Characterization and Cause Analysis of Shallow Groundwater Hydrochemistry in the Plains of Henan Province, China
Abstract
:1. Introduction
2. Data and Research Methodology
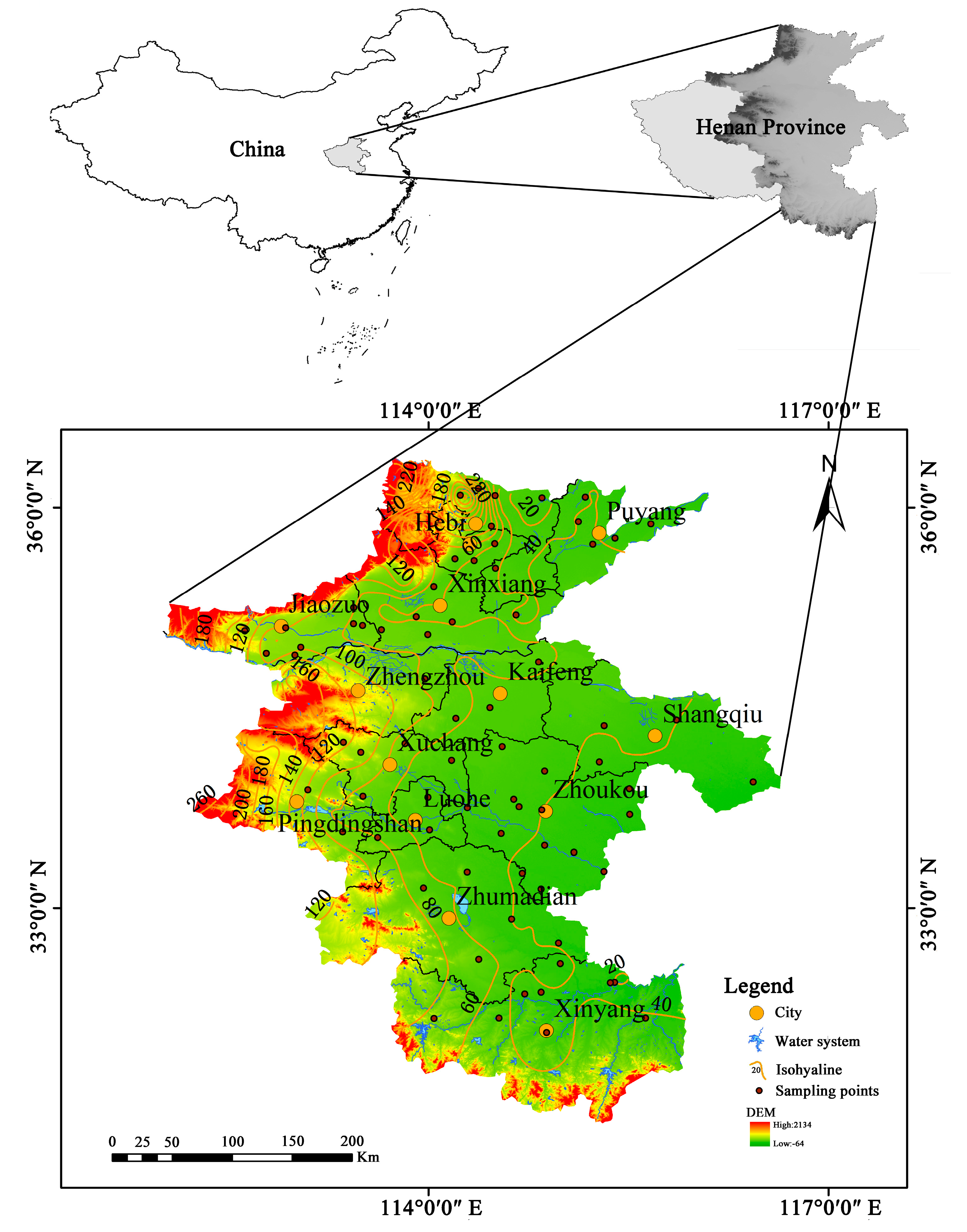
2.1. Data Sampling
2.2. Research Methodology
3. Water Chemistry Component Characteristics
3.1. Statistical Characteristics of Groups
