Abstract
In plant cells, ammonium is considered the most convenient nitrogen source for cell metabolism. However, despite ammonium being the preferred N form for microalgae, at higher concentrations, it can be toxic, and can cause growth inhibition. Microalgae’s tolerance to ammonium depends on the species, with various taxa showing different thresholds of tolerability and symptoms of toxicity. In the environment, ammonium at high concentrations represents a dangerous pollutant. It can affect water quality, causing numerous environmental problems, including eutrophication of downstream waters. For this reason, it is important to treat wastewater and remove nutrients before discharging it into rivers, lakes, or seas. A valid and sustainable alternative to conventional treatments could be provided by microalgae, coupling the nutrient removal from wastewater with the production of valuable biomass. This review is focused on ammonium and its importance in algal nutrition, but also on its problematic presence in aquatic systems such as wastewaters. The aim of this work is to provide recent information on the exploitation of microalgae in ammonium removal and the role of ammonium in microalgae metabolism.
1. Introduction
Microalgae are single-cell photosynthetic organisms, ranging in size from a few µm to a few hundred µm; they are considered the greatest primary producer of any aquatic ecosystem. Although over 300,000 microalgal species exist, only around 30,000 have been studied or documented [1]. Phylogenetically, microalgae include many different groups, existing in various aquatic and terrestrial habitats, and are tolerant of a wide range of temperatures, salinities, pH values, and different light intensities. Therefore, they represent a huge group of organisms that can live in an extensive range of environments.
Recently, microalgae have attracted considerable interest worldwide due to their potential extensive applications in the renewable energy, biopharmaceutical, and nutraceutical industries [2,3]. A wide spectrum of biologically active compounds has been found in algal biomass; for example, proteins, polyunsaturated fatty acids (PUFAs), pigments, vitamins and minerals, and extracellular compounds such as oligosaccharides [4,5,6,7]. In particular, microalgae accumulate a high lipid content, normally ranging from 10% to 50% of dry weight [8,9]; but in some genera, such as Botryococcus, the lipid content can reach 60% to 90% of dry weight [10,11]. Due to their fast growth rate, these microorganisms can be easily cultured in closed bioreactors or open systems and achieve high biomass yields [12], and their cultivation does not compete for resources used in conventional agriculture [13]. Photosynthesis in microalgae is similar to that in higher plants, although characterized by a higher yield compared to terrestrial crops, as it is more efficient in transforming solar energy into chemical energy [14,15]. However, microalgal photosynthesis and growth can be affected by several factors, including light supply, temperature, pH, inorganic carbon availability, salinity, and nutrients [15,16,17,18]. Particularly, among nutrients, nitrogen (N) is considered one of the most critical for plant-cell growth, since it is a constituent of proteins such as peptides, enzymes, chlorophylls, and energy-transfer molecules [16,19]. In fact, N is the second most abundant element in microalgal cells following carbon (C), ranging from 1% to 14% of their dry weight [20]. Due to nitrogen’s important roles in cell metabolism, its restriction is often considered a limiting resource for microalgae, and in general for plant growth. Microalgae can utilize different nitrogen forms such as nitrate, nitrite, ammonium, or organic nitrogen (e.g., urea and amino acids). However, the favorable N-form not only differs from species to species, but the diverse N-source also can influence the biochemical composition of the algal cells in different ways [21,22]. In recent years, due to microalgae’s ability to use inorganic and organic nitrogen for their growth, their use in treating wastewaters, particularly those rich in ammonium, has been studied [23]. Excess N discharge into water can lead to eutrophication phenomena in natural aquatic environments, as well as a decline in shellfish habitats and aquatic plant life [24]. The effect of using microalgae in wastewater treatments has been studied by many researchers to make N removal and the process of eliminating contaminants and pollutants more sustainable [24]. Moreover, when exploiting their metabolic flexibility to grow photo-, hetero- or mixo-trophically, microalgae represent an interesting system for treating a wide range of wastewaters [25].
Water is a very important resource for human development. It is been estimated that a shortage of 40% of water resources could occur by 2030 [26]. Therefore, the sustainable treatment of wastewater and water reutilization represent an important challenge globally. Ammonium is the one of the most common pollutants in wastewaters. Studies on ammonium-containing wastewater are not limited to its removal, but are also centered on nutrient recycling. The high resistance of some microalgae to high ammonium concentrations makes it possible to use these organisms for bioremediation and wastewater treatment. Furthermore, wastewaters from industrial processes can be characterized not only by high N concentration, but also by extreme pH and temperature; in these kinds of effluents, extremophilic microalgae, which are able to grow well in intolerably hostile or even lethal habitats, can be employed. The utilization of microalgae to remove the ammonium content in wastewater, as well as their high-value biomass production, are becoming attractive solutions.
In our review, we explore several issues related to the use of ammonium from microalgae and their sustainable utilization in wastewater treatments (WWT). In particular, the aim of this study is to review recent advancements in microalgal application for ammonium removal from wastewaters. We critically review ammonium recovery in wastewater, especially its biochemical and biological significance in microalgal cultivation. This review seeks to provide new knowledge on the role of microalgae in ammonium removal and the utilization of this nutrient in algal metabolism.
2. Nitrogen Sources for Microalgae and Ammonium Utilization
Nitrogen is an essential component in many molecules of all living matter. In the environment, N actively cycles among water, atmosphere, and soil in different concentrations and forms, such as dinitrogen gas (N2), ammonium (NH4+), nitrate (NO3−), nitrite (NO2−), and organic nitrogen (e.g., urea, amino acids, and peptides) [27].
Dinitrogen gas is the most abundant form of N on Earth (~78% in the atmosphere), but it can only be used by a limited number of bacteria and archaea; therefore, it represents an inaccessible source for microalgae and plants in general. The exploitable inorganic N sources in the environment are NH4+, NO3−, and NO2−, which show a diverse concentration based on different habitats [28]. In the environment, the most abundant N source for plant cells is nitrate; however, microalgae are often able to utilize different nitrogen sources based not only on its availability in the environment, but also based on what species they are. In aerated soil, nitrate concentrations can be variable (from 10 to 100 mM), but the ammonium concentration is generally quite low (<1 mg Kg−1), because it is rapidly converted by bacteria to nitrate [29]. In some acidic and/or anaerobic environments, the ammonium can represent the dominant inorganic form of N [30]. In ocean waters, the estimated concentration of nitrate is between 7–31 µM; of ammonium, 0.001–0.3 µM; and of nitrite, about 0.006–0.1 µM [28].
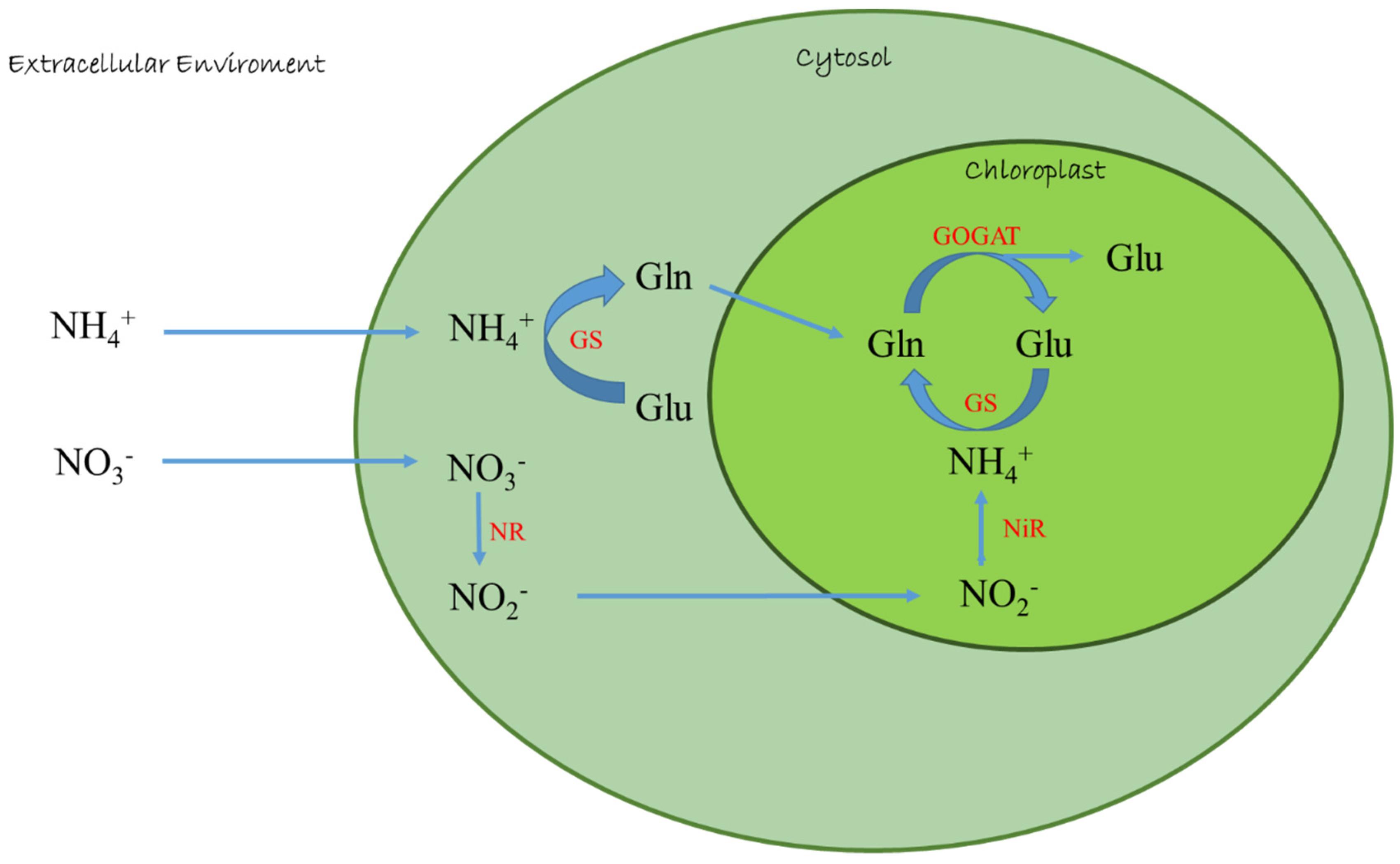
In plant cell, the assimilation of inorganic nitrogen into amino acids and proteins requires energy and organic skeletons; microalgae such as plants prefer NH4+ because the metabolic cost to reduce ammonium to organic matter is lower than the cost for other nitrogen forms reduction [27]. By using ammonium, the microalgae avoid energy consumption due to nitrate/nitrite reduction, but also due to nitrate reductase (NR) and nitrite reductase (NiR) enzymes production [31,32]. In fact, in contrast to nitrate and ammonium, subsequent to its transport into the cell, is directly incorporated into amino acids via GS-GOGAT cycle by glutamine synthetase (GS)-glutamate synthase (GOGAT) enzymes (Figure 1), and in some green algae, also under certain conditions, via the NADP-glutamate dehydrogenase (GDH) pathway [32,33].
