Effect of Fluid Chemistry on the Consolidation and Hydraulic Conductivity of Sand-Clay Liners
Abstract
:1. Introduction and Objectives
2. Materials and Methods
2.1. General
2.1.1. Al-Qatif Clay
2.1.2. Sand
2.1.3. NaCl
2.1.4. CaCl2
2.1.5. Cement Material
2.1.6. Bentonite
2.2. Compaction Tests
2.3. Compressibility Tests
2.4. Hydraulic Conductivity
3. Results
3.1. General Test Results
3.2. Influence of NaCl Concentration
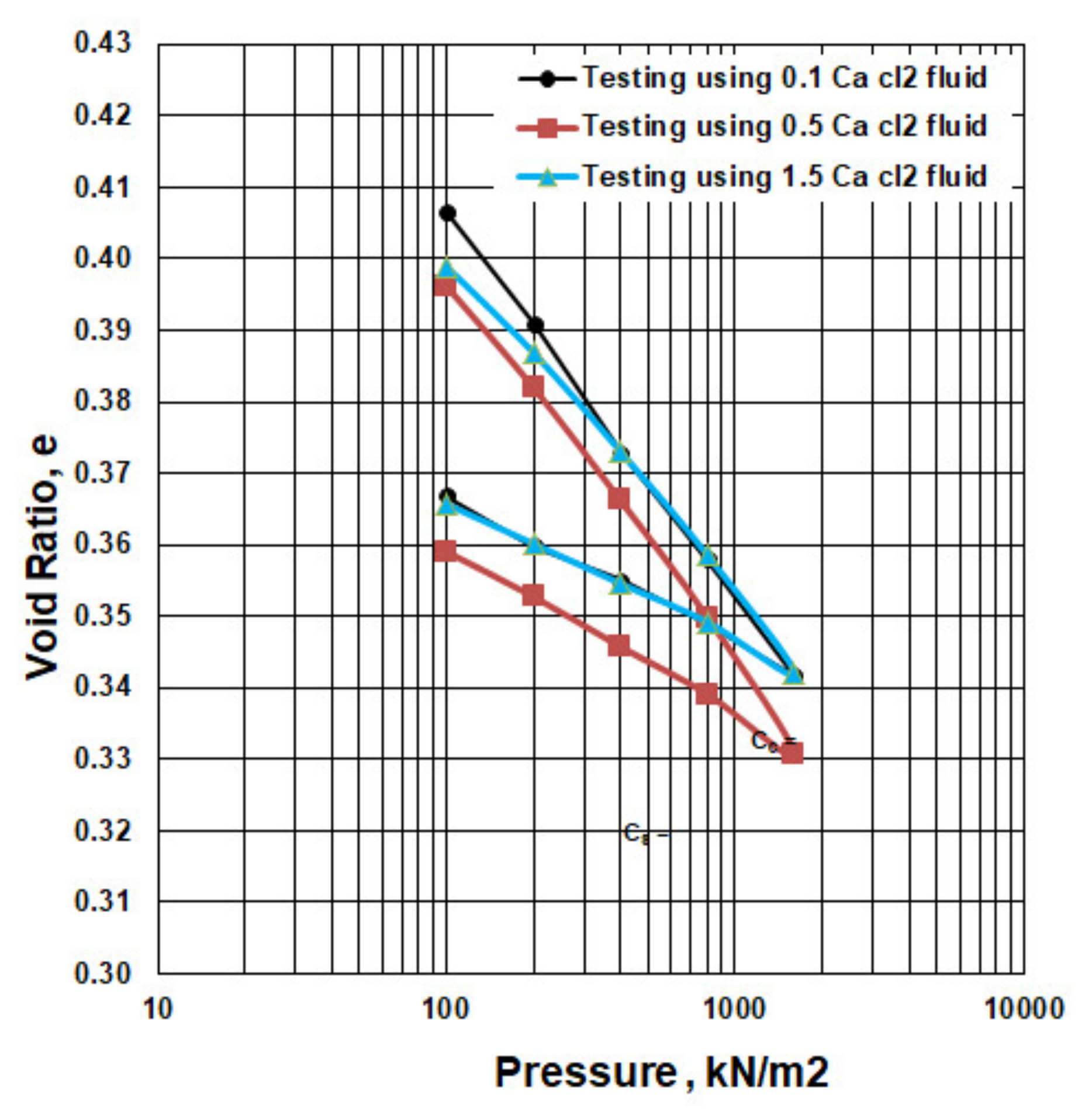
3.3. Influence of CaCl2 Concentration
3.4. Influence of pH
3.5. Influence of Cement Grout Addition
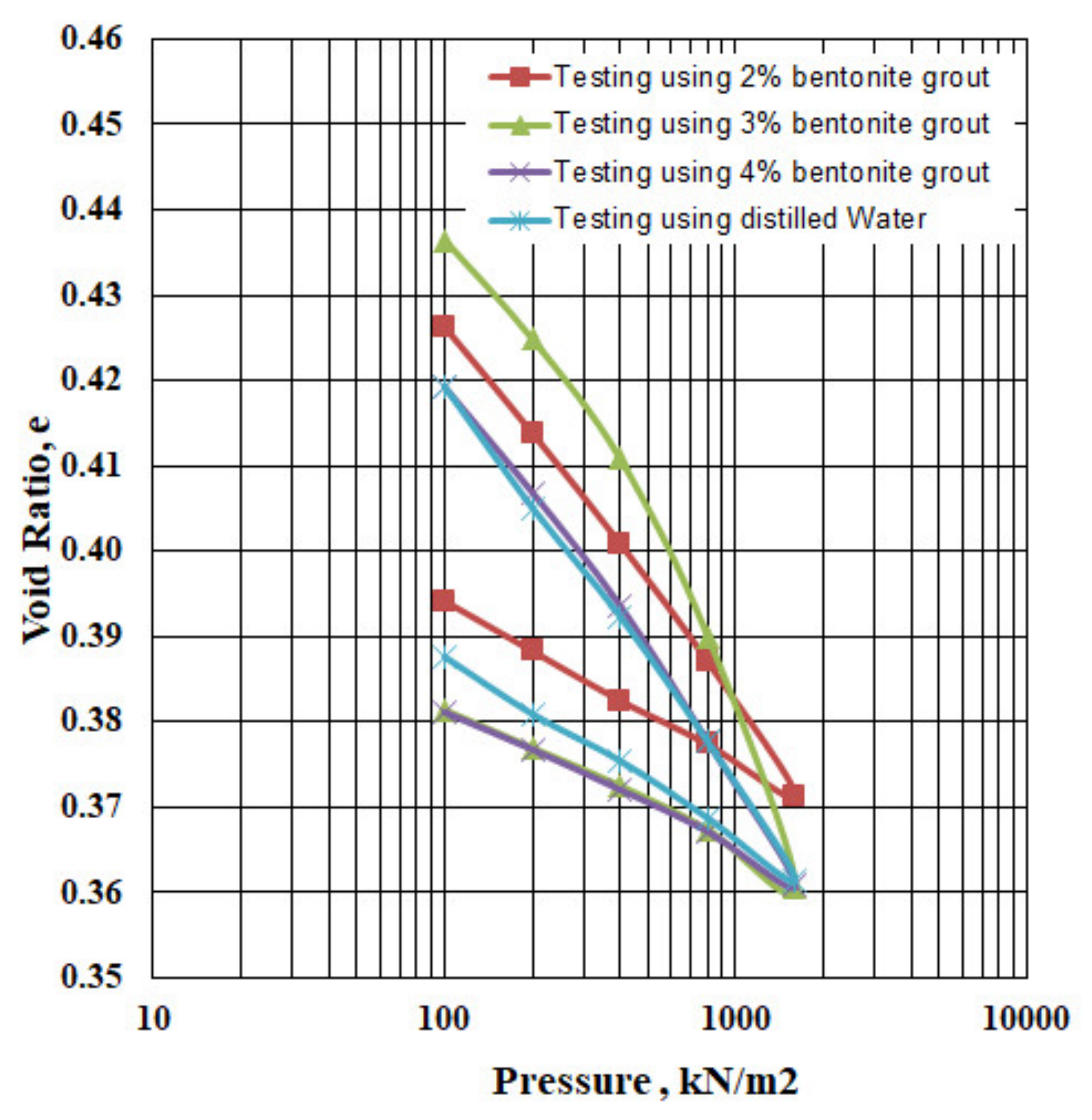
3.6. Influence of Bentonite Grout Addition
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolt, G.H. Physico-chemical analysis of the compressibility of pure clays. Géotechnique 1956, 6, 86–93. [Google Scholar] [CrossRef]
- Mitchell, J.K. Fundamentals of Soil Behavior; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Zein, A.K.M. Comparison of measured and predicted swelling behavior of a compacted black cotton soil. In Proceedings of the Sixth International Conference on Expansive Soils, New Delhi, India, 1–4 December 1987; pp. 121–126. [Google Scholar]
- Chen, F.H. Foundations on Expansive Soils; Elsevier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Dafalla, M.A.; Al-Sharmrani, M.A. Performance-based solutions for foundations on expansive soils—Al Ghatt Region, Saudi Arabia. In Proceedings of the GEO-CHIANGMAI 2008: An International Conference on Geotechnical Engineering, Chiangmai, Thailand, 10–12 December 2008. [Google Scholar]
- Wigger, C.; Van Loon, L.R. Effect of the pore water composition on the diffusive anion transport in argillaceous, low permeability sedimentary rocks. J. Contam. Hydrol. 2018, 213, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Langdon, N.J.; Al Hussaini, M.J.; Walden, P.J.; Sangha, C.M. An assessment of permeability of clay liners: Two case histories. In Engineering Geology Special Publications; Geological Society: London, UK, 2004; Volume 3. [Google Scholar]
