Recycling of Blast Furnace Slag and Fluorite Tailings into Diopside-Based Glass-Ceramics with Various Nucleating Agents’ Addition
Abstract
:1. Introduction
2. Raw Materials and Experimental Design
3. Preparation Process of Glass-Ceramics
4. Characterization Methods and Performance Testing
5. Results and Discussion
5.1. Effect of Blast Furnace Slag Percentage on Diopside-Based Glass-Ceramics
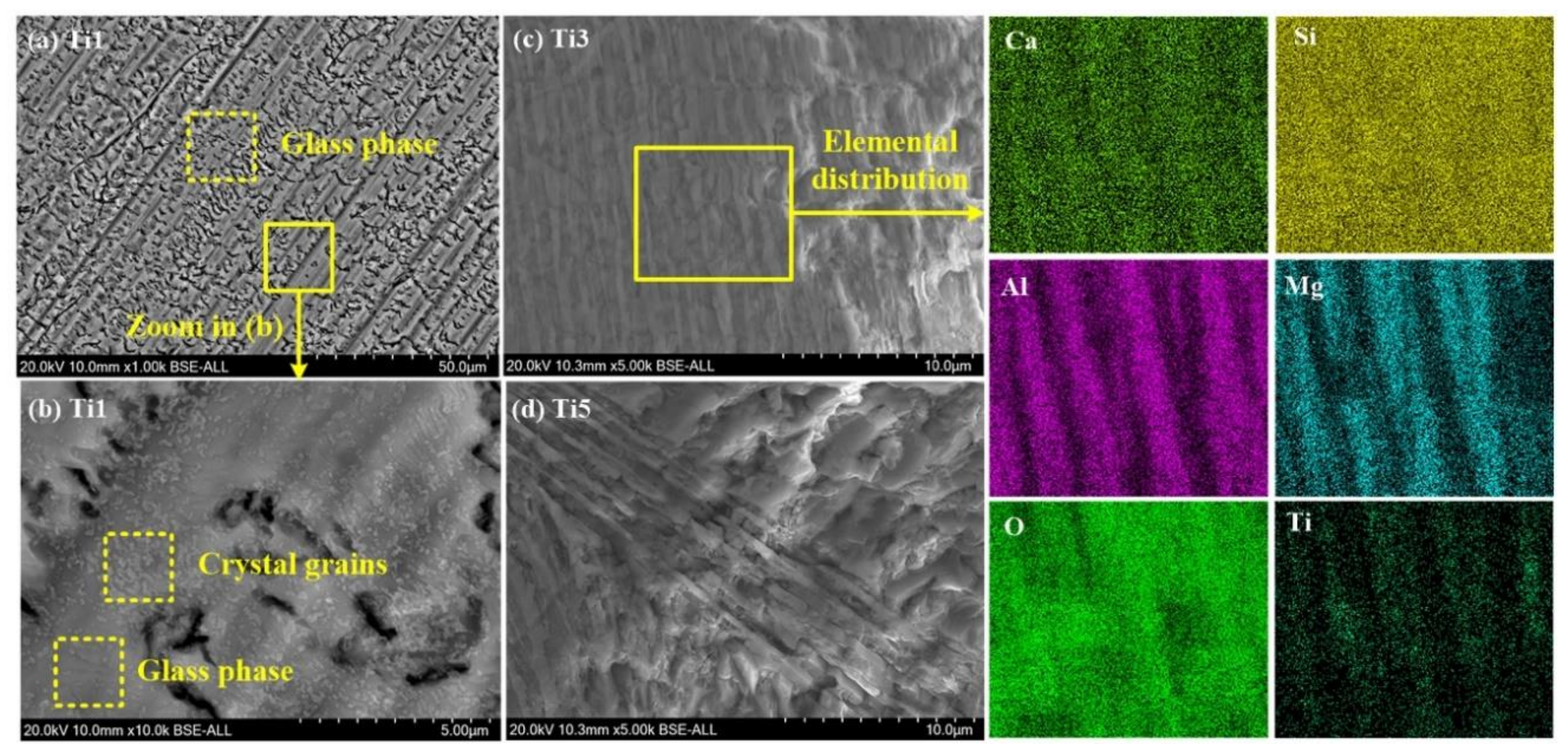