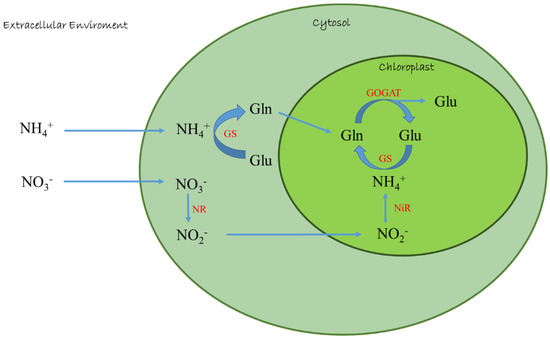
Figure 1.
Ammonium and nitrate assimilation in microalgae. NR: Nitrate reductase; NiR: Nitrite reductase; GS: Glutamine synthetase; GOGAT: Glutamate synthase; Glu: Glutamic acid; Gln: Glutamine.
However, some microalgae, such as Botryococcus braunii and Dunaliella tertiolecta, prefer nitrate as an inorganic N source, showing a reduced growth in the presence of ammonium [22,34].
In addition, ammonium is crucial not only as nutrient, but also as an environmental signal for cellular response [35,36]. In fact, in Chlorella vulgaris, the expression level of GS was up-regulated 6.4-fold under 10 mg L−1 of ammonium compared to that in 4 mg L−1, confirming the key role of GS in ammonium assimilation [36].
According to Kronzucker et al. [37], there is a short-term inhibition effect of NH4+ on nitrate-uptake due to the direct consequences of ammonium on the plasma membrane; this prompt effect is apparent within minutes of NH4+ exposure. Inhibition of nitrate uptake is a highly variable process, depending on the species, their physiological status, and on environmental conditions [38]. In fact, for some species of phytoplankton, ammonium concentrations between 100–300 nmol L−1 can be enough to totally suppress nitrate uptake [39], but concentrations up to 1–2 µmol L−1 are sometimes necessary for the same effect in other aquatic microorganisms [38]. It was reported that the inhibition of nitrate transport is probably due to the accumulation of a product of ammonium assimilation such as glutamine [40]. In cyanobacteria, ammonium availability results in an immediate inhibition of nitrate uptake; in particular, the bispecific nitrate/nitrite transporter NRT (ABC-type transporter NrtABCD) is repressed, as well as the NR and NiR proteins [41]. On the contrary, in Chlamydomonas acidophila grown in a medium containing NH4+ as unique source of inorganic N, the NR activity was low, but not absent; therefore, NH4+ replete cells after N-starvation show an NR activity higher than that of NO3− grown cells [32].
Although the uptake of ammonium is usually fast and preferred to other forms of nitrogen, at important concentrations and prolonged exposure, it may be toxic for algal cells and cause growth inhibition [26,42]. In the follow section, the toxic effect and the tolerance to high ammonium concentration will be discussed.
3. The Equilibrium Ammonium/Ammonia and Effect on Microalgae
In the environment, ammonia represents a volatile molecule with very high solubility (~35% w/w at 25 °C), and for this reason, it is easily found as a liquid solution. In water, the sum of NH3 and NH4+ represents the total ammonia nitrogen (TAN) that constitutes a buffer system ammonia/ammonium, as explained by the following formula:
NH4+ + OH− ↔ NH3 + H2O
This equilibrium between ammonium (NH4+; ionized form) and ammonia (NH3; unionized form) depends on some parameters; in fact, the two chemical forms are readily interchangeable depending upon the pH, temperature, and salinity of water [20,43].
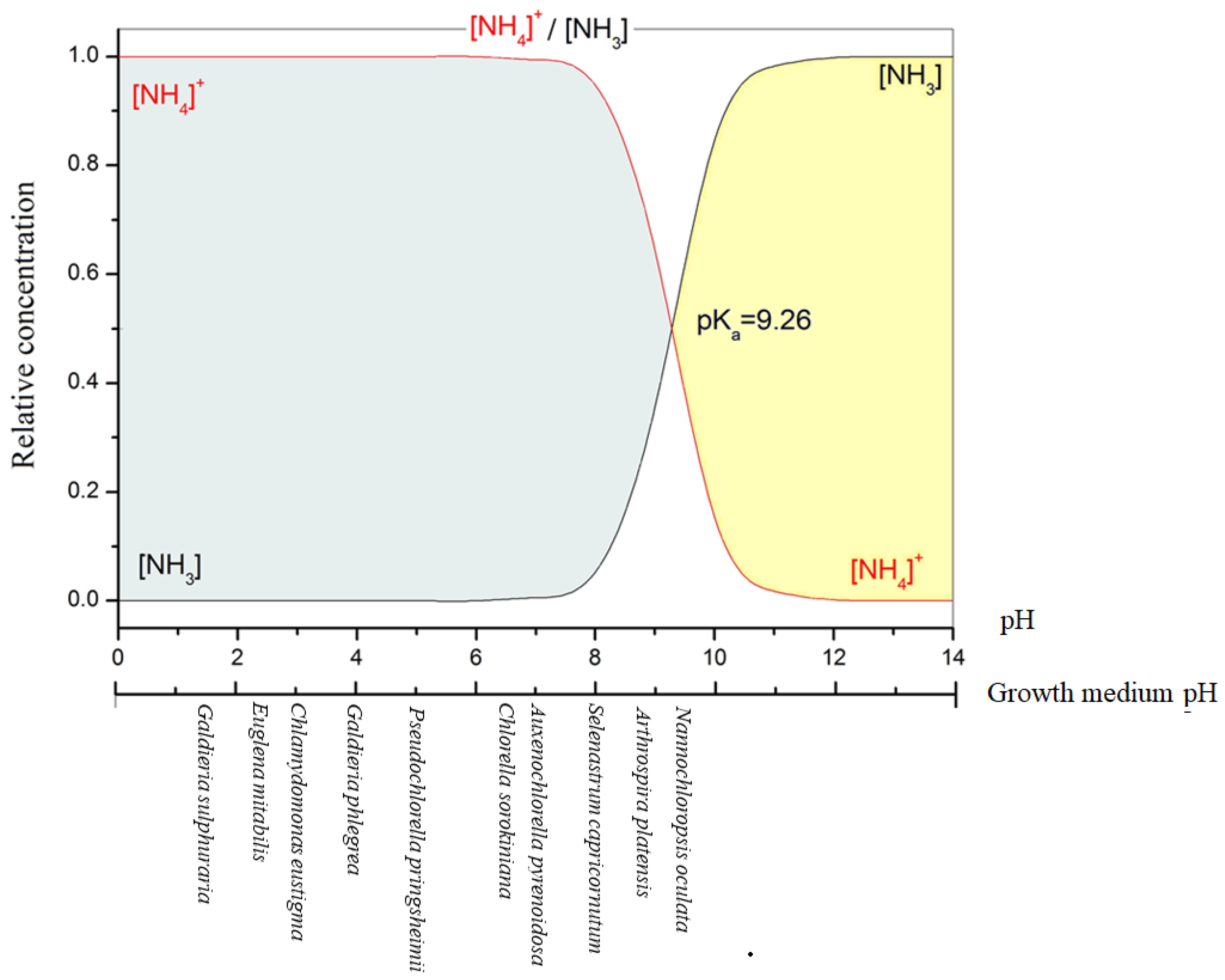
Being the ion dissociation constant (pKa) of the NH4+/NH3 buffer system 9.26 at 25 °C, when the pH of the medium is less than 9.26, hydrogen ions are incorporated into ammonia to produce ammonium ions that become the dominant species in the medium (Figure 2) [44]. Therefore, as pH rises, the ammonia concentration increases noticeably.
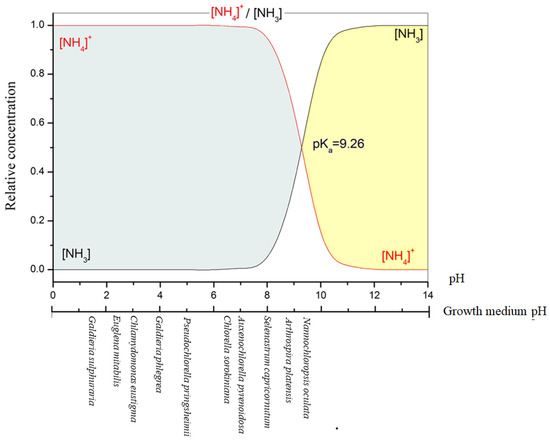
Figure 2.
Influence of the pH on NH4+/NH3 dissociation equilibrium in water at 25 °C, and the optimum pH growth of some microalgae.
In natural waters, ammonium is present in much greater concentrations than ammonia due to the prevalence of circum-neutral pH. In fact, it was estimated that in seawater (pH 8.00, 20 °C), ~90% of TAN is present as ammonium ions [45]. According to Erikson [46], the ratio NH4+/NH3 increases 10-fold for each rise of pH unity and 2-fold for each 10 °C rise of temperature between 0–30 °C.
