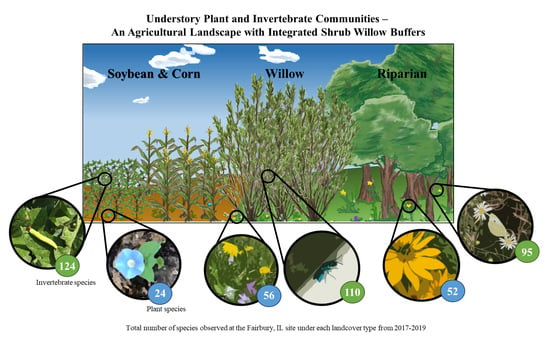
Invertebrate and Plant Community Diversity of an Illinois Corn–Soybean Field with Integrated Shrub Willow Bioenergy Buffers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Planting and Management
2.3. Invertebrate Sampling
2.4. Understory Vegetation Sampling
2.5. Data Analysis
2.5.1. Diversity and Species Richness
2.5.2. Community Composition
2.5.3. Plant and Invertebrate Community Relationship
3. Results
3.1. Invertebrate Community
3.2. Understory Plant Community
3.3. Vegetation Community Influence on Invertebrate Community
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Power, A.G. Ecosystem services and agriculture: Tradeoffs and synergies. Phil. Trans. R. Soc. B 2010, 365, 2959–2971. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.J.; Vickery, J.A.; Norris, K. Farmland Biodiversity and the Footprint of Agriculture. Science 2007, 315, 381–384. [Google Scholar] [CrossRef]
- Baum, S.; Bolte, A.; Weih, M. High value of short rotation coppice plantations for phytodiversity in rural landscapes. GCB Bioenergy 2012, 4, 728–738. [Google Scholar] [CrossRef]
- Pollinator Partnership. Available online: http://pollinator.org/pollinators (accessed on 13 September 2018).
- Saunders, M.E. Ecosystem services in agriculture: Understanding the multifunctional role of invertebrates. Agric. For. Èntomol. 2017, 20, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Garcia, J.M.; Weller, J. Conservation Reserve Program (CRP) – Maintaining Beehives on CRP Acreage. USDA memo 2013. Available online: https://www.fsa.usda.gov/Internet/FSA_File/7722287_honey_bees.pdf (accessed on 15 July 2021).
- Perttu, K.L. Environmental justification for short-rotation forestry in Sweden. Biomass Bioenergy 1998, 15, 1–6. [Google Scholar] [CrossRef]
- Volk, T.; Abrahamson, L.; Nowak, C.; Smart, L.; Tharakan, P.; White, E. The development of short-rotation willow in the northeastern United States for bioenergy and bioproducts, agroforestry and phytoremediation. Biomass Bioenergy 2006, 30, 715–727. [Google Scholar] [CrossRef]
- Blank, P.J.; Williams, C.L.; Sample, D.W.; Meehan, T.D.; Turner, M.G. Alternative scenarios of bioenergy crop production in an agricultural landscape and implications for bird communities. Ecol. Appl. 2016, 26, 42–54. Available online: http://www.jstor.org/stable/24701219 (accessed on 30 May 2021). [CrossRef] [PubMed]
- Dauber, J.; Bolte, A. Bioenergy: Challenge or support for the conservation of biodiversity? GCB Bioenergy 2014, 6, 180–182. [Google Scholar] [CrossRef] [Green Version]
- Rowe, R.L.; Hanley, M.E.; Goulson, D.; Clarke, D.J.; Doncaster, C.P.; Taylor, G. Potential benefits of commercial willow Short Rotation Coppice (SRC) for farm-scale plant and invertebrate communities in the agri-environment. Biomass Bioenergy 2011, 35, 325–336. [Google Scholar] [CrossRef]
- Verheyen, K.; Buggenhout, M.; Vangansbeke, P.; De Dobbelaere, A.; Verdonckt, P.; Bonte, D. Potential of short rotation coppice plantations to reinforce functional biodiversity in agricultural landscapes. Biomass Bioenergy 2014, 67, 435–442. [Google Scholar] [CrossRef]
- Ostaff, D.; Mosseler, A.; Johns, R.; Javorek, S.; Klymko, J.; Ascher, J. Willows (Salix spp.) as pollen and nectar sources for sustaining fruit and berry pollinating insects. Can. J. Plant Sci. 2015, 95, 505–516. [Google Scholar] [CrossRef]
