The Production of Biogenic Silica from Different South African Agricultural Residues through a Thermo-Chemical Treatment Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Biogenic Silica
2.2.2. Characterization of the Biogenic Silica
3. Results and Discussion
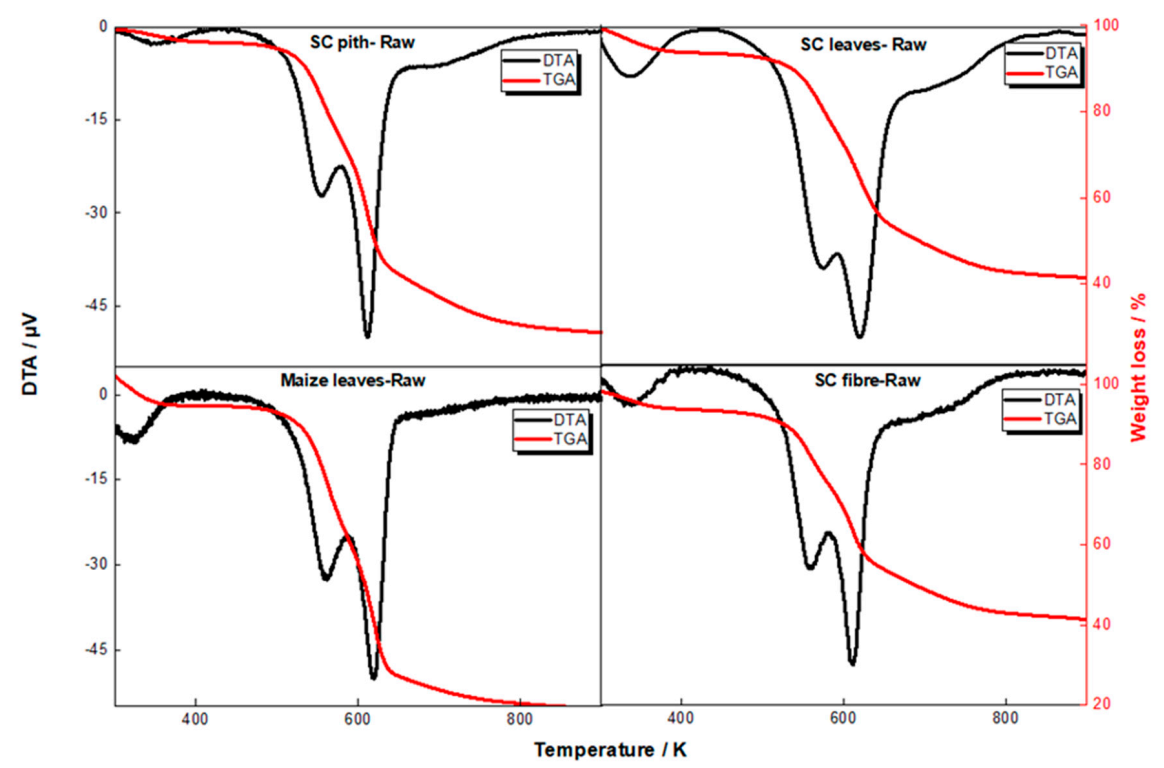
3.1. Thermal Analysis and Assessment of the Combustion Protocol
3.2. The Influence of Leaching on the Chemical and Elemental Composition
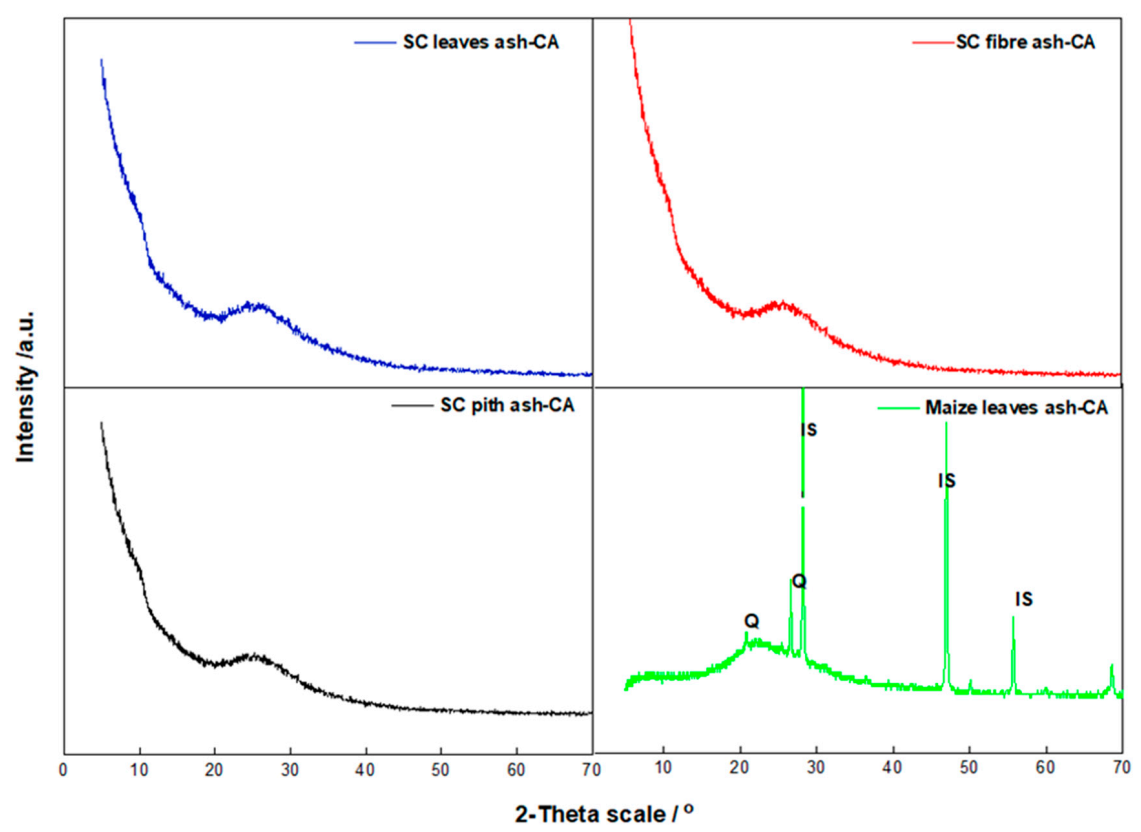
3.3. Phase Identification
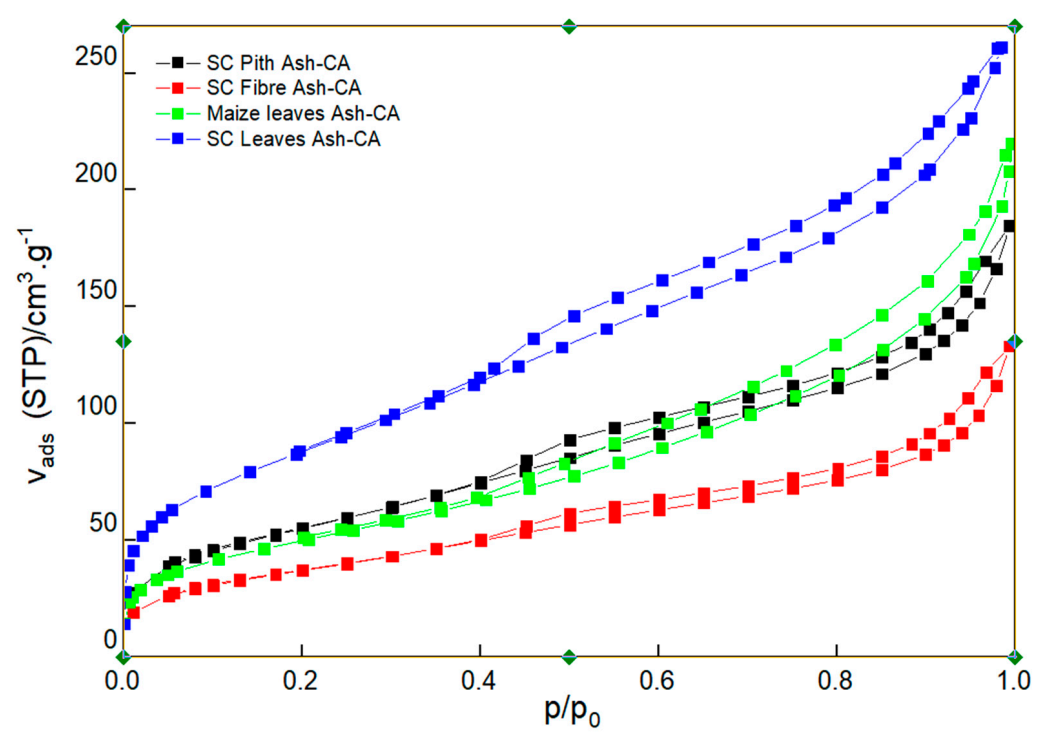
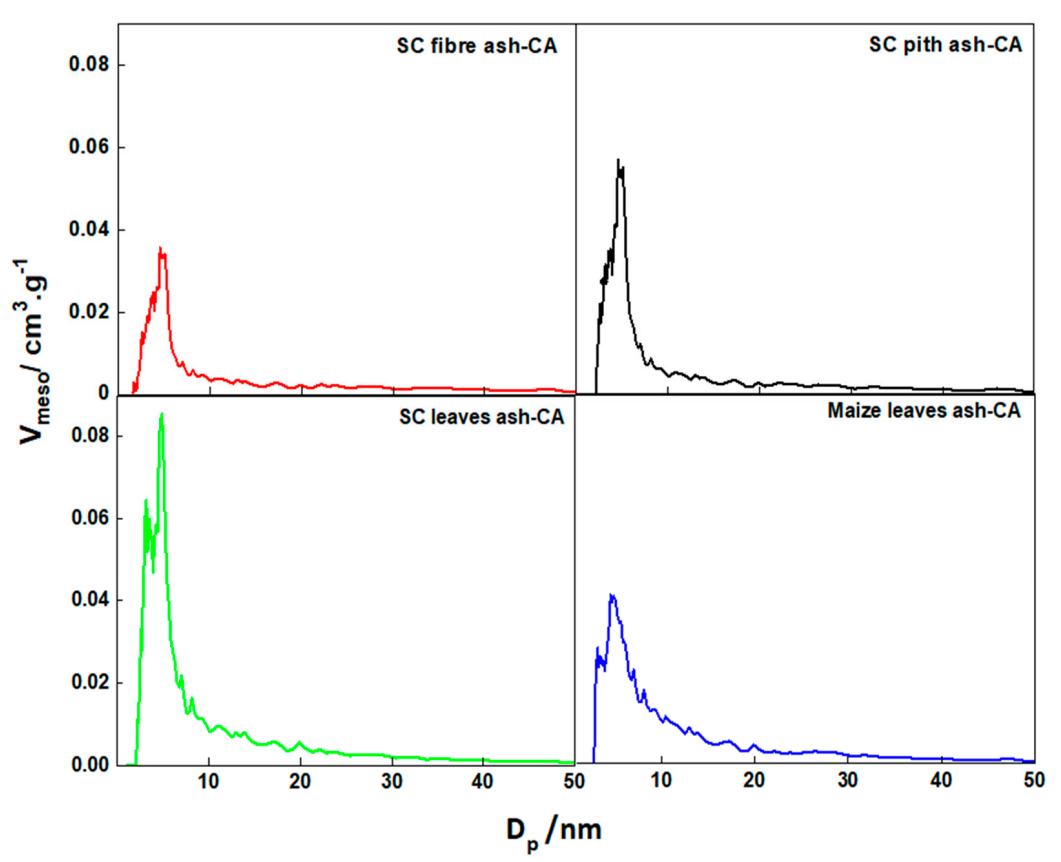
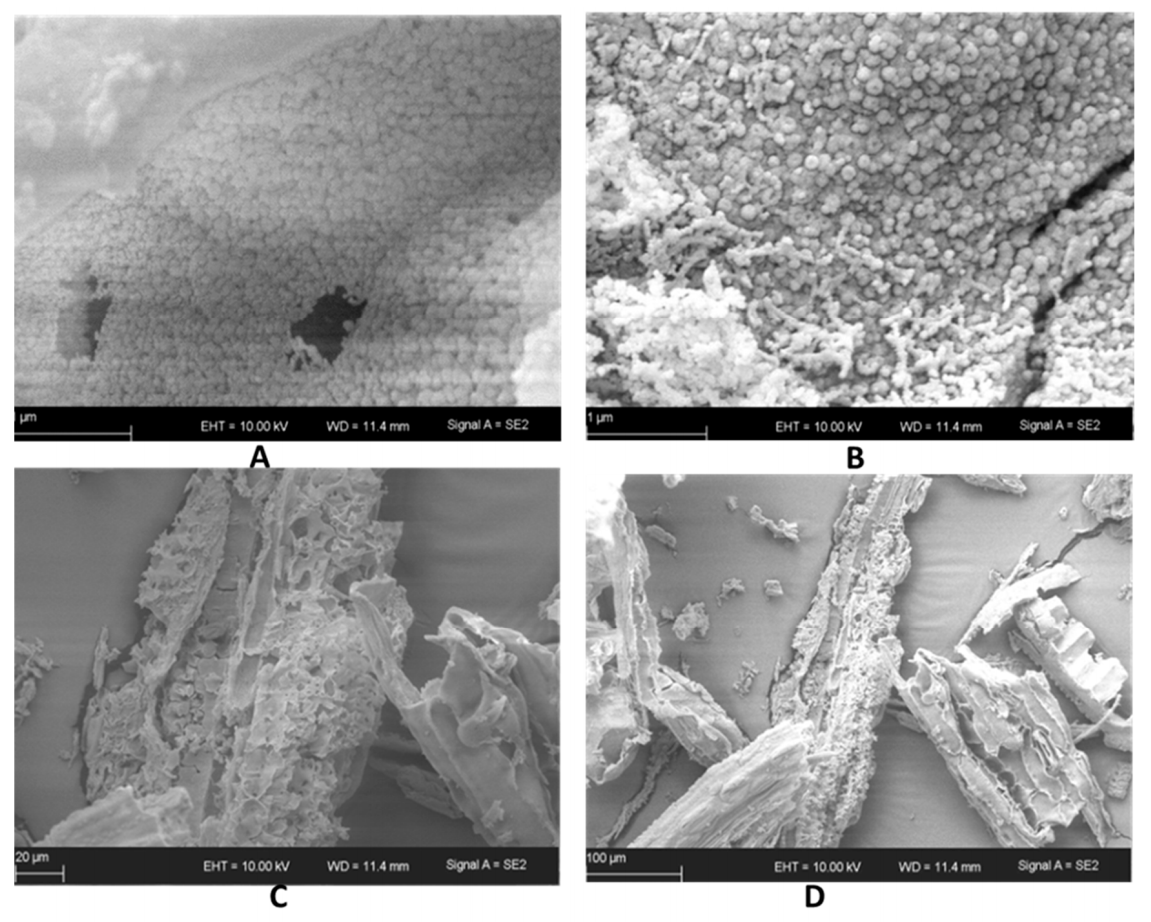
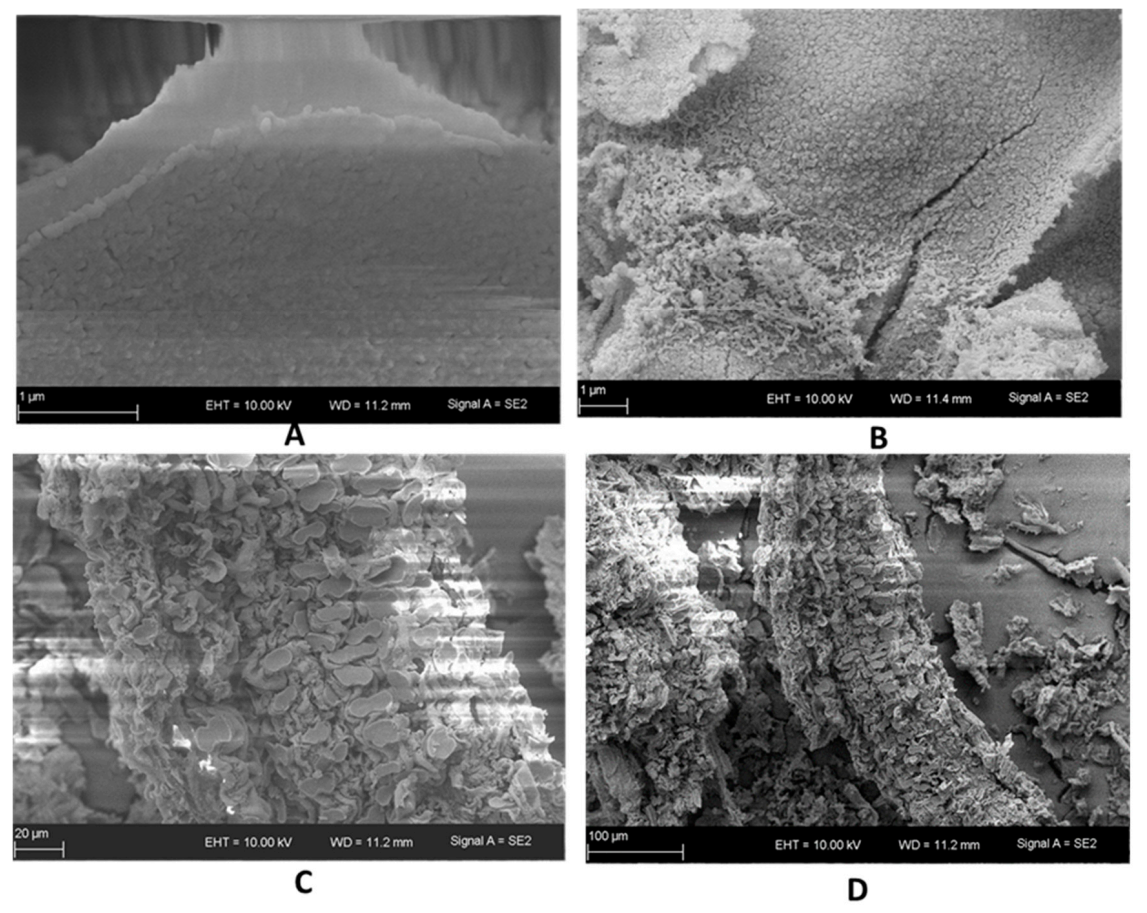
3.4. Textural and Structural Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Umeda, J.; Kondoh, K.; Michiura, Y. Process Parameters Optimization in Preparing High-Purity Amorphous Silica Originated from Rice Husks. Mater. Trans. 2007, 48, 3095–3100. [Google Scholar] [CrossRef] [Green Version]
- Alyosef, H.A.; Eilert, A.; Welscher, J.; Ibrahim, S.S.; Denecke, R.; Schwieger, W.; Enke, D. Characterization of Biogenic Silica Generated by Thermo Chemical Treatment of Rice Husk. Part. Sci. Technol. 2013, 31, 524–532. [Google Scholar] [CrossRef]
- Alyosef, H.A.; Schneider, D.; Wassersleben, S.; Roggendorf, H.; Weiß, M.; Eilert, A.; Denecke, R.; Hartmann, I.; Enke, D. Meso/Macroporous Silica from Miscanthus, Cereal Remnant Pellets, and Wheat Straw. ACS Sustain. Chem. Eng. 2015, 3, 2012–2021. [Google Scholar] [CrossRef]
