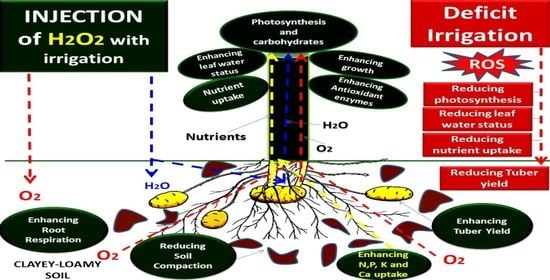
Hydrogen Peroxide Supplementation in Irrigation Water Alleviates Drought Stress and Boosts Growth and Productivity of Potato Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Treatments and Design
2.3. Data Recorded
2.3.1. Biomass
2.3.2. Root Respiration (μmol CO2 m−2 s−1)
2.3.3. Leaf Relative Chlorophyll Content (LRCC)
2.3.4. Leaf Area (LA)
2.3.5. Leaf Dry Weight
2.3.6. Nutrient Analysis
2.3.7. Leaf Relative Water Content (LRWC) and Leaf Relative Water Deficit (LRWD)
2.3.8. Leaf Osmotic Potential (Mpa)
2.3.9. Catalase Enzyme (CAT) Activity
2.3.10. Proline Content
2.3.11. Soluble Carbohydrates Content
2.3.12. Measurements on Tuber and Tuber Yield
2.4. Statistical Analysis
3. Results
3.1. Analysis of Variance (ANOVA) Procedure Considering H202 Application with Irrigation Level on Potato Plants
3.2. Effect of H2O2 Application (Irrigation) on Plant Growth, Biomass and Root Respiration
3.3. Effect of H2O2—Treatment (Irrigation) on Osmotic Status and Nutrient Acquisition in Potato Plants
3.3.1. Leaf Water Status (LWS)
3.3.2. Analysis of Osmolyte Content in Leaf
3.3.3. Catalase (CAT) Enzyme Activity in Leaf
3.3.4. Nutrient Analysis
3.4. Effect of H2O2—Induced Oxygenated Irrigation on Yield Attributes and Water Use Efficiency in Potato
3.4.1. Yield Attributes
3.4.2. Water Use Efficiency (WUE)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement.
Informed Consent Statement.
Data Availability Statement.
Acknowledgments
Conflicts of Interest
References
- Ibrahim, M.; Abd El-Gawad, H.; Bondok, A. Physiological impacts of potassium citrate and folic acid on growth, yield and some viral diseases of potato plants. Middle East J. Agric. Res. 2015, 4, 577–589. [Google Scholar]
- Mullins, E.; Milbourne, D.; Petti, C.; Doyle-Prestwich, B.M.; Meade, C. Potato in the age of biotechnology. Trends Plant Sci. 2006, 11, 254–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haverkort, A.; De Ruijter, F.; Van Evert, F.; Conijn, J.; Rutgers, B. Worldwide sustainability hotspots in potato cultivation. 1. Identification and mapping. Potato Res. 2013, 56, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Soltys-Kalina, D.; Plich, J.; Strzelczyk-Żyta, D.; Śliwka, J.; Marczewski, W. The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of ‘Katahdin’-derived potato cultivars. Breed Sci 2016, 66, 328–331. [Google Scholar] [CrossRef] [Green Version]
- Samak, D.H.; El-Sayed, Y.S.; Shaheen, H.M.; El-Far, A.H.; Abd El-Hack, M.E.; Noreldin, A.E.; El-Naggar, K.; Abdelnour, S.A.; Saied, E.M.; El-Seedi, H.R.; et al. Developmental Toxicity of Carbon Nanoparticles during Embryogenesis in Chicken. Environ. Sci. Pollut. Res 2020, 27, 19058–19072. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Elkelish, A.; Soliman, M.; Elansary, H.O.; Zaid, A.; Wani, S.H. Serratia Marcescens BM1 Enhances Cadmium Stress Tolerance and Phytoremediation Potential of Soybean Through Modulation of Osmolytes, Leaf Gas Exchange, Antioxidant Machinery, and Stress-Responsive Genes Expression. Antioxidants 2020, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Gemenet, D.C.; Villordon, A. Root system architecture and abiotic stress tolerance: Current knowledge in root and tuber crops. Front. Plant Sci. 2016, 7, 1584. [Google Scholar] [CrossRef] [Green Version]