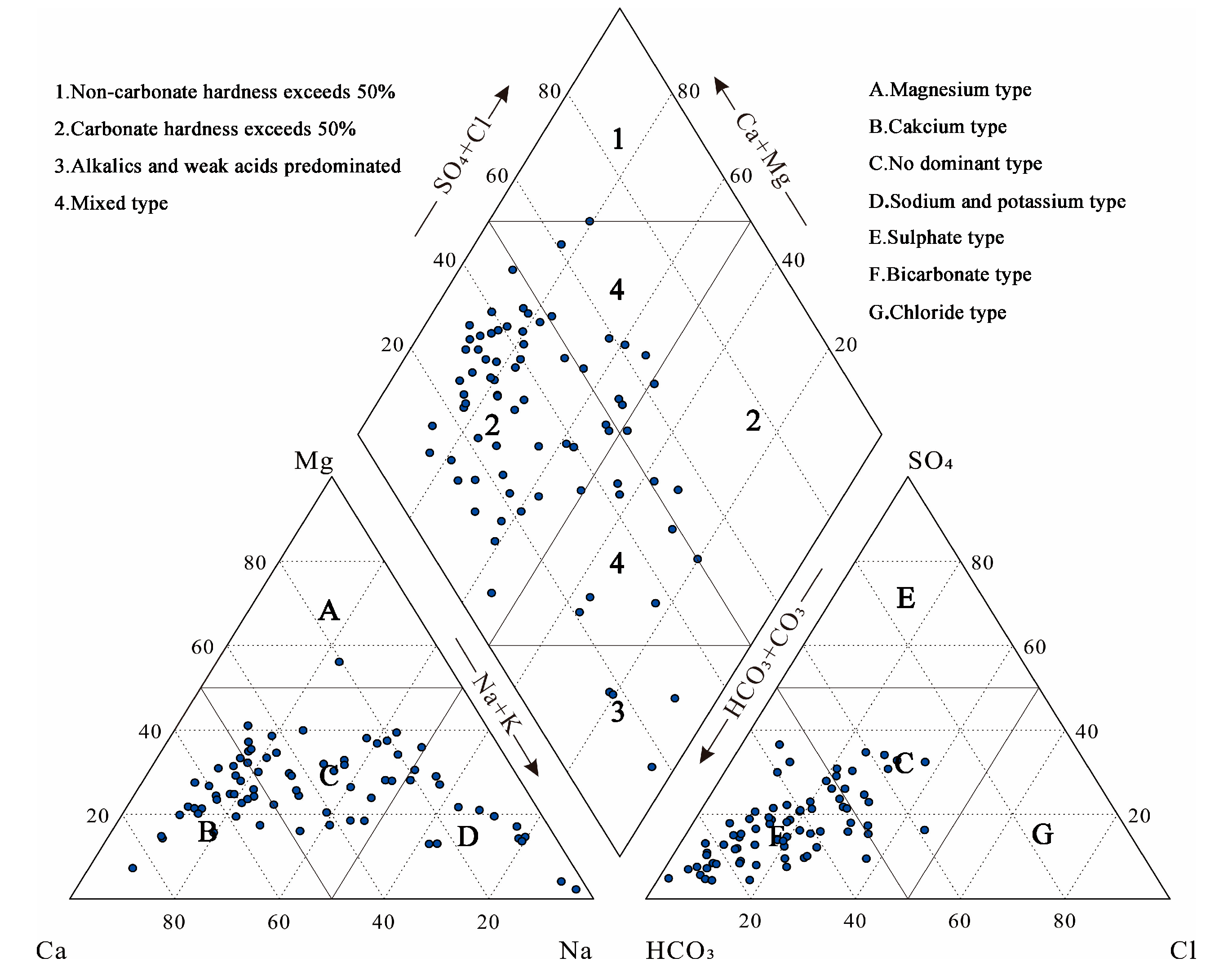
3.2. Distribution of Hydrochemistry Types
3.3. Groundwater Quality Assessment Results
4. Cause Analysis of Groundwater Hydrochemistry
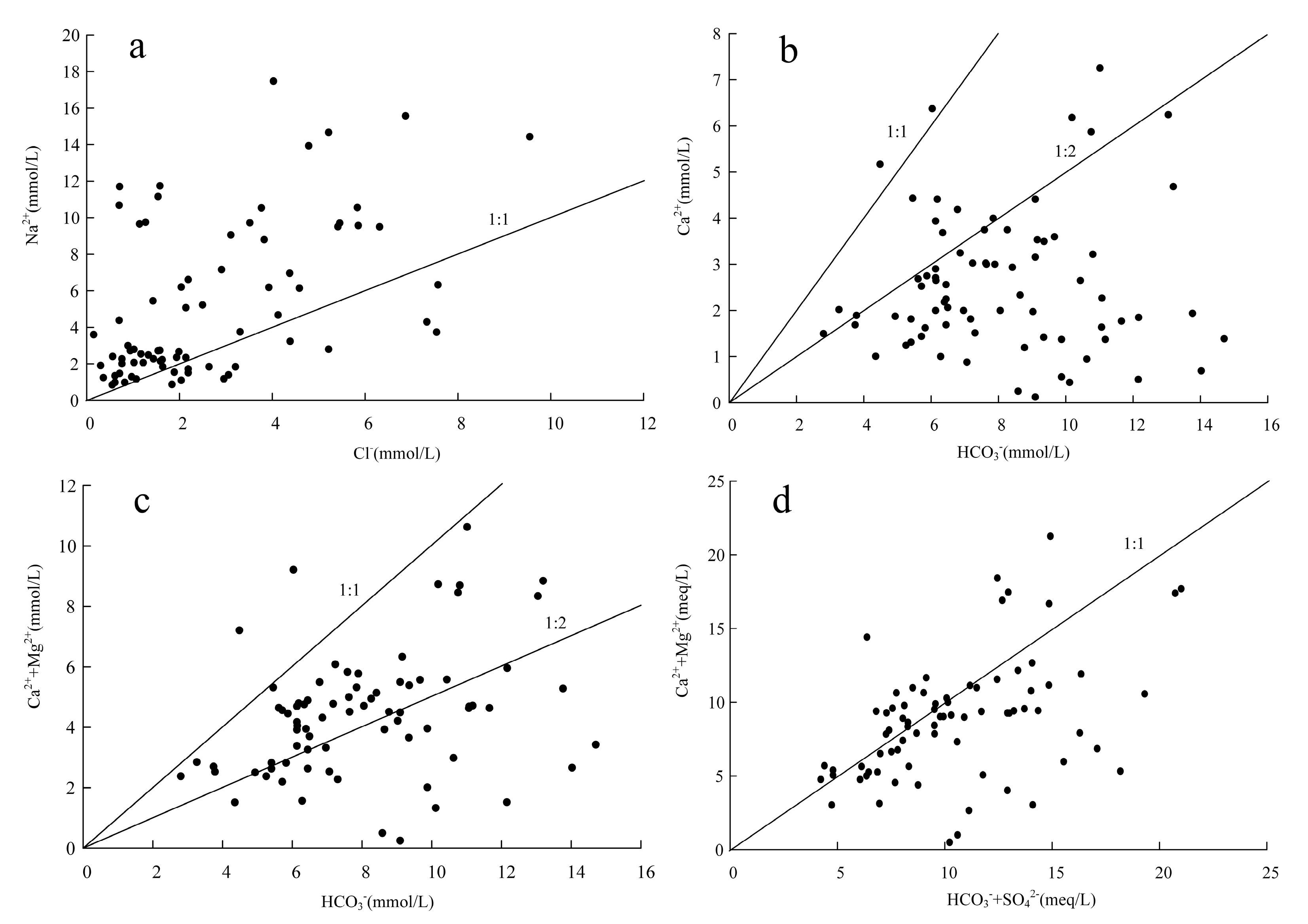
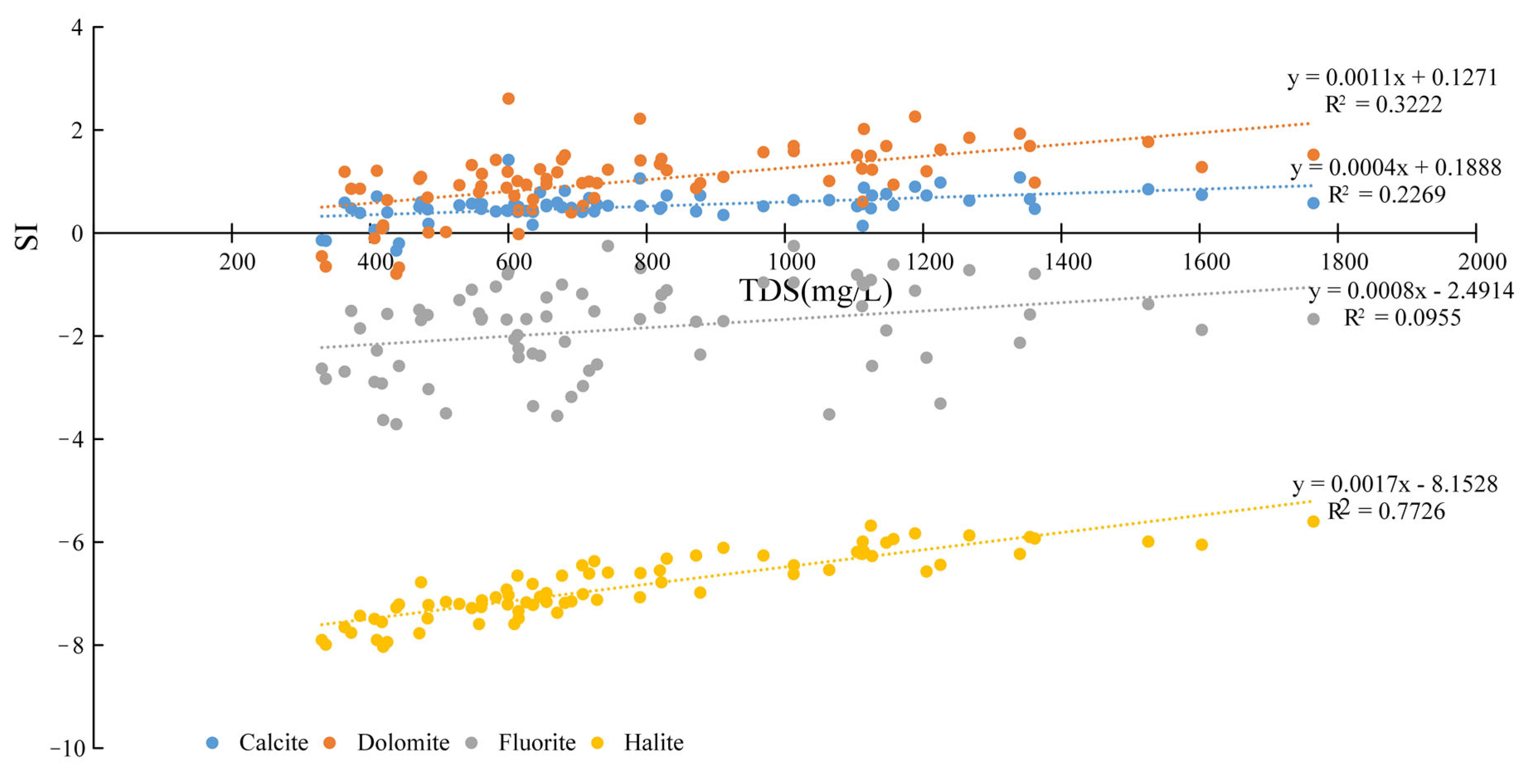
4.1. Ionic Change Pattern
4.2. Analysis of Ion Sources
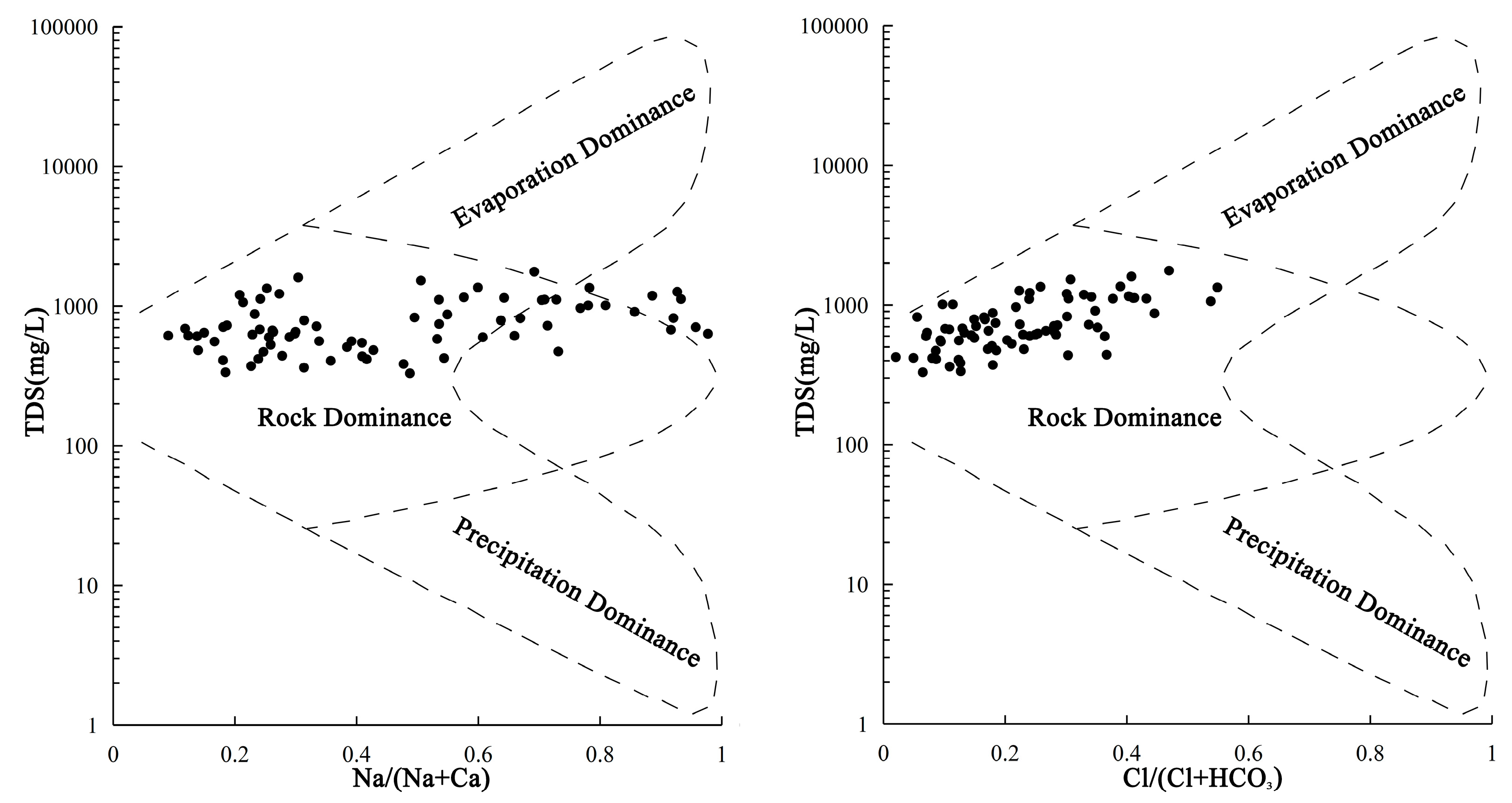
4.3. Mechanisms of Hydrochemical Formation
5. Discussions
6. Conclusions
- (1)
- A combination of the entropy weighting and subjective assignment methods was used to determine the relative weights of each evaluation index not only reflecting the data characteristics of the evaluation index itself but also to some extent combining the subjective judgment of the importance of the evaluation index. According to the results of groundwater quality assessment, the water quality in most areas of the study area is above medium to good, and the water quality is poor in areas with long runoff paths or strong human activities, mainly distributed locally in Jiaozuo City, Xinxiang City, Zhoukou City, and Puyang City, in a patchy distribution.