- Schneider, D.; Wassersleben, S.; Weiß, M.; Denecke, R.; Stark, A.; Enke, D. A Generalized Procedure for the Production of High-Grade, Porous Biogenic Silica. Waste Biomass Valorization 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Okoronkwo, E.A.; Imoisili, P.E.; Olubayode, S.A.; Olusunle, S.O.O. Development of Silica Nanoparticle from Corn Cob Ash. Adv. Nanopart. 2016, 5, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Alyosef, H.A.; Ibrahim, S.; Welscher, J.; Inayat, A.; Eilert, A.; Denecke, R.; Schwieger, W.; Münster, T.; Kloess, G.; Einicke, W.D.; et al. Effect of acid treatment on the chemical composition and the structure of Egyptian diatomite. Int. J. Miner. Process. 2014, 132, 17–25. [Google Scholar] [CrossRef]
- Kootstra, A.M.J.; Beeftink, H.H.; Scott, E.L.; Sanders, J.P. Comparison of dilute mineral and organic acid pretreatment for enzymatic hydrolysis of wheat straw. Biochem. Eng. J. 2009, 46, 126–131. [Google Scholar] [CrossRef]
- Yalçin, N.; Sevinç, V. Studies on silica obtained from rice husk. Ceram. Int. 2001, 27, 219–224. [Google Scholar] [CrossRef]
- Mupa, M.; Hungwe, C.B.; Witzleben, S.; Mahamadi, C.; Muchanyereyi, N. Extraction of silica gel from Sorghum bicolour (L.) moench bagasse ash. Afr. J. Pure Appl. Chem. 2015, 9, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Umeda, J.; Kondoh, K. High-purity amorphous silica originated in rice husks via carboxylic acid leaching process. J. Mater. Sci 2008, 43, 7084–7090. [Google Scholar] [CrossRef]