- Habib, N.; Ali, Q.; Ali, S.; Javed, M.T.; Zulqurnain Haider, M.; Perveen, R.; Shahid, M.R.; Rizwan, M.; Abdel-Daim, M.M.; Elkelish, A. Use of Nitric Oxide and Hydrogen Peroxide for Better Yield of Wheat (Triticum aestivum L.) under Water Deficit Conditions: Growth, Osmoregulation, and Antioxidative Defense Mechanism. Plants 2020, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Saied, E.M.; Banhart, S.; Bürkle, S.E.; Heuer, D.; Arenz, C. A Series of Ceramide Analogs Modified at the 1-Position with Potent Activity against the Intracellular Growth of Chlamydia Trachomatis. Future Med. Chem 2015, 7, 1971–1980. [Google Scholar] [CrossRef]
- Zappi, M.; White, K.; Hwang, H.-M.; Bajpai, R.; Qasim, M. The fate of hydrogen peroxide as an oxygen source for bioremediation activities within saturated aquifer systems. J. Air Waste Manag. Assoc. 2000, 50, 1818–1830. [Google Scholar] [CrossRef] [Green Version]
- Quan, L.J.; Zhang, B.; Shi, W.W.; Li, H.Y. Hydrogen peroxide in plants: A versatile molecule of the reactive oxygen species network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Elkelish, A.A.; Alhaithloul, H.A.S.; Qari, S.H.; Soliman, M.H.; Hasanuzzaman, M. Pretreatment with Trichoderma Harzianum Alleviates Waterlogging-Induced Growth Alterations in Tomato Seedlings by Modulating Physiological, Biochemical, and Molecular Mechanisms. Environ. Exp. Bot. 2020, 171. [Google Scholar] [CrossRef]
- Abd El-Gawad, H.; El-Azm, N.A.; Hikal, M. Effect of potassium silicate on tuber yield and biochemical constituents of potato plants grown under drought stress conditions. Middle East J. Agric. Res 2017, 6, 718–731. [Google Scholar]
- Ibrahim, M.; Ibrahim, H.A. Assessment of Selenium Role in Promoting or Inhibiting Potato Plants under Water Stress. J. Hortic. Sci. Ornam. Plants 2016, 8, 125–139. [Google Scholar]
- Elkelish, A.; Qari, S.H.; Mazrou, Y.S.A.; Abdelaal, K.A.A.; Hafez, Y.M.; Abu-Elsaoud, A.M.; Batiha, G.E.-S.; El-Esawi, M.A.; Nahhas, N.E. Exogenous Ascorbic Acid Induced Chilling Tolerance in Tomato Plants through Modulating Metabolism, Osmolytes, Antioxidants, and Transcriptional Regulation of Catalase and Heat Shock Proteins. Plants 2020, 9, 431. [Google Scholar] [CrossRef] [Green Version]
- El-Esawi, M.A.; Alayafi, A.A. Overexpression of Rice Rab7 Gene Improves Drought and Heat Tolerance and Increases Grain Yield in Rice (Oryza Sativa L.). Genes 2019, 10, 56. [Google Scholar] [CrossRef] [Green Version]
- Sarker, K.K.; Hossain, A.; Timsina, J.; Biswas, S.K.; Kundu, B.C.; Barman, A.; Murad, K.F.I.; Akter, F. Yield and quality of potato tuber and its water productivity are influenced by alternate furrow irrigation in a raised bed system. Agric. Water Manag. 2019, 224, 105750. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Ahmad, M. Overexpression of AtWRKY30 Transcription Factor Enhances Heat and Drought Stress Tolerance in Wheat (Triticum Aestivum L.). Genes 2019, 10, 163. [Google Scholar] [CrossRef] [Green Version]
