Abstract
Farmland species face many threats, including habitat loss and malnutrition during key periods of their life cycle. This is aggravated in conventionally managed monocultures, leading to nutrient deficiencies that impair the survival and reproduction of farmland wildlife. For instance, protein deficiencies in wheat or vitamin B3 deficiency in maize reduce by up to 87% the reproductive success of the critically endangered common hamster (Cricetus cricetus), a flagship species of European farmlands. It is urgent to identify and implement agricultural practices that can overcome these deficiencies and help restoring hamsters’ reproductive success. As part of a conservation program to diversify farming habitats in collaboration with farmers, we tested whether associations between wheat or maize and three supplemental crops (soybean, sunflower and fodder radish) supported hamsters’ performance during hibernation and reproduction. We observed that maize–sunflower, maize–radish and wheat–soybean associations minimized hamsters’ body mass loss during hibernation. The wheat–soybean association led to the highest reproductive success (N = 2 litters of 4.5 ± 0.7 pups with a 100% survival rate to weaning), followed by maize–sunflower and maize–radish. These crop associations offer promising opportunities to overcome nutritional deficiencies caused by cereal monocultures. Their agronomic potential should promote their implementation on a large scale and benefit farmland biodiversity beyond the common hamster.
1. Introduction
Current biodiversity loss [1,2] is especially pronounced in farmlands, where bird populations have decreased by more than 50% since the 1970s [3]. Although less studied than birds, populations of farmland invertebrates and mammals are also drastically declining [4,5,6], although the severity of the decline may have been overestimated [7,8]. Among mammals, the common hamster (Cricetus cricetus) is a flagship species of European farmlands [9], classified as critically endangered by the IUCN [10]. In Europe, its distribution area has decreased by 74% in only 50 years [11]. In France, this area has been reduced by 94% since the 1960s [12].
Intensive agriculture is considered one of the main causes of hamsters’ decline, along with habitat fragmentation and climate change [11,13,14,15]. In Western Europe, agriculture intensification has led to landscape homogenization and a lack of protective and nutritional cover during most of the year [14,15,16]. In Alsace, the only French area of presence of this species, wheat (Triticum aestivum) and maize (Zea mays) alone can cover more than 80% of arable land some years [17]. These two crops are deficient in essential nutrients, which can reduce hamster reproduction by up to 87% [18,19]. Maize is especially deficient in tryptophan and vitamin B3, leading to reproductive and behavioral disorders [18]. We thus urgently need to identify sustainable agricultural practices that can compensate for such deficiencies, while being of agro-economic interest for farmers to guarantee their implementation on a large scale.
The common hamster is a hibernating species, which uses torpor (i.e., phases of hypometabolism and hypothermia) to cope with the reduction in food resources and temperatures in winter [20]. In small mammalian hibernating species, individuals with the best body condition emerge earlier in spring and have more time to reproduce, leading to a greater number of litters for females and more mating opportunities for males (Cricetus cricetus [21]; Spermophilus citellus [22]; Cynomys ludovicanus [23]). Some species store internal fat reserves (“fat-storers”), whereas other species hoard large amounts of food in their burrow (“food-hoarders”) [20]. In all cases, hibernation quality and reproductive success depend on the quantity and the quality of the reserves amassed during the pre-hibernation stage (reviewed in [24]). Common hamsters are food-hoarding hibernators [25], and the composition and quality of their hoards can have a strong impact on their hibernation quality and subsequent reproduction [26,27]. Global protein and lipid content of the diet can, for instance, shape hibernation patterns [26]. In addition, polyunsaturated fatty acids (PUFAs) are crucial for hibernation, as they modulate the duration and depth of torpor bouts [28,29,30]. Food-hoarding hibernators can modulate their PUFA intake when their hoards allow them to do so [29,31,32,33]. While the nutritional needs of animals for optimal hibernation and reproduction are well known [20,24,29,33], we know little about how hibernating species respond to the reduced diversity of food and the dominance of crops in their environment.