- Le Blond, J.S.; Horwell, J.C.; Williamson, B.J.; Oppenheimer, C. Generation of crystalline silica from sugarcane burning. J Env. Monit. 2010, 12, 1459–1470. [Google Scholar] [CrossRef]
- Musić, S.; Filipović-Vinceković, N.; Sekovanić, L. Precipitation of amorphous SiO2 particles and their properties. Braz. J. Chem. Eng. 2011, 28, 89–94. [Google Scholar] [CrossRef]
- Sisuka, W. Annual South African Sugar Production Forecast to Grow Despite Revenue Pressures. Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Sugar%20Annual_Pretoria_South%20Africa%20-%20Republic%20of_4-15-2019.pdf (accessed on 11 January 2021).
- Chandel, A.; Da Silva, S.; Carvalho, W.; Singh, O. Sugarcane bagasse and leaves: Foreseeable biomass of biofuel and bio-products. J. Chem. Technol. Biotech. 2011, 87, 11–20. [Google Scholar] [CrossRef]
- Smithers, J. Review of sugarcane trash recovery systems for energy cogeneration in South Africa. Renew. Sustain. Energy Rev. 2014, 32, 915–925. [Google Scholar] [CrossRef]
- Du Plessis, J. Maize Production; Department of Agriculture South Africa: Pretoria, South Africa, 2003. [Google Scholar]
- Wilkonson, D. Industrial guidelines for burning sugarcane. In SASRI Information Sheets; Sugar Research Institute: Durban, South Africa, 2013; pp. 1–2. [Google Scholar]