5.2. Effect of TiO2 Addition on Diopside-Based Glass-Ceramics
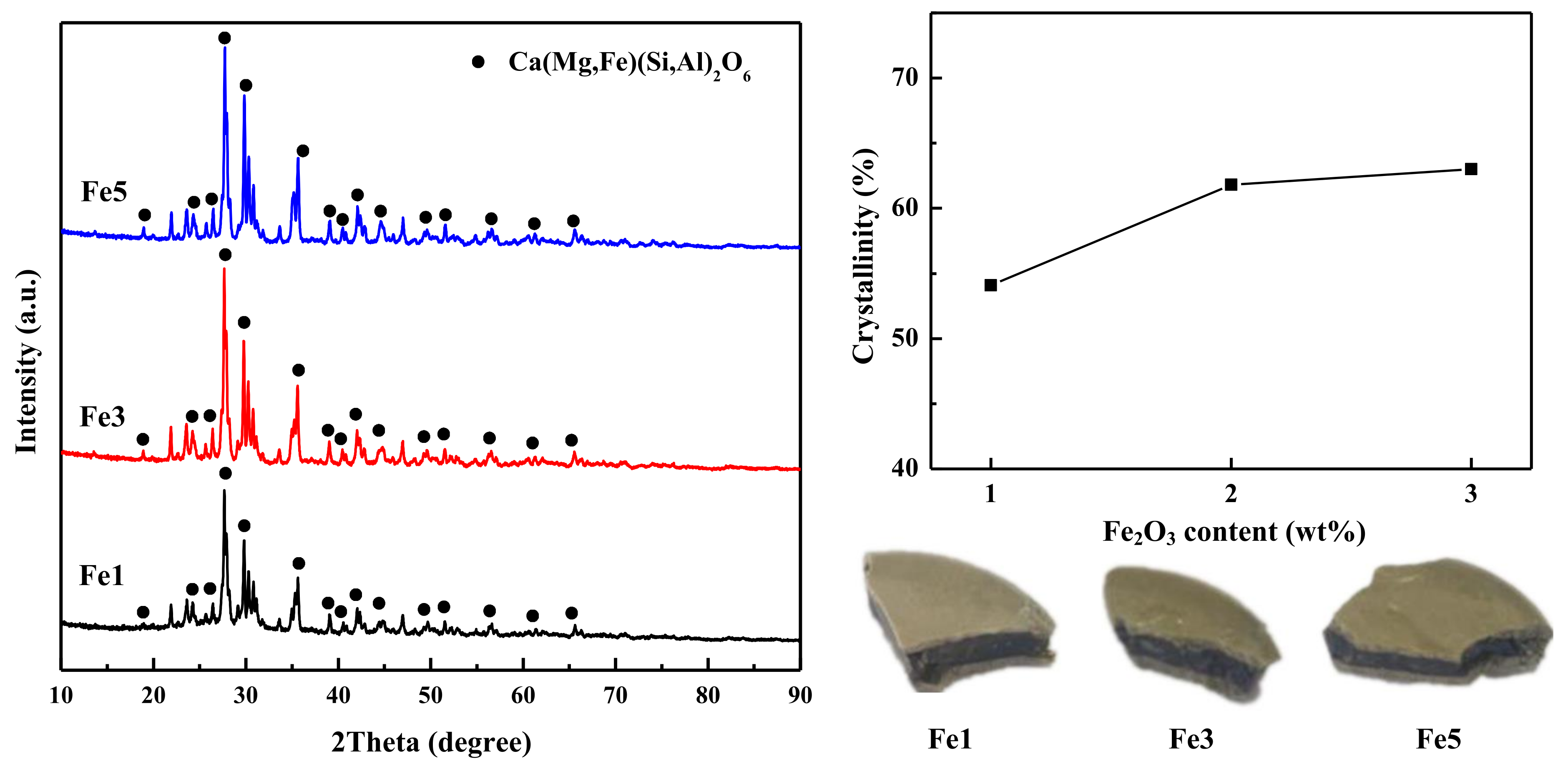
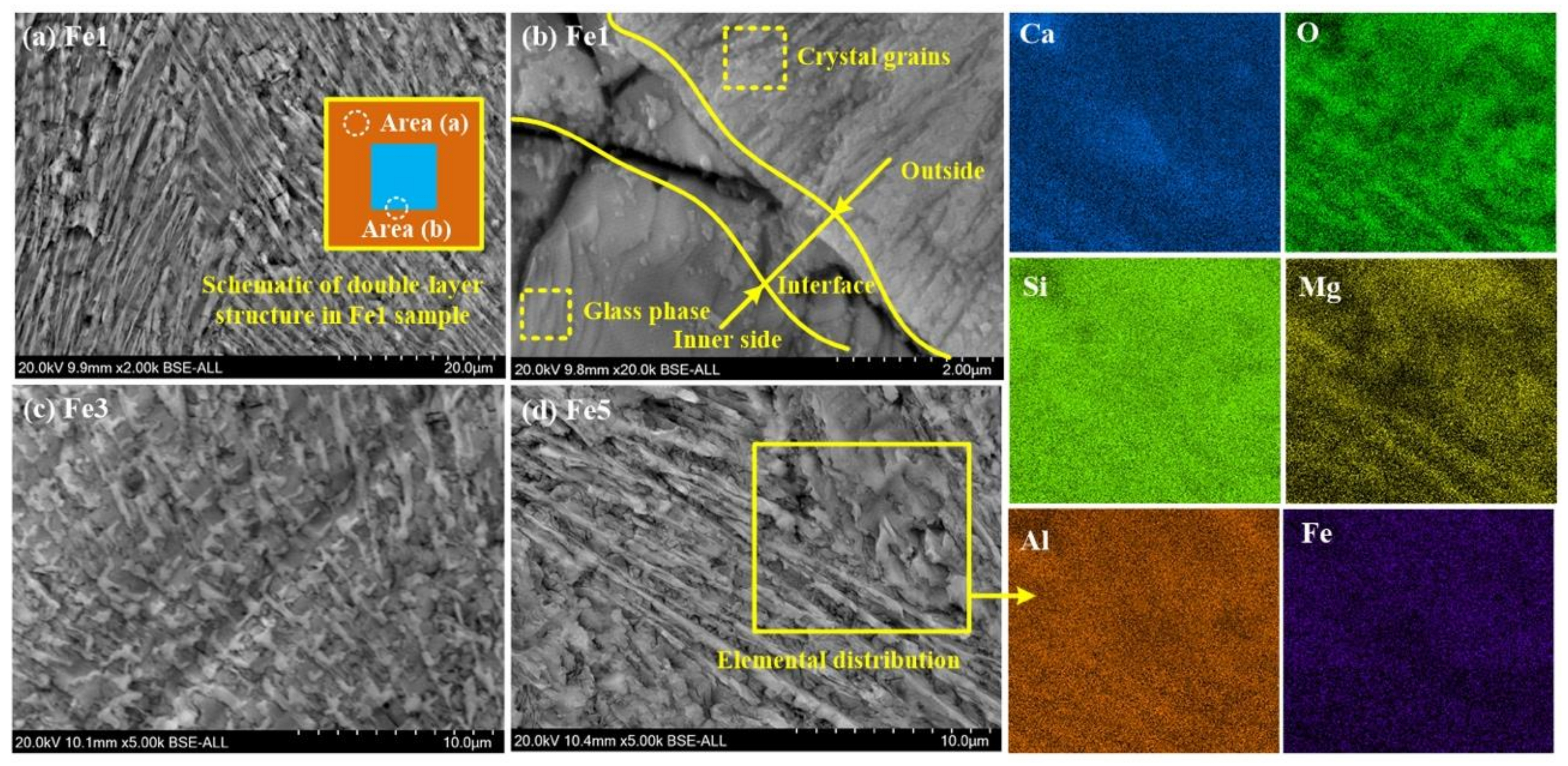
5.3. Effect of Fe2O3 Addition on Diopside-Based Glass-Ceramics
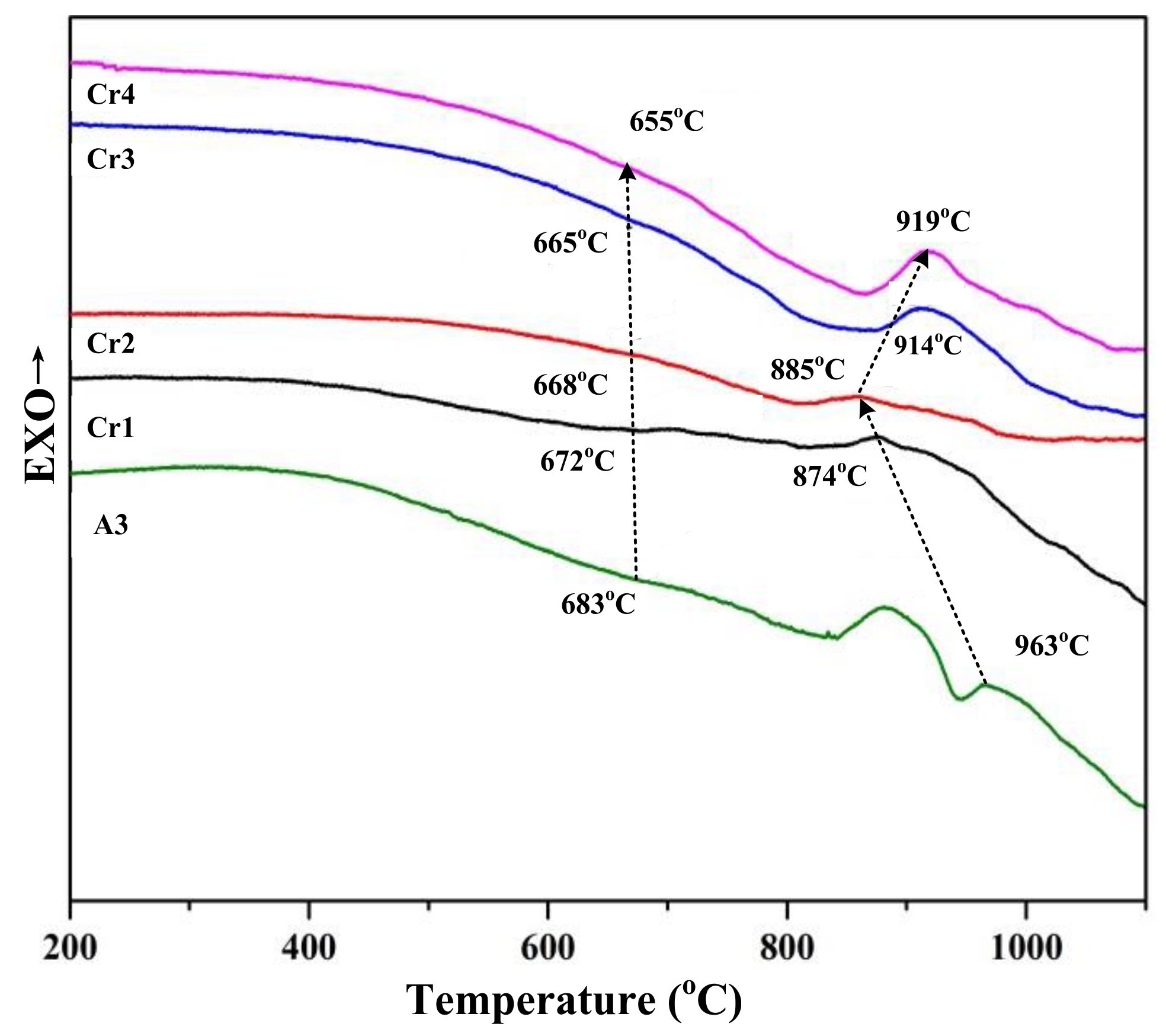
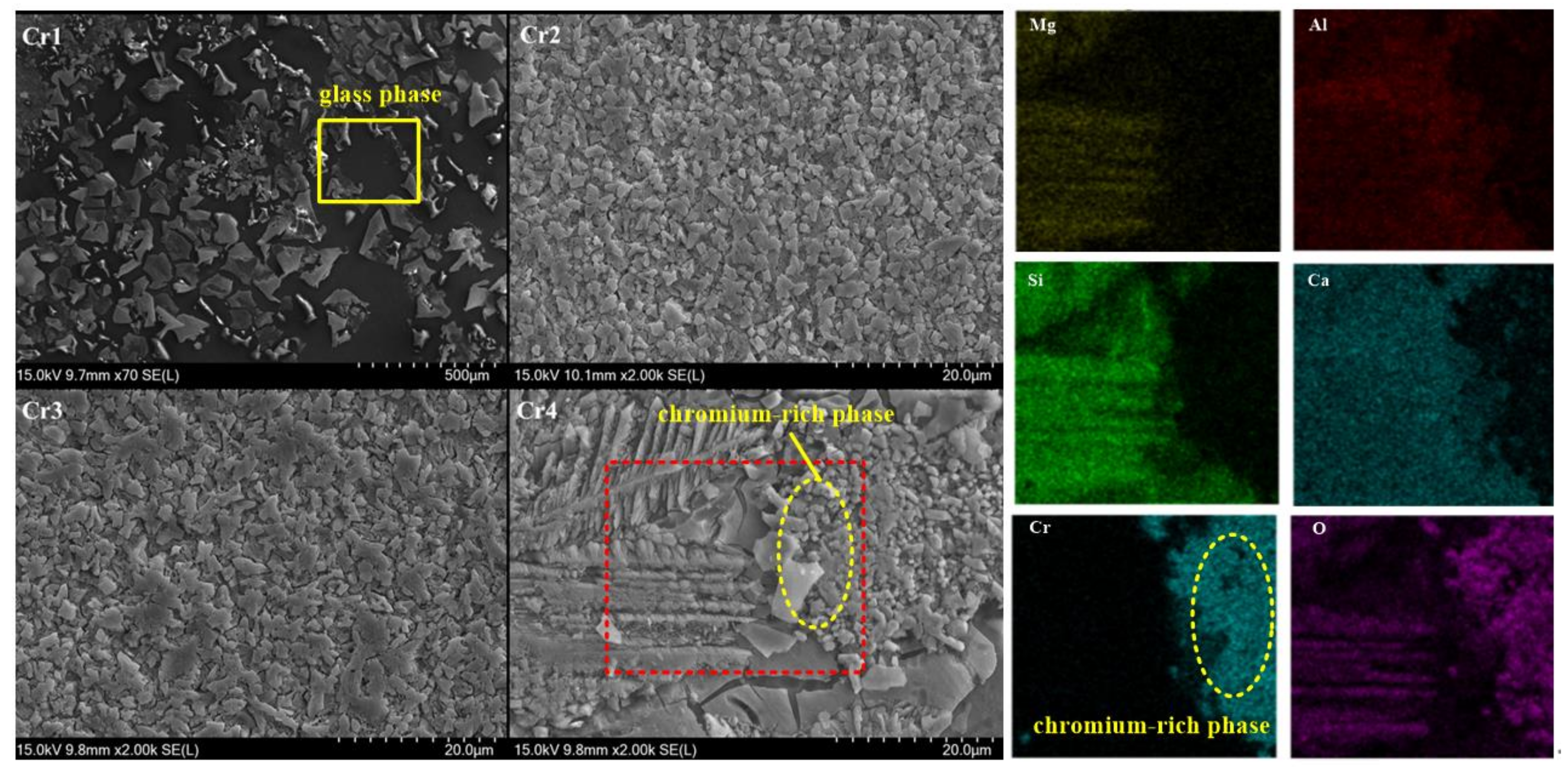
5.4. Effect of Cr2O3 Addition on Diopside-Based Glass-Ceramics
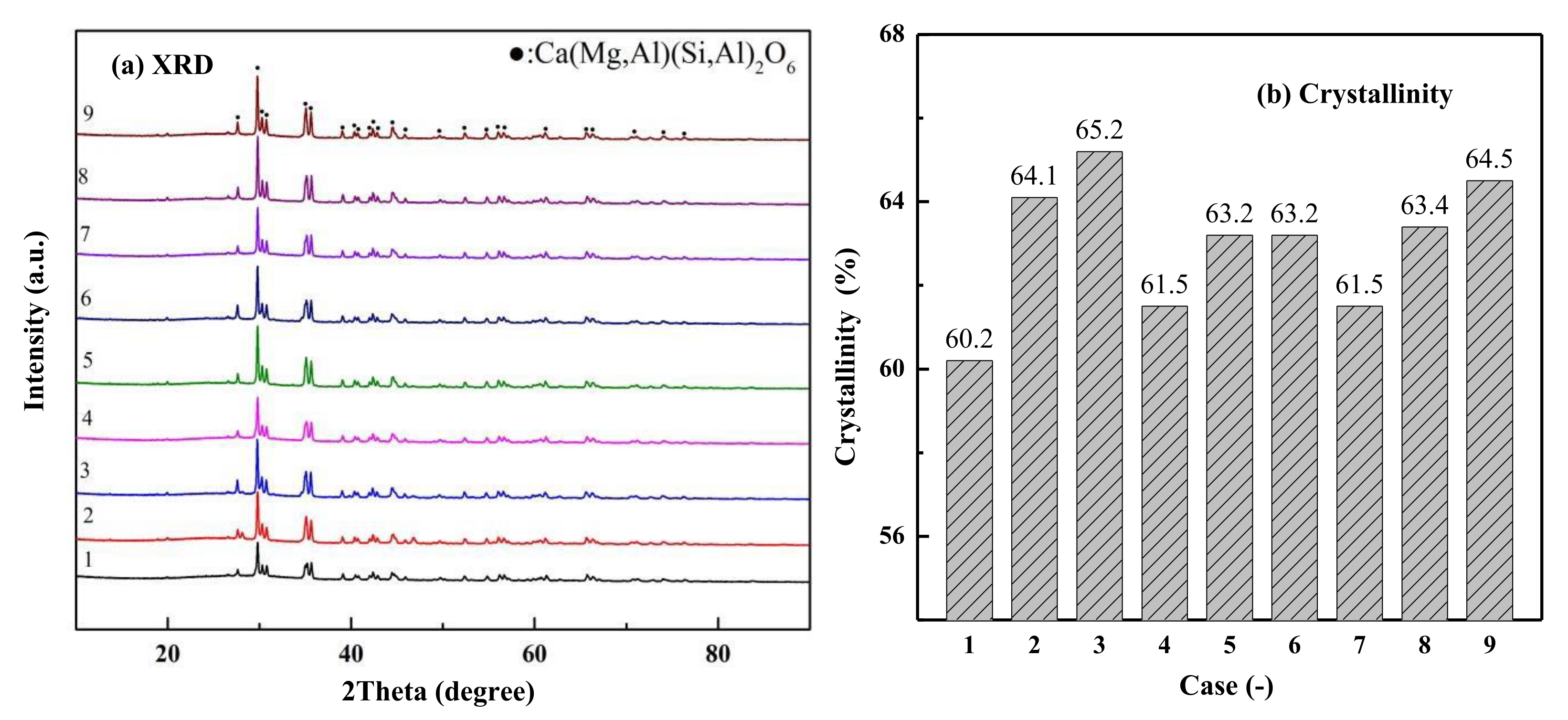
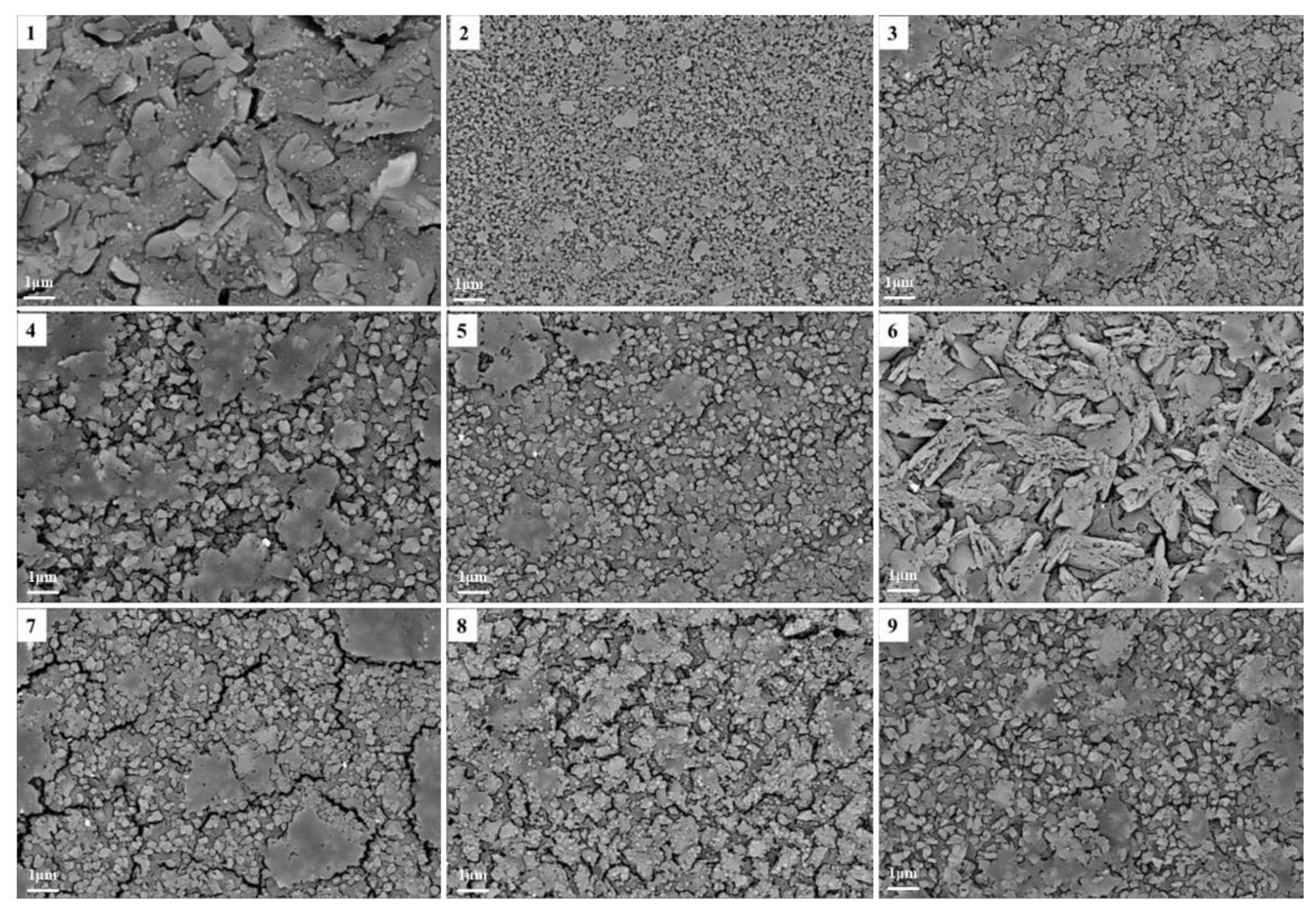
5.5. Optimization of Composite Nucleating Agent Addition
6. Conclusions
- (1)
- The crystallization characteristics and mechanisms correlate to the addition of various nucleating agents. With the addition of Fe2O3 (ranging between 1 wt% and 5 wt%) or TiO2 (ranging between 1 wt% and 5 wt%), the parent glass realized surface crystallization, while the addition of Cr2O3 (ranging between 1 wt% and 4 wt%) promoted the transformation of the crystallization mode to three-dimensional volumetric crystallization.
- (2)
- The diopside-based glass-ceramics could be prepared from 55% blast furnace and 45% fluorite tailing with the addition of composite nucleating agents consisting of 1.5% Cr2O3, 2% TiO2 and 3% Fe2O3. The heat treatment was conducted at a nucleation temperature of 720 °C and a crystallization temperature of 920 °C, and the nucleation and crystallization durations were 1.0 h and 1.5 h, respectively.
