Effects of Different Ingredients and Stabilisers on Properties of Mixes Based on Almond Drink for Vegan Ice Cream Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrolysates Preparation
2.2. Ice Crem Mixes
2.2.1. Materials for Use for Different Ice-Cream Mix Recipes
2.2.2. The Ice-Cream Mix Production
2.3. Ice-Cream Mix Properties Analysis
2.3.1. Density
2.3.2. Particle Size Distribution
2.3.3. Emulsion Stability Evaluation
2.3.4. Rheological Properties
2.3.5. Microstructural Analysis of Ice-Cream Mix
2.3.6. Statistical Analysis
3. Results and Discussion
3.1. The Density of Ice-Cream Mix
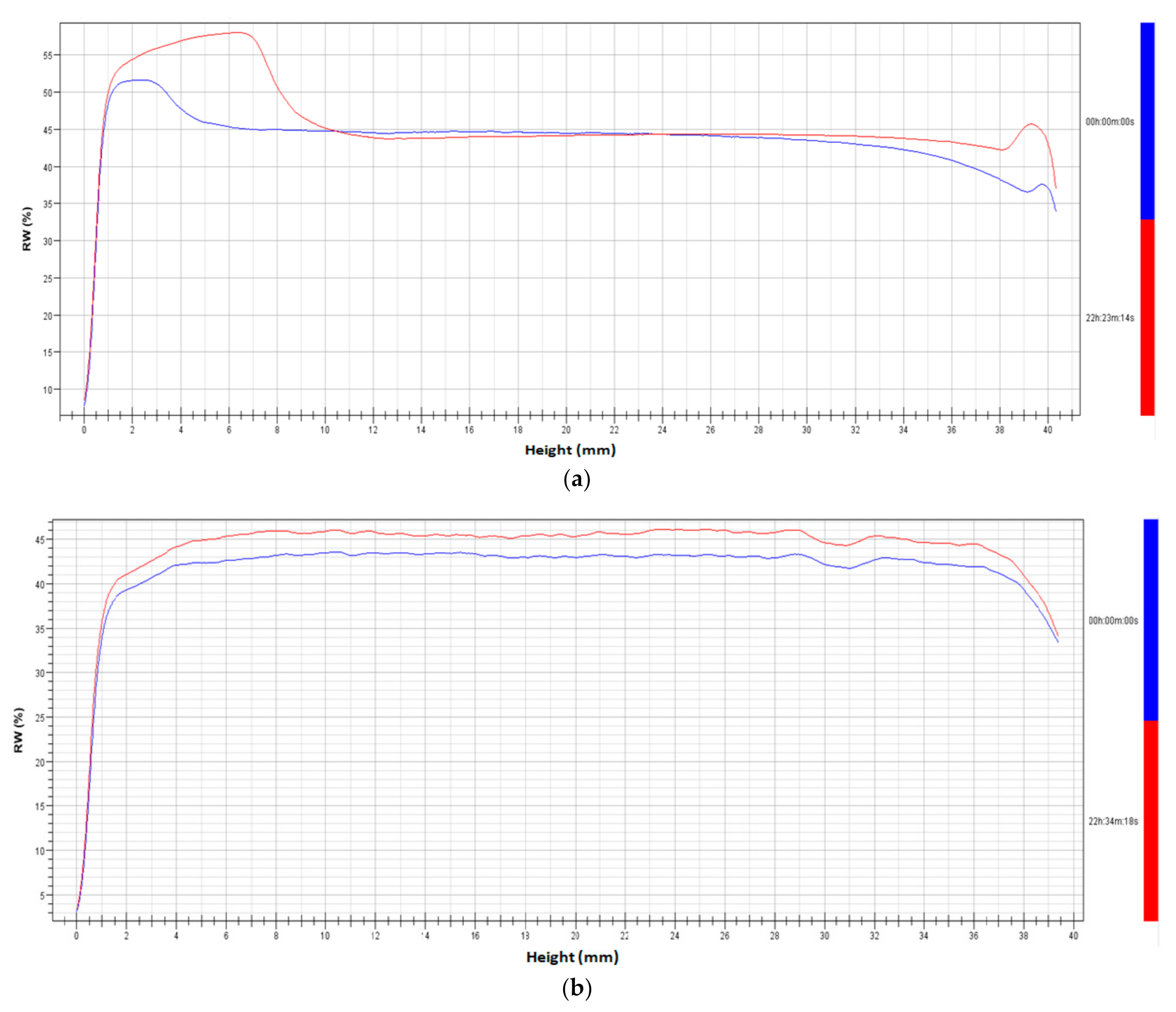
3.2. The Stability of Ice-Cream Mix
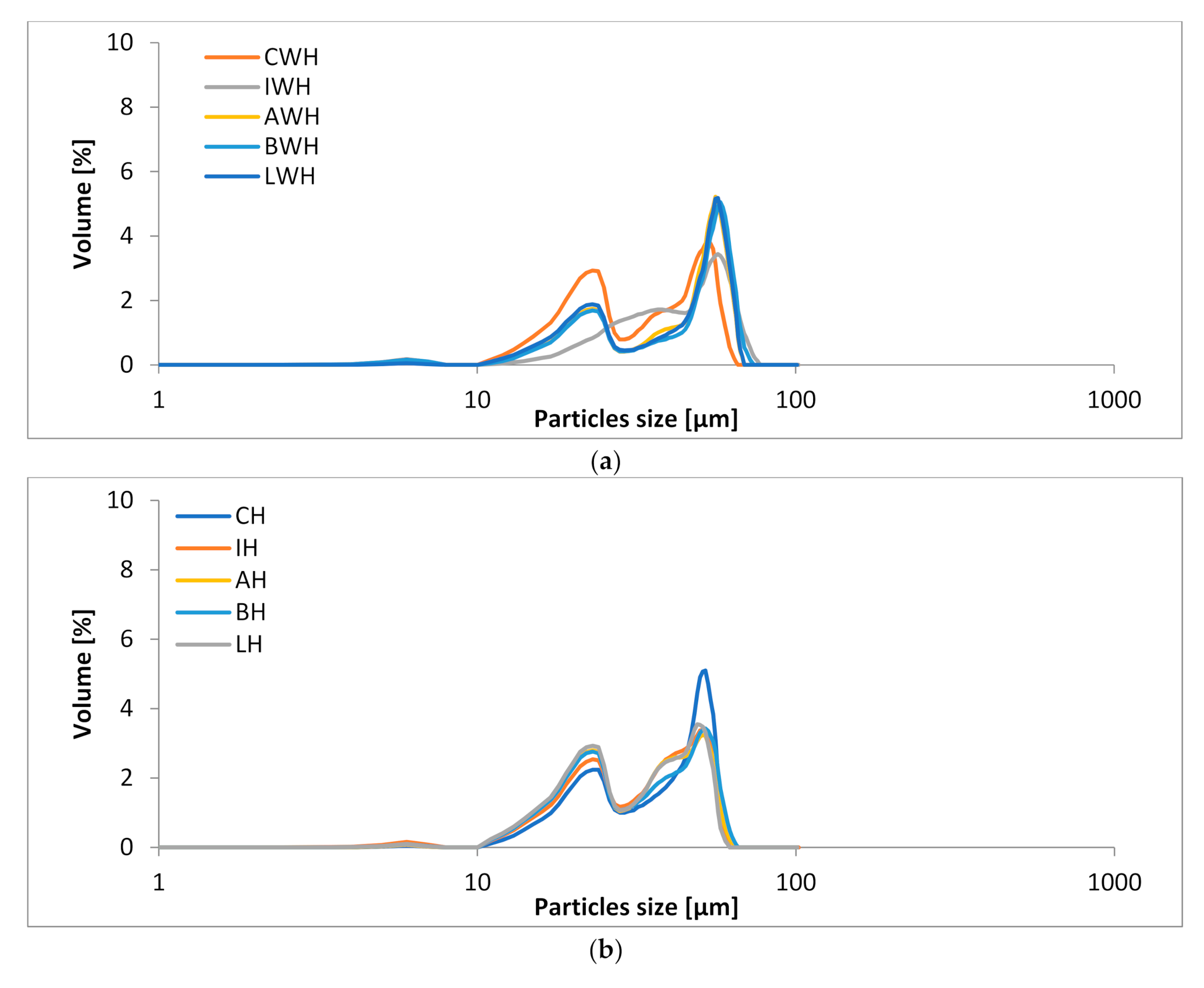
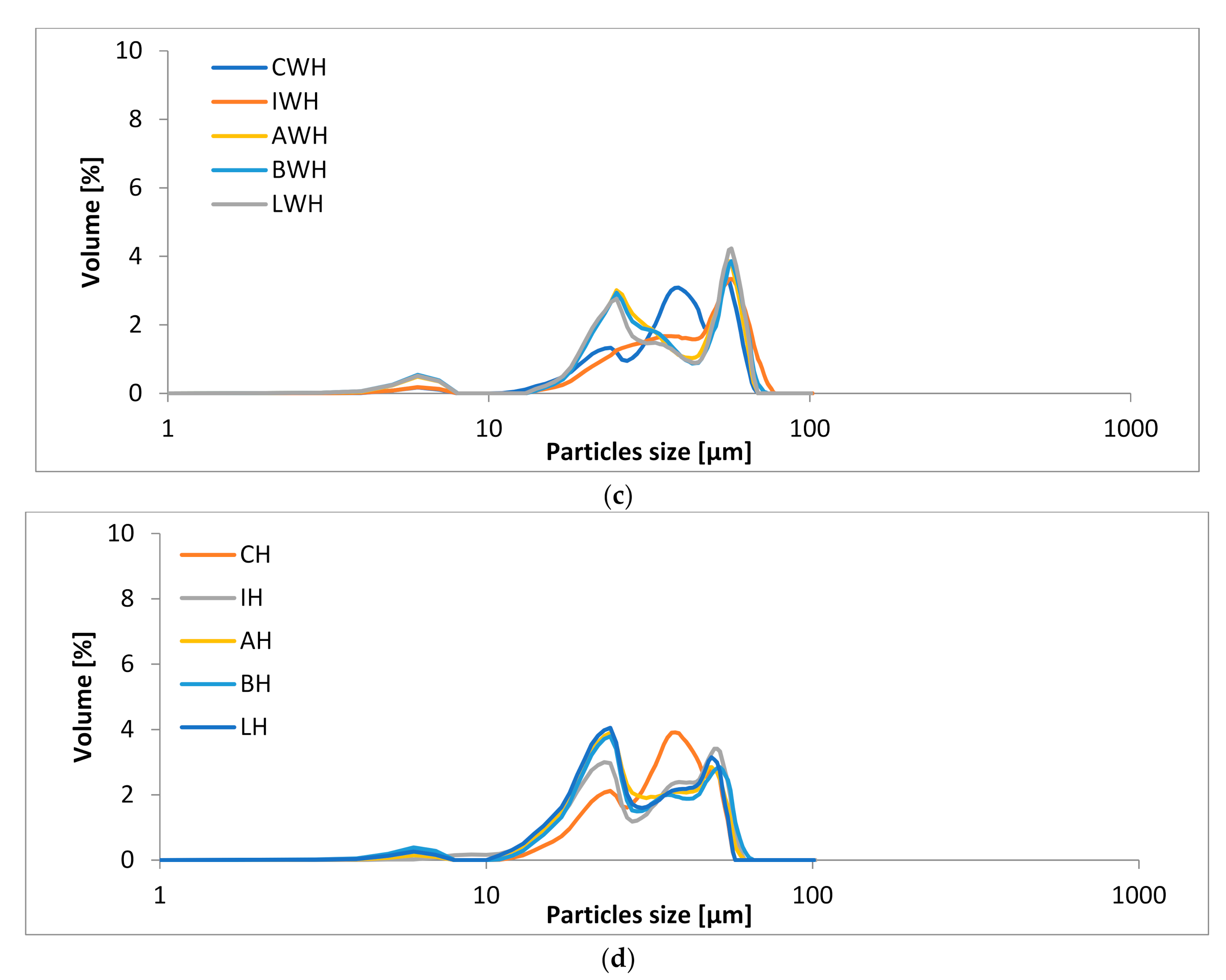
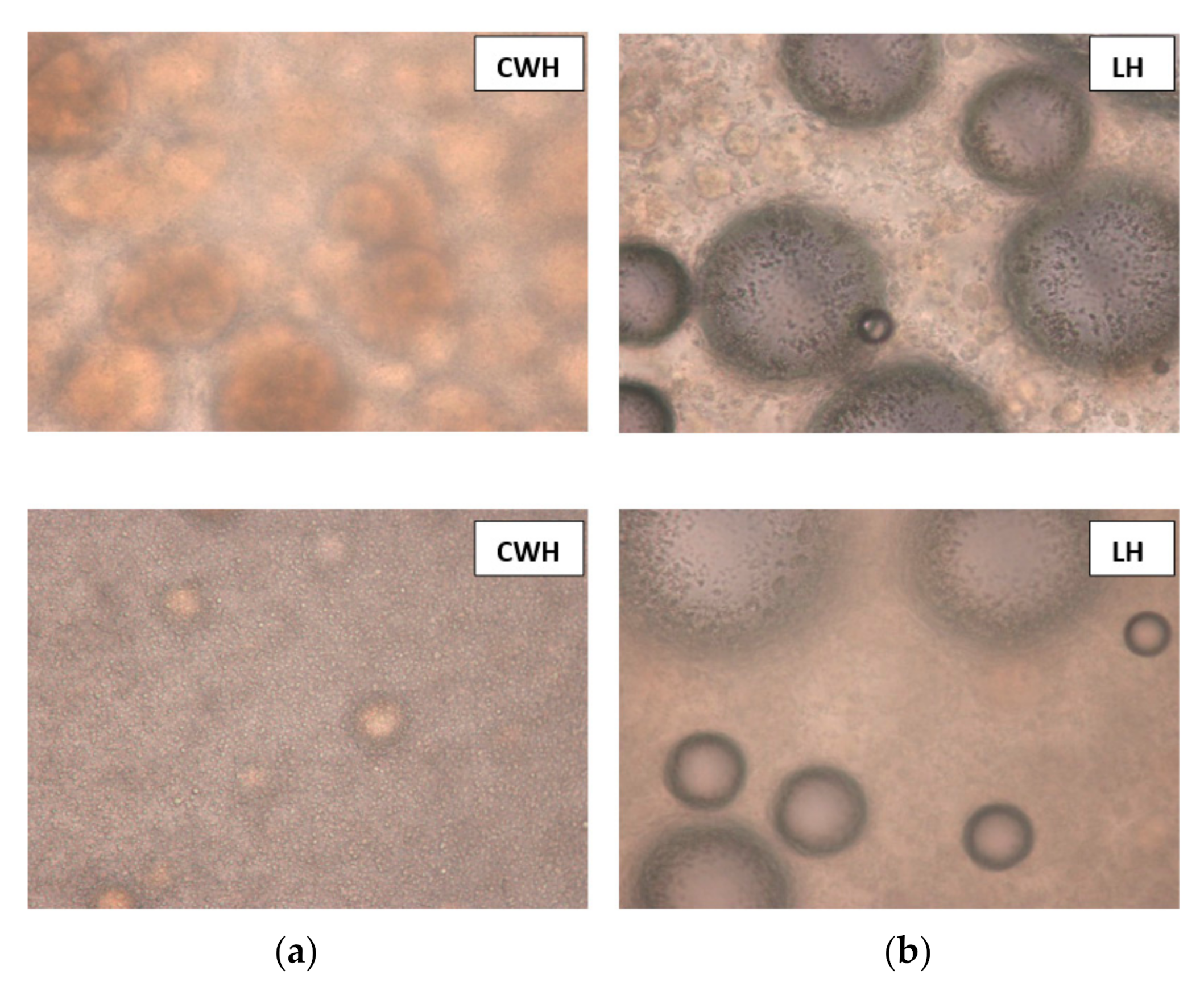
3.3. The Particle Sizes of Ice-Cream Mixes
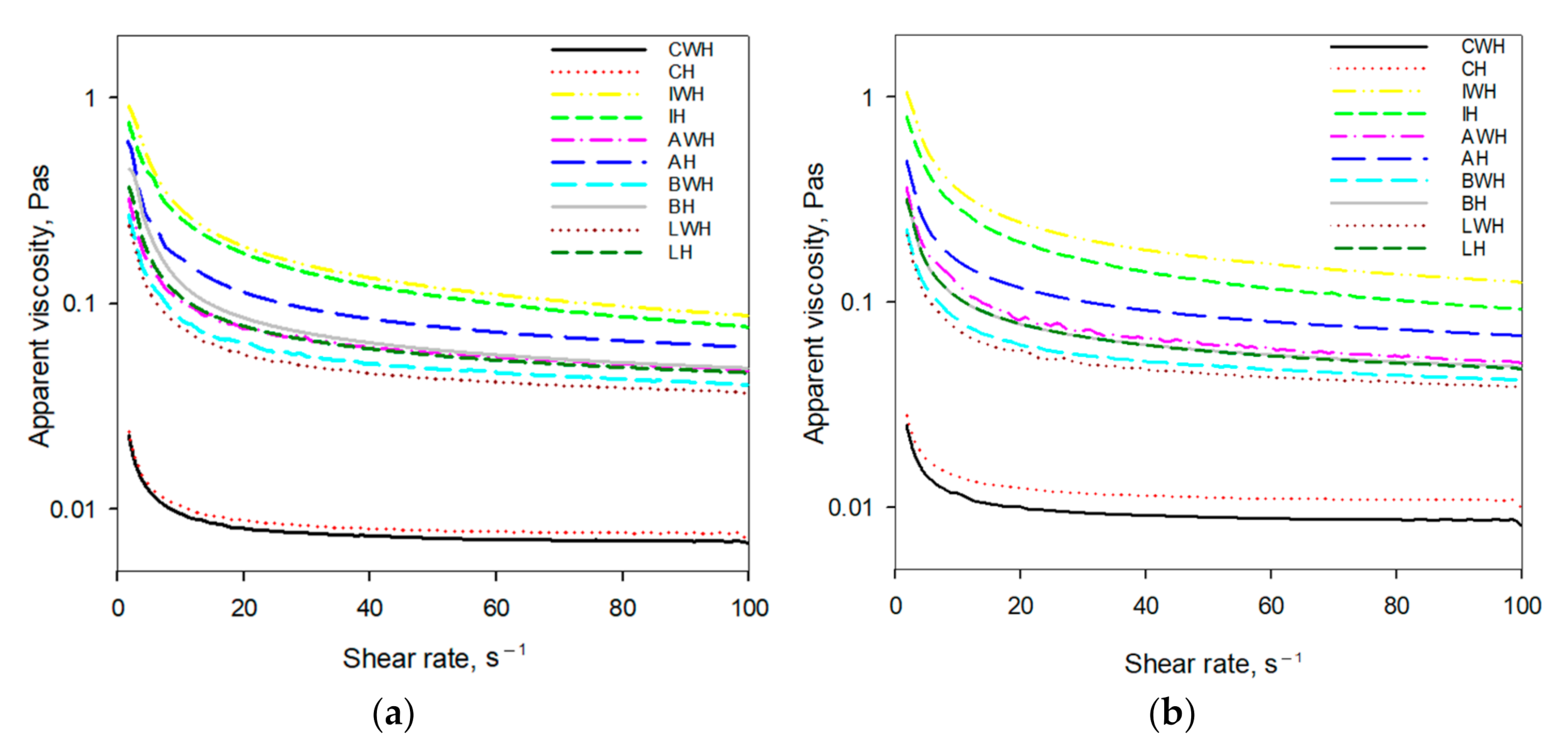
3.4. The Rheological Properties
3.5. The Microscopic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konstantas, A.; Stamford, L.; Azapagic, A. Environmental impacts of ice cream. J. Clean. Prod. 2019, 209, 259–272. [Google Scholar] [CrossRef]
- Sandström, V.; Valin, H.; Krisztin, T.; Havlik, P.; Herrero, M.; Kastner, T. The role of trade in the greenhouse gas footprints of EU diets. Glob. Food Secur. 2018, 19, 48–55. [Google Scholar] [CrossRef]
- Saget, S.; Costa, M.; Santos, C.S.; Vasconcelos, M.W.; Gibbons, J.; Styles, D.; Williams, M. Substitution of beef with pea protein reduces the environmental footprint of meat balls whilst supporting health and climate stabilization goals. J. Clean. Prod. 2021, 297, 126447. [Google Scholar] [CrossRef]
- Krizanova, J.; Rosenfeld, L.D.; Tomiyama, J.A.; Guardiola, J. Pro-environmental behaviour predicts adherence to plant-based diets. Appetite 2021, 163, 105243. [Google Scholar] [CrossRef] [PubMed]
- Baukid, F.; Rosell, C.M.; Castellari, M. Pea protein ingredients: A mainstream ingredient to (re)formulate innovative foods and beverages. Trends Food Sci. Technol. 2021, 110, 729–742. [Google Scholar] [CrossRef]
- Vanga, S.K.; Raghavan, V. How well do plant based alternatives fare nutritionally compared to cow’s milk? J. Food Sci. Technol. 2018, 55, 10–20. [Google Scholar] [CrossRef]