- Street, R.A. Technology and Applications of Amorphous Silicon; Springer: Berlin, Germany, 2000. [Google Scholar]
- Joshi, H.H.; Gertz, R.E.; Carvalho, M.D.G.; Beall, B.W. Use of silica desiccant packets for specimen storage and transport to evaluate pneumococcal nasopharyngeal carriage among nepalese children. J. Clin. Microbiol. 2008, 46, 3175–3176. [Google Scholar] [CrossRef] [Green Version]
- Joiner, A. A silica toothpaste containing blue covarine: A new technological breakthrough in whitening. Int. Dent. J. 2009, 59, 284–288. [Google Scholar] [CrossRef]
- Yu, D.P.; Hang, Q.L.; Ding, Y.; Zhang, H.Z.; Bai, Z.G.; Wang, J.J.; Zou, Y.H.; Qian, W.; Xiong, G.C.; Feng, S.Q. Amorphous silica nanowires: Intensive blue light emitters. Appl. Phys. Let. 1998, 73, 3076–3078. [Google Scholar] [CrossRef]
- Carmona, V.; Oliveira, R.; Silva, W.; Mattoso, L.; Marconcini, J. Nanosilica from rice husk: Extraction and characterization. Ind. Crop. Prod. 2013, 43, 291–296. [Google Scholar] [CrossRef]
- Barrett, E.; Joyner, L.; Halenda, P. The determination of pore volume and area distributions in Porous Substances. I. computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Kumar, A.; Wang, L.; Dzenis, Y.A.; Jones, D.D.; Hanna, M.A. Thermogravimetric characterization of corn stover as gasification and pyrolysis feedstock. Biomass Energy 2008, 32, 460–467. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Liou, T. Preparation and characterization of nano-structured silica from rice husk. Mater. Sci. Eng. 2004, 364, 313–323. [Google Scholar] [CrossRef]