The salinity also affects the NH4+/NH3 equilibrium, but only with minor influence. In fact, a raise in the ionic strength of the medium cause a very small decrease in the ammonia content. An enhancement of salinity from 20 to 34% leads to a little diminution from 3.41 to 2.98% of ammonia [45,47].
Usually, cultivation systems of microalgae are enhanced with gaseous inorganic carbon source (CO2) to improve the photosynthetic activity and the biomass production. The pH of the medium and then the NH4+/NH3/availability can also depend on the CO2 assimilation rate of the algae [44]. If the CO2 dissolution rate is superior to that of assimilation, the continuous CO2 insufflation leads to an HCO3− and H+ accumulation, resulting in the acidification of the culture medium. On the contrary, in the presence of an elevated microalgal assimilation rate, the CO2 assimilation is not enough to satisfy the cells’ demand of inorganic carbon; in this case, the carbonate would be assimilated as a C-source by the cell, which makes the K+/Na+ accumulation and the medium alkalization [44].
In an alkaline environment, the ammonia represents the main form; it can diffuse rapidly through membranes different to the ammonium that at low concentrations is taken up by AMT high affinity transporters [28]. Therefore, the ammonia represents the most toxic N form, having a direct impact on the photosynthetic apparatus of microalgae [42].
For plant cells, the NH3 results are dangerous because it is uncharged and lipid soluble, and thus passes through biological membranes more easily than the ionized form NH4+ [48]. The toxicity of ammonia in autotrophic microalgae at first occurs through disrupting the thylakoid transmembrane proton gradient [42,49,50]. Ammonia, diffusing through cell membranes, affects not only the ΔpH component of the thylakoid proton gradient, but also brings oxidative stress. When the NH3 increases, it crosses membranes, leading to enhanced flux through the chloroplast membrane into the thylakoid lumen [49]. Once in the thylakoid lumen, NH3 in the acidic environment is converted to NH4+, thereby decreasing the transmembrane proton gradient needed to power ATP to ADP conversion [49]. Moreover, according to Markou et al. [50], NH4+ could affect the Oxygen Evolving Complex (OEC) by shifting a water ligand to the outer Mn cluster of the OEC.
Wang et al. [44] proposed a model of ammonium nitrogen competition between N assimilation and PSII damage. According to this model, when the adsorbed ammonium was transported into the chloroplast, it could be taken as an inorganic N-source and assimilated by the GS-GOGAT cycle and/or as a dangerous molecule, which primarily damages the OEC and afterward blocks electron transport from QA− (primary quinone) to QB (secondary quinone). Therefore, the negative effects on PSII are dependent on the assimilation rate, which is further up to the GS-GOGAT cycle activity [44]. Ammonia compromises the enzymatic activity of some proteins and the lipid peroxidation in membranes, and induces a general cell-level disorder [51].
In ammonium tolerance, the GS/GOGAT activity plays an important role. In Chlorella strains with high performance of the GS/GOGAT cycle, the NH4+ is rapidly converted into N-organic molecules, avoiding the ammonium accumulation and toxic effect for the cells [44].
As stated before, the microalgae tolerance to NH4+/NH3 varies from species to species [44,49]. In fact, while Neochloris oleoabundans and Dunaliella tertiolecta showed an inhibiting growth effect toward ammonia already at 2.3 and 3.3 mg L−1, respectively, Chlorella sorokiniana and Nannochloropsis oculata were largely unaffected by ammonia concentrations (0.2–16.7 mg L−1) [49]. According to Zheng et al. [52], the threshold of NH4+ toxicity in Chlorella vulgaris is around 110 mg L−1, and Collos and Harrison [45] found comparable results. At NH4+ 110 mg L−1 Chlorella cells maintain high Fv/Fm values and a good photosynthetic efficiency [52]. A higher concentration of ammonium (220 mg L−1) corresponds in C. vulgaris to a low cell viability of 61% [52].
Katayama et al. [13] demonstrated the ammonium tolerance might be acquired as a result of the acclimatization; in fact, two strains of Bacillariophyceae (Thalassiosira weissflogii TRG10-p and TRG10-p105) acclimated up to 180 mg L−1 NH4+, which significantly surpassed the tolerance levels of 65 mg L−1 previously reported by Collos and Harrison [45]. According to Chuka-Ogwude [53], under high levels of ammonia, Fv/Fm values decreased with the rising NH3-N concentration due to the disruption of the photosynthetic apparatus. In acclimated microalgae, cells restore their homeostasis avoiding the toxic effects of the stressor on photosystems.
The establishment of the inhibition of photosynthetic activities occurring in microalgae is of particular importance in algae-based wastewater treatment processes. The resistance or acclimation of some microalgae to high concentrations of ammonium makes it possible to use these organisms for bioremediation and for treatment of wastewater usually rich in ammonium/ammonia content.
4. Wastewater and Ammonium Content
Water is the most precious resource of our planet, and according to Adam et al. [26], it could face a global shortage of 40% by 2030; for these reasons, the WWT represents a universal issue for a sustainable solution for water reutilization. Following the world’s growth, the spread of wastewater production has become an important environmental problem.
Wastewaters must be treated for environmental protection before being discharged into rivers, lakes, or seas [54]. The variability of the wastewaters (e.g., scales, contaminants, pH, temperature) imposes different managements [25], but in general, wastewater may be dealt with by 1. primary treatment: It removes sedimentable solid fractions; 2. secondary treatment: It provides physical/chemical/biological processes, leading to the consumption of organic matter and oxidation of the major nutrients; 3. tertiary treatment: It allows disinfection and removes nitrogen, phosphorous, and traces of organic molecules [23].
Ammonia nitrogen represents the most common N-containing pollutants usually found in the tertiary stage of conventional wastewater treatment. The removal of macronutrients as nitrogen represents one of the principal criteria for tertiary treatment [23]. A complete and efficient tertiary process, aimed at removing TAN, but also phosphate in wastewater, is usually more expansive than primary treatment [55]. The TAN concentrations vary according to the nature of the wastewater, as shown in the Table 1. In industrial-based wastewater, the ammonium concentrations may be in the range of 5–1000 mg L−1 [26]. Typically, activities such as food processing, rubber processing, textile and leather manufacturing, fertilizer plant, agricultural and zootechnical industries, etc., release high levels of ammonium concentration [56]. Among the high-strength ammonium wastewaters, there is the manure-free piggery wastewater, with a concentration of NH4+ of ~220–2945 mg L−1, and wastewaters from coal gasification with 130–280 mg L−1 [52,57,58]. Concentrations of NH4+/NH3− up to 100 mg L−1 are generally derived from anaerobic digestion, when ammonium is produced by degradation of the N-matter in the feedstock, primarily in the form of proteins [59,60]. On the other hand, ammonium concentration in the municipal wastewater ranges between 10–200 mg L−1 [26].

Table 1.
NH3-NH4+ concentration (mg L−1) in different wastewater sources.
Ammonium pollution affects the water quality of water bodies causing numerous environmental problems such as oxygen depletion, pH shift, cyanotoxin production, and eutrophication of downstream waters: When N level is higher than 1.9 mg L−1, the water body is considered eutrophic [76,77]. In the last past decades, elevated N-nutrient contents in the water layer have seriously compromised the biodiversity of freshwater ecosystems worldwide [78]. In recent years, many sustainable technologies and methods are studied to remove the TAN from water stream, leading its content under the recommended threshold level from the World Health Organization (WHO) [56].
The TAN abatement in wastewater treatment is usually divided into different approaches: Physical (e.g., membrane separation), chemical (e.g., coagulation, solvent extraction, ion exchange,), and/or biological [23,56,79]. The high effective cost and energy consumption of technologies for one-step tertiary treatment of WWT represent a problem for industries and municipalities [80]. A valid alternative to conventional treatments may be provided by microalgae, coupling the nutrient removal from wastewater with the production of valuable biomass.
5. Microalgae in Wastewater Treatment for Ammonium Removal
In the last decade, microalgae have become important organisms for biological purification of wastewater due their ability to utilize and/or accumulate nutrients, heavy metals, and organic and inorganic substances in their cells/bodies. The use of microalgae for WWT requires a minimum of mechanical equipment and reasonably little energy consumption for their operations [81,82]. In wastewaters, N concentration must be reduced to acceptable limits (generally ˂10–15 mg L−1 depending on discharge point, population, and region regulation as described by regulation 91/271/CEE) before being released into the water body [83]. Many studies demonstrated that microalgae have a great potential for N removal and reported successful cultivations [13,23,24,25,52,80]. Green microalgae (Chlorophyceae) represent the more exploited algae in WWT (Table 2).

Table 2.
An overview of recent WWT research on microalgae.
It was calculated that for 1-ton microalgal production, about 40–100 Kg of inorganic N-compound and 11–13 ML/Ha/year of water are required. It was also estimated that about 2500 m3 of wastewater to obtain 1-ton of microalgal biomass could be treated [80]. Therefore, at the same time, the utilization of wastewater for algal cultivation could allow the removal nutrients from effluents and reduce the use of precious freshwater. According to Yang and colleagues [84], using wastewater for microalgal cultivation would reduce ~90% of the water demand and eliminate the requirement for nutrients. Wastewater could afford most essential resources for large-scale of microalgal cultivation by providing inorganic nutrients and organic matter for mixo- or heterotrophic cultures. In autotrophic microalgae cultivation, algae can give a further possibility of integrating the nutrient removal with carbon capture: CO2 can be incorporated into biomolecules as proteins, carbohydrates, and lipids by photosynthetic reactions [55]. Therefore, phototrophic cultivations could represent a single-step solution to reduce the CO2 emission as well as the eutrophication by nitrogen [76,85].