- Akdeniz, V.; Akalin Sibel, A. New approach for yoghurt and ice cream production: High intensity ultrasound. Trends Food Sci. Technol. 2019, 86, 392–398. [Google Scholar] [CrossRef]
- Fiol, C.; Prado, D.; Romero, C.; Laburu, N.; Mora, M.; Alava, I.J. Introduction of a new family of ice creams. Int. J. Gastron. Food Sci. 2017, 7, 5–10. [Google Scholar] [CrossRef]
- Innocente, N.; Biasutti, M.; Venir, E.; Spaziani, M.; Marchesini, G. Effect of high-pressure homogenization on droplet size distribution and rheological properties of ice cream mixes. J. Dairy Sci. 2009, 92, 1864–1875. [Google Scholar] [CrossRef]
- Tolstoguzov, V. Some thermodynamic considerations in food formulation. Food Hydrocoll. 2003, 17, 1–23. [Google Scholar] [CrossRef]
- Yüksel, A.; Şat, İ.; Yüksel, M.; Çakmakçı, S. A comparative study on the physicochemical characteristics, organic acid profiles, mineral compositions and sensory properties of ice creams produced with different types of nuts. Carpathian J. Food Sci. Technol. 2017, 9, 137–151. [Google Scholar]
- O’Neli, C.E.; Nicklas, T.A.; Flugoni, V.L., III. Almond consumption is associated with better nutrient intake, nutrient adequacy, and diet quality in adult: National health and nutrition examination survey 2001–2010. Food Sci. Nutr. 2016, 7, 504–515. [Google Scholar]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef]
- Watzl, B.; Girrbach, S.; Roller, M. Inulin, oligofructose and immunomodulation. Br. J. Nutr. 2005, 93 (Suppl. 1), S49–S55. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Beevers, J.; Park, M.; Greenwood, R.; Norton, I. Comparative assessment of the effect of ultrasound treatment on protein functionality pre- and post-emulsification. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 89–98. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, J.; Murray, B.; Flynn, C.R.; Norton, I. The effect of ultrasound on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016, 53, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Voronin, G.L.; Roberts, R.; Felix, T.L.; Coupland, J.N.; Harte, F.M. Effect of high-pressure-jest processing on the physiochemical properties of low-fat ice cream mix. J. Dairy Sci. 2020, 103, 6003–6014. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Saxena, A. Bacterial carrageenans: An overview of production and biotechnological applications. 3 Biotech 2016, 6, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiran-Yildirim, B.; Hale, J.; Wefers, D.; Gaukel, V. Ice recrystallisation inhibition of commercial κ-, ι-, and λ-carrageenans. J. Food Eng. 2021, 290, 110269. [Google Scholar] [CrossRef]
- Weiner, L.M. Toxicological properties of carrageenan. Agents Actions 1991, 32, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Thrimawithana, T.; Young, S.; Dunstan, D.; Alany, R. Texture and rheological characterisation of kappa and iota carrageenan in the presence of counter ions. Carbohydr. Polym. 2010, 82, 69–77. [Google Scholar] [CrossRef]
- Henares, B.M.; Enriquez, E.P.; Dayrit, F.M.; Rojas, N.R.L. Iota-carrageenan hydrolysis by Pseudoalteromonas carrageenovora IFO12985. Philipp. J. Sci. 2010, 139, 131–138. [Google Scholar]
- Kamińska-Dwórznicka, A.; Antczak, A.; Samborska, K.; Lenart, A. Acid hydrolysis of kappa-carrageenan as a way of gaining new substances for freezing process modification and protection from excessive recrystallisation of ice. Int. J. Food Sci. Technol. 2015, 50, 1799–1806. [Google Scholar] [CrossRef]
- Kamińska-Dwórznicka, A.; Skrzypczak, P.; Gondek, E. Modification of kappa carrageenan by β-galactosidase as a new method to inhibit recrystallization of ice. Food Hydrocoll. 2016, 61, 31–35. [Google Scholar] [CrossRef]
- Dłużewska, E.; Gazda, B.; Leszczyński, K. Wpływ wybranych hydrokoloidów polisacharydowych na jakość koncentratów lodów owocowych. Acta Sci. Pol. Technol. Aliment. 2003, 2, 97–107. [Google Scholar]
- Goff, H.D.; Hartel, R.W. Ice Cream, 7th ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Zambrano-Mayorga, L.; Ramirez-Navas, J.; Ochoa-Martinez, C.I. Influence of the formulation on the thermophysical properties and the quality parameters of diary ice cream. DYNA 2019, 86, 117–125. [Google Scholar] [CrossRef]