- Fang, F. Pretreatment Techniques for Biofuels and Biorefineries, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 158–159. [Google Scholar]
- Umeda, J.; Imai, H.; Kondoh, K. Polysaccharide hydrolysis and metallic impurities removal behavior of rice husks in citric acid leaching treatment. Trans. JWRI 2009, 38, 13–18. [Google Scholar]
- Faizul, C.; Chik, A.; Bari, M.; Noorina, H. Extraction of silica from palm ash using organic acid leaching treatment. Key Eng. Mat. 2013, 7, 3690–3695. [Google Scholar] [CrossRef]
- Boochapun, S.; Lamamorphanth, W.; Kamwilaisak, K. The acid hydrolysis of sugarcane leaves as a biofeedstook for bioethanol production. Adv. Mater. Res. 2014, 931–932, 194–199. [Google Scholar] [CrossRef]
- Worathanakul, P.; Payubnop, W.; Muangpet, A. Characterization for post-treatment effect of bagasse ash for silica extraction. World Acad. Sci. Eng. Technol. 2009, 3, 339–341. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U. Eco-production of silica from sugarcane bagasse ash for use as a photochromic pigment filler. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Sholeh, M.; Rochmadi, R.; Sulistyo, H.; Budhijanto, B. Synthesis of precipitated silica from bagasse ash as reinforcing filler in rubber. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Kuala Lumpur, Malaysia, 2020; Volume 778, p. 012012. [Google Scholar] [CrossRef]