In a culture medium, an ammonium concentration higher than 100 mg L−1 inhibits the photosynthesis in some microalgal species [42,45,66]. Recently, several studies have been conducted to alleviate TAN toxicity and to optimize the cell ammonium assimilation in microalgal cultures and wastewater treatment [20,42,45]. It was reported that a high concentration of TAN could be mitigated by increasing the initial culture cells concentration, modulating light intensity, and/or monitoring the pH of their medium [20,42,50,86]. In addition, microalgal growth and biomass yield are less affected by high ammonium concentration using a mixotrophic cultivation; in fact, mixotrophic cells can provide more energy for fast ammonium assimilation, reducing ammonium inhibition and increasing the microalgal biomass [42].
According to recent studies (Table 2), microalgal systems can efficiently treat many kinds of ammonium-containing-wastewaters: Domestic and urban wastewaters, livestock wastes, agro-industrial wastewater, piggery effluent, effluent from food processing factories, and so on.
To optimize the nutrient removal, microalgae can be employed in WWT as monoculture, consortia, or combined systems. Monoculture or consortia systems refer to systems using only a single or a consortium of microalgae for nutrient removal without the support of other organisms [82]. Otherwise, combined systems use both microalgae and bacteria for an efficient removal of nutrients (such as ammonium) and organic matter [82]. An interesting symbiosis for ammonium abatement is represented by nitrifying bacteria (e.g., Nitromonas and Nitrobacter) and microalgae. Bacteria oxidizes ammonium into nitrite and then to nitrate, and from the other side, microalgae by photosynthesis supply O2 for oxidation and utilize CO2, deriving from bacterial respiration, for the Calvin–Benson cycle [82,87].
Definitively, microalgae exploited in WWT represent an extensive area of research and development with enormous potential. Although many studies were conducted regarding the possibility of using microalgae as nutrient removers from wastewater, today only a few studies regard algal cultivation under continuous wastewater administration [88]. Wastewater treatment is a global issue on which much research is still ongoing. In fact, freshwater has become a limited resource in many areas of the world. Continuous cultivation of microalgae with wastewater could not only provide a continuous supply of microalgae biomass, but it would also represent a great sustainable process for recycling wastewater.
6. Extremophilic Microalgae in WWT
Extremophile microalgae are organisms that are able to grow well in intolerably hostile or even lethal habitats, and which could represent a good solution for treatment of “extreme wastewaters”. They can grow well under extreme pH, temperatures, salinity, light intensities, nutrients available, and heavy metal concentrations. However, pH and temperature are the major constraints affecting NH4+/NH3 equilibrium in wastewater, and for this reason, only these two will be discussed below.
In specific cases, wastewaters from industrial processes are characterized by high TAN concentration and extreme pH (4.00 > pH > 8.00) and/or temperature (10 °C > C > 40 °C) that are not compatible with the metabolism of most microalgae species [25]. For example, water draining from the sites of mines is frequently rich in sulphate (100 to > 5000 mg dm−3), and it is acid with pH < 4 [104]. These kinds of wastewaters are highly polluting and toxic to most life forms; if untreated, they may devastate the streams and rivers into which they flow [104]. Acid wastewaters are usually derived from ammunition industries/labs, pharmaceutical industries, mining sites, steel industry, electroplating and phosphorous industries and, in many cases, their drainage is treated so as to be neutralized before being released into the environment [105,106]. The low pH wastewater causes numerous problems in the effluent treatment as well as in the water body in which it is discharged [105]. Furthermore, a highly acidic medium breaks down organic matter and eradicates microbial organisms able to treat the water naturally [105].
In environments with low pH, the number of algae able to survive are limited compared to a neutrophilic environment. Microalgae described to be metabolically active in highly acidic environments include some Chlorophyta, such as Chlamydomonas acidophila and Dunaliella acidophila, Chrysophyta, such as Ochromonas sp., and Euglenophyta, such as Euglena mutabilis [107]. Poliextremophiles Rhodophyta (Cyanidium caldarium, Galdieria sulphuraria, Galdieria phlegrea, and Galdieria maxima), frequently encountered in acidic waters and in geothermal areas of the world [19,107,108,109], can be employed in the treatment of these “extreme wastewaters”.
Red microalgae of the genus Galdieria (Cyanidiaceae) represent a very interesting organism, growing in cryptoendolithic habitats with pH ranging 0.50–4.00 [17,107,108,109]. All Galdieria species are able to grow in the dark (heterotrophically) or in the light (mixotrophically) by using numerous carbon sources as organic substrates, with about 50 different carbon sources such as sugars, sugar alcohols, tricarboxylic-acid-cycle intermediates, and amino acids [17,108,110]. Galdieria sp., being acidophilic organisms, colonize habitats with very low pH in which the more available nitrogen form is present as ammonium; for this reason, these organisms are well adapted to utilize NH4+ as a principal source of inorganic nitrogen and to tolerate prolonged exposure to this nutrient. According to Henkanatte-Gederaet et al. [97], Galdieria sulphuraria is able to grow well in filtered primary-settled urban wastewater (pH 2.5), considerably reducing organic carbon (46–72%) and ammoniacal nitrogen (63–89%), and it is able to grow on primary effluents, showing a high nutrient removal rate [98].
In WWT, another important parameter for NH4+/NH3 equilibrium is the temperature. The wastewater temperature can depend on the production process of derivation, but also on the climate of the region in which it is produced. In fact, according to Bugajski et al. [111], a significant correlation between the temperatures of wastewater with the air temperatures exists. Therefore, in the coldest and hottest regions, the wastewater is strongly influenced by seasonal and night/day temperatures.
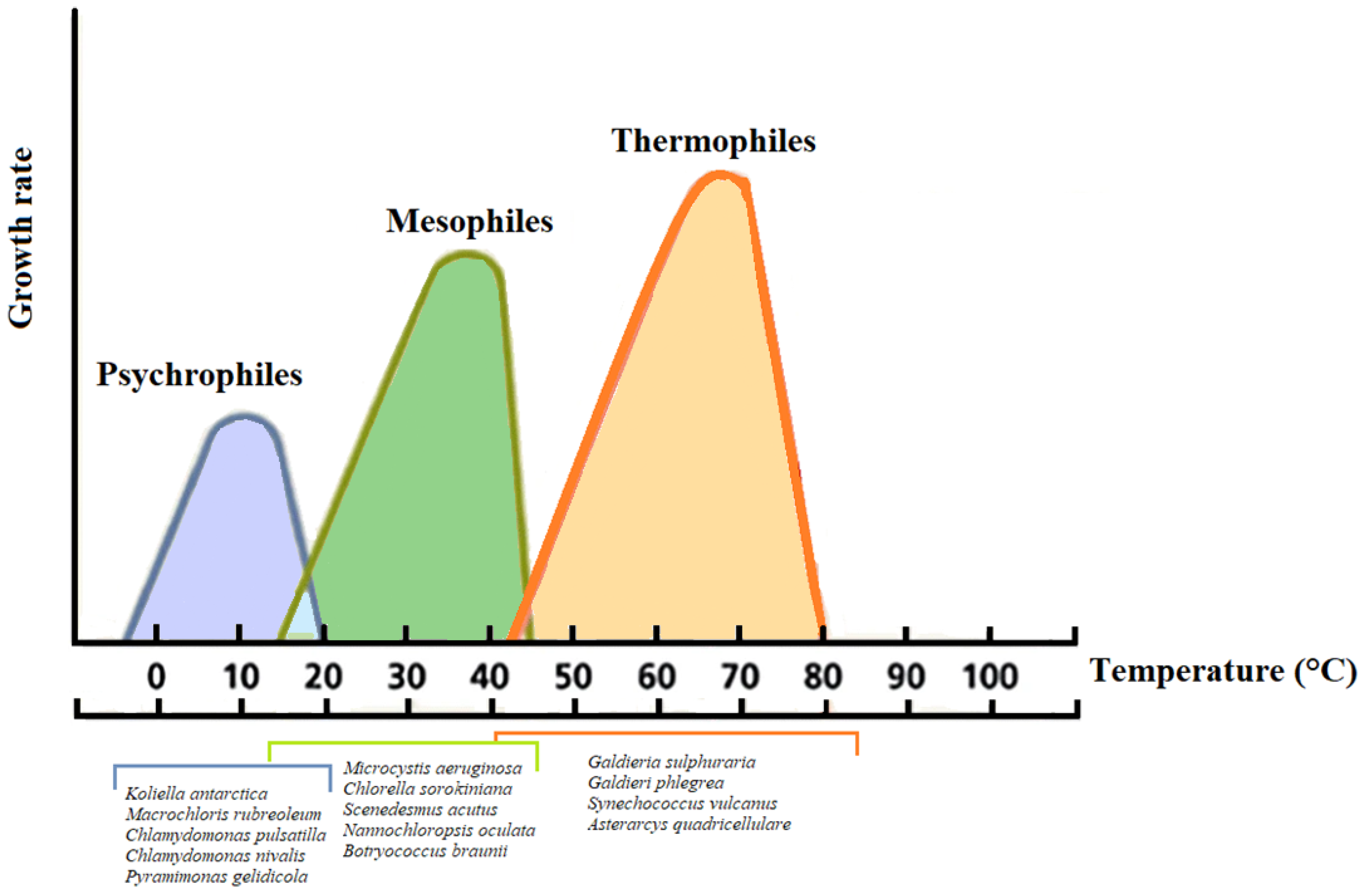
The increase in the temperature of the wastewater causes a change in the solubility of oxygen, an acceleration of the oxygen adsorption process, the rate of bacteria activity, and of some chemical and biological processes [112,113]. In particular, the free ammonia proportion at 25 °C relative to the TAN is about two-fold compared with 15 °C [112,114]. There are three types of microalgae concerning the supporting temperature (Figure 3): 1. Mesophiles: Living at temperatures ranging between 15–45 °C (T °C optimum ~32.5 °C); 2. thermophiles: Growing at temperatures ranging between 40 to 75 °C; 3. psychrophiles: Growing in temperatures ranging between 0 and 20 °C. Thermophiles and psychrophiles represent the extremophile organisms, which are not only able to tolerate prohibitive conditions, but require them for their metabolism [113].