- Florowska, A.; Wójcik, E.; Florowski, T.; Dłużewska, E. Wpływ dodatków preparatów błonnikowych na wybrane wyróżniki jakości lodów. Zesz. Probl. Postępów Nauk Rol. 2013, 574, 11–18. [Google Scholar]
- Warren, M.M.; Hartel, R.W. Structural, compositional, and sensorial properties of United States commercial ice cream products. J. Food Sci. 2014, 79, E2005-13. [Google Scholar] [CrossRef]
- Alves, A.B.; Santos, W.F.D., Jr.; Faria, W.C.S.; Nascimento, E.; Lanzarin, M.; Siqueria, P.B.; Siqueria, N.F.M.P.; Barros, W.M.D. Developmnet, characterization, and shelf-life of lactose-free artisan ice cream produces with different bases. Res. Soc. Dev. 2021, 10, 1–16. [Google Scholar]
- Hu, Y.; Ting, Y.; Hu, J.; Hsieh, S. Techniques and methods to study functional characteristics of emulsion systems. J. Food Drug Anal. 2017, 25, 16–26. [Google Scholar] [CrossRef]
- Mengual, O.; Meunier, G.; Cayre, I.; Puech, K.; Snabre, P. Characterisation of instability of concentrated dispersion by a new optical analyser: The TURBISCAN MA 1000. Colloids Surf. A Physicochem. Eng. Asp. 1999, 152, 111–123. [Google Scholar] [CrossRef]
- Cheng, J.; Ma, Y.; Li, X.; Yan, T.; Cui, J. Effects of milk protein-polysaccharide interactions on the stability of ice cream mix model systems. Food Hydrocoll. 2015, 45, 327–336. [Google Scholar] [CrossRef]
- Keeney, P.G. The fat stability problem in ice cream. Ice Cream Rev. 1958, 8, 42–45. [Google Scholar]
- Berger, K.G.; White, G.W. An electron microscopical investigation of fat destabilization in ice cream. J. Food Technol. 1971, 6, 285–294. [Google Scholar] [CrossRef]
- Berger, K.G.; Bullimore, B.K.; White, G.W.; Wright, W.B. The structure of ice cream. Dairy Ind. 1972, 419–425, 493–497. [Google Scholar]
- Koxholt, M.; Eisenmann, B.; Hinrichs, J. Effect of the fat globule size on the meltdown of ice cream. J. Dairy Sci. 2001, 84, 31–37. [Google Scholar] [CrossRef]
- Biasutti, M.; Venir, E.; Marino, M.; Maifreni, M.; Innocente, N. Effects of high pressure homogenisation of ice cream mix on the physical and structural properties of ice cream. Int. Dairy J. 2013, 32, 40–45. [Google Scholar] [CrossRef]
- Warren, M.M.; Hartel, R.W. Effects of emulsifier, overrun and dasher speed on ice cream microstructure and melting properties. J. Food Sci. 2018, 83, 639–647. [Google Scholar] [CrossRef]