- (3)
- Under optimized components and heat treatment parameters, the obtained diopside-based glass-ceramics displayed a Vickers hardness of 7.12 GPa, density of 2.95 g·cm−3, water absorption of 0.02%, acid resistance of 0.23% and alkali resistance of 0.02%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shew, H. RPR Cement Technology; Wuhan University of Technology Press: Wuhan, China, 1991; pp. 35–54. [Google Scholar]
- He, D.F.; Gao, C.; Pan, J.T.; Xu, A.J. Preparation of glass-ceramics with diopside as the main crystalline phase from low and me-dium titanium-bearing blast furnace slag. Ceram. Int. 2018, 44, 1384–1393. [Google Scholar] [CrossRef]
- Deng, L.; Jia, R.; Yun, F.; Zhang, X.; Li, H.; Zhang, M.; Jia, X.; Ren, D.; Li, B. Influence of Cr2O3 on the viscosity and crystallization behavior of glass ceramics based on blast furnace slag. Mater. Chem. Phys. 2020, 240, 122212. [Google Scholar] [CrossRef]
- Helbig, C.; Baldauf, H.; Mahnke, J.; Stöckelhuber, K.W.; Schulze, H.J. Investigation of Langmuir monofilms and flotation experi-ments with anionic/cationic collector mixtures. Int. J. Miner. Process. 1998, 53, 135–144. [Google Scholar] [CrossRef]
- Strnad, Z. Glass-Ceramic Materials; Elsevier Science Publishers: Amsterdam, The Netherlands, 1986; pp. 76–88. [Google Scholar]
- Pioro, L.; Pioro, I. Reprocessing of metallurgical slag into materials for the building industry. Waste Manag. 2004, 24, 371–379. [Google Scholar] [CrossRef]
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef]
- Boltakova, N.; Faseeva, G.; Kabirov, R.; Nafikov, R.; Zakharov, Y. Utilization of inorganic industrial wastes in producing construction ceramics. Review of Russian experience for the years 2000–2015. Waste Manag. 2017, 60, 230–246. [Google Scholar] [CrossRef]
- Teo, P.T.; Seman, A.A.; Basu, P.; Sharif, N.M. Recycling of Malaysia’s electric arc furnace (EAF) slag waste into heavy-duty green ceramic tile. Waste Manag. 2014, 34, 2697–2708. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Ni, W.; Jia, Y.; Zhu, L.-P.; Huang, X.-Y. Crystallization behavior of glass ceramics prepared from the mixture of nickel slag, blast furnace slag and quartz sand. J. Non-Cryst. Solids 2010, 356, 1554–1558. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, D.; Bi, Y.; Long, M. Preparation of low cost glass–ceramics from molten blast furnace slag. Ceram. Int. 2012, 38, 2495–2500. [Google Scholar] [CrossRef]
