38, 314–323. [Google Scholar] [CrossRef]
- Rao, K.H.; Cases, J.M.; Barres, O.; Forssberg, K.S.E. Flotation, Electrokinetic and FT–IR Studies of Mixed anionic/Cationic Col–Lectors in muscovite–biotite System. In Mineral Processing: Recent Advances and Future Trends.; Mehrotra, S.P., Shekhar, R., Eds.; Allied Publishing Ltd.: New Dehli, India, 1995; pp. 29–44. [Google Scholar]
- Rao, K.H.; Forssberg, K. Mixed Collector Systems in Flotation. Int. J. Miner. Process. 1997, 51, 67–79. [Google Scholar] [CrossRef]
- Teng, Q.; Wen, Q.; Yang, Z.; Liu, S. Evaluation of the Biological Flotation Reagent Obtained from Paenibacillus Amylolyticus in Magnetite and Phlogopite Flotation System. Colloids Surfaces A: Physicochem. Eng. Asp. 2021, 610, 125930. [Google Scholar] [CrossRef]
- Ma, M. Froth Flotation of Iron Ores. Int. J. Min. Eng. Miner. Process. 2012, 1, 56–61. [Google Scholar] [CrossRef]
- Gaudin, A.M.; Morrow, J.G. Adsorption of Dodecylamine on Hematite and Its Flotation Effect. Trans. Metall. Soc. AIME 1954, 12, 1196–1202. [Google Scholar]
- Papini, R.M.; Brandão, P.R.G.; Peres, A. Cationic Flotation of Iron Ores: Amine Characterization and Performance. Mining Met. Explor. 2001, 18, 5–9. [Google Scholar] [CrossRef]
- Fuerstenau, D.W. Streaming Potential Studies on Quartz in Solutions of Aminium Acetates in Relation to the Formation of Hemi– Micelles at the Quartz–Solution Interface. J. Phys. Chem. 1956, 60, 981–985. [Google Scholar] [CrossRef]
- Pattanaik, A.; Venugopal, R. Investigation of Adsorption Mechanism of Reagents (Surfactants) System and Its Applicability in Iron Ore Flotation – An Overview. Colloid Interface Sci. Commun. 2018, 25, 41–65. [Google Scholar] [CrossRef]
- Somasundaran, P.; Lou, A. Iron Oxide Mineral Flotation Fundamentals; Parekh, B.K., Miller, J.D., Eds.; Advances in Flotation Technology, SME: New York, NY, USA, 1999; pp. 23–43. [Google Scholar]
- Cassola, M.S.; Silva, W.C.; Bartalini, N.M. Development and Applications on Collectors at the Iron Ore Flotation, Technical Report; Application & Development Laboratory of Clariant Co.: Sao Paulo, Brazil, 2015. [Google Scholar]
- Calgaroto, S.; Azevedo, A.; Rubio, J. Separation of Amine–Insoluble Species by Flotation With Nano and Microbubbles. Miner. Eng. 2016, 89, 24–29. [Google Scholar] [CrossRef]
- Ren, L.; Qiu, H.; Zhang, Y.; Nguyen, A.V.; Zhang, M.; Wei, P.; Long, Q. Effects of Alkyl Ether Amine and Calcium Ions on Fine Quartz Flotation and Its Guidance for Upgrading Vanadium from Stone Coal. Powder Technol. 2018, 338, 180–189. [Google Scholar] [CrossRef]
- Lelis, D.F.; Da Cruz, D.G.; Lima, R.M.F. Effects of Calcium and Chloride Ions in Iron Ore Reverse Cationic Flotation: Fundamental Studies. Miner. Process. Extr. Met. Rev. 2019, 40, 402–409. [Google Scholar] [CrossRef]
- Tohry, A.; Dehghan, R.; Oliveira, A.V.; Chelgani, S.C.; Filho, L.D.S.L. Enhanced Washburn Method (EWM): A Comparative Study for the Contact Angle Measurement of Powders. Adv. Powder Technol. 2020, 31, 4665–4671. [Google Scholar] [CrossRef]
- KRÜSS GmbH. Processor Tensiometer K12: User’s Manual; KRÜSS GmbH: Hamburg, Germany, 1994; p. 84. [Google Scholar]
- Jiang, J.; Shang, X.; Wang, H.; Xu, Y.–B.; Gao, Y.; Zhou, Q. Diagnostic value of contrast–enhanced ultrasound in thyroid nodules with calcification. Kaohsiung J. Med. Sci. 2015, 31, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Bailey, S.W. CLASSIFICATION and STRUCTURES of the MICAS. Micas 1984, 13, 1–12. [Google Scholar] [CrossRef]
- Kramer, A.; Gaulocher, S.; Martins, M.; Filho, L.D.S.L. Surface Tension Measurement for Optimization of Flotation Control. Procedia Eng. 2012, 46, 111–118. [Google Scholar] [CrossRef] [Green Version]