- Kurt, A.; Atalar, I. Effects of quince seed on the rheological, structural and sensory characteristics of ice cream. Food Hydrocoll. 2018, 82, 186–195. [Google Scholar] [CrossRef]
- Javidi, F.; Razavi, S.M.; Behrouzian, F.; Alghooneh, A. The influence of basil seed gum, guar gum and their blend on the rheological, physical and sensory properties of low fat ice cream. Food Hydrocoll. 2016, 52, 625–633. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Tehrani, M.M.; Mohebbi, M. Rheological and sensory properties of fat reduced vanilla ice creams containing milk protein concentrate (MPC). J. Food Meas. Charact. 2017, 11, 567–575. [Google Scholar] [CrossRef]
- BahramParvar, M.; Razavi, S.M.; Khodaparast, M.H. Rheological characterization and sensory evaluation of a typical soft ice cream made with selected food hydrocolloids. Food Sci. Technol. Int. 2010, 16, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Atalar, I.; Kurt, A.; Gul, O.; Yazici, F. Improved physicochemical, rheological and bioactive properties of ice cream: Enrichment with high pressure homogenized hazelnut milk. Int. J. Gastron. Food Sci. 2021, 24, 100358. [Google Scholar] [CrossRef]
- Dogan, M.; Kayacier, A. The effect of ageing at a low temperature on the rheological properties of Kahramanmaras-type ice cream mix. Int. J. Food Prop. 2007, 10, 19–24. [Google Scholar] [CrossRef]
- Rao, M.A. Rheology of Fluid And Semisolid Foods: Principles and Applications; Springer Science & Business Media: New York, NY, USA, 2007. [Google Scholar]
| Sample | Characteristics |
|---|---|
| CWH | control sample without the stabilisers and without the homogenisation treatment |
| CH | control sample without the mix of stabilisers and with the homogenisation treatment |
| IWH | sample with the addition of the stabilisers (ɩ-carrageenan, locust bean gum, xanthan gum) and without the homogenisation treatment |
| IH | sample with the addition of the stabilisers (ɩ-carrageenan, locust bean gum, xanthan gum) and with the homogenisation treatment |