- Collenburg, L.; Beyersdorf, N.; Wiese, T.; Arenz, C.; Saied, E.M.; Becker-Flegler, K.A.; Schneider-Schaulies, S.; Avota, E. The Activity of the Neutral Sphingomyelinase Is Important in T Cell Recruitment and Directional Migration. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Koller, H. Leaf Area-Leaf Weight Relationships in the Soybean Canopy 1. Crop Sci. 1972, 12, 180–183. [Google Scholar] [CrossRef]
- Azab, E.; Elsalam, H.; Sharnouby, M. Performance of Catharanthus Roseus Plants in Response to Gamma Irradiation. J. Bio. Chem. Res. 2016, 33, 130–140. [Google Scholar]
- Azab, E.; Soror, A.-F.S. Physiological Behavior of the Aquatic Plant Azolla Sp. in Response to Organic and Inorganic Fertilizers. in Response to Organic and Inorganic Fertilizers. Plants 2020, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Bovay, E.; Cossy, A. Determination of potassium, calcium, magnesium, and sodium in flashed vegetable material by flame spectrophotometry. Mitt. Geb. Lebensmitt. Hyg 1955, 46, 540–568. [Google Scholar]
- Tourneux, C.; Devaux, A.; Camacho, M.R.; Mamani, P.; Ledent, J.-F. Effect of water shortage on six potato genotypes in the highlands of Bolivia (II): Water relations, physiological parameters. Agronomie 2003, 23, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Barr, H.; Weatherley, P. A re-examination of the relative turgidity technique for estimating water deficit in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Shackel, K.A. Direct measurement of turgor and osmotic potential in individual epidermal cells: Independent confirmation of leaf water potential as determined by in situ psychrometry. Plant Physiol. 1987, 83, 719–722. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.M.; Knowles, N.R. Changes in lipid peroxidation and lipolytic and free-radical scavenging enzyme activities during aging and sprouting of potato (Solanum tuberosum) seed-tubers. Plant Physiol. 1993, 102, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Chinard, F.P. Photometric estimation of proline and ornithine. J. Biol. Chem. 1952, 199, 91–95. [Google Scholar] [CrossRef]
- Ábrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for determination of proline in plants. Methods Mol. Biol. 2010, 639, 317–331. [Google Scholar] [CrossRef]
- Yemm, E.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roper, T.R.; Keller, J.D.; Loescher, W.H.; Rom, C.R. Photosynthesis and carbohydrate partitioning in sweet cherry: Fruiting effects. Physiol. Plant. 1988, 72, 42–47. [Google Scholar] [CrossRef]
- Passioura, J. Increasing crop productivity when water is scarce—from breeding to field management. Agric. Water Manag. 2006, 80, 176–196. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hao, Y.; Cui, X.Y.; Zhao, H.; Xu, C.; Zhou, X.; Xu, Z. Responses of soil respiration and its components to drought stress. J. Soils Sediments 2014, 14, 99–109. [Google Scholar] [CrossRef]
- Nawaz, M.F.; Bourrie, G.; Trolard, F. Soil compaction impact and modelling. A review. Agron. Sustain. Dev. 2013, 33, 291–309. [Google Scholar] [CrossRef] [Green Version]