- Obrike, S.E.; Osadebe, C.C.; Omoniyi, S.S. Geotechnical analysis of two Nigerian soils for use as clay liners. Bull. Eng. Geol. Environ. 2009, 68, 417–419. [Google Scholar] [CrossRef]
- Zumrawi, M.M.; Mahjoub, A.M.; Alnour, I.N. Effect of some chloride salts on swelling properties of expansive soil. Univ. Khartoum Eng. J. 2016, 6, 35–41. [Google Scholar]
- Alonso, E.E.; Vaunat, J.; Gens, A. Modelling the mechanical behaviour of expansive clays. Eng. Geol. 1999, 54, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Mollins, L.H. The Design of Bentonite-Sand Mixtures. Ph.D. Thesis, University of Leeds, Leeds, UK, 1996. [Google Scholar]
- Mollins, L.H.; Stewart, D.I.; Cousens, T.W. Predicting the properties of bentonite-sand mixtures. Clay Miner. 1996, 31, 243–252. [Google Scholar] [CrossRef]
- Studds, P.G. The Effect of Ionic Solutions on the Properties of Soil. Ph.D. Thesis, University of Leeds, Leeds, UK, 1997. [Google Scholar]
- Studds, P.G.; Stewart, D.I.; Cousens, T.W. The effects of salt solutions on the properties of bentonite-sand mixtures. Clay Miner. 1998, 33, 651–660. [Google Scholar] [CrossRef]
- Di Maio, C.; Santoli, L.; Schiavone, P. Volume Change Behaviour of Clays: The Influence of Mineral Composition, Pore Fluid Composition and Stress State. Mech. Mater. 2004, 36, 435–451. [Google Scholar] [CrossRef]
- Gajo, A.; Maines, M. Mechanical Effects of Aqueous Solutions of Inorganic Acids and Bases on a Natural Active Clay. Géotechnique 2007, 57, 687–699. [Google Scholar] [CrossRef]
- Deng, Y.F.; Yue, X.B.; Cui, Y.J.; Shao, G.H.; Liu, S.Y.; Zhang, D.W. Effect of Pore Water Chemistry on the Hydro-Mechanical Behaviour of Lianyungang Soft Marine Clay. Appl. Clay Sci. 2014, 95, 167–175. [Google Scholar] [CrossRef]