- Lanning, F.; Ponnaiya, B.; Crumpton, C. The chemical nature of silica in plants. Plant Physiol. 1958, 33, 339–343. [Google Scholar] [CrossRef] [Green Version]
- Chisholm, J. Comparison of Quartz Standards for X-ray Diffraction Analysis: HSE A9950 (Sikron F600) and NIST SRM 1878. Ann. Occup. Hyg. 2005, 49, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Bhattacharjee, B. Porosity, pore size distribution and in situ strength of concrete. Cem. Concr. Res. 2003, 33, 155–164. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Ferch, H. Pulverförmige amorphe synthetische Kieselsäure-Produkte Herstellung und Charakterisierung. Chem. Ing. Tech. 1976, 48, 922–933. [Google Scholar] [CrossRef]
| Type of Biomass | Pre-Treatment Acid | Pre-Treatment Temperature (K) | Silica Content (wt%) | BET Surface Area (m2 g−1) | References |
|---|---|---|---|---|---|
| Rice husk | 10% (v/v) citric acid | 423 | 98.8 | n.d. | [22] |
| Rice husk | 5 wt% citric acid | 323 | 99.1 | n.d. | [10] |
| Rice husk | 5 wt% citric acid | 353 | 97.7 | 313 | [2] |
| Rice Straw | 3.25 M citric acid | 323 | 99.1 | 264 | [4] |
| Spelt husk | 95.8 | 185 | |||
| Oat husk | 99.1 | 248 | |||
| Horsetail | 91.6 | 301 |
| Biomass | Nitrogen (%) | Carbon (%) | Hydrogen (%) |
|---|---|---|---|
| SC leaves −Raw | 0.40 ± 0.00 | 39.50 ± 0.00 | 5.42 ± 0.10 |
| SC leaves Ash-HW | 0.031 ± 0.01 | 0.19 ± 0.00 | 0.42 ± 0.01 |