| AWH | sample with the addition of the stabilisers (the acid hydrolysates of ɩ-carrageenan, locust bean gum, xanthan gum) and without the homogenisation treatment |
| AH | sample with the addition of the stabilisers (the acid hydrolysates of ɩ-carrageenan, locust bean gum, xanthan gum) and with the homogenisation treatment |
| BWH | sample with the addition of the stabilisers (the enzymatic β-galactosidase hydrolysates of ɩ-carrageenan, locust bean gum, xanthan gum) and without the homogenisation treatment |
| BH | sample with the addition of the stabilisers (the enzymatic β-galactosidase hydrolysates of ɩ-carrageenan, locust bean gum, xanthan gum) and with the homogenisation treatment |
| LWH | sample with the addition of the stabilisers (the enzymatic commercial lactase hydrolysates of ɩ-carrageenan, locust bean gum, xanthan gum) without the homogenisation treatment |
| LH | sample with the addition of the stabilisers (the enzymatic commercial lactase hydrolysates of ɩ-carrageenan, locust bean gum, xanthan gum) and with the homogenisation treatment |
| Sample | Density [g/cm3] | Density after 24 h of Maturation [g/cm3] |
|---|---|---|
| CWH | 1.00 ± 0.03 a,b | 1.00 ± 0.03 a |
| CH | 1.00 ± 0.03 a,b | 1.00 ± 0.03 a |
| IWH | 1.09 ± 0.11 b | 1.09 ± 0.11 a |
| IH | 0.98 ± 0.06 a,b | 0.98 ± 0.06 a |
| AWH | 1.00 ± 0.03 a,b | 0.98 ± 0.03 a |
| AH | 0.94 ± 0.03 a | 0.98 ± 0.02 a |
| BWH | 1.00 ± 0.03 a,b | 1.01 ± 0.02 a |
| BH | 0.94 ± 0.05 a | 0.99 ± 0.03 a |
| LWH | 0.99 ± 0.03 a,b | 1.01 ± 0.04 a |
| LH | 0.92 ± 0.03 a | 0.97 ± 0.03 a |
| Sample | TSI |
|---|---|
| CWH | 2.1 |
| CH | 3.0 |
| IWH | 2.3 |
| IH | 4.1 |
| AWH | 2.1 |
| AH | 6.4 |
| BWH | 3.6 |
| BH | 6.5 |
| LWH | 1.7 |
| LH | 5.2 |
| Sample | D50 | D50 after 24 h of Maturation |
|---|---|---|
| CWH | 15.65 ± 0.62 c | 17.23 ± 0.49 d |
| CH | 20.54 ± 0.45 d | 11,35 ± 0.48 b |
| IWH | 30.40 ± 0.44 e | 28.50 ± 0.43 e |
| IH | 13.77 ± 0.19 b | 11.51 ± 0.94 b |
| AWH | 38.36 ± 0.49 f | 11.41 ± 0.97 b |
| AH | 13.06 ± 0.53 a,b | 6.33 ± 0.12 a |
| BWH | 42.15 ± 0.19 g | 11.29 ± 0.53 b |
| BH | 13.42 ± 0.40 b | 6.88 ± 0.33 a |
| LWH | 38.31 ± 0.66 f | 13.20 ± 0.45 c |
| LH | 11.91 ± 0.16 a | 6.37 ± 0.48 a |