- Ashmawy, A.; Darwish, E.; Sotelo, N.; Muhammad, N. Hydraulic performance of untreated and polymer-treated bentonite in inorganic landfill leachates. Clays Clay Miner. 2002, 50, 546–552. [Google Scholar] [CrossRef]
- Tong, S.; Sample-Lord, K.M.; Bohnhoff, G.L. Diffusion through sodium and polymer enhanced bentonites exposed to dilute and aggressive solutions. Can. Geotech. J. 2021. [Google Scholar] [CrossRef]
- Zhang, F.; Ye, W.; Chen, Y.; Chen, B.; Cui, Y.J. Influences of Salt Solution Concentration and Vertical Stress During Saturation on the Volume Change Behavior of Compacted GMZ01 Bentonite. Eng. Geol. 2016, 207, 48–55. [Google Scholar] [CrossRef]
- Daniel, D.E. Geotechnical Practice for Waste Disposal; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Rowe, R.K.; Quigley, R.M.; Booker, J.R. Clayey Barrier Systems for Waste Disposal Facilities; E & FN Spon: London, UK, 1995; 404p. [Google Scholar]
- Azam, S.; Abduljauwad, S.N.; Al-Shayea, N.A.; Al-Amoudi, O.S.B. Expansive Characteristics of gypsiferous/Hydritic Formations. Eng. Geol. 1998, 51, 89–107. [Google Scholar] [CrossRef]
- Azam, S. Influence of Mineralogy on Swelling and Consolidation of Soils in Eastern Saudi Arabia. Can. Geotech. J. 2003, 40, 964–975. [Google Scholar] [CrossRef] [Green Version]
- Dafalla, M.A. The compressibility and swell of mixtures for sand-clay liners. Adv. Mater. Sci. Eng. 2017, 2017, 3181794. [Google Scholar] [CrossRef] [Green Version]
- ASTM D2435/D2435M-11. Standard Test Methods for One-Dimensional Consolidation Properties of Soils Using Incremental Loading; ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM D4546-96. Standard Test Methods for One-Dimensional Swell or Settlement Potential of Cohesive Soils; ASTM International: West Conshohocken, PA, USA, 1996.