- (2)
- The predominance of HCO3 type anions in the groundwater types in the study area indicates that carbonate dissolution plays a dominant role in regulating hydrochemistry. The presence of ions dominated by Cl− and SO42− in local areas indicates that the weathering dissolution of rock salt and mannite as well as human activities also influence the hydrochemistry components simultaneously. From the perspective of cations, the distribution of hydrochemistry types has a certain zonation, and the precipitation of calcite and dolomite, the weathering dissolution of rock salt and fluorite, and the cation exchange and adsorption are the main factors affecting the hydrochemistry distribution of cations.
- (3)
- Human activities can directly or indirectly affect changes in the chemical components of groundwater. For example, inappropriate behaviors such as overuse of fertilizers, industrial and agricultural wastewater discharge, manure accumulation, groundwater over-exploitation, and unreasonable irrigation can lead to the abnormal distribution of certain ions and deteriorate the pristine groundwater environment. Therefore, it is necessary to continuously monitor groundwater to provide scientific data for sustainable groundwater quality management and to develop corresponding measures to prevent further degradation of the groundwater environment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Hanbali, A.; Kondoh, A. Groundwater vulnerability assessment and evaluation of human activity impact (HAI) within the Dead Sea groundwater basin, Jordan. Hydrogeol. J. 2008, 16, 499–510. [Google Scholar] [CrossRef]
- Li, P.; He, S.; Yang, N.; Xiang, G. Groundwater quality assessment for domestic and agricultural purposes in Yan’an City, northwest China: Implications to sustainable groundwater quality management on the Loess Plateau. Environ. Earth Sci. 2018, 77, 775. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Lee, G.; Hong, J.; Bae, J.H.; Lim, K.J. Prediction of Aquatic Ecosystem Health Indices through Machine Learning Models Using the WGAN-Based Data Augmentation Method. Sustainability 2021, 13, 10435. [Google Scholar] [CrossRef]
- Gleick, P.H. Dirty-Water: Estimated Deaths from Water-Related Diseases 2000–2020; Pacific Institute for Studies in Development, Environment, and Security: Oakland, CA, USA, 2002; pp. 1–12.
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Von Gunten, U.; Wehrli, B. Global water pollution and human health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Saraswat, C.; Kumar, P.; Dasgupta, R.; Avtar, R.; Bhalani, P. Sustainability assessment of the groundwater quality in the Western India to achieve urban water security. Appl. Water Sci. 2019, 9, 73. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liu, Y.; Jiang, L.; Wang, J.; Wu, W.; Bi, J. Evaluating the economic loss induced by water pollution based on multi-regional CGE model: A case study of Yangtze River Delta Basin. China Environ. Sci. 2016, 36, 2849–2856. [Google Scholar]
- Guo, H.; Wang, Y. Geochemical characteristics of shallow groundwater in Datong basin, northwestern China. J. Geochem. Explor. 2005, 87, 109–120. [Google Scholar] [CrossRef]
- Jeevanandam, M.; Kannan, R.; Srinivasalu, S.; Rammohan, V. Hydrogeochemistry and Groundwater Quality Assessment of Lower Part of the Ponnaiyar River Basin, Cuddalore District, South India. Environ. Monit. Assess. 2006, 132, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Oateng, T.K.; Opoku, F.; Akoto, O. Heavy metal contamination assessment of groundwater quality: A case study of Oti landfill site, Kumasi. Appl. Water Sci. 2019, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Khalaj, M.; Kholghi, M.; Saghafian, B.; Bazrafshan, J. Impact of climate variation and human activities on groundwater quality in northwest of Iran. J. Water Supply Res. Technol. 2019, 68, 121–135. [Google Scholar] [CrossRef]
- McGill, B.M.; Altchenko, Y.; Hamilton, S.K.; Kenabatho, P.K.; Sylvester, S.R.; Villholth, K.G. Complex interactions between climate change, sanitation, and groundwater quality: A case study from Ramotswa, Botswana. Hydrogeol. J. 2019, 27, 997–1015. [Google Scholar] [CrossRef] [Green Version]