- Mostafa, N.Y.; Shaltout, A.; Abdel-Aal, M.S.; El-Maghraby, A. Sintering mechanism of blast furnace slag–kaolin ceramics. Mater. Des. 2010, 31, 3677–3682. [Google Scholar] [CrossRef]
- Yang, S.M.; Zhang, W. Research on glass ceramics of multi-solid waste slag by blast furnace slag and fly ash. Bull. Chin. Ceram. Soc. 2015, 34, 487–491. [Google Scholar]
- Gong, X.C.; Chen, Q.W.; Guo, H.W.; Yan, B.J.; Li, H.W.; Pei, F.J. Preparation of sintered glass ceramics with blast furnace slag and waste glass. Metal World 2018, 3, 13–17. [Google Scholar]
- Deng, L.; Zhang, X.; Zhang, M.; Jia, X.; Zhang, Z.; Li, B. Structure and properties of in situ synthesized FeSi2-diopside glass ceramic composites from Bayan Obo tailings, blast furnace slag, and fly ash. J. Alloy. Compd. 2019, 785, 932–943. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Hirano, S.; Hirai, N.; Tanaka, T. Preparation of Porous Ceramics by the Hydrothermal Reaction of Blast Furnace Slag for Use in a Water-retentive Material. ISIJ Int. 2008, 48, 1322–1324. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Xin, W.-B.; Huo, X.-G.; Luo, G.-P.; Zhang, F. Preparation and Properties of Blast Furnace Slag Glass Ceramics Containing Cr2O3. High Temp. Mater. Process. 2019, 38, 726–732. [Google Scholar] [CrossRef]
- Karamanov, A.; Hamzawy, E.M.A.; Karamanov, E.; Jordanov, N.B.; Darwish, H. Sintered glass-ceramics and foams by metal-lurgical slag with addition of CaF2. Ceram. Int. 2020, 46, 6507–6516. [Google Scholar] [CrossRef]
- Sun, R.J.; He, F.; Wang, L.G.; Zhang, W.T.; Liu, X.Q.; Yang, H.; Xie, J.L. Influence of TiO2 content on structure and properties of blast furnace slag glass-ceramics. Bull. Chin. Ceram. Soc. 2019, 38, 2542–2548. [Google Scholar]
- Zhang, W.T.; He, F.; Xiao, Y.L.; Xie, M.Q.; Xie, J.L.; Li, F.X.; Yang, H.; Luo, Z.H. Structure, crystallization mechanism, and prop-erties of glass ceramics from molten blast furnace slag with different B2O3/Al2O3. Mater. Chem. Phys. 2020, 243, 122664. [Google Scholar] [CrossRef]
- Zhang, W.; He, F.; Xiao, Y.; Xie, M.; Xie, J.; Sun, R.; Yang, H.; Luo, Z. Effects of Al/Na and heat treatment on the structure and properties of glass ceramics from molten blast furnace slag. Ceram. Int. 2019, 45, 13692–13700. [Google Scholar] [CrossRef]
- Khater, G. Influence of Cr2O3, LiF, CaF2 and TiO2 nucleants on the crystallization behavior and microstructure of glass-ceramics based on blast-furnace slag. Ceram. Int. 2011, 37, 2193–2199. [Google Scholar] [CrossRef]