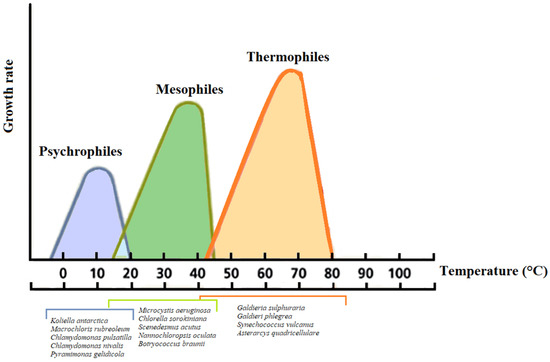
Figure 3.
Relation of temperature and growth rates for representative psychrophilic, mesophilic, and thermophilic microalgae.
In a wastewater pond in India, where the temperature ranges between 37–43 °C, two green algae, Asterarcys quadricellulare and Chlorella sorokiniana, were isolated [113]. Most of the reported strains of Chlorella grow in an optimum range between 25–32 °C, however, some strains, after acclimation, become thermotolerant and can tolerate temperatures of 40 °C or higher [89,113].
Another challenge is the treatment of low temperature wastewaters. In fact, a large variety of wastewaters, including domestic sewage and industrial wastewater, such as those from bottling, malting, brewery, and soft drink manufacturing plants, are characterized by low temperatures, and their heating is required, thus raising the costs of this waste treatment [115,116]. Systems based on psychrophilic microalgae able to operate at low temperatures (10–20 °C) offer the possibility to reduce the energy costs.
Koliella antarctica is a genus of psychrophilic unicellular green alga belonging to the class of Trebouxiophyceae [117]. Koliella cells can be grown in a laboratory at a temperature as low as 2 °C, but can adapt to different physical environmental parameters such as temperature (from −2 to 20 °C) and light (from 8 to 60 µmol photons m−2 s−1); they represent an interesting potential biological system for treatment of low temperature wastewater, such as water from fresh fruit processing industries [25,117,118,119].
The extremophiles have shown to be promising for WWT, and for bioremediation. Many of industrial wastes have harsh conditions, which make extremophiles and polyextremophiles a good choice for their treatment before releasing them into the environment [120].
7. High-Value Molecules from Microalgae Exploited in WWT
The integration of microalgae with WWT offers promising opportunities to reduce the treatment costs, to recycle the nutrients, and to obtain sustainable useful bioproducts (proteins, pigments, lipids, carbohydrates, biofuel, etc.) [25]. The employment of appropriate microalgal species in WWT allows for the hyperaccumulation of valuable bioproducts without compromising the cells’ growth and their biomass increase.
The microalgae Scenedesmus strain SDEC8, grown in anaerobically digested effluent kitchen waste, can accumulate more biomass than Scenedesmus SDEC13 grown in the same culture medium [121]. This result indicates the importance of a proper choice of the algal species and of the strain for WWT aiming to biomass or biomolecules production. In fact, the ability to grow in the various wastewaters varies from species to species, and it is surely strain dependent.
Prospectively, to reduce the economic costs of biofuel from algae, wastewater has been proposed as an alternative nutrient source for microalgae cultivation [25,103]. According to Rinna et al. [103], Botryococcus braunii (strain LB572), grown in domestic wastewater effluent, shows not only an efficient nitrogen consumption, but also an effective intracellular lipid production and accumulation, in particular regarding saturated fatty acids. Growing algae in a low-cost medium is necessary to diminish the cost of microalgal cultivation and to make the biofuel production a more economic and environment-friendly process [122]. In Chlorella vulgaris, the biomass and lipid contents are appreciably higher for cells cultivated in urban wastewater than in the basal medium since the lipid productivity that results is about 1.5 times higher in cells cultivated in wastewater [122]. In contrast to biomass production and productivity, the highest lipid accumulation was reached in microalgae such as Chlorella ellipsoidea and Scenedesmus sp., cultivated in domestic secondary effluent, characterized by low N content [8,123,124]. In fact, in some species of microalgae, lipid content can reach up to 80%, usually under N-deficiency conditions [10,11]. For this reason, in wastewaters, lipid productivity can be enhanced by two-stage cultivation, where lipid content increases during the second stage characterized by N starvation [125].
The algal biomass also constitutes a valuable source of pigments, mainly chlorophylls and carotenoids, which have been correlated with several health benefits [125]. From Phormidium autumnale, grown under heterotrophic cultivation in slaughterhouse waste, carotenoids at industrial scale to 108-ton year are produced [125,126]. In Thermosynechococcus sp., grown in aerobic treated swine wastewater, satisfactory phycobiliprotein and carotenoid contents were obtained [102].
The cyanobacterium Arthrospira platensis and the Rhodophytes Porphyridium sp. and Galdieria sp. are the principal producers of phycobiliproteins [19,100,108]. Phycobiliproteins, in particular phycocyanin, have high commercial value as antioxidant and anti-inflammatory molecules and represent safer ingredients for food, nutraceutical, and pharmaceutical purposes [3,108,109]. The production of these pigments from microalgae cultivated in wastewater has not been significantly explored. According to Arashiro and colleagues [100], the cultivations of Nostoc sp., Arthrospira platensis, and Porphyridium purpureum in food-industry wastewater showed efficient treatment of the wastewater, reaching high removal of nutrients and interesting phycobiliproteins accumulation in algal biomass.
Several microalgal species, such as Chlorella ssp., Arthrospira platensis, Scenedesmus sp., Botryococcus braunii, and many others, show high nutrient removal capacity in wastewater and return valuable molecules [100,103,123,124]. The application of these strategies for microalgae utilization in WWT needs further study, but opens great possibilities in the ammonium mitigation from effluents and in sustainable biomolecules production.
8. Conclusions
An excess of ammonium in water bodies can lead to an eutrophication phenomenon in natural environments. For this, WWT represent an important global issue. The modern WWT technologies in use are considered efficient in processing, but they necessitate a lot of energy and do not contemplate the recycling of useful nutrients such as ammonium. Microalgae are photo-, mixo-, and heterotrophic organisms that can be used in WWT for removal of the inorganic nitrogen and of other pollutants. To improve the conventional WWT, the utilization of microalgae for nutrient removal could represent a sustainable solution. In fact, being algae producers of biologically active compounds, their biomass obtained by WWT can be exploited as biomolecule sources.
Further research needs to focus on continuous microalgae cultivation in wastewater that could not only provide a continuous supply of biomass, but would also represent a great sustainable process for recycling wastewater.
Author Contributions
The authors contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- De Morais, M.G.; de Fontoura Prates, D.; Moreira, J.B.; Duarte, J.H.; Costa, J.A.V. Phycocyanin from microalgae: Properties, extraction and purification, with some recent applications. Ind. Biotechnol. 2018, 14, 30–37. [Google Scholar] [CrossRef]
- Bottone, C.; Camerlingo, R.; Miceli, R.; Salbitani, G.; Sessa, G.; Pirozzi, G.; Carfagna, S. Antioxidant and anti-proliferative properties of extracts from heterotrophic cultures of Galdieria sulphuraria. Nat. Prod. Res. 2019, 15, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Vidanarachchi, J.K.; Kurukulasuriya, M.S.; Malshani Samaraweera, A.; Silva, K.F.S.T. Applications of Marine Nutraceuticals in Dairy Products. Adv. Food Nutr. Res. 2012, 65, 457–478. [Google Scholar] [PubMed]
- De Jesus Raposo, M.F.; De Morais, R.M.S.C.; De Morais, A.M.M.B. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Camacho, F.; Macedo, A.; Malcata, F. Potential Industrial Applications and Commercialization of Microalgae in the Functional Food and Feed Industries: A Short Review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef]
- Napolitano, G.; Fasciolo, G.; Salbitani, G.; Venditti, P. Chlorella sorokiniana Dietary Supplementation Increases Antioxidant Capacities and Reduces ROS Release in Mitochondria of Hyperthyroid Rat Liver. Antioxidants 2020, 9, 883. [Google Scholar] [CrossRef]
- Wu, H.Y.; Hu, H.Y.; Yu, Y.; Zhang, T.Y.; Zhu, S.F.; Zhuang, L.L.; Zhang, X.; Lu, Y. Microalgal species for suitable biomass/lipid production using wastewater as resources: A review. Renew. Sustain. Energy Rev. 2014, 33, 675–688. [Google Scholar] [CrossRef]
- Sun, X.M.; Ren, L.J.; Zhao, Q.Y.; Ji, X.J.; Huang, H. Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef]
- Metting, F.B. Biodiversity and application of microalgae. J. Microbiol. Bioech. 1996, 17, 477–489. [Google Scholar] [CrossRef]
