Reduction of Lead and Antimony Ions from the Crystal Glass Wastewaters Utilising Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater Composition
- (i)
- Cooling water for abrasives from the grinding of crystal glass, contaminated with Pb, Sb, and abraded glass of size 0.001–0.07 mm,
- (ii)
- Water from acid polishing of glass products, composed of residues of sulphuric acid (H2SO4), hydrofluoric acid (HF), hexafluorosilicic acid (H2SiF6) and their salts, neutralised in an industrial plant by Ca(OH)2. It also contains Pb and Sb ions´ residues.
2.2. Adsorbents
2.3. Adsorption Trials
- (1)
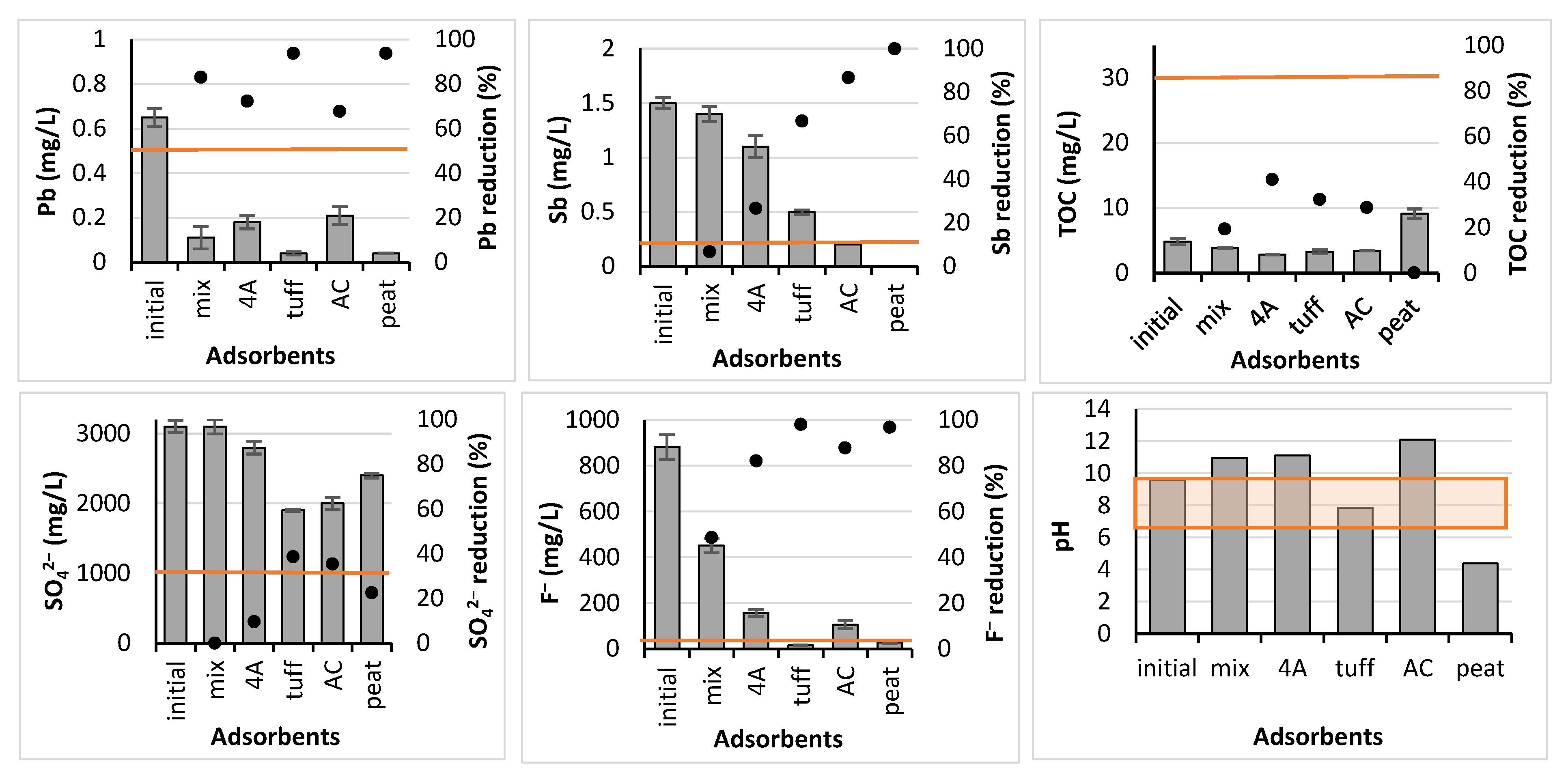
- A preliminary screening trial, where five adsorbents, i.e., mix, 4A, tuff, AC, and peat, were employed in the batch adsorption experiments;
- (2)
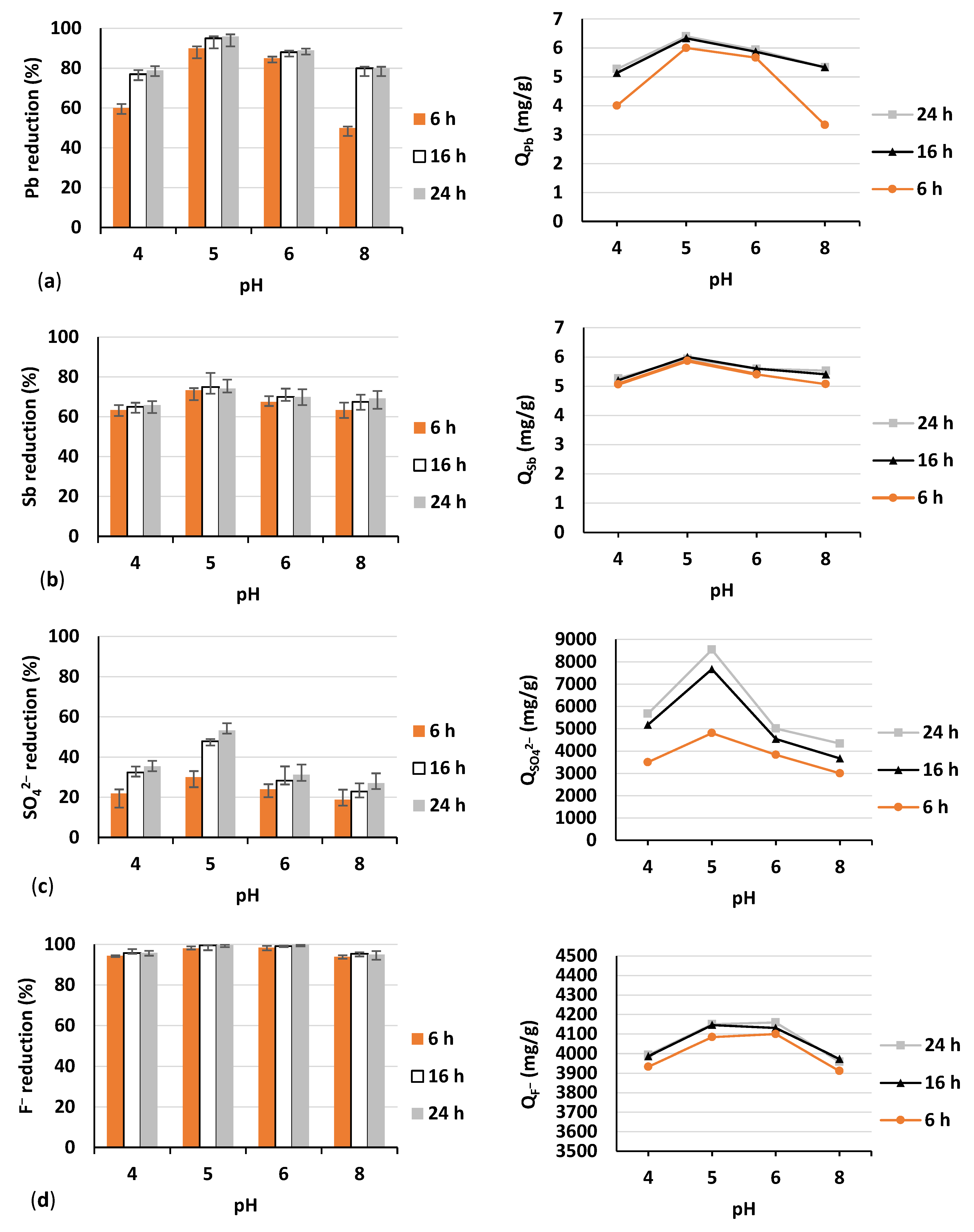
- A batch adsorption experiment with the most efficient adsorbent, namely tuff, selected in the preliminary phase; herein, pH and adsorption time were changed,
- (3)
- A laboratory-scale column experiment with tuff; herein, wastewater flow was changed.