- Baum, S.; Bolte, A.; Weih, M. Short Rotation Coppice (SRC) Plantations Provide Additional Habitats for Vascular Plant Species in Agricultural Mosaic Landscapes. BioEnergy Res. 2012, 5, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Seifert, C.; Leuschner, C.; Culmsee, H. Chapter 6: Short rotation coppice as habitat for vascular plants. In Bioenergy from Dendromass for the Sustainable Development of Rural Areas; Manning, D.B., Bemmann, A., Bredemeier, M., Lamersdorf, N., Ammer, C., Eds.; Wiley-Verlag: Weinheim, Germany, 2015; Volume 16, pp. 63–78. [Google Scholar]
- Wheelock, M.J.; O’Neal, M.E. Insect Pollinators in Iowa Cornfields: Community Identification and Trapping Method Analysis. PLoS ONE 2016, 11, e0143479. [Google Scholar] [CrossRef] [Green Version]
- Vanbeveren, S.P.; Ceulemans, R. Biodiversity in short-rotation coppice. Renew. Sustain. Energy Rev. 2019, 111, 34–43. [Google Scholar] [CrossRef]
- Graham, J.B.; Nassauer, J.I.; Currie, W.S.; Ssegane, H.; Negri, M.C. Assessing wild bees in perennial bioenergy landscapes: Effects of bioenergy crop composition, landscape configuration, and bioenergy crop area. Landsc. Ecol. 2017, 32, 1023–1037. [Google Scholar] [CrossRef]
- Adegbidi, H.G.; Volk, T.A.; White, E.H.; Abrahamson, L.P.; Briggs, R.D.; Bickelhaupt, D.H. Biomass and nutrient removal by willow clones in experimental bioenergy plantations in New York State. Biomass Bioenergy 2001, 20, 399–411. [Google Scholar] [CrossRef]
- Kuzovkina, Y.A.; Quigley, M.F. Willows Beyond Wetlands: Uses of Salix L. Species for Environmental Projects. Water Air Soil Pollut. 2005, 162, 183–204. [Google Scholar] [CrossRef]
- Abrahamson, L.; Volk, T.; Smart, L.; White, E. Short-rotation willow for bioenergy, bioproducts, agroforestry and phytoremediation in the northeastern United States. IEA Bioenergy Task 43. 2012. Available online: https://www.ieabioenergy.com/wp-content/uploads/2018/01/IEA_Bioenergy_Task43_PR2012_01.pdf (accessed on 30 June 2021).
- Hénault-Ethier, L.; Lucotte, M.; Smedbol, É.; Gomes, M.P.; Maccario, S.; Laprise, M.E.L.; Perron, R.; Larocque, M.; Lepage, L.; Juneau, P.; et al. Potential Efficiency of Grassy or Shrub Willow Buffer Strips against Nutrient Runoff from Soybean and Corn Fields in Southern Quebec, Canada. J. Environ. Qual. 2019, 48, 352–361. [Google Scholar] [CrossRef]
- Ssegane, H.; Negri, M.C.; Quinn, J.; Urgun-Demirtas, M. Multifunctional landscapes: Site characterization and field-scale design to incorporate biomass production into an agricultural system. Biomass Bioenergy 2015, 80, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Zumpf, C.; Ssegane, H.; Negri, M.C.; Campbell, P.; Cacho, J. Yield and water quality impacts of field-scale integration of willow into a continuous corn rotation system. J. Environ. Qual. 2017, 46, 811–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NOAA. Available online: https://www.ncdc.noaa.gov/cdoweb/search;jsessionid=55E88BDD157E7DB5822944FB334B6C10 (accessed on 31 January 2019).
- U.S. Climate Data. Available online: https://www.usclimatedata.com/climate/bloomington/illinois/united-states/usil1523 (accessed on 29 January 2020).
- Abrahamson, L.P.; Volk, T.A.; Smart, L.B.; Cameron, K.D. Shrub Willow Biomass Producer’s Handbook; College of Environmental Science and Forestry: Syracuse, NY, USA, 2010; Available online: http://www.esf.edu/willow/documents/ProducersHandbook.pdf (accessed on 4 March 2019).