| SC leaves Ash-CA | 0.02 ± 0.00 | 0.14 ± 0.01 | 0.31 ± 0.00 |
| SC leaves Ash-SA | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.12 ± 0.01 |
| SC Pith -Raw | 0.10 ± 0.01 | 45.30 ± 0.10 | 5.80 ± 0.05 |
| SC Pith Ash-CA | 0.01 ± 0.01 | 0.04 ± 0.01 | 0.38 ± 0.04 |
| SC Fiber −Raw | 0.03 ± 0.00 | 45.20 ± 0.10 | 5.90 ± 0.00 |
| SC Fiber Ash-CA | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.14 ± 0.03 |
| Maize leaves −Raw | 0.10 ± 0.00 | 45.30 ± 0.10 | 5.80 ± 0.10 |
| Maize leaves Ash-CA | 0.03 ± 0.01 | 0.05 ± 0.01 | 0.37 ± 0.04 |
| Constituent | SC Leaves-Raw (wt%) | SC Leaves Ash-HW (wt%) | SC Leaves Ash-CA (wt%) | SC Leaves Ash-SA (wt%) | SC Pith -Raw (wt%) | SC Pith Ash-CA (wt%) | SC Fiber -Raw (wt%) | SC Fiber Ash-CA (wt%) | Maize Leaves -Raw (wt%) | Maize Leaves Ash-CA (wt%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 63.8 | 87.6 | 95.4 | 99.3 | 39.2 | 94.8 | 33.7 | 94.3 | 51.4 | 93.0 | |
| P2O5 | 1.4 | 0.7 | 0.4 | 0.1 | 1.3 | 1.1 | 4.0 | 2.6 | 5.3 | 3.3 | |
| K2O | 3.8 | 1.4 | 0.2 | <LOD | 2.4 | 0.1 | 12.0 | 0.1 | 5.8 | 0.3 | |
| CaO | 14.9 | 7.5 | 2.6 | 0.2 | 19.0 | 0.4 | 14.9 | 0.6 | 21.4 | 1.3 | |
| MgO | 1.5 | 1.2 | 0.4 | <LOD | 2.2 | 0.3 | 2.9 | 0.9 | 2.0 | 0.4 | |
| SO3 | 4.5 | 0.9 | 0.8 | 0.3 | 4.4 | 0.3 | 7.3 | 0.4 | 5.5 | 0.4 | |
| Fe2O3 | 2.3 | 0.2 | 0.1 | <LOD | 5.7 | 0.9 | 2.5 | 0.2 | 2.4 | 0.3 | |
| Al2O3 | 3.1 | 0.1 | 0.1 | 0.1 | 11.7 | 0.8 | 12.4 | 0.2 | 1.7 | 0.6 | |