2.4. Analytical Procedure
2.4.1. Characterisation of Adsorbents
2.4.2. Analysis of Wastewaters
3. Results and Discussion
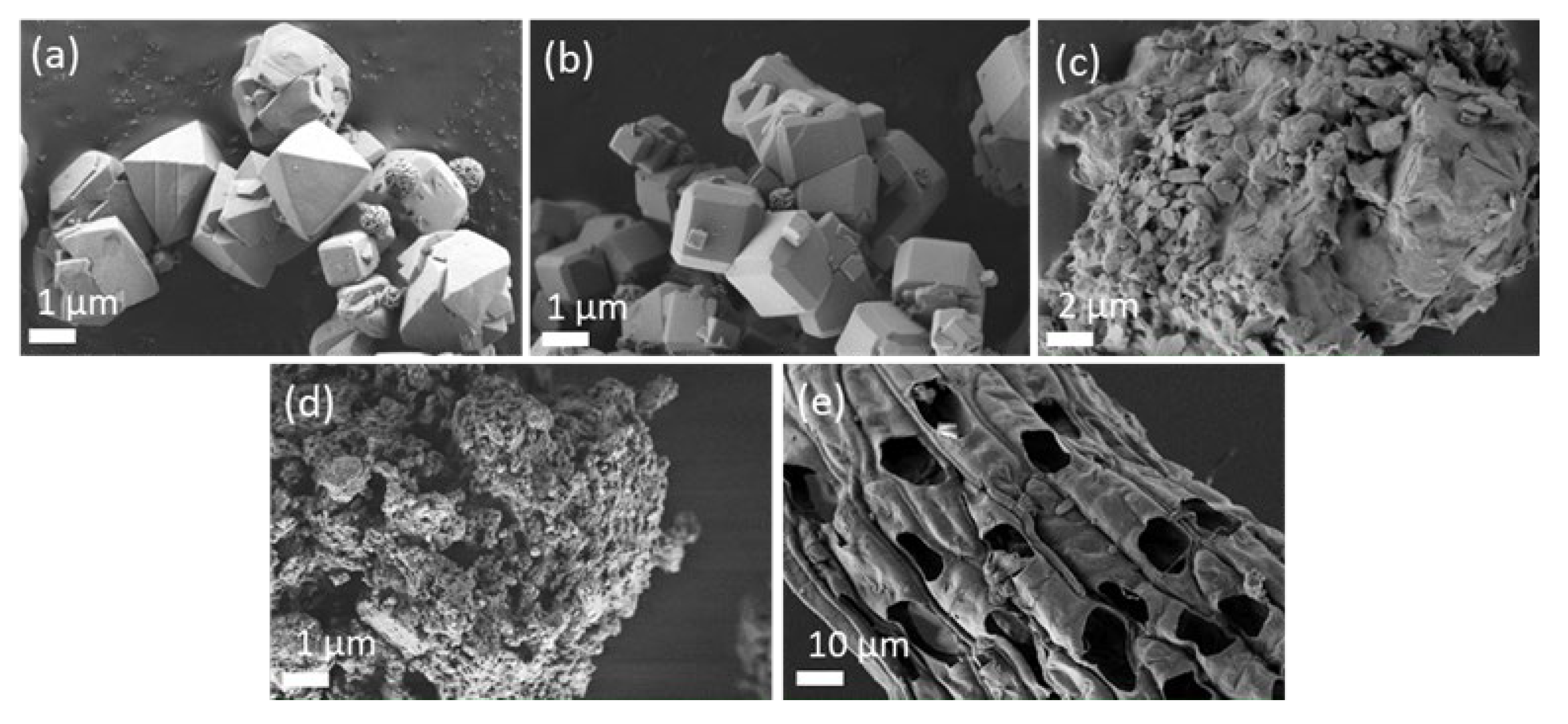
3.1. Characterisation of Adsorbents
3.2. Batch Experiment
3.3. Laboratory-Scale Column Trial
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bulc, T.G.; Ojstršek, A. The use of constructed wetland for dye-rich textile wastewater treatment. J. Hazard. Mater. 2008, 155, 76–82. [Google Scholar] [CrossRef]
- Thomas, M.; Kozik, V.; Bąk, A.; Barbusiński, K.; Jampilek, J. Removal of Heavy Metal Ions from Wastewaters: An Application of Sodium Trithiocarbonate and Wastewater Toxicity Assessment. Materials 2021, 14, 655. [Google Scholar] [CrossRef] [PubMed]
- Gholipour, A.; Zahabi, H.; Stefanakis, A.I. A novel pilot and full-scale constructed wetland study for glass industry wastewater treatment. Chemosphere 2020, 247, 125966. [Google Scholar] [CrossRef] [PubMed]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Ahmad, S.Z.N.; Salleh, W.N.W.; Ismail, A.F.; Yusof, N.; Yusop, M.Z.M.; Aziz, F. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: Toxicity, roles of functional groups and mechanisms. Chemosphere 2020, 248, 126008. [Google Scholar] [CrossRef] [PubMed]
- Atkovska, K.; Lisichkov, K.; Ruseska, G.; Dimitrov, A.T.; Grozdanov, A. Removal of heavy metal ions from wastewater using conventional and nanosorbents: A review. J. Chem. Technol. Metall. 2018, 53, 202–219. [Google Scholar]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2018, 17, 729–754. [Google Scholar] [CrossRef]
- Giwa, A.; Dindi, A.; Kujawa, J. Membrane bioreactors and electrochemical processes for treatment of wastewaters con-taining heavy metal ions, organics, micropollutants and dyes: Recent developments. J. Hazard. Mater. 2019, 370, 172–195. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. NPJ Clean Water 2021, 4, 1–15. [Google Scholar] [CrossRef]
- Abdullah, N.; Yusof, N.; Lau, W.; Jaafar, J.; Ismail, A. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Bhateria, R.; Singh, R. A review on nanotechnological application of magnetic iron oxides for heavy metal removal. J. Water Process. Eng. 2019, 31, 100845. [Google Scholar] [CrossRef]
- Burakov, A.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Jaspal, D.; Malviya, A. Composites for wastewater purification: A review. Chemosphere 2019, 246, 125788. [Google Scholar] [CrossRef]