- Bouderbala, A.; Gharbi, B.Y. Hydrogeochemical characterization and groundwater quality assessment in the intensive agricultural zone of the Upper Cheliff plain, Algeria. Environ. Earth Sci. 2017, 76, 744. [Google Scholar] [CrossRef]
- Lin, Y.; Zou, S.; Wang, J.; Li, J. Hrdrochemical characteristics and water quality assessment of Emei Mountain basalt groundwater. Yangtze River 2021, 52, 20–24. [Google Scholar]
- Maerhubai, Y.; Ma, L.; Jilili, A.; Zhang, W. Hydrochemical characteristics and their influence on rivers in the Western part of the Tianshan Mountains, Xinjiang, China. Arid Zone Res. 2021, 38, 600–609. [Google Scholar]
- Cui, Y.; Liu, J.; Gao, Z.; Cai, W. Hydrochemical Characteristics and Water Quality Evaluation of Groundwater in Huixian County. Water Sav. Irrig. 2021, 5, 96–102. [Google Scholar]
- Boateng, T.K.; Opoku, F.; Acquaah, S.O.; Akoto, O. Groundwater quality assessment using statistical approach and water quality index in Ejisu-Juaben Municipality, Ghana. Environ. Earth Sci. 2016, 75, 489. [Google Scholar] [CrossRef]
- Kong, L.; Wang, Z.; Wang, B. An analysis of the hydro-chemical characteristics and causes of drinking water source of concentrated deep groundwater in Fuyang City. China Rural Water Hydropower 2020, 3, 78–82. [Google Scholar]
- Lumb, A.; Sharma, T.C.; Bibeault, J.F. A review of genesis and evolution of water quality index (WQI) and some future directions. Water Qual. Expo. Health 2011, 3, 11–24. [Google Scholar] [CrossRef]
- Verma, P.; Singh, P.K.; Sinha, R.R.; Tiwari, A.K. Assessment of groundwater quality status by using water quality index (WQI) and geographic information system (GIS) approaches: A case study of the Bokaro district, India. Appl. Water Sci. 2020, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Şener, Ş.; Şener, E.; Davraz, A. Evaluation of water quality using water quality index (WQI) method and GIS in Aksu River (SW-Turkey). Sci. Total Environ. 2017, 584, 131–144. [Google Scholar] [CrossRef]
- Shil, S.; Singh, U.K.; Mehta, P. Water quality assessment of a tropical river using water quality index (WQI), multivariate statistical techniques and GIS. Appl. Water Sci. 2019, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Tian, Y.; Liu, Y.; Xu, D.; Liu, J.; Jiang, Y. Characteristics of two comprehensive assessment methods for water quality based on different evaluation criteria and their applications in aquatic environment management. Acta Ecol. Sin. 2019, 39, 7538–7546. [Google Scholar]
- Ma, H.; Xiao, Y.; Yan, F. Evaluation Method and Application of Modified CCME WQI Based on Fuzzy Variable Set Theory. Water Resour. Power 2018, 36, 35–38. [Google Scholar]
- Bun, S.; Sek, S.; Oeurng, C.; Fujii, M.; Ham, P.; Painmanakul, P. A Survey of Household Water Use and Groundwater Quality Index Assessment in a Rural Community of Cambodia. Sustainability 2021, 13, 10071. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, Z.; Yang, L.; Ma, Y.; Li, B.; Gong, L.; Liu, H. Phreatic water quality assessment and associated hydrogeochemical processes in an Irrigated Region along the Upper Yellow River, Northwestern China. Water 2020, 12, 463. [Google Scholar] [CrossRef] [Green Version]
- Chaurasia, A.K.; Pandey, H.K.; Tiwari, S.K.; Prakash, R.; Pandey, P.; Ram, A. Groundwater quality assessment using water quality index (WQI) in parts of Varanasi district, Uttar Pradesh, India. J. Geol. Soc. India 2018, 92, 76–82. [Google Scholar] [CrossRef]
- Deep, A.; Gupta, V.; Bisht, L.; Kumar, R. Application of WQI for water quality assessment of high-altitude snow-fed sacred Lake Hemkund, Garhwal Himalaya. Sustain. Water Resour. Manag. 2020, 6, 89. [Google Scholar] [CrossRef]
- Jehan, S.; Ullah, I.; Khan, S.; Muhammad, S.; Khattak, S.A.; Khan, T. Evaluation of the Swat River, Northern Pakistan, water quality using multivariate statistical techniques and water quality index (WQI) model. Environ. Sci. Pollut. Res. 2020, 27, 38545–38558. [Google Scholar] [CrossRef]