| Cl | 1.7 | <LOD | <LOD | <LOD | 5.6 | <LOD | 6.2 | <LOD | 0.8 | <LOD | |
| Others a | 3.0 | 0.4 | 0.0 | 0.0 | 8.5 | 1.3 | 4.1 | 0.7 | 3.7 | 0.4 | |
| Sample | ABET (m2 g−1) | Dp (nm) | Vmeso (cm3 g−1) |
|---|---|---|---|
| SC leaves Ash-CA | 323 | 5.0 | 0.41 |
| SC leaves Ash-SA | 326 | 4.7 | 0.47 |
| SC leaves Ash-HW | 133 | 8.0 | 0.25 |
| SC Pith Ash-CA | 203 | 6.0 | 0.29 |
| SC Fiber Ash-CA | 136 | 6.0 | 0.21 |
| Maize leaves Ash-CA | 182 | 7.0 | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maseko, N.N.; Schneider, D.; Wassersleben, S.; Enke, D.; Iwarere, S.A.; Pocock, J.; Stark, A. The Production of Biogenic Silica from Different South African Agricultural Residues through a Thermo-Chemical Treatment Method. Sustainability 2021, 13, 577. https://doi.org/10.3390/su13020577
Maseko NN, Schneider D, Wassersleben S, Enke D, Iwarere SA, Pocock J, Stark A. The Production of Biogenic Silica from Different South African Agricultural Residues through a Thermo-Chemical Treatment Method. Sustainability. 2021; 13(2):577. https://doi.org/10.3390/su13020577
Chicago/Turabian StyleMaseko, Ncamisile Nondumiso, Denise Schneider, Susan Wassersleben, Dirk Enke, Samuel Ayodele Iwarere, Jonathan Pocock, and Annegret Stark. 2021. "The Production of Biogenic Silica from Different South African Agricultural Residues through a Thermo-Chemical Treatment Method" Sustainability 13, no. 2: 577. https://doi.org/10.3390/su13020577