- Raghuvir, P.B.; Prabhu, P.P.; Prabhu, B.; Mathew, T.M. A Review on removal of heavy metal ions from wastewater using natural/modified bentonite. MATEC Web Conf. 2018, 144, 02021. [Google Scholar]
- Bartczak, P.; Norman, M.; Klapiszewski, Ł.; Karwańska, N.; Kawalec, M.; Baczyńska, M.; Wysokowski, M.; Zdarta, J.; Ciesielczyk, F.; Jesionowski, T. Removal of nickel(II) and lead(II) ions from aqueous solution using peat as a low-cost adsorbent: A kinetic and equilibrium study. Arab. J. Chem. 2018, 11, 1209–1222. [Google Scholar] [CrossRef] [Green Version]
- Abdelrehiem, A.A.; Razek, T.M.A.; Rabbah, M.A. Lead recovery from crystal glass solid wastes. J. Environ. Sci. 2019, 48, 1–18. [Google Scholar] [CrossRef]
- Kumar, V. Impact of glass industry effluent disposal on soil characteristics in haridwar region, india. J. Environ. Health Sci. 2016, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Besisa, D.H.; Ewais, E.M.; Ahmed, Y.M. Optical, magnetic and electrical properties of new ceramics/lead silicate glass composites recycled from lead crystal wastes. J. Environ. Manag. 2021, 285, 112094. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Wang, H.; Li, J.; Li, F. An Effective Method to Remove Antimony in Water by Using Iron-Based Coagulants. Water 2019, 12, 66. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, R.M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalin. 2017, 7, 387–419. [Google Scholar] [CrossRef]
- Kang, S.-K.; Choo, K.-H. Use of MF and UF membranes for reclamation of glass industry wastewater containing colloidal clay and glass particles. J. Membr. Sci. 2003, 223, 89–103. [Google Scholar] [CrossRef]
- Kang, S.-K.; Choo, K.-H. Use of submerged microfiltration membranes for glass industry wastewater reclamation: Pilot-scale testing and membrane cleaning. Desalination 2006, 189, 170–180. [Google Scholar] [CrossRef]
- Lee, S.; Park, H.; Lee, C.-H.; Lee, M.; Pak, H.; Chung, K. Clean technology in the crystal glass industry. J. Clean. Prod. 1997, 5, 207–210. [Google Scholar] [CrossRef]
- Doma, H.S.; Abou-Taleb, E.M.; Nazih, M.; El-Qelish, M. Zero-discharge technology in the crystal glass industry. J. Adv. Environ. Biol. 2015, 9, 66–75. [Google Scholar]
- Lee, S.-J.; Park, J.H.; Ahn, Y.; Chung, J.W. Comparison of Heavy Metal Adsorption by Peat Moss and Peat Moss-Derived Biochar Produced Under Different Carbonization Conditions. Water Air Soil Pollut. 2015, 226, 1–11. [Google Scholar] [CrossRef]