| Sample | Coefficients | Bingham Model | Ostwald de Waele Model | Herschel–Bulkley Model |
|---|---|---|---|---|
| CWH | χ2 | 1511.1 | 5629.7 | 26.4 |
| R | 0.9998 | 0.9992 | 0.9999 | |
| CH | χ2 | 3794.6 | 7782.7 | 41.0 |
| R | 0.9991 | 0.9988 | 0.9999 | |
| IWH | χ2 | 6,400,003.1 | 653,333.3 | 5252 |
| R | 0.9848 | 0.9844 | 0.9999 | |
| IH | χ2 | 9,023,333.3 | 656,333.3 | 7259 |
| R | 0.9840 | 0.9989 | 0.9999 | |
| AWH | χ2 | 897,667.6 | 402,666.7 | 7436.5 |
| R | 0.9970 | 0.9987 | 0.9993 | |
| AH | χ2 | 1,393,333.3 | 1,823,333.3 | 8200.3 |
| R | 0.9961 | 0.9958 | 0.9998 | |
| BWH | χ2 | 476,000.0 | 497,667.0 | 16,984.1 |
| R | 0.9978 | 0.9977 | 0.9984 | |
| BH | χ2 | 826,333.3 | 131,000.0 | 5919.7 |
| R2 | 0.9979 | 0.9951 | 0.9998 | |
| LWH | χ2 | 252,666.7 | 281,666.7 | 7536.3 |
| R | 0.9983 | 0.9985 | 0.9996 | |
| LH | χ2 | 522,666.7 | 611,100.0 | 831.3 |
| R | 0.9973 | 0.9978 | 1.0000 |
| Sample | Coefficients | Bingham Model | Ostwald De Waele Model | Herschel–Bulkley Model |
|---|---|---|---|---|
| CWH | χ2 | 13,470.0 | 28,005.6 | 5101.1 |
| R | 0.9988 | 0.9974 | 0.9997 | |
| CH | χ2 | 4144.7 | 9231.7 | 19.4 |
| R | 0.9996 | 0.9995 | 1.0000 | |
| IWH | χ2 | 122,000.0 | 798,333.3 | 9784.3 |
| R | 0.9871 | 0.9991 | 0.9997 | |
| IH | χ2 | 13,900,000.0 | 2,810,000.0 | 2787.7 |
| R | 0.9925 | 0.9985 | 1.0000 | |
| AWH | χ2 | 862,000 | 604,666.7 | 21,495.1 |
| R | 0.9975 | 0.9982 | 0.9990 | |
| AH | χ2 | 2,513,333.3 | 611,666.7 | 383.0 |
| R | 0.9958 | 0.9989 | 1.0000 | |
| BWH | χ2 | 495,666.7 | 327,000.0 | 1537.1 |
| R | 0.9980 | 0.9921 | 0.9999 | |
| BH | χ2 | 852,333.3 | 327,333.3 | 125.0 |
| R | 0.9972 | 0.9989 | 1.0000 | |
| LWH | χ2 | 291,114.0 | 187,333.3 | 4069 |
| R | 0.9987 | 0.9991 | 0.9997 | |
| LH | χ2 | 939,000.0 | 230,666.7 | 166.7 |
| R | 0.9968 | 0.9990 | 1.0000 |
| Sample | Ageing Time, h | τo, 10−3 Pa | K, 10−3 Pa sn | n |
|---|---|---|---|---|
| CWH | 0 | 26.6 ± 0.6 a | 6.8 ± 2.0 a | 0.991 ± 0.005 i |
| 24 | 28.6 ± 1.9 a | 9.4 ± 1.8 a | 0.978 ± 0.004 g,h,i | |
| CH | 0 | 27.6± 0.8 a | 7.8 ± 1.3 a | 0.979 ± 0.010 h,i |
| 24 | 29.9 ± 1.2 a | 11.8 ± 3.2 a | 0.949 ± 0.036 f,g,h,i | |
| IWH | 0 | 1080.3 ± 49.9 f | 336.0 ± 14.7 h,i | 0.653 ± 0.011 a |