- Ben-Noah, I.; Friedman, S.P. Review and evaluation of root respiration and of natural and agricultural processes of soil aeration. Vadose Zone J. 2018, 17, 1–47. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.-P.; Cheng, Y.-J.; Wu, X.-B.; Kwak, S.-S.; Chen, W.; Eneji, A.E. Exogenous hydrogen peroxide positively influences root growth and metabolism in leaves of sweet potato seedlings. Aust. J. Crop Sci. 2012, 6, 1572. [Google Scholar]
- Li, Y.; Niu, W.; Dyck, M.; Wang, J.; Zou, X. Yields and nutritional of greenhouse tomato in response to different soil aeration volume at two depths of subsurface drip irrigation. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Ismail, S.Z.; Khandaker, M.M.; Mat, N.; Boyce, A.N. Effects of hydrogen peroxide on growth, development and quality of fruits: A review. J. Agron. 2015, 14, 331–336. [Google Scholar] [CrossRef] [Green Version]
- Monneveux, P.; Ramírez, D.A.; Pino, M.-T. Drought tolerance in potato (Solanum tuberosum L.): Can we learn from drought tolerance research in cereals? Plant Sci. 2013, 205, 76–86. [Google Scholar] [CrossRef]
- Sariyev, A.; Barutcular, C.; Acar, M.; Hossain, A.; El Sabagh, A. Sub-surface drip irrigation in associated with H2O2 improved the productivity of maize under clay-rich soil of Adana, Turkey. Phyton 2020, 89, 519. [Google Scholar] [CrossRef]
- Bhattarai, S.P.; Huber, S.; Midmore, D.J. Aerated subsurface irrigation water gives growth and yield benefits to zucchini, vegetable soybean and cotton in heavy clay soils. Ann. Appl. Biol. 2004, 144, 285–298. [Google Scholar] [CrossRef]
- Bhattarai, S.P.; Dhungel, J.; Midmore, D.J. Oxygation Improves Yield and Quality and Minimizes Internal Fruit Crack of Cucurbits on a Heavy Clay Soil in the Semi-arid Tropics. J. Agric. Sci. 2010, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Gil, P.M.; Ferreyra, R.; Barrera, C.; Zúñiga, C.; Gurovich, L. Effect of injecting hydrogen peroxide into heavy clay loam soil on plant water status, net CO2 assimilation, biomass, and vascular anatomy of avocado trees. Chil. J. Agric. Res. 2009, 69, 97–106. [Google Scholar] [CrossRef]
- Dat, J.; Vandenabeele, S.; Vranova, E.; Van Montagu, M.; Inzé, D.; Van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. CMLS 2000, 57, 779–795. [Google Scholar] [CrossRef]
- Cheeseman, J.M. Hydrogen peroxide concentrations in leaves under natural conditions. J. Exp. Bot. 2006, 57, 2435–2444. [Google Scholar] [CrossRef] [Green Version]
- Mazuela, P. Effect of oxygen supply on water uptake in a melon crop under soilless culture. Interciencia 2010, 35, 769–771. [Google Scholar]






| Soil Depth | Texture | Soil Particle Distribution % | F.C.% | P.W.P.% | B.d. | Soil pH | Soil EC | ||
|---|---|---|---|---|---|---|---|---|---|
| cm | Sand | Silt | Clay | θ at −33 kPa | θ at −1500 kPa | g cm−3 | |||
| 0–30 | Clay-loamy | 24 | 36 | 40 | −31.05 | −1481 | 1.32 | 6.84 | 2.64 |
| 30–60 | Clay-loamy | 27 | 32 | 41 | −32.71 | −1768 | 1.34 | 6.82 | 2.67 |
| pH | TDS | HCO3− | CO3−2 | Cl− | SO4−2 | Na+ |
|---|---|---|---|---|---|---|
| mg/L | Meq/L | |||||
| 7.2 | 403 | 0.5 | 0 | 2.5 | 1.28 | 1.7 |
| Irrigation Level | Hydrogen Peroxide Injection Rate | ||
|---|---|---|---|
| Deficit irrigation | Zero (T1) | 300 ppm (T2) | 600 ppm (T3) |