- Ojstršek, A.; Fakin, D.; Hribernik, S.; Fakin, T.; Bračič, M.; Kurečič, M. Electrospun nanofibrous composites from cellulose acetate/ultra-high silica zeolites and their potential for VOC adsorption from air. Carbohydr. Polym. 2020, 236, 116071. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Krukowska, J.; Thomas, P. Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon. Chem. Eng. J. 2017, 307, 353–363. [Google Scholar] [CrossRef]
- Rinaldi, P.S.; Akromah, Z.N.; Ramadhan, H.; Husna, S.; Syamsudin, D.L.; Panggabean, P.B.; Murdianti, R.A.; Fatahillah, M.H.; Perala, I.; Rizqia, E.K.; et al. Physical and chemical analysis of land in forest peat swamp in resort Pondok soar, Tanjung Puting National Park, Central Kalimantan. IOP Conf. Ser. Earth Environ. Sci. 2019, 394, 012037. [Google Scholar] [CrossRef]
- Ojstršek, A.; Fakin, D. Colour and TOC reduction using biofilter packed with natural zeolite for the treatment of textile wastewaters. Desalin. Water Treat. 2011, 33, 147–155. [Google Scholar] [CrossRef]
- Kasiuliene, A.; Carabante, I.; Bhattacharya, P.; Kumpiene, J. Treatment of metal (loid) contaminated solutions using iron-peat as sorbent: Is landfilling a suitable management option for the spent sorbent? Environ. Sci. Pollut. Res. Int. 2019, 26, 21425–21436. [Google Scholar] [CrossRef] [Green Version]
- Runtti, H.; Tynjälä, P.; Tuomikoski, S.; Kangas, T.; Hu, T.; Rämö, J.; Lassi, U. Utilisation of barium-modified analcime in sulphate removal: Isotherms, kinetics and thermodynamics studies. J. Water Process Eng. 2017, 16, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Waghmare, S.; Arfin, T.; Rayalu, S.; Lataye, D.; Dubey, S.; Tiwari, S. Adsorption behavior of modified zeolite as novel adsorbents for fluoride removal from drinking water: Surface phenomena, kinetics and thermodynamics studies. Int. J. Sci. Eng. Technol. Res. 2015, 4, 4114–4124. [Google Scholar]
- Lim, W.-R.; Kim, S.W.; Lee, C.-H.; Choi, E.; Oh, M.H.; Seo, S.N.; Park, H.-J.; Hamm, S.-Y. Performance of composite mineral adsorbents for removing Cu, Cd, and Pb ions from polluted water. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, L.; Jun, B.-M.; Flora, J.R.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Ogata, F.; Saenjum, C.; Nakamura, T.; Kawasaki, N. Removal of Pb2+ from Aqueous Solutions Using K-Type Zeolite Synthesized from Coal Fly Ash. Water 2020, 12, 2375. [Google Scholar] [CrossRef]