- Salbitani, G.; Barone, C.M.A.; Carfagna, S. Effect of bicarbonate on growth of the oleaginous microalga Botryococcus braunii. Int. J. Plant. Biol. 2019, 10, 8273. [Google Scholar] [CrossRef]
- Serive, B.; Kaas, R.; Bérard, J.B.; Pasquet, V.; Picot, L.; Cadoret, J.P. Selection and optimisation of a method for efficient metabolites extraction from microalgae. Bioresour. Technol. 2012, 124, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Nagao, N.; Kasan, N.A.; Khatoon, H.; Rahman, N.A.; Takahashi, K.; Furuya, K.; Yamada, Y.; Wahid, M.E.A.; Jusoh, M. Bioprospecting of indigenous marine microalgae with ammonium tolerance from aquaculture ponds for microalgae cultivation with ammonium-rich wastewaters. J. Biotechnol. 2020, 323, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, G.; Demurtas, O.; Ferrante, P. Le microalghe come bio-fabbriche per composti ad elevato valore aggiunto. ENEA Mag. EAI Speciale Biotecnologie per lo Sviluppo Sostenibile 2013, I, 61–66. [Google Scholar]
- Salbitani, G.; Bolinesi, F.; Affuso, M.; Carraturo, F.; Mangoni, O.; Carfagna, S. Rapid and positive effect of bicarbonate addition on growth and photosynthetic efficiency of the green microalgae Chlorella sorokiniana (Chlorophyta, Trebouxiophyceae). Appl. Sci. 2020, 10, 4515. [Google Scholar] [CrossRef]
- Kim, G.; Mujtaba, G.; Lee, K. Effects of nitrogen sources on cell growth and biochemical composition of marine chlorophyte Tetraselmis sp. for lipid production. Algae 2016, 31, 257–266. [Google Scholar] [CrossRef]
- Carfagna, S.; Bottone, C.; Cataletto, P.R.; Petriccione, M.; Pinto, G.; Salbitani, G.; Vona, V.; Pollio, A.; Ciniglia, C. Impact of sulfur starvation in autotrophic and heterotrophic cultures of the Extremophilic Microalga Galdieria phlegrea (Cyanidiophyceae). Plant Cell Physiol. 2016, 57, 1890–1898. [Google Scholar] [CrossRef]
- Salbitani, G.; Del Prete, S.; Bolinesi, F.; Mangoni, O.; De Luca, V.; Carginale, V.; Donald, W.A.; Supuran, C.T.; Carfagna, S.; Capasso, C. Use of an immobilized thermostable α-CA (SspCA) for enhancing the metabolic efficiency of the freshwater green microalga Chlorella sorokiniana. J. Enzym. Inhib. Med. Chem. 2020, 35, 913–920. [Google Scholar] [CrossRef]
- Salbitani, G.; Carfagna, S. Different behavior between autotrophic and heterotrophic Galdieria sulphuraria (Rhodophyta) cells to nitrogen starvation and restoration. Impact on pigment and free amino acid contents. Int. J. Plant. Biol. 2020, 11, 8567. [Google Scholar] [CrossRef]
- Markou, G.; Vandamme, D.; Muylaert, K. Microalgal and cyanobacterial cultivation: The supply of nutrients. Water Res. 2014, 65, 186–202. [Google Scholar] [CrossRef]
- Arumugam, M.; Agarwal, A.; Arya, M.C.; Ahmed, Z. Influence of nitrogen sources on biomass productivity of microalgae Scenedesmus bijugatus. Bioresour. Technol. 2013, 131, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Ruangsomboon, S. Effects of different media and nitrogen sources and levels on growth and lipid of green microalga Botryococcus braunii KMITL and its biodiesel properties based on fatty acid composition. Bioresour. Technol. 2015, 191, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Molazadeh, M.; Ahmadzadeh, H.; Pourianfar, H.R.; Lyon, S.; Rampelotto, P.H. The Use of Microalgae for Coupling Wastewater Treatment with CO2 Biofixation. Front. Bioeng. Biotechnol. 2019, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Yuan, Q. Removal of nitrogen from wastewater using microalgae and microalgae–bacteria consortia. Cogent. Environ. Sci. 2016, 2, 1275089. [Google Scholar] [CrossRef]
- Wollmann, F.; Dietze, S.; Ackermann, J.U.; Bley, T.; Walther, T.; Steingroewer, J.; Krujatz, F. Microalgae wastewater treatment: Biological and technological approaches. Eng. Life Sci. 2019, 19, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.R.; Othman, M.H.D.; Samah, R.A.; Puteh, M.H.; Ismail, A.F.; Mustafa Rahman, M.A.; Jaafar, J. Current trends and future prospects of ammonia removal in wastewater: A comprehensive review on adsorptive membrane development. Sep. Purif. Technol. 2019, 213, 114–132. [Google Scholar] [CrossRef]
- Mandal, S.; Shurin, J.B.; Efroymson, R.A.; Mathews, T.J. Functional divergence in nitrogen uptake rates explains diversity–productivity relationship in microalgal communities. Ecosphere 2018, 9, e02228. [Google Scholar] [CrossRef]
- Sanz-Luque, E.; Chamizo-Ampudia, A.; Llamas, A.; Galvan, A.; Fernandez, E. Understanding nitrate assimilation and its regulation in microalgae. Front. Plant. Sci. 2015, 26, 899. [Google Scholar] [CrossRef]
- Ouyang, Y.; Norton, J.M.; Stark, J.M.; Reeve, J.R.; Habteselassie, M.Y. Ammonia-oxidizing bacteria are more responsive than archaea to nitrogen source in an agricultural soil. Soil Biol. Biochem. 2016, 96, 4–15. [Google Scholar] [CrossRef]
- Hachiya, T.; Sakakibara, H. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. J. Exp. Bot. 2017, 68, 2501–2512. [Google Scholar] [CrossRef]
- Pritchard, D.W.; Hurd, C.L.; Beardall, J.; Hepburn, C.D. Restricted use of nitrate and a strong preference for ammonium reflects the nitrogen ecophysiology of a light-limited red alga. J. Phycol. 2015, 51, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Lachman, S.; Mettler-Altmann, T.; Wacker, A. Nitrate or ammonium: Influences of nitrogen source on the physiology of a green alga. Ecol. Evol. 2018, 9, 1070–1082. [Google Scholar] [CrossRef] [PubMed]
- Hellebust, J.A.; Ahmad, I. Regulation of Nitrogen Assimilation in Green Microalgae. Biol. Oceanogr. 1989, 6, 241–255. [Google Scholar]
- Chen, M.; Tang, H.; Ma, H.; Holland, T.C.; Ng, K.Y.; Salley, S.O. Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour. Technol. 2011, 102, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Müller, C.; Matt, P.; Gibon, Y.; Carillo, P.; Morcuende, R.; Scheible, W.R.; Krapp, A. Steps towards an integrated view of nitrogen metabolism. J. Exp. Bot. 2002, 53, 959–970. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, X.; Wu, K.; Fu, X. Nitrogen signaling and use efficiency in plants: What’s new? Curr. Opin. Plant. Biol. 2015, 27, 192–198. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Glass, A.D.; Yaeesh Siddiqi, M. Inhibition of nitrate uptake by ammonium in barley. Analysis Of component fluxes. Plant. Physiol. 1999, 120, 283–292. [Google Scholar] [CrossRef]
- L’Helguen, S.; Maguer, J.F.; Caradec, J. Inhibition kinetics of nitrate uptake by ammonium in size-fractionated oceanic phytoplankton communities: Implications for new production and f-ratio estimates. J. Plankton Res. 2008, 30, 1179–1188. [Google Scholar] [CrossRef]
- Wheeler, P.A.; Kokkinakis, S.A. Ammonium recycling limits nitrate use in the oceanic subarctic. Pacific. Limnol. Oceanogr. 1990, 35, 1267–1278. [Google Scholar] [CrossRef]
- Holzer, H. Regulation of enzymes by enzyme-catalized chemical modification. Advanc. Enzymol. 1969, 32, 297–326. [Google Scholar]
- Watzer, B.; Forchhammer, K. Cyanophycin synthesis optimizes nitrogen utilization in the unicellular cyanobacterium Synechocystis sp. PCC6803. Appl. Environ. Microbiol. 2018, 84, e01298-18. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, W.; Zhai, J.; Wei, H.; Wang, Q. Effect of ammonium nitrogen on microalgal growth, biochemical composition and photosynthetic performance in mixotrophic cultivation. Bioresour Technol. 2019, 273, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kankanamge, N.R.; Chow, C.; Welsh, D.T.; Li, T.; Teasdale, P.R. Removing ammonium from water and wastewater using cost-effective adsorbents: A review. J. Environ. Sci. 2018, 63, 174–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, W.; Chen, H.; Zhan, J.; He, C.; Wang, Q. Ammonium Nitrogen Tolerant Chlorella Strain Screening and Its Damaging Effects on Photosynthesis. Front. Microbiol. 2019, 9, 3250. [Google Scholar] [CrossRef]
- Collos, Y.; Harrison, P.J. Acclimatation and toxicity of high ammonium concentrations to unicellular algae. Mar. Pollut. Bull. 2014, 80, 8–23. [Google Scholar] [CrossRef]
- Erikson, R.J. An evaluation of mathematical models for the effects of pH and temperature on ammonia toxicity to aquatic organisms. Water Res. 1985, 19, 1047–1058. [Google Scholar] [CrossRef]
- Bower, C.E.; Bidwell, J.P. Ionization of Ammonia in Seawater: Effects of Temperature, pH, and Salinity. JFRBC 1978, 35, 1012–1016. [Google Scholar] [CrossRef]
- Körner, S.; Das, S.K.; Veenstra, S.; Vermaat, J.E. The effect of pH variation at the ammonium/ammonia equilibrium in wastewater and its toxicity to Lemna gibba. Aquatic Bot. 2001, 71, 71–78. [Google Scholar] [CrossRef]
- Gutierrez, J.; Kwan, T.A.; Zimmerman, J.B.; Peccia, J. Ammonia inhibition in oleaginous microalgae. Algal Res. 2016, 19, 123–127. [Google Scholar] [CrossRef]
- Markou, G. Effect of light intensity on the degree of ammonia toxicity on PSII activity of Arthrospira platensis. Bioresour. Technol. 2016, 216, 453–461. [Google Scholar] [CrossRef]