We sought to understand how storable crop-based food (e.g., seeds) might affect hamsters’ hibernation and subsequent reproduction while identifying crops that could be associated with the main monocultures of Western Europe, i.e. maize and wheat, to improve hamsters’ reproductive success in farmlands. We thus investigated the effects of six crop-based diets on the hibernation and subsequent first reproduction of 42 pairs of captive-reared hamsters. Diets were composed of wheat or maize seeds (main food) and supplemented with seeds of one of the following crops: sunflower (Helianthus annuus), soybean (Glycine max) or fodder radish (Raphanus sativus oleiformis). Considering that farmers are more inclined to implement innovative practices when there is a two-way exchange and when their concerns are considered from the initiation of research projects [34,35], the crops were selected through oral communications with farmers and conservation practitioners.
We predicted that sunflower, followed by radish, would reduce the use of torpors [26,29,31,36] since they have higher lipid and PUFA contents than soybean [36,37]. Soybean, on the other hand, should promote longer torpor bouts and be favorable for reproduction based on its protein, tryptophan and vitamin B3 content [18,26,36,37]. Finally, we did not predict differences between wheat- and maize-based diets given that grains of these two crops have similar macronutrients and energy values and that the supplemental crops should prevent the incidence of the vitamin B3 deficiency caused by maize consumption [18].
2. Materials and Methods
2.1. Selection of Supplemental Crops Based on Consultation with Farmers
As part of a LIFE+ Nature and Biodiversity Project, i.e., projects supported by the EU that develop and implement environmental and nature conservation solutions, oral communications were initiated with farmers in 2013 to identify innovative crops that may be favorable for common hamsters (LIFE+ ALISTER). Following the discovery of the negative impact of maize [14,18] and monocultures [19] on hamsters’ body mass, reproduction and survival, results were presented on several occasions to most stakeholders between 2013 and 2016. Stakeholders included farmers (from the AFSAL and CUMA de la Plaine, Alsace, France), agronomists (Chambre d’Agriculture d’Alsace) and conservation practitioners from France (French Biodiversity Agency, OFB) and 5 other countries (Germany, The Netherlands, Austria, Ukraine, Poland). All of them were invited to provide suggestions of crops of ecological or agro-economic interest to be associated with maize and wheat. Based on the suggestions that emerged from those oral communications, we considered both the number of farmers that mentioned a similar crop and whether this crop provided complementary nutrients to wheat or maize (e.g., crops that were suggested but had similar nutritional values to wheat or maize, such as other cereals, were not included). Soybean was selected for its economic interest for farmers, being suggested by most farmers surveyed. Sunflower was selected as a historically common crop in the region and because it was previously identified as a proper supplemental crop to maize and wheat in exclosures [19]. Moreover, sunflower and soybean were considered for their high tryptophan and vitamin B3 contents, as well as for their respective PUFA and protein contents (see Table S1; [36,37]). Regarding fodder radish, high and stable populations of hamsters were recorded in those fields (La Haye M.J.J, The Netherlands, personal communication). Moreover, radish is a good intercultural crop for wheat and especially favorable for invertebrates and farmland birds [38]. It is also used as a cover crop for soil decompaction [39]. Furthermore, lipid and PUFA contents are relatively high in radish, though lower than in sunflower [40].
2.2. Study Species and Housing Conditions
This study was carried out on 84 one-year-old hamsters (42 ♂ and 42 ♀) from November 2015 to the beginning of June 2016 in our captive breeding unit (CNRS, IPHC-DEPE, Strasbourg, France). Animals were maintained under photoperiodic and thermal conditions that mimicked an annual cycle [26] in three different rooms, ensuring an equal distribution of hamsters in each diet group in each room. Hamsters were housed individually (W ×H × D: 265 × 237 × 420 mm) for the entire period except for reproduction, during which they were housed as breeding pairs for two weeks in larger cages (W × H × D: 380 × 257 × 590 mm) equipped with a shelter box (W × H × D: 140 × 230 × 230 mm) and paper to make a nest.
2.3. Diet Composition
A total of 6 diets were tested, with 14 individuals in each group (7 ♂ and 7 ♀) that had access to wheat or maize seeds (main food) and a supplemental seed type (radish, soybean or sunflower) as follows: maize–soybean (Msoy), maize–sunflower (Msunf), maize–radish (Mrad), wheat–soybean (Wsoy), wheat–sunflower (Wsunf) or wheat–radish (Wrad; Table 1). Animals had free access to water and food (both the main food and the supplement) throughout the experiments. To meet hamsters’ requirements for proteins during reproduction [18,26], and considering that they are omnivorous [16], they were supplemented with 5g of earthworm (Lumbricus terrestris) every other day from 8 March to 10 June.