- Elboughdiri, N.; Arellano-Garcia, H. The use of natural zeolite to remove heavy metals Cu (II), Pb (II) and Cd (II), from industrial wastewater. Cog. Eng. 2020, 7, 1782623. [Google Scholar] [CrossRef]
- Ciosek, A.L.; Luk, G.K. An Innovative Dual-Column System for Heavy Metallic Ion Sorption by Natural Zeolite. Appl. Sci. 2017, 7, 795. [Google Scholar] [CrossRef] [Green Version]
- Mokgehle, T.M.; Richards, H.; Chimuka, L.; Gitari, W.M.; Tavengwa, N.T. Sulphates removal from AMD using CFA hy-drothermally treated zeolites in column studies. Miner. Eng. 2019, 141, 105851. [Google Scholar] [CrossRef]
- Ojstršek, A.; Fakin, D. Layered compact textiles applied in fixed-bed column as filters for dye-rich textile wastewaters treatment—A case study. Desalin. Water Treat. 2013, 51, 3060–3068. [Google Scholar] [CrossRef]

| Cooling Wastewaters | Acid Polishing Wastewaters | ||||||
|---|---|---|---|---|---|---|---|
| Pb (mg/L) | Sb (mg/L) | pH | Pb (mg/L) | Sb (mg/L) | pH | SO42− (mg/L) | |
| Minimal | 0.2 | ˂DL * | 8.32 | 0.1 | ˂DL * | 7.3 | 1500 |
| Maximal | 4 | 1.8 | 10.44 | 2 | 0.4 | 9.1 | 3300 |
| Average | 1.08 | 0.45 | 9.18 | 0.6 | 0.2 | 7.8 | 2150 |
| Limit value ** | 0.5 | 0.3 | 6.5–9.5 | 0.5 | 0.3 | 6.5–9.5 | 1000 |
| Pb (mg/L) | Sb (mg/L) | SO42− (mg/L) | F− (mg/L) | pH | |
|---|---|---|---|---|---|
| Initial | 0.95 ± 0.05 | 0.9 ± 0.08 | 2750 ± 112 | 450 ± 15 | 9.92 ± 0.40 |
| Velocity flow | |||||
| 10 mL/h | 0.029 ± 0.01 | 0.18 ± 0.02 | 2115 ± 23 | 17 ± 2.7 | 7.82 ± 0.08 |
| 30 mL/h | 0.095 ± 0.01 | 0.29 ± 0.02 | 2270 ± 46 | 89 ± 3.5 | 7.95 ± 0.07 |
| 90 mL/h | 0.11 ± 0.04 | 0.37 ± 0.06 | 2390 ± 72 | 109 ± 10.4 | 8.05 ± 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojstršek, A.; Gorjanc, N.; Fakin, D. Reduction of Lead and Antimony Ions from the Crystal Glass Wastewaters Utilising Adsorption. Sustainability 2021, 13, 11156. https://doi.org/10.3390/su132011156
Ojstršek A, Gorjanc N, Fakin D. Reduction of Lead and Antimony Ions from the Crystal Glass Wastewaters Utilising Adsorption. Sustainability. 2021; 13(20):11156. https://doi.org/10.3390/su132011156
Chicago/Turabian StyleOjstršek, Alenka, Natalija Gorjanc, and Darinka Fakin. 2021. "Reduction of Lead and Antimony Ions from the Crystal Glass Wastewaters Utilising Adsorption" Sustainability 13, no. 20: 11156. https://doi.org/10.3390/su132011156