| Optimum irrigation | Zero (T4) | 300 ppm (T5) | 600 ppm (T6) |
| Trait | Df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|
| Branches number | 5 | 0.4888889 | 0.5246 ns | 0.0618 ns |
| Stem length | 5 | 113.99647 | 53.396328 | 0.0000 *** |
| Shoot dry weight | 5 | 96.660173 | 44.827823 | 0.0000 *** |
| Leaf chlorophyll reading | 5 | 20.373142 | 17.662396 | 0.0001 *** |
| Leaf dry weight | 5 | 0.38621 | 69.535184 | 0.0000 *** |
| Leaf area | 5 | 550.09037 | 72.896609 | 0.0000 *** |
| Root Dry Weight | 5 | 4.0805433 | 24.369697 | 0.0000 *** |
| Root to Shoot ratio | 5 | 0.0007021 | 17.674965 | 0.0001 *** |
| Root Respiration | 5 | 10.378019 | 29.256672 | 0.0000 *** |
| Nitrogen cont. % | 5 | 0.51705 | 203.83049 | 0.0000 *** |
| Phosphorus cont. % | 5 | 0.0234161 | 14.341091 | 0.0003 *** |
| K cont. % | 5 | 0.7247689 | 39.993378 | 0.0000 *** |
| Calcium cont. % | 5 | 0.0056523 | 4.039521 | 0.0288 * |
| Leaf Relative Water Cont. | 5 | 227.92749 | 130.07828 | 0.0000 *** |
| Leaf Relative Water Deficit | 5 | 179.2018 | 158.90676 | 0.0000 *** |
| Leaf Osmotic Potential. | 5 | 0.11945 | 14.311102 | 0.0003 *** |
| Leaf Proline Cont. | 5 | 9.5617022 | 34.723668 | 0.0000 *** |
| Leaf Total Carbohydrates | 5 | 59.783157 | 137.98024 | 0.0000 *** |
| Catalase activity | 5 | 16.576276 | 45.765672 | 0.0000 *** |
| Tuber number/plant | 5 | 6.1473789 | 29.013189 | 0.0000 *** |
| Average tuber weight | 5 | 489.48241 | 84.385808 | 0.0000 *** |
| Tuber yield/plant | 5 | 145942.99 | 66.534393 | 0.0000 *** |
| Tuber yield/hectare | 5 | 525.42987 | 169.00871 | 0.0000 *** |
| Water productivity | 5 | 20.350223 | 88.33329 | 0.0000 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Elhady, S.A.; El-Gawad, H.G.A.; Ibrahim, M.F.M.; Mukherjee, S.; Elkelish, A.; Azab, E.; Gobouri, A.A.; Farag, R.; Ibrahim, H.A.; El-Azm, N.A. Hydrogen Peroxide Supplementation in Irrigation Water Alleviates Drought Stress and Boosts Growth and Productivity of Potato Plants. Sustainability 2021, 13, 899. https://doi.org/10.3390/su13020899
Abd Elhady SA, El-Gawad HGA, Ibrahim MFM, Mukherjee S, Elkelish A, Azab E, Gobouri AA, Farag R, Ibrahim HA, El-Azm NA. Hydrogen Peroxide Supplementation in Irrigation Water Alleviates Drought Stress and Boosts Growth and Productivity of Potato Plants. Sustainability. 2021; 13(2):899. https://doi.org/10.3390/su13020899
Chicago/Turabian StyleAbd Elhady, Salama A., Hany G. Abd El-Gawad, Mohamed F. M. Ibrahim, Soumya Mukherjee, Amr Elkelish, Ehab Azab, Adil A. Gobouri, Reham Farag, Huda A. Ibrahim, and Nashwa Abu El-Azm. 2021. "Hydrogen Peroxide Supplementation in Irrigation Water Alleviates Drought Stress and Boosts Growth and Productivity of Potato Plants" Sustainability 13, no. 2: 899. https://doi.org/10.3390/su13020899
APA StyleAbd Elhady, S. A., El-Gawad, H. G. A., Ibrahim, M. F. M., Mukherjee, S., Elkelish, A., Azab, E., Gobouri, A. A., Farag, R., Ibrahim, H. A., & El-Azm, N. A. (2021). Hydrogen Peroxide Supplementation in Irrigation Water Alleviates Drought Stress and Boosts Growth and Productivity of Potato Plants. Sustainability, 13(2), 899. https://doi.org/10.3390/su13020899