- Zhang, T.; Wang, S. Explanation of the Influence of Sodium Chloride Solution on Volume Deformation and Permeability of Normally Consolidated Clays. Materials 2019, 12, 1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gueddouda, M.K.; Goual, I.; Lamara, M.; Smaida, A.; Mekarta, B. Chemical stabilization of expansive clays from Algeria. Glob. J. Res. Eng. 2011, 11, 1–8. [Google Scholar]
- He, Y.; Chen, Y.; Ye, W.; Chen, B.; Cui, Y. Influence of Salt Concentration on Volume Shrinkage and Water Retention Characteristics of Compacted GMZ Bentonite. Environ. Earth. Sci. 2016, 75, 535. [Google Scholar] [CrossRef]
- Belabbaci, Z.; Mamoune, S.M.A.; Bekkouche, A. Laboratory study of the influence of mineral salts on swelling (KCl, MgCl2). Earth Sci. Res. 2013, 2, 135–142. [Google Scholar] [CrossRef]
- Reddy, N.G.; Tahasildar, J.; Rao, B.H. Evaluating the Influence of Additives on Swelling Characteristics of Expansive Soils. Int. J. Geosynth. Ground Eng. 2015, 1, 40891. [Google Scholar] [CrossRef] [Green Version]
- Kolaventi, S.S.; Venigalla, S.G.; Rakesh, D. Stabilization of Black Cotton Soil using Salts and Their Comparative Analysis. Int. J. Eng. Dev. Res. 2016, 4, 797–800. [Google Scholar]
- Bouazza, A.; Gates, W.P. Overview of performance compatibility issues of GCLs with respect to leachates of extreme chemistry. Geosynth. Int. 2014, 21, 151–167. [Google Scholar] [CrossRef]
- Alkaya, D.; Esener, A.B. Usability of sand-bentonite-cement mixture in the construction of unpermeable layer. Scientific Res. Essays 2011, 6, 4492–4503. [Google Scholar]
- Yoon, J.; El Mohtar, C.S. Groutability of granular soils using sodium pyrophosphate modified bentonite suspensions. Tunn. Undergr. Space Technol. 2013, 37, 135–145. [Google Scholar] [CrossRef]
- Dawar, A.; Shah, Z.; Tassaddiq, A.; Islam, S.; Kumam, P. Joule heating in magnetohydrodynamic micropolar boundary layer flow past a stretching sheet with chemical reaction and microstructural slip. Case Stud. Therm. Eng. 2021, 25, 100870. [Google Scholar] [CrossRef]
- Zafar, Z.U.A.; Shah, Z.; Ali, N.; Kumam, P.; Alzahrani, E.O. Numerical study and stability of the Lengyel–Epstein chemical model with diffusion. Adv. Differ. Equ. 2020, 2020, 427. [Google Scholar] [CrossRef]
- Shah, Z.; Kumam, P.; Deebani, W. Radiative MHD Casson Nanofluid Flow with Activation energy and chemical reaction over past nonlinearly stretching surface through Entropy generation. Sci. Rep. 2020, 10, 4402. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Property | Range |
|---|---|
| Material passing sieve number 200 | >90% |
| Liquid Limit (%) | 130–150% |
| Plastic Limit (%) | 60–70% |
| Plasticity Index | 70–80 |
| Maximum Dry density (kN/m3) | 11.5–12 |