- Tumminello, G.; Volk, T.A.; McArt, S.H.; Fierke, M.K. Maximizing pollinator diversity in willow biomass plantings: A comparison between willow sexes and among pedigrees. Biomass Bioenergy 2018, 117, 124–130. [Google Scholar] [CrossRef]
- The R Development Core Team. R: A language and environment for statistical computing, Vienna, R Foundation for Statistical Computing. Available online: http://www.R-project.org2009 (accessed on 10 October 2021).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R package Version 2.5–7. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 30 April 2021).
- Baxter, J. Vegetation Sampling Using the Quadrat Method. Lecture: Methods in ECC (BIO221B). Available online: https://www.csus.edu/indiv/b/baxterj/bio%20221b/vegetation%20sampling%20quadrat.pdf (accessed on 12 March 2019).
- Vahdati, F.B.; Mehrvarz, S.S.; Dey, D.C.; Naqinezhad, A. Environmental factors-ecological species group relationships in the Surash lowland-mountain forests in northern Iran. Nord. J. Bot. 2016, 35, 240–250. [Google Scholar] [CrossRef]
- Fletcher, D.; MacKenzie, D.; Villouta, E. Modelling skewed data with many zeros: A simple approach combining ordinary and logistic regression. Environ. Ecol. Stat. 2005, 12, 45–54. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: New York, NY, USA, 2002. [Google Scholar]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. Available online: https://cogns.northwestern.edu/cbmg/LiawAndWiener2002.pdf (accessed on 30 May 2021).
- Meyer, D.; Dimitriadou, E.; Hornik, K.; Weingessel, A.; Leisch, F. e1071: Misc Functions of the Department of Statistics, Probability Theory Group (Formerly: E1071); R Package Version 1.7-2. Available online: https://CRAN.R-project.org/package=e1071 (accessed on 3 March 2020).
- Max Kuhn. caret: Classification and Regression Training. R Package Version 6.0-86. 2020. Available online: https://CRAN.R-project.org/package=caret (accessed on 10 October 2021).
- Lefcheck, J. A Practical Guide to Machine Learning in Ecology. Sample (Ecology). Available online: https://jonlefcheck.net/2015/02/06/a-practical-guide-to-machine-learning-in-ecology/ (accessed on 7 December 2019).
- Simpson, G.L. Cocorresp: Co-Correspondence Analysis Ordination Methods. R Package Version 0.4-1. Available online: https://cran.r-project.org/package=cocorresp (accessed on 6 June 2019).
- Simpson, G.L. Introduction to Co-Correspondence Analysis. Available online: https://cran.r-project.org/web/packages/cocorresp/vignettes/cocorresp-intro.html (accessed on 22 October 2019).
- van der Maarel, E. Transformation of cover-abundance values for appropriate numerical treatment-Alternatives to the proposals by Podani. J. Veg. Sci. 2007, 18, 767–770. [Google Scholar] [CrossRef]
- Valtonen, A.; Malinga, G.M.; Nyafwono, M.; Nyeko, P.; Owiny, A.; Roininen, H. The successional pathway of the tree community and how it shapes the fruit-feeding butterfly community in an Afrotropical forest. J. Trop. Ecol. 2017, 33, 12–21. [Google Scholar] [CrossRef]
- Haughton, A.J.; Bohan, D.A.; Clark, S.J.; Mallott, M.D.; Mallott, V.; Sage, R.; Karp, A. Dedicated biomass crops can enhance biodiversity in the arable landscape. GCB Bioenergy 2015, 8, 1071–1081. [Google Scholar] [CrossRef] [Green Version]
- Reddersen, J. SRC-willow (Salixviminalis) as a resource for flower-visiting insects. Biomass Bioenergy 2001, 20, 171–179. [Google Scholar] [CrossRef]
- Cunningham-Minnick, M.J.; Peters, V.E.; Crist, T.O. Nesting habitat enhancement for wild bees within soybean fields increases crop production. Apidologie 2019, 50, 833–844. [Google Scholar] [CrossRef]
- Fry, D.; Slater, F. The Biodiversity of Short Rotation Willow Coppice in the Welsh Landscape: A Report to the Institute of Biological, Environmental and Rural Sciences, Aberystwyth University for EU Project “Willows for Wales”; Aberystwyth University: Penglais, UK, 2009; Available online: https://www.aber.ac.uk/en/media/departmental/ibers/research/willowforwales/Biodiversity-of-src-coppice-in-the-Welsh-Landscape.pdf (accessed on 14 February 2012).