- Guo, X.Z.; Cai, X.B.; Song, J.; Yang, G.Y.; Yang, H. Crystallization and microstructure of CaO-MgO-Al2O3-SiO2 glass-ceramics containing complex nucleation agents. J. Non-Cryst. Solids 2014, 405, 63–67. [Google Scholar] [CrossRef]
- Hang, H.N.; Cheng, Y.; Yang, Q.Q.; Send, T. Mechanical and tribological properties of calcia-magnesia-alumina-silica-based glass-ceramics prepared by in situ crystallization. Mater. Sci. Eng. A-Struct. 2006, 423, 170–174. [Google Scholar]
- Xiao, H.; Cheng, Y.; Yu, L.; Liu, H. A study on the preparation of CMAS glass–ceramics by in situ crystallization. Mater. Sci. Eng. A 2006, 431, 191–195. [Google Scholar] [CrossRef]
- Xie, C.S.; Gui, Y.L.; Song, C.Y.; Hu, B.S. Effect of CaO/SiO2; and heat treatment on the microstructure of glass-ceramics from blast furnace slag. Ceram. Silikaty 2016, 60, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Likitvanichkul, S.; LaCourse, W.C. Effect of fluorine content on crystallization of canasite glass-ceramics. J. Mater. Sci. 1995, 30, 6151–6155. [Google Scholar] [CrossRef]
- Mukherjee, D.P.; Das, S.K. SiO2–Al2O3–CaO glass-ceramics: Effects of CaF2 on crystallization, microstructure and properties. Ceram. Int. 2013, 39, 571–578. [Google Scholar] [CrossRef]
- Chen, J.X.; Yan, B.J.; Li, H.W.; Li, P.; Guo, H.W. Vitrification of blast furnace slag and fluorite tailings for giving diop-side-fluorapatite glass-ceramics. Mater. Lett. 2018, 218, 309–312. [Google Scholar] [CrossRef]
- Yu, B.; Liang, K.; Gu, S. Effect of crystallization of CaO-P2O5-SiO2-MgO-F- glass-ceramics on its mechanical properties. Int. J. Miner. Metall. Mater. 2002, 9, 212–215. [Google Scholar]
- Liu, H.-P.; Huang, X.-F.; Ma, L.-P.; Chen, D.-L.; Shang, Z.-B.; Jiang, M. Effect of Fe2O3 on the crystallization behavior of glass-ceramics produced from naturally cooled yellow phosphorus furnace slag. Int. J. Miner. Metall. Mater. 2017, 24, 316–323. [Google Scholar] [CrossRef]
- Rezvani, M.; Eftekhari-Yekta, B.; Solati-Hashjin, M.; Marghussian, V.K. Effect of Cr2O3, Fe2O3 and TiO2 nucleants on the crystalli-zation behaviour of SiO2-Al2O3-CaO-MgO(R2O) glass-ceramics. Ceram. Int. 2005, 31, 75–80. [Google Scholar] [CrossRef]
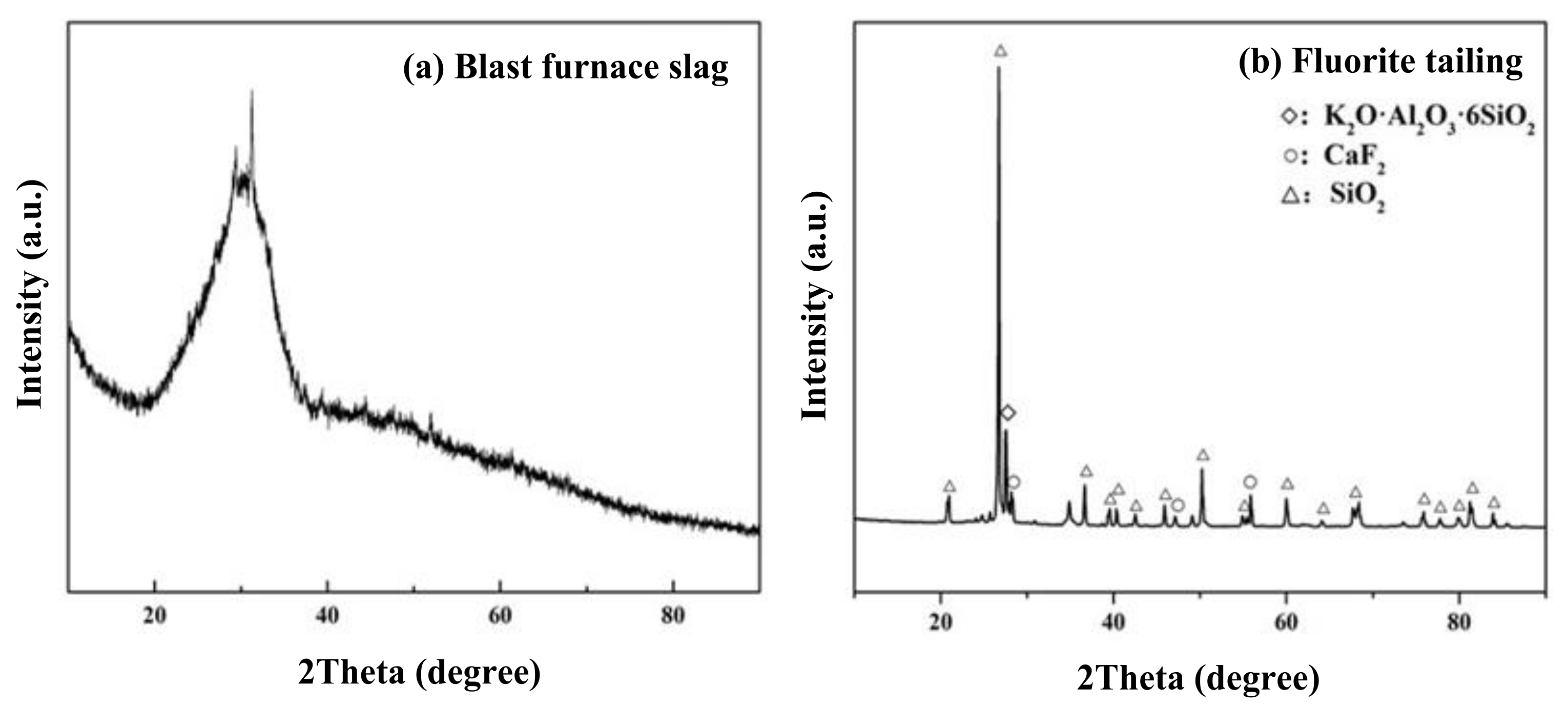
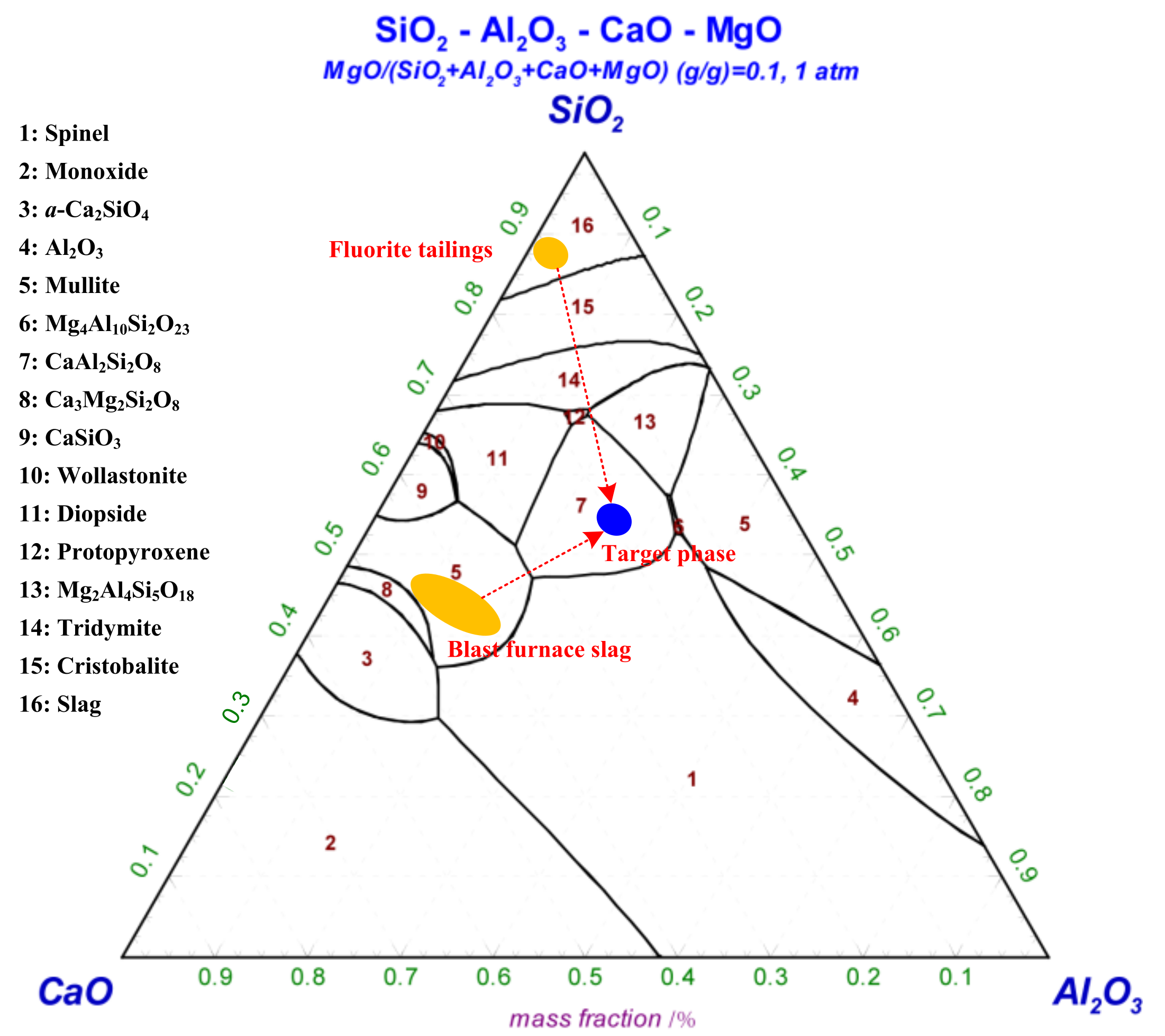


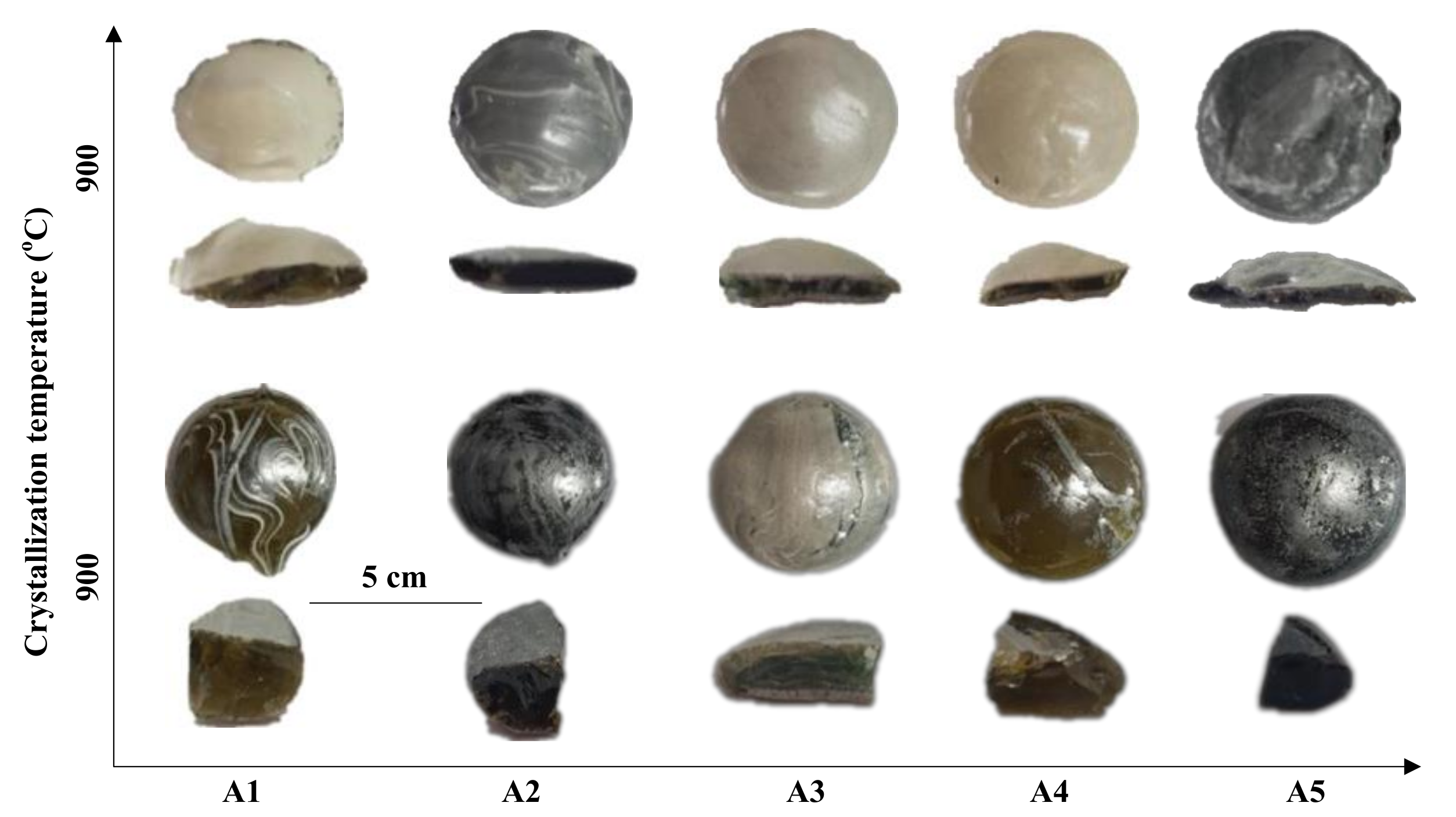


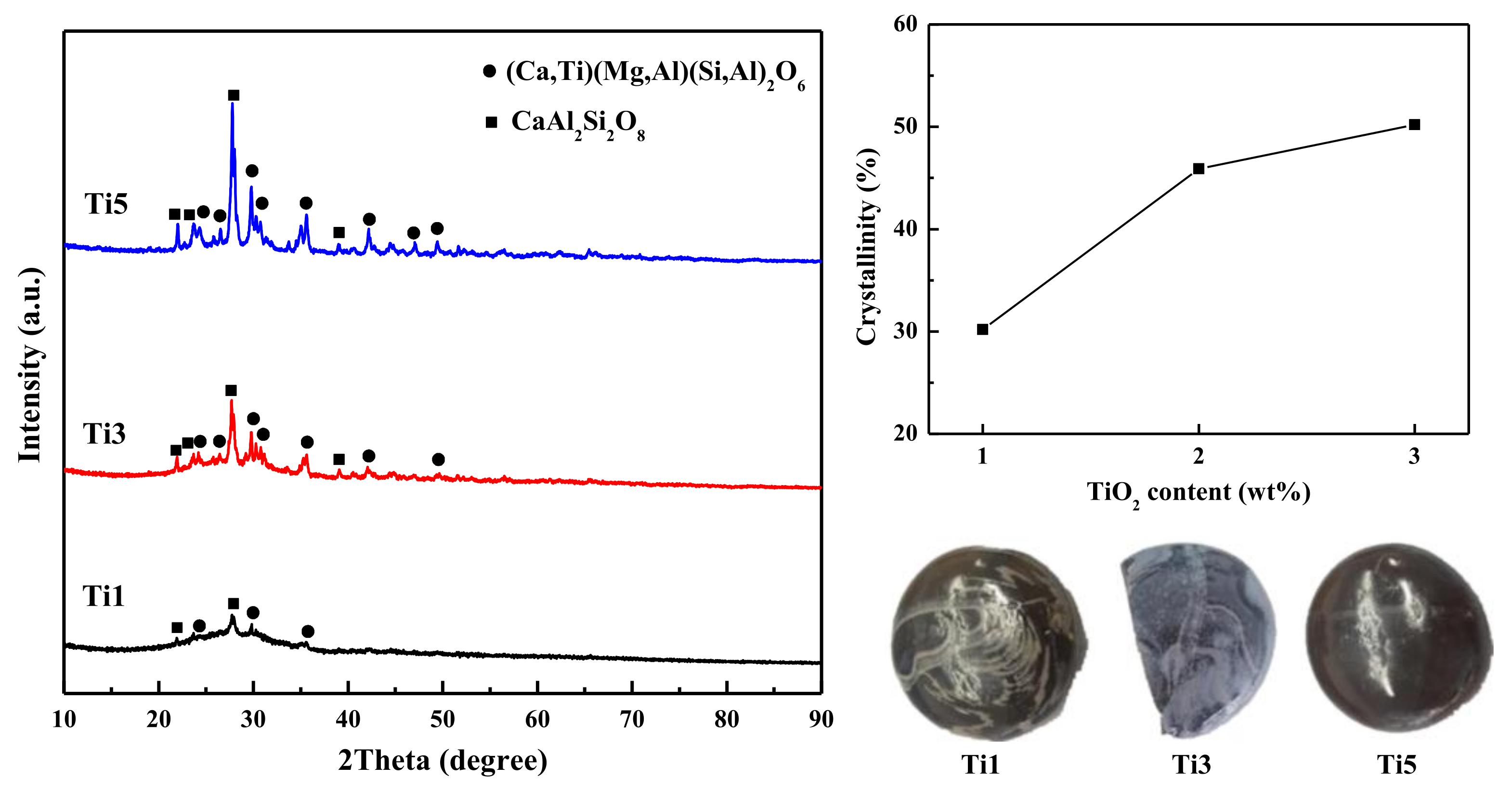



| Items | SiO2 | CaO | MgO | Al2O3 | Fe2O3 | Na2O | K2O | TiO2 | F− |