- de Matos, V.E.; Nogueira, S.D.C.S.; Kowalczuk, P.B.; da Silva, G.R.; Peres, A.E.C. Differences in Etheramines Froth Properties and the Effects on Iron Ore Flotation. Part I: Two–Phase Systems. Miner. Process. Extr. Met. Rev. 2021, 1–8. [Google Scholar] [CrossRef]
- Ruan, Y.; Zhang, Z.; Luo, H.; Xiao, C.; Zhou, F.; Chi, R. Effects of Metal Ions on the Flotation of Apatite, Dolomite and Quartz. Minerals 2018, 8, 141. [Google Scholar] [CrossRef] [Green Version]
- Abaka–Wood, G.B.; Addai–Mensah, J.; Skinner, W. A Study of Flotation Characteristics of Monazite, Hematite, and Quartz Using Anionic Collectors. Int. J. Miner. Process. 2017, 158, 55–62. [Google Scholar] [CrossRef]
- Fan, G.; Wang, L.; Cao, Y.; Li, C. Collecting Agent–Mineral Interactions in the Reverse Flotation of Iron Ore: A Brief Review. Minerals 2020, 10, 681. [Google Scholar] [CrossRef]
- Rath, R.; Subramanian, S. Studies on Adsorption of Guar Gum onto Biotite Mica. Miner. Eng. 1997, 10, 1405–1420. [Google Scholar] [CrossRef]
- De Carvalho, J.A.E.; Brandão, P.R.G.; Henriques, A.B.; De Oliveira, P.S.; Cançado, R.Z.L.; Da Silva, G.R. Selective Flotation of Apatite from Micaceous Minerals Using Patauá Palm Tree Oil Collector. Miner. Eng. 2020, 156, 106474. [Google Scholar] [CrossRef]
- Wang, Q.; Heiskanen, K. Dispersion Selectivity and Heterocoagulation in Apatite–Hematite–Phlogopite Fine Particle Suspensions II. Dispersion Selectivities of the Mineral Mixtures. Int. J. Miner. Process. 1992, 35, 133–145. [Google Scholar] [CrossRef]
- Bai, Y.; Li, C.; Song, W.; An, H.; Zhao, J. Application of Sodium Dodecyl Glycinate to the Flotation of Deslimed Molybdenum Tailings. Physicochem. Prob. Miner. Proc. 2019, 55, 1120–1131. [Google Scholar] [CrossRef]
- Churaev, N.; Sergeeva, I.; Sobolev, V.; Jacobasch, H.–J.; Weidenhammer, P.; Schmitt, F.–J. Modification of Quartz Surfaces Using Cationic Surfactant Solutions. Colloids Surfaces A: Physicochem. Eng. Asp. 2000, 164, 121–129. [Google Scholar] [CrossRef]
- Smith, R.W.; Scott, J.L. Mechanisms of Dodecylamine Flotation of Quartz. Miner. Process. Extr. Met. Rev. 1990, 7, 81–94. [Google Scholar] [CrossRef]
- Kosmulski, M. Chemical Properties of Material Surfaces; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar]




| Pure Mineral | Chemical Composition (%) | BET Specific Surface (m2/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe2O3 | K2O | Al2O3 | SiO2 | MgO | CaO | TiO2 | Na2O | ||
| Quartz | 0.9 | – | 0.05 | 98.8 | 0.05 | 0.05 | – | 0.1 | 0.67 |
| Phlogo-pite | 6.3 | 13.3 | 15.1 | 39.2 | 22.7 | 1.7 | 0.9 | 0.3 | 1.84 |
| Biotite | 11 | 11 | 11.8 | 38.2 | 19.3 | 1.5 | 2.9 | 0.3 | 2.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tohry, A.; Dehghan, R.; Mohammadi-Manesh, H.; Filho, L.d.S.L.; Chelgani, S.C. Effect of Ether Mono Amine Collector on the Cationic Flotation of Micaceous Minerals—A Comparative Study. Sustainability 2021, 13, 11066. https://doi.org/10.3390/su131911066
Tohry A, Dehghan R, Mohammadi-Manesh H, Filho LdSL, Chelgani SC. Effect of Ether Mono Amine Collector on the Cationic Flotation of Micaceous Minerals—A Comparative Study. Sustainability. 2021; 13(19):11066. https://doi.org/10.3390/su131911066
Chicago/Turabian StyleTohry, Arash, Reza Dehghan, Hossein Mohammadi-Manesh, Laurindo de Salles Leal Filho, and Saeed Chehreh Chelgani. 2021. "Effect of Ether Mono Amine Collector on the Cationic Flotation of Micaceous Minerals—A Comparative Study" Sustainability 13, no. 19: 11066. https://doi.org/10.3390/su131911066
APA StyleTohry, A., Dehghan, R., Mohammadi-Manesh, H., Filho, L. d. S. L., & Chelgani, S. C. (2021). Effect of Ether Mono Amine Collector on the Cationic Flotation of Micaceous Minerals—A Comparative Study. Sustainability, 13(19), 11066. https://doi.org/10.3390/su131911066