- Deepa, S.; Venkateswaran, S. Appraisal of groundwater quality in upper Manimuktha sub basin, Vellar river, Tamil Nadu, India by using Water Quality Index (WQI) and multivariate statistical techniques. Modeling Earth Syst. Environ. 2018, 4, 1165–1180. [Google Scholar] [CrossRef]
- Zou, Z.; Yi, Y.; Sun, J. Entropy method for determination of weight of evaluating indicators in fuzzy synthetic evaluation for water quality assessment. J. Environ. Sci. 2006, 18, 1020–1023. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, X.; Xiao, C. Assessing the influence of land use on groundwater pollution based on coefficient of variation weight method: A case study of Shuangliao City. Environ. Sci. Pollut. Res. 2019, 26, 34964–34976. [Google Scholar] [CrossRef]
- Guo, W.; Zuo, Q.; Ma, J. Spatial and temporal change analysis of harmony development among population-water resources-economics in Henan. China. Resour. Sci. 2015, 37, 2251–2260. [Google Scholar]
- Chen, P. Analysis of Water Consumption of Water Resources in Henan Province Based on Cloud Model. J. Shantou Univ. 2021, 36, 41–49. [Google Scholar]
- Shi, X.; Dong, W.; Li, M.; Zhang, Y. Evaluation of groundwater renewability in the Henan Plains, China. Geochem. J. 2012, 46, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Wei, X.; Du, J.L.; Jin, M.G. The GIS-based function evaluation and regional planning of shallow groundwater in Henan Province. China Rural Water Hydropower 2007, 9, 36–40. [Google Scholar]
- Ma, Y.G.; Cheng, J.P.; Jiao, F.; Duo, K.X.; Rong, Z.; Li, M.; Wang, W.H. Distribution, sources, and potential risk of polycyclic aromatic hydrocarbons (PAHs) in drinking water resources from Henan Province in middle of China. Environ. Monit. Assess. 2008, 146, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Li, D. Construction of Early Waring Index System of Water Resources Security in Henan Province; Henan University: Kaifeng, China, 2019. [Google Scholar]
- Du, Q.; Song, Q. Study on overexploitation of groundwater in Henan Province and control measures. Water Resour. Dev. Manag. 2021, 1, 33–37. [Google Scholar]
- Wang, G.; Shang, L.; Liu, X.; Tang, L.; Cheng, Q. Study on causes of poisoning and asphyxiation in roadway opening by AHP-entropy weight method. China Saf. Sci. J. 2021, 31, 187–192. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Li, S.; Yu, B.; Chen, F.; Wang, Y. Analysis of hydrochemical and isotopic characteristics in Pishan river oasis zone. J. Arid Land Resour. Environ. 2021, 35, 116–122. [Google Scholar]
- Meng, Q. Hydrochemical characteristics and controlling factors of the shallow groundwater in the midstream and downstream areas of Shiyang river basin. J. Arid Land Resour. Environ. 2021, 35, 80–87. [Google Scholar]
- Han, C.; Gao, Z.; Liu, J. Chemical Characteristics of Groundwater and Health Risk Assessment of Nitrate in Tancheng Area. Earth Environ. 2021, 49, 436–446. [Google Scholar]
- Lei, W.; Tang, C.; Jiang, L. Discussion on Iron and Manganese Transport and Concentrate in Underground Water. Jiangxi Sci. 2006, 1, 80–82. [Google Scholar]
- Jiang, J.; Xian, H.; Li, Q.; Tao, H.; Jiang, Y. Groundwater geochemistry and its implications for arsenic mobilization in Kuitun river basin, Xinjiang. Environ. Chem. 2021, 40, 1775–1786. [Google Scholar]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Eslami, F.; Yaghmaeian, K.; Mohammadi, A.; Salari, M.; Faraji, M. An integrated evaluation of groundwater quality using drinking water quality indices and hydrochemical characteristics: A case study in Jiroft, Iran. Environ. Earth Sci. 2019, 78, 314. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, W.H.; Li, M.Z.; Zheng, Z.X.; Shi, X.F.; Guo, Z.X. Study on distribution characteristics of TDS and hydrochemical type of shallow groundwater in Henan Plain. J. China Hydrol. 2011, 31, 79–83. [Google Scholar]
- Hu, B.; Liu, X.; Wang, N.; Jiang, X. Analysis of groundwater quality in the shallow aquifers of the east plain in Henan. Ground Water 2021, 43, 33–36. [Google Scholar]