Table 1.
Timetable and details of the experimental design and diets. The natural photoperiods followed the Ephemerides of Strasbourg, France (Lat: 48°34′48.0072″ N, Long: 7°45′0.0000″ E).
2.4. Macronutrient Content of the Diets
We recorded the total protein, lipid, mineral, and energy content of each of the six diets. Grains were freeze-dried to constant mass and ground to obtain a homogenous powder for analysis. Just before analysis, the powder was freeze-dried again for 48 h to eliminate any remaining traces of water. Nitrogen content was determined in triplicate using 150–200 mg aliquots according to the Kjeldahl method [41]. Protein content was calculated as nitrogen content × 6.25 [42]. Lipid content was determined in duplicate using 1 g aliquots according to a procedure adapted from the Folch method [43] with a chloroform/methanol (2/1, v/v) solution as extraction solvent. Mineral content was determined gravimetrically in duplicate from 1–2 g samples ignited in a muffle furnace at 400 °C for 24 h. Total seed water was then calculated by subtracting total dry seed mass from fresh seed mass. Finally, energy content was determined on dry 0.7–1.4 g aliquots by using an isoperibol bomb calorimeter Parr 6200, with benzoic acid as a standard. The carbohydrate ratio was equal to 100% of the dry matter value minus the lipid, protein, and mineral percentages.
2.5. Hamster Body Mass, Food Intake and Food Preferences
Adult hamsters were weighed at the onset of hibernation (between 25 November and 9 December) and post-hibernation (23–25 March). These data were used to calculate changes in hamsters’ body mass during hibernation (hereafter referred to as Δbody mass), often used as a proxy of body condition and hibernation quality [26,33]. During hibernation, cages were cleaned only when hamsters were awake to avoid any disturbance. The litter was collected and sieved to collect uneaten food that was subsequently dried and weighed to calculate hamsters’ food intake over hibernation. This information was then used to estimate different parameters of hamsters’ daily food intake (g·day−1 of dry matter, water, proteins, lipids, carbohydrates, minerals and kJ·day−1 of energy intake) and food preferences.
2.6. Geometrical Representation of Nutrient Intake
We represented nutrient consumption in a geometric space whose different dimensions correspond to various macronutrients (lipids, proteins and carbohydrates), following the framework proposed by Raubenheimer and Simpson [44,45]. We then represented the macronutrient intake and the ratio between each macronutrient for each hamster in the six diets. This geometric representation enabled to represent the ratio of macronutrients contained in each type of seed, i.e., the lines on the nutrient space, called “food rails”. If a hamster ate only one of the two foods in the diet, the macronutrient intake point would be on the food rail of the considered food. The further the point is from the origin of the graph, the higher the consumption.
2.7. Activity Index
Over the entire hibernation period, the use of torpor was estimated three times a week between 3 and 5 p.m., starting on the 21 December and ending on the 8 March. A score was attributed to each hamster according to its breath movements and position in the cage, as adapted from [46]. A score of 1 was assigned to hamsters with a tightly curved body and displaying less than 1 breath per 30 s (i.e., considered being in deep torpor). A score of 2 was assigned to hamsters with a curved body and breathing 1–7 times in 30 seconds (i.e., in shallow torpor or in a deep sleep). Finally, a score of 3 was attributed to hamsters that were either moving in the cage or displaying more than 7 breaths in 30 seconds (i.e., not in torpor, either active or in a light sleep). Over the entire hibernation period, we calculated an index of activity for each hamster by averaging raw scores (see analyses below), ranging from 1 (mostly inactive, in torpor) to 3 (mostly active, never in torpor). This activity index was used in graphical representations, while raw scores were used in statistical analysis.
2.8. Reproductive Success and Litter Size
We monitored the number of females that initiated parturition and the litter size twice a day (at 8:00 a.m. and 7:00 p.m.) from the end of April to the end of May. We recorded pups’ body mass (±0.01 g) at 8 and 30 days of age [18,26]. We also recorded the presence of dead pups at parturition. Females whose litters were stillborn were not considered to have successfully delivered.
2.9. Data Analyses
Activity index: We looked at the effect of the diet on the hamsters’ activity scores between December and the end of March. Because these scores are ordinal quantitative variables, we used the cumulative link model (CLM) with a logit link for non-binary data (R, v.3.4.3.). The main food (wheat or maize), the