- Capinera, J. Handbook of Vegetable Pests, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Stannard, L.J. The Thrips, or Thysanoptera, of Illinois. Ill. Nat. Hist. Surv. Bull. 1968, 29, 215–552. [Google Scholar] [CrossRef]
- Patterson, R. Beneficial True Bugs: Minute Pirate Bugs. UTAH Pests Fact Sheet. 2017. Available online: https://digitalcommons.usu.edu/cgi/viewcontent.cgi?article=2816&context=extension_curall(ENT-188-17PR) (accessed on 30 May 2021).
- Sarwar, M.; Salman, M. From Production to Field Application Methodology of Generalist Predator Green Lacewing, Chrysoperla carnea [Stephens] (Neuroptera: Chrysopidae). Zool Stud. 2016, 1, 35–40. [Google Scholar]
- Taylor, R.L.; Maxwell, B.D.; Boik, R.J. Indirect effects of herbicides on bird food resources and beneficial arthropods. Agric. Ecosyst. Environ. 2006, 116, 157–164. [Google Scholar] [CrossRef]
- Hawkinson, C. Beneficials in the Garden. Available online: https://aggie-horticulture.tamu.edu/galveston/beneficials/beneficial-46_harvestmen.htm (accessed on 4 June 2019).
- Landis, D.A.; Werling, B.P. Arthropods and biofuel production systems in North America. Insect Sci. 2010, 17, 220–236. [Google Scholar] [CrossRef]









| Year | Cluster | Number of Samples from Each Landcover | Number of Species Associated with Each Cluster | Description | ||
|---|---|---|---|---|---|---|
| Riparian | Grain | Willow | ||||
| 2017 | 1 | 0 | 6 | 0 | 20 | Only soybean samples from the August sampling date; dominated by black flies (Sarcophagidae) |
| 2 | 3 | 5 | 9 | 37 | 94% August sampling dates; dominated by Japanese beetles (Popillia japonica), flower flies (Syrphidae), and fungus gnats (Sciaridae) | |
| 3 | 4 | 4 | 11 | 24 | Higher number of willow samples; all August samples; dominated by black flies, carpenter bees (Ceratina calcarata), and mosquitoes (Culicidae) | |
| 4 | 0 | 7 | 1 | 15 | Mainly soybean samples; all June samples; dominated by Japanese beetles, flower flies, and fungus gnats | |
| 5 | 2 | 5 | 4 | 44 | Split among all landcovers; all August samples; dominated by black flies, longhorn bees (Melissodes bimaculate), carpenter bees, mosquitoes, and greenbottle and bluebottle flies (Calliphoridae) | |
| 2018 | 1 | 0 | 9 | 11 | 10 | Both corn and willow; 55% April samples, 30% June samples; dominated by black flies |
| 2 | 7 | 10 | 10 | 27 | Split across all three landcovers; 60% April samples; dominated by black flies and mosquitoes | |
| 3 | 9 | 29 | 22 | 55 | Both corn and willow; 22% June, 33% July, 43% September; dominated by Thrips palmi, marsh flies (Sciomyzidae), flower flies, and black flies | |
| 2019 | 1 | 9 | 18 | 8 | 65 | High number of soybean samples; 40% of 18 July samples, 11–17% from each of the 4 other dates; dominated by springtails (Entomobryidae), marsh flies, and black flies |
| 2 | 3 | 14 | 22 | 39 | High number of willow samples; 85% from April sampling dates; dominated by click beetles (Elateridae), mosquitoes, and pigweed flea beetles (Disonycha glabrata) | |
| 3 | 2 | 14 | 13 | 72 | Mainly soybean and willow; 80% from July and September; dominated by Thrips palmi | |
| 4 | 5 | 9 | 15 | 58 | High number of willow samples; 60% from 12 July; dominated by flower flies and longhorn bees | |
| Landcover Type | Invertebrate Community | Plant Community |
|---|---|---|
| Grain | White caterpillar–Noctuidae family Green stink bug–Chinavia hilaris Burrowing bug–Cydnidae family Plant bug–Miridae family Noctuid caterpillar–Noctuidae family Cocklebur weevil–Rhodobaenus quinquepunctatus American bird grasshopper–Schistocerca americana Spur-throated grasshopper–Melanoplus spp. | Spotted spurge–Euphorbia maculata |