|---|---|---|---|---|---|---|---|---|---|
| Blast furnace slag | 33.91 | 35.13 | 10.08 | 16.51 | 0.04 | 0.54 | 0.61 | 0.73 | 0.00 |
| Fluorite tailing | 72.00 | 7.64 | 0.13 | 5.34 | 1.04 | 0.30 | 2.58 | 0.07 | 7.83 |
| Case | Blast Furnace Slag (wt%) | Fluorite Tailing (wt%) | TiO2 (wt%) | Fe2O3 (wt%) | Cr2O3 (wt%) |
|---|---|---|---|---|---|
| A1 | 45.0 | 55.0 | / | / | / |
| A2 | 50.0 | 50.0 | / | / | / |
| A3 | 55.0 | 45.0 | / | / | / |
| A4 | 60.0 | 40.0 | / | / | / |
| A5 | 65.0 | 35.0 | / | / | / |
| Ti1 | m | n | 1.0 | / | / |
| Ti3 | m | n | 3.0 | / | / |
| Ti5 | m | n | 5.0 | / | / |
| Fe1 | m | n | / | 1.0 | / |
| Fe3 | m | n | / | 3.0 | / |
| Fe5 | m | n | / | 5.0 | / |
| Cr1 | m | n | / | / | 1.0 |
| Cr2 | m | n | / | / | 2.0 |
| Cr3 | m | n | / | / | 3.0 |
| Cr4 | m | n | / | / | 4.0 |
| Case | Cr2O3 | TiO2 | Fe2O3 |
|---|---|---|---|
| 1 | 1.5 | 1.0 | 2.0 |
| 2 | 1.5 | 2.0 | 3.0 |
| 3 | 1.5 | 3.0 | 4.0 |
| 4 | 2.0 | 1.0 | 3.0 |
| 5 | 2.0 | 2.0 | 4.0 |
| 6 | 2.0 | 3.0 | 2.0 |
| 7 | 2.5 | 1.0 | 4.0 |
| 8 | 2.5 | 2.0 | 2.0 |
| 9 | 2.5 | 3.0 | 3.0 |
| Case | Vickers Hardness (GPa) | Density (g·cm−3) | Water Absorption (%) | Acid Resistance (%) | Alkali Resistance (%) |
|---|---|---|---|---|---|
| 1 | 5.91 | 2.47 | 0.06 | 0.48 | 0.05 |
| 2 | 7.12 | 2.95 | 0.02 | 0.23 | 0.02 |
| 3 | 6.96 | 2.81 | 0.03 | 0.32 | 0.03 |
| 4 | 6.61 | 2.71 | 0.04 | 0.42 | 0.04 |
| 5 | 6.85 | 2.87 | 0.03 | 0.29 | 0.02 |
| 6 | 6.01 | 2.55 | 0.05 | 0.44 | 0.04 |
| 7 | 6.73 | 2.83 | 0.03 | 0.31 | 0.03 |
| 8 | 6.11 | 2.58 | 0.05 | 0.44 | 0.04 |
| 9 | 6.52 | 2.75 | 0.04 | 0.39 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Huang, X.; Yan, B.; Hu, S.; Guo, H.; Chen, D. Recycling of Blast Furnace Slag and Fluorite Tailings into Diopside-Based Glass-Ceramics with Various Nucleating Agents’ Addition. Sustainability 2021, 13, 11144. https://doi.org/10.3390/su132011144
Zhao W, Huang X, Yan B, Hu S, Guo H, Chen D. Recycling of Blast Furnace Slag and Fluorite Tailings into Diopside-Based Glass-Ceramics with Various Nucleating Agents’ Addition. Sustainability. 2021; 13(20):11144. https://doi.org/10.3390/su132011144
Chicago/Turabian StyleZhao, Wei, Xiaofeng Huang, Bingji Yan, Shaoyan Hu, Hongwei Guo, and Dong Chen. 2021. "Recycling of Blast Furnace Slag and Fluorite Tailings into Diopside-Based Glass-Ceramics with Various Nucleating Agents’ Addition" Sustainability 13, no. 20: 11144. https://doi.org/10.3390/su132011144