- Nimptsch, J.; Pflugmacher, S. Ammonia triggers the promotion of oxidative stress in the aquatic macrophyte Myriophyllum mattogrossense. Chemosphere 2007, 66, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Zengh, H.; Wu, X.; Zou, G.; Zhou, T.; Liu, Y.; Ruan, R. Cultivation of Chlorella vulgaris in manure-free piggery wastewater with high-strength ammonium for nutrients removal and biomass production: Effect of ammonium concentration, carbon/nitrogen ratio and pH. Bioresour Technol. 2019, 273, 203–211. [Google Scholar]
- Chuka-ogwude, D.; Ogbonna, J.; Moheimani, N.R. A review on microalgal culture to treat anaerobic digestate food waste effluent. Algal Res. 2020, 47, 101841. [Google Scholar] [CrossRef]
- Arbib, Z.; Ruiz, J.; Alvarez, P.; Garrido, C.; Barragan, J.; Perales, J.A. Chlorella stigmatophora for urban wastewater nutrient removal and CO2 abatement. Int. J. Phytoremediat. 2012, 14, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Karri, R.R.; Sahu, J.N.; Chimmiri, V. Critical review of abatement of ammonia from wastewater. J. Mol. Liq. 2018, 261, 21–31. [Google Scholar] [CrossRef]
- Ramakrishnan, A.; Surampalli, R.Y. Performance of anaerobic hybrid reactors for the treatment of complex phenolic wastewa-ters with biogas recirculation. Bioresour. Technol. 2013, 129, 26–32. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.; Gong, Z.; Yang, F. Removal of COD, phenols and ammonium from Lurgi coal gasification wastewater using A2O-MBR system. J. Hazard. Mater. 2012, 235, 78–84. [Google Scholar] [CrossRef]
- Jiang, Y.; McAdam, E.; Zhang, Y.; Heaven, S.; Banks, C.; Longhurst, P. Ammonia inhibition and toxicity in anaerobic digestion: A critical review. J. Water Process. Eng. 2019, 32, 100899. [Google Scholar] [CrossRef]
- Kotsyurbenko, O.R.; Chin, K.J.; Glagolev, M.V.; Stubner, S.; Simankova, M.V.; Nozhevnikova, A.N.; Conrad, R. Acetoclastic and hydrogenotrophic methane production and methanogenic populations in an acidic West-Siberian peat bog. Environ. Microbiol. 2004, 6, 1159–1173. [Google Scholar] [CrossRef]
- Ruìz-Marìn, A.; Mendoza-Espinosa, L.G.; Stephenson, T. Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour Technol. 2010, 101, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Lee, S.A.; Ko, S.R.; Oh, H.M.; Ahn, C.Y. Effects of photoperiod on nutrient removal, biomass production, and algal-bacterial population dynamics in lab-scale photobioreactors treating municipal wastewater. Water Res. 2015, 68, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, S.; Cao, S.; Miao, Y.; Jia, F.; Du, R.; Peng, Y. Biological nitrogen removal from sewage via anammox: Recent advances. Bioresour. Technol. 2016, 200, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Jafarinejad, S.; Park, H.; Mayton, H.; Walker, S.L.; Jiang, S.C. Concentrating ammonium in wastewater by forward osmosis using a surface modified nanofiltration membrane. Environ. Sci. Water Res. Technol. 2019, 5, 246–255. [Google Scholar] [CrossRef]
- Cruz, H.; Luckman, P.; Seviour, T.; Verstraete, W.; Laycock, B.; Pikaar, I. Rapid removal of ammonium from domestic wastewater using polymer hydrogels. Sci. Rep. 2018, 8, 2912. [Google Scholar] [CrossRef]
- Posadas, E.; Alcántara, C.; García-Encina, P.A.; Gouveia, L.; Guieysse, B.; Norvill, Z.; Acién, F.G.; Markou, G.; Congestri, R.; Koreiviene, J.; et al. 3—microalgae cultivation in wastewater. In Microalgae-Based Biofuels and Bioproducts; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Lincolnshire, IL, USA, 2017; pp. 67–91. [Google Scholar]
- García, D.; de Godos, I.; Domínguez, C.; Turiel, S.; Bolado, S.; Muñoz, R. A systematic comparison of the potential of microalgae-bacteria and purple phototrophic bacteria consortia for the treatment of piggery wastewater. Bioresour Technol. 2019, 276, 18–27. [Google Scholar] [CrossRef]
- Lee, C.; An, J.; Lee, Y.S.; Choi, K.; Kim, J.Y. Uncertainty-based concentration estimation of chlortetracycline antibiotics in swine farms and risk probability assessment for agricultural application of manure. J. Hazard. Mater. 2021, 402, 123763. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D.; Metcalf Eddy, Inc.; Burton, F. Wastewater Engineering: Treatment and Reuse; McGraw-Hill Education: New York, NY, USA, 2003. [Google Scholar]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Yalei, Z.; Chunmin, Z.; Xuefei, L.; Jinpeng, L. Cultivation of Chlorella pyrenoidosa in soybean processing wastewater. Bioresour Technol. 2011, 102, 9884–9890. [Google Scholar]
- Muylaert, K.; Beuckels, A.; Depraetere, O.; Foubert, I.; Markou, G.; Vandamme, D. Wastewater as a Source of Nutrients for Microalgae Biomass Production. In Biomass and Biofuels from Microalgae; Moheimani, N., McHenry, M., de Boer, K., Bahri, P., Eds.; Biofuel and Biorefinery Technologies; Springer: Cham, Switzerland, 2015; p. 2. [Google Scholar]
- Raper, E.; Fisher, R.; Anderson, D.R.; Stephenson, T.; Soares, A. Nitrogen removal from coke making wastewater through a pre-denitrification activated sludge process. Sci. Total Environ. 2019, 666, 31–38. [Google Scholar] [CrossRef]
- Reges, K.D.; Costa, A.G.; Cunha, V.T.; Portela, J.C.; Batista, R.; Mendonça, V.; Pereira, J.O.; Gurgel, G.C.; Oliveira, J.F.; Freire, F.G.; et al. Growing, Production and Quality of Thornless Cactus Irrigated With Dairies Effluent. J. Agric. Sci. 2019, 11, 175. [Google Scholar] [CrossRef]
- Gil-Pulido, B.; Tarpey, E.; Almeida, E.L.; Finnegan, W.; Zhan, X.; Dobson, A.; O’Leary, N. Evaluation of dairy processing wastewater biotreatment in an IASBR system: Aeration rate impacts on performance and microbial ecology. Biotechnol. Rep. 2018, 19, e00263. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Chen, P.H.; Yu, R.F.; Ho, W.N. Treating ammonium-rich wastewater with sludge from water treatment plant to produce ammonium alum. Sustain. Environ. Res. 2016, 26, 63–69. [Google Scholar] [CrossRef]
- Brown, T.; Simpson, J. Managing phosphorous inputs to urban lakes: I. Determining the trophic state of your lake. Watershed Prot. Tech. 2001, 3, 771–781. [Google Scholar]
- Kastner, F.; Buchwald, R.; Biedermann, R. Occurrence of Aeshna viridis in marsh ditches in relation to habitat conditions (Odonata: Aeshnidae). Int. J. Odonatol. 2018, 21, 205–219. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Liu, Y.; Chang, S.W.; Nguyen, D.D.; Liang, H.; Wang, J. A critical review on ammonium recovery from wastewater for sustainable wastewater management. Bioresour. Technol. 2018, 268, 749–758. [Google Scholar] [CrossRef]
- Khanzada, Z.T.; Övez, S. Growing Fresh Water Microalgae in High Ammonium Landfill Leachate. Am. AJMA 2018, 6, 40–51. [Google Scholar] [CrossRef]
- Mayo, A.W.; Hanai, E.E. Dynamics of nitrogen transformation and removal in a pilot high rate pond. J. Water Resour. Prot. 2014, 6, 433–445. [Google Scholar] [CrossRef]
- Fito, J.; Alemu, K. Microalgae–bacteria consortium treatment technology for municipal wastewater management. Nanotechnol. Environ. Eng. 2019, 4, 4. [Google Scholar] [CrossRef]
- Freed, T. Wastewater industry moving toward enhanced nutrient removal standards. Water World 2007, 23, 1–3. [Google Scholar]
- Yang, J.; Xu, M.; Zhang, X.; Hu, Q.; Sommerfeld, M.; Chen, Y. Life-cycle analysis on biodiesel production from microalgae: Water footprint and nutrients balance. Bioresour. Technol. 2011, 102, 6633. [Google Scholar] [CrossRef]
- Judd, S.; van den Broeke, L.J.P.; Shurair, M.; Kuti, Y.; Znad, H. Algal remediation of CO2 and nutrient discharges: A review. Water Res. 2015, 87, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Depraetere, O.; Muylaert, K. Effect of ammonia on the photosynthetic activity of Arthrospira and Chlorella: A study on chlorophyll fluorescence and electron transport. Algal Res. 2016, 16, 449–457. [Google Scholar] [CrossRef]
- Vargas, G.; Donoso-Bravo, A.; Vergara, C.; Ruiz-Filippi, G. Assessment of microalgae and nitrifiers activity in a consortium in a continuous operation and the effect of oxygen depletion. Electron. J. Biotechnol. 2016, 23, 63–68. [Google Scholar] [CrossRef]
- Praveen, P.; Loh, K. Nutrient removal in an algal membrane photobioreactor: Effects of wastewater composition and light/dark cycle. Appl. Microbiol. Biotechnol. 2019, 103, 3571–3580. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Trejo, A.; Huss, V.A.R.; Hernandez, J.-P.; Bashan, Y. Chlorella sorokiniana UTEX 2805, a heat and intense, sunlight-tolerant microalga with potential for removing ammonium from wastewater. Bioresour Technol. 2008, 99, 4980–4989. [Google Scholar]
- Kobayashi, N.; Noel, E.A.; Barnes, A.; Watson, A.; Rosenberg, J.N.; Erickson, G.; Oyler, G.A. Characterization of three Chlorella sorokiniana strains in anaerobic digested effluent from cattle manure. Bioresour Technol. 2013, 150, 377–386. [Google Scholar] [CrossRef]