| Willow | Tan jumping spider–Salticidae family Green lacewing fly–Chrysopidae family Whitefly–Aleyrodidae family | Clover–Trifolium spp. Oxalis–Oxalis spp. Rugel’s plantain–Plantago rugelii Poision ivy–Toxicodendron radicans Tall fescue–Festuca arundinacea Alumroot–Heuchera spp. Black medick–Medicago lupulina Grape–Vitis spp. Beggarticks–Bidens spp. |
| Riparian | Ichneumon parasitic wasp–Ichneumonidae family Flower longhorn beetle–Strangalia famelica Black horse fly–Tabanus atratus Weevil wasp–Cerceris species Nettle pollen beetle–Brachypterus urticae | Avens spp.–Geum spp. Swamp buttercup–Ranunculus septentrionalis Onion spp.–Allium spp. |
| Year | Cluster | Number of Samples from Each Landcover | Number of Species Associated with Each Cluster | Description | ||
|---|---|---|---|---|---|---|
| Riparian | Grain | Willow | ||||
| 2018 | 1 | 0 | 8 | 0 | 3 | Corn only; primarily from the southern (marginal) soils; dominated by yellow foxtail (Setaria glauca) and chickweed (Stellaria media) |
| 2 | 0 | 21 | 0 | 6 | Corn only; spring and fall sampling dates; dominated by chickweed (Stellaria media) and henbit (Lamium amplexicaule) | |
| 3 | 19 | 18 | 12 | 30 | All landcovers; primarily samples from southern willow plots but a mixture of both soil types for riparian and corn plots; dominated by common cocklebur (Xanthium strumarium) and morning glory (Convolvulaceae family) | |
| 4 | 0 | 0 | 48 | 17 | Willow only; all sampling dates; dominated by red-seeded dandelion (Taraxacum erythrospermum) | |
| 5 | 0 | 13 | 0 | 4 | Corn only; primarily from the northern (non-marginal) soils; dominated by giant ragweed (Ambrosia trifida) and giant goldenrod (Solidago gigantea) | |
| 2019 | 1 | 0 | 24 | 0 | 7 | Soybean only; April sampling dates; dominated by chickweed (Stellaria media) |
| 2 | 0 | 0 | 53 | 48 | Willow only; all dates; all locations; dominated by red-seeded dandelion (Taraxacum erythrospermum) | |
| 3 | 0 | 14 | 0 | 8 | Soybean only; July and September only; dominated by common cocklebur (Xanthium strumarium) and bluevine (Ampelamus albidus) | |
| 4 | 20 | 22 | 7 | 48 | All landcovers; dominated by morning glory (Convolvulaceae family) and Virginia wild rye (Elymus virginicus) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zumpf, C.; Quinn, J.; Cacho, J.; Grasse, N.; Negri, M.C.; Lee, D. Invertebrate and Plant Community Diversity of an Illinois Corn–Soybean Field with Integrated Shrub Willow Bioenergy Buffers. Sustainability 2021, 13, 12280. https://doi.org/10.3390/su132112280
Zumpf C, Quinn J, Cacho J, Grasse N, Negri MC, Lee D. Invertebrate and Plant Community Diversity of an Illinois Corn–Soybean Field with Integrated Shrub Willow Bioenergy Buffers. Sustainability. 2021; 13(21):12280. https://doi.org/10.3390/su132112280
Chicago/Turabian StyleZumpf, Colleen, John Quinn, Jules Cacho, Nora Grasse, Maria Cristina Negri, and DoKyoung Lee. 2021. "Invertebrate and Plant Community Diversity of an Illinois Corn–Soybean Field with Integrated Shrub Willow Bioenergy Buffers" Sustainability 13, no. 21: 12280. https://doi.org/10.3390/su132112280
APA StyleZumpf, C., Quinn, J., Cacho, J., Grasse, N., Negri, M. C., & Lee, D. (2021). Invertebrate and Plant Community Diversity of an Illinois Corn–Soybean Field with Integrated Shrub Willow Bioenergy Buffers. Sustainability, 13(21), 12280. https://doi.org/10.3390/su132112280