| 24 | 910.8 ± 113.1 e,f | 462.3 ± 65.7 j | 0.633 ± 0.029 a | |
| IH | 0 | 1410.3 ± 316.1 g | 255.6 ± 78.4 g,h | 0.742 ± 0.056 b |
| 24 | 1508.3 ± 119.1 g | 349.2 ± 21.7 i | 0.757 ± 0.011 b,c | |
| AWH | 0 | 444.3 ± 65.7 c | 75.7 ± 9.7 c,d | 0.889 ± 0.030 e,f,g |
| 24 | 509.8 ± 7.3 c,d | 67.7 ± 1.8 c | 0.927 ± 0.006 e,f,g,h,i | |
| AH | 0 | 815.8 ± 99.1 d,e,f | 106.5 ± 18.0 e,f | 0.855 ± 0.037 d,e,f |
| 24 | 510.2 ± 32.0 c,d | 176.9 ± 7.7 g | 0.777 ± 0.010 b,c,d | |
| BWH | 0 | 396.9 ± 13.1 c | 55.5 ± 2.3 b | 0.918 ± 0.008 e,f,g,h |
| 24 | 321.0 ± 42.4 b,c | 56.6 ± 4.5 b | 0.925 ± 0.020 e,f,g,h | |
| BH | 0 | 737.3 ± 105.1 d,e | 56.9 ± 13.9 b,c | 0.940 ± 0.051 f,g,h,i |
| 24 | 360.2 ± 34.8 b,c | 97.7 ± 3.2 e | 0.830 ± 0.010 c,d,e | |
| LWH | 0 | 315.8 ± 22.1 b,c | 53.6 ± 3.6 b | 0.907 ± 0.010 e,f,g,h |
| 24 | 265.0 ± 43.3 b,c | 55.2 ± 6.9 b | 0.913 ± 0.027 e,f,g,h | |
| LH | 0 | 469.2 ± 36.9 c,d | 87.5 ± 7.9 d,e | 0.884 ± 0.018 d,e |
| 24 | 330.8 ± 23.9 b,c | 108.5 ± 1.0 f | 0.806 ± 0.019 b,c,d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kot, A.; Kamińska-Dwórznicka, A.; Galus, S.; Jakubczyk, E. Effects of Different Ingredients and Stabilisers on Properties of Mixes Based on Almond Drink for Vegan Ice Cream Production. Sustainability 2021, 13, 12113. https://doi.org/10.3390/su132112113
Kot A, Kamińska-Dwórznicka A, Galus S, Jakubczyk E. Effects of Different Ingredients and Stabilisers on Properties of Mixes Based on Almond Drink for Vegan Ice Cream Production. Sustainability. 2021; 13(21):12113. https://doi.org/10.3390/su132112113
Chicago/Turabian StyleKot, Anna, Anna Kamińska-Dwórznicka, Sabina Galus, and Ewa Jakubczyk. 2021. "Effects of Different Ingredients and Stabilisers on Properties of Mixes Based on Almond Drink for Vegan Ice Cream Production" Sustainability 13, no. 21: 12113. https://doi.org/10.3390/su132112113
APA StyleKot, A., Kamińska-Dwórznicka, A., Galus, S., & Jakubczyk, E. (2021). Effects of Different Ingredients and Stabilisers on Properties of Mixes Based on Almond Drink for Vegan Ice Cream Production. Sustainability, 13(21), 12113. https://doi.org/10.3390/su132112113