- Hernández, D.; Riaño, B.; Coca, M.; García-González, M.C. Treatment of agro-industrial wastewater using microalgae–bacteria consortium combined with anaerobic digestion of the produced biomass. Bioresour Technol. 2013, 135, 598–603. [Google Scholar] [CrossRef]
- Moondra, N.; Jariwala, N.D.; Christian, R.A. Sustainable treatment of domestic wastewater through microalgae. Int. J. Phytoremediat. 2020, 22, 1480–1486. [Google Scholar] [CrossRef]
- Sydney, E.B.; da Silva, T.E.; Tokarski, A.; Novak, A.C.; de Carvalho, J.C.; Woiciecohwski, A.L.; Larroche, C.; Soccol, C.R. Screening of microalgae with potential for biodiesel production and nutrient removal from treated domestic sewage. Appl. Energy 2011, 88, 3291–3294. [Google Scholar] [CrossRef]
- Kang, J.; Wen, Z. Use of microalgae for mitigating ammonia and CO2 emissions from animal production operations-Evaluation of gas removal efficiency and algal biomass composition. Algal Res. 2015, 11, 204–210. [Google Scholar] [CrossRef]
- Uggetti, E.; Sialve, E.; Latrille, E.; Steyer, J.P. Anaerobic digestate as substrate for microalgae culture: The role of ammonium concentration on the microalgae productivity. Bioresour Technol. 2014, 152, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.L.; Bui, M.H. Removal of Nutrients from Fertilizer Plant Wastewater Using Scenedesmus sp.: Formation of Bioflocculation and Enhancement of Removal Efficiency. J. Chem. 2020, 2020, 9. [Google Scholar] [CrossRef]
- Henkanatte-Gedera, S.M.; Selvaratnam, T.; Karbakhshravari, M.; Myint, M.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Removal of dissolved organic carbon and nutrients from urban wastewaters by Galdieria sulphuraria: Laboratory to field scale demonstration. Algal Res. 2017, 24, 450–456. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Pegallapati, A.K.; Montelya, F.; Rodriguez, G.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Evaluation of a thermo-tolerant acidophilic alga, Galdieria sulphuraria, for nutrient removal from urban wastewaters. Bioresour Technol. 2014, 156, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Sloth, J.K.; Jensen, H.C.; Pleissner, D.; Eriksen, N.T. Growth and phycocyanin synthesis in the heterotrophic microalga Galdieria sulphuraria on substrates made of food waste from restaurants and bakeries. Bioresour Technol. 2017, 238, 296–305. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Boto-Ordóñez, M.; Van Hulle, S.W.H.; Ferrer, I.; Garfí, M.; Rousseau, D.P.L. Natural pigments from microalgae grown in industrial wastewater. Bioresour Technol. 2020, 303, 122894. [Google Scholar] [CrossRef]
- Hernández, D.; Riaño, B.; Coca, M.; Solana, M.; Bertucco, A.; García-González, M.C. Microalgae cultivation in high rate algal ponds using slaughterhouse wastewater for biofuel applications. Chem. Eng. J. 2016, 285, 449–458. [Google Scholar] [CrossRef]
- Winayu, B.N.R.; Lai, K.T.; Hsueh, H.T.; Chu, H. Production of phycobiliprotein and carotenoid by efficient extraction from Thermosynechococcus sp. CL-1 cultivation in swine wastewater. Bioresour Technol. 2021, 319, 124125. [Google Scholar] [CrossRef]
- Rinna, F.; Buono, S.; Cabanelas, I.T.D.; Nascimento, I.A.; Sansone, G.; Barone, C.M.A. Wastewater treatment by microalgae can generate high quality biodiesel feedstock. J. Water Process. Eng. 2017, 18, 144–149. [Google Scholar] [CrossRef]
- Kolmert, A.; Johnson, D.B. Remediation of acidic waste waters using immobilised, acidophilic sulfate-reducing bacteria. J. Chem. Technol. Biotechnol. 2001, 76, 836–843. [Google Scholar] [CrossRef]
- Goyal, A.; Srivastava, V.C. Treatment of highly acidic wastewater containing high energetic compounds using dimensionally stable anode. Chem. Engin. J. 2017, 325, 289–299. [Google Scholar] [CrossRef]
- Hirooka, S.; Miyagishima, S. Cultivation of Acidophilic Algae Galdieria sulphuraria and Pseudochlorella sp. YKT1 in Media Derived from Acidic Hot Springs. Front. Microbiol. 2016, 7, 2022. [Google Scholar] [CrossRef] [PubMed]
- Nancucheo, I.; Johnson, D.B. Acidophilic algae isolated from mine-impacted environments and their roles in sustaining heterotrophic acidophiles. Front. Microbial. 2012, 3, 325. [Google Scholar] [CrossRef] [PubMed]
- Carfagna, S.; Landi, V.; Coraggio, F.; Salbitani, G.; Vona, V.; Pinto, G.; Pollio, A.; Ciniglia, C. Different characteristics of C-phycocyanin (C-PC) in two strains of the extremophilic Galdieria phlegrea. Algal Res. 2018, 31, 406–412. [Google Scholar] [CrossRef]
- Salbitani, G.; Cipolletta, S.; Vona, V.; Di Martino, C.; Carfagna, S. Heterotrophic Cultures of Galdieria phlegrea Shift to Autotrophy in the Presence or Absence of Glycerol. J. Plant. Growth Regul. 2020. [Google Scholar] [CrossRef]
- Barone, R.; De Napoli, L.; Mayol, L.; Paolucci, M.; Volpe, M.G.; D’Elia, L.; Pollio, A.; Guida, M.; Gambino, E.; Carraturo, F.; et al. Autotrophic and Heterotrophic Growth Conditions Modify Biomolecole Production in the Microalga Galdieria sulphuraria (Cyanidiophyceae, Rhodophyta). Mar. Drugs 2020, 18, 169. [Google Scholar] [CrossRef]
- Bugajski, P.; Kurek, K.; Jóźwiakowski, K. Effect of wastewater temperature and concentration of organic compounds on the efficiency of ammonium nitrogen removal in a household treatment plant servicing a school building. Arch. Environ. Prot. 2019, 45, 31–37. [Google Scholar]
- Alisawi, H.A.O. Performance of wastewater treatment during variable temperature. Appl. Water Sci. 2020, 10, 89. [Google Scholar] [CrossRef]
- Varshney, P.; Mikulic, P.; Vonshak, A.; Beardall, J.; Wangikar, P.P. Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour. Technol. 2015, 184, 363–372. [Google Scholar] [CrossRef]
- Von Sperling, M. Wastewater Characteristics, Treatment and Disposal; IWA Publishing: London, UK, 2007. [Google Scholar]
- Langenhoff, A.A.M.; Stuckey, D.C. Treatment of dilute wastewater using an anaerobic baffled reactor: Effect of low temperature. Water Res. 2000, 34, 3867–3875. [Google Scholar] [CrossRef]
- Lettinga, G.; Rebac, S.; Zeeman, G. Challenge of psychrophilic anaerobic wastewater treatment. Trends Biotechnol. 2001, 19, 363–370. [Google Scholar] [CrossRef]
- Vona, V.; Di Martino Rigano, V.; Andreoli, C.; Lobosco, O.; Caiazzo, M.; Martello, A.; Carfagna, S.; Salbitani, G.; Rigano, C. Comparative analysis of photosynthetic and respiratory parameters in the psychrophilic unicellular green alga Koliella antarctica, cultured in indoor and outdoor photo-bioreactors. Physiol. Mol. Biol. Plants 2018, 24, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, C.; Lokhrost, G.M.; Mani, A.M.; Scarabel, L.; Moro, I.; La Rocca, N.; Tognetto, L. Koliella antarctica sp. nov. (Klebsormidiales) a new marine green microalga from the Ross Sea. Algological Studies Archiv für Hydrobiologie 1998, 125, 1–8. [Google Scholar]
- Fogliano, V.; Andreoli, C.; Martello, A.; Caiazzo, M.; Lobosco, O.; Formisano, F.; Carlino, P.A.; Meca, G.; Graziani, G.; Di Martino Rigano, V.; et al. Functional ingredients produced by culture of Koliella antartica. Aquaculture 2010, 299, 115–120. [Google Scholar] [CrossRef]
- Alavi, S.; Rafieyan, S.; Yavari-Bafghi, M.; Amoozegar, M.A. Extremophiles: A Powerful Choice for Bioremediation of Toxic Oxyanions. In Microbial Bioremediation & Biodegradation; Shah, M., Ed.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Yu, Z.; Song, M.; Pei, H.; Han, F.; Jiang, L.; Hou, Q. The growth characteristics and biodiesel production of ten algae strains cultivated in anaerobically digested effluent from kitchen waste. Alga Res. 2017, 24, 265–275. [Google Scholar] [CrossRef]
- Sharma, J.; Kumar, V.; Kumar, S.S.; Malyan, S.K.; Mathimani, T.; Bishnoi, N.R.; Pugazhendhi, A. Microalgal consortia for municipal wastewater treatment—Lipid augmentation and fatty acid profiling for biodiesel production. J. Photochem. Photobiol. B 2020, 202, 111638. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Hu, H.Y.; Zhang, X.; Yu, Y.; Chen, Y.S. Growth and lipid accumulation properties of a freshwater microalga, Chlorella ellipsoidea Yj1, in domestic secondary effluents. Appl. Energy 2011, 88, 3295–3299. [Google Scholar] [CrossRef]
- Li, X.; Hu, H.Y.; Yang, J. Lipid accumulation and nutrient removal properties of a newly isolated freshwater microalga, Scenedesmus sp. LX1, growing in secondary effluent. New Biotechnol. 2010, 27, 59–63. [Google Scholar]
- Meng, T.K.; Kassim, M.A.; Cheirsilp, B. Chapter 4—Mixotrophic cultivation: Biomass and biochemical biosynthesis for biofuel production. In Microalgae Cultivation for Biofuels Production; Yousuf, A., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 51–67. [Google Scholar]
- Rodrigues, D.B.; Flores, É.M.M.; Barin, J.S.; Mercadante, A.Z.; Jacob-Lopes, E.; Zepka, L.Q. Production of carotenoids from microalgae cultivated using agroindustrial wastes. Food Res. Int. 2014, 65, 144–148. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).