- Marandi, A.; Shand, P. Groundwater chemistry and the Gibbs Diagram. Appl. Geochem. 2018, 97, 209–212. [Google Scholar] [CrossRef]







| Evaluation Indicators | Minimum Value | Maximum Value | Average Value | Coefficient of Variation | WHO Limit Values |
|---|---|---|---|---|---|
| K+ (mg/L) | 0.220 | 5.770 | 2.803 | 2.696 | 12 |
| Na+ (mg/L) | 19.690 | 402.000 | 118.234 | 0.826 | 200 |
| Ca2+ (mg/L) | 5.010 | 29.180 | 103.086 | 0.589 | 200 |
| Mg2+ (mg/L) | 3.040 | 131.580 | 43.666 | 0.555 | 150 |
| Fe3+ (mg/L) | 0.010 | 6.130 | 0.493 | 2.062 | 0.3 |
| Cl− (mg/L) | 5.320 | 338.900 | 94.246 | 0.784 | 250 |
| SO42− (mg/L) | 14.410 | 476.460 | 119.393 | 0.786 | 250 |
| HCO3− (mg/L) | 17.860 | 896.990 | 489.196 | 0.333 | 600 |
| F− (mg/L) | 0.040 | 3.500 | 0.748 | 1.043 | 1.5 |
| NO3–N (mg/L) | 0.005 | 69.320 | 7.689 | 1.791 | 50 |
| Mn (mg/L) | 0.001 | 2.060 | 0.182 | 1.797 | 0.1 |
| As (mg/L) | 0.001 | 0.062 | 0.004 | 2.534 | 0.01 |
| TH (mg/L) | 25.000 | 1057.500 | 436.901 | 0.464 | 500 |
| TDS (mg/L) | 33.090 | 1764.690 | 779.659 | 0.422 | 1000 |
| pH | 7.000 | 8.300 | 7.423 | 0.040 | 6.5–8.5 |
| Evaluation Indicators | Entropy Method | Literature [26] | Literature [27] | Average Value |
|---|---|---|---|---|
| K+ | 0.0357 | 0.0455 | 0.1490 | 0.0767 |
| Na+ | 0.0536 | 0.0909 | 0.0483 | 0.0643 |
| Ca2+ | 0.0357 | 0.0455 | 0.0194 | 0.0335 |
| Mg2+ | 0.0357 | 0.0455 | 0.0183 | 0.0332 |
| Fe3+ | 0.0714 | 0.1364 | 0.1234 | 0.1104 |
| Cl− | 0.0714 | 0.0455 | 0.0328 | 0.0499 |
| SO42− | 0.0893 | 0.0455 | 0.0366 | 0.0571 |
| HCO3− | 0.0536 | 0.0455 | 0.0141 | 0.0377 |
| F− | 0.0893 | 0.0909 | 0.0552 | 0.0785 |
| NO3–N | 0.0893 | 0.0909 | 0.1167 | 0.0990 |
| Mn | 0.0714 | 0.0909 | 0.1013 | 0.0879 |
| As | 0.0893 | 0.0909 | 0.2184 | 0.1329 |
| TH | 0.0714 | 0.0455 | 0.0129 | 0.0433 |
| TDS | 0.0714 | 0.0455 | 0.0274 | 0.0481 |
| pH | 0.0714 | 0.0455 | 0.0262 | 0.0477 |
| GWQI Index | <50 | 50–100 | 100–200 | 200–300 | >300 |
|---|---|---|---|---|---|
| Grade | Excellent | Good | Moderate | Poor | Very poor |
| K+ | Na+ | Ca2+ | Mg2+ | Fe3+ | Cl− | SO42− | HCO3− | F− | NO3–N | Mn | As | TH | TDS | pH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K+ | 1 | ||||||||||||||
| Na+ | 0.37 | 1 | |||||||||||||
| Ca2+ | −0.17 | −0.23 | 1 | ||||||||||||
| Mg2+ | 0.18 | 0.33 | 0.17 | 1 | |||||||||||
| Fe3+ | 0.25 | 0.17 | −0.08 | 0.16 | 1 | ||||||||||
| Cl− | 0.15 | 0.33 | 0.29 | 0.42 | 0.01 | 1 | |||||||||
| SO42− | 0.31 | 0.42 | 0.15 | 0.43 | 0.06 | 0.54 | 1 | ||||||||
| HCO3− | 0.16 | 0.47 | 0.00 | 0.42 | 0.26 | 0.23 | 0.24 | 1 | |||||||
| F− | 0.16 | 0.44 | −0.39 | 0.19 | 0.16 | 0.09 | 0.17 | 0.38 | 1 | ||||||
| NO3–N | −0.07 | −0.27 | 0.45 | −0.05 | −0.33 | 0.13 | 0.08 | −0.28 | −0.38 | 1 | |||||
| Mn | 0.04 | 0.14 | 0.06 | 0.23 | 0.43 | 0.08 | 0.05 | 0.27 | 0.14 | −0.30 | 1 | ||||
| As | 0.29 | 0.28 | −0.13 | 0.26 | 0.50 | 0.06 | 0.12 | 0.32 | 0.27 | −0.37 | 0.37 | 1 | |||
| TH | −0.01 | 0.02 | 0.66 | 0.51 | 0.05 | 0.49 | 0.34 | 0.24 | −0.16 | 0.29 | 0.13 | 0.05 | 1 | ||
| TDS | 0.26 | 0.51 | 0.23 | 0.49 | 0.11 | 0.66 | 0.62 | 0.51 | 0.21 | 0.03 | 0.13 | 0.18 | 0.48 | 1 | |
| pH | 0.28 | 0.30 | −0.40 | 0.02 | 0.08 | −0.01 | 0.13 | 0.12 | 0.45 | −0.28 | −0.05 | 0.19 | −0.27 | 0.08 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Yan, B.; Li, Y.; Sun, H.; Wang, Y.; Chen, J. Characterization and Cause Analysis of Shallow Groundwater Hydrochemistry in the Plains of Henan Province, China. Sustainability 2021, 13, 12586. https://doi.org/10.3390/su132212586
Sun J, Yan B, Li Y, Sun H, Wang Y, Chen J. Characterization and Cause Analysis of Shallow Groundwater Hydrochemistry in the Plains of Henan Province, China. Sustainability. 2021; 13(22):12586. https://doi.org/10.3390/su132212586
Chicago/Turabian StyleSun, Jian, Baizhong Yan, Yao Li, Huixiao Sun, Yahui Wang, and Jiaqi Chen. 2021. "Characterization and Cause Analysis of Shallow Groundwater Hydrochemistry in the Plains of Henan Province, China" Sustainability 13, no. 22: 12586. https://doi.org/10.3390/su132212586