| Optimum Moisture Content | 32–40%. |
| Swell percent (ASTM D4546) | 16–18% |
| Swelling pressure (ASTM D4546) | 500–800 kN/m2 (γ = 12 kN/m2) |
| Al-Qatif Clay | |||||||
|---|---|---|---|---|---|---|---|
| K+ (%) | K2O (%) | Al3+ (%) | Al2O3 (%) | Si (%) | SiO2 (%) | Ca2+ (%) | CaO (%) |
| 1.8 | 2.2 | 3.3 | 6.3 | 8.1 | 17.3 | 0.7 | 0.9 |
| HY OCMA Bentonite | |||||||
| FeO3 (%) | K2O (%) | Na2O (%) | Al2O3 (%) | MgO (%) | SiO2 (%) | TiO2 (%) | CaO (%) |
| 2.9 | 0.1 | 1.9 | 17.0 | 4.6 | 55.2 | <0.1 | 0.9 |
| Chemical Media | Concentration | e1 (100 kN/m2) | e2 (200 kN/m2) | mv (m2/kN) | Cv (200) (m2/Year) | k (cm/s × 10−8) | Cc (100–200) kN/m2 | Cr (100–200 kN/m2) |
|---|---|---|---|---|---|---|---|---|
| NaCl | ||||||||
| 0.1 M | 0.407 | 0.394 | 9.71 × 10−5 | 1.3198 | 0.399 | 0.0452 | 0.021 | |
| 0.5 M | 0.404 | 0.392 | 8.37 × 10−5 | 1.1252 | 0.293 | 0.0389 | 0.019 | |
| 1.5 M | 0.374 | 0.361 | 9.21 × 10−5 | 1.6622 | 0.477 | 0.0419 | 0.016 | |
| CaCl2 | ||||||||
| 0.1 M | 0.4067 | 0.391 | 0.000112235 | 2.445 | 0.853 | 0.0522 | 0.0236 | |
| 0.5 M | 0.3962 | 0.382 | 0.000102224 | 1.670 | 0.531 | 0.0472 | 0.0209 | |
| 1.5 M | 0.3989 | 0.3869 | 8.61512 × 10−5 | 2.986 | 0.800 | 0.0399 | 0.0183 | |
| Acidic Water | ||||||||
| pH = 4 | 0.4065 | 0.3942 | 8.78352 × 10−5 | 2.9968 | 0.819 | 0.049 | 0.021 | |
| pH = 5 | 0.4097 | 0.3978 | 8.47729 × 10−5 | 3.0114 | 0.794 | 0.039 | 0.020 | |
| pH = 6 | 0.4047 | 0.3895 | 0.000108797 | 2.0640 | 0.699 | 0.051 | 0.022 | |
| DW (Distilled) | pH = 7 | 0.4193 | 0.4051 | 0.000100552 | 2.1989 | 0.688 | 0.047 | 0.022 |
| Cement grout | ||||||||
| 2.5% by wt | 0.4297 | 0.4196 | 7.08946 × 10−5 | 1.6397 | 0.362 | 0.034 | 0.012 | |
| 5.0% by wt | 0.4289 | 0.4186 | 7.23442 × 10−5 | 3.6469 | 0.821 | 0.034 | 0.014 | |
| 7.5% by wt | 0.4303 | 0.4211 | 6.45297 × 10−5 | 4.4287 | 0.889 | 0.031 | 0.008 | |
| Bentonite grout | ||||||||
| 2% by wt | 0.4263 | 0.4139 | 8.73178 × 10−5 | 1.5329 | 0.416 | 0.041 | 0.020 | |
| 3% by wt | 0.4365 | 0.4249 | 8.10792 × 10−5 | 2.1958 | 0.554 | 0.038 | 0.015 | |
| 4% by wt | 0.4192 | 0.4069 | 8.70458 × 10−5 | 1.8745 | 0.506 | 0.041 | 0.014 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dafalla, M. Effect of Fluid Chemistry on the Consolidation and Hydraulic Conductivity of Sand-Clay Liners. Sustainability 2021, 13, 11213. https://doi.org/10.3390/su132011213
Dafalla M. Effect of Fluid Chemistry on the Consolidation and Hydraulic Conductivity of Sand-Clay Liners. Sustainability. 2021; 13(20):11213. https://doi.org/10.3390/su132011213
Chicago/Turabian StyleDafalla, Muawia. 2021. "Effect of Fluid Chemistry on the Consolidation and Hydraulic Conductivity of Sand-Clay Liners" Sustainability 13, no. 20: 11213. https://doi.org/10.3390/su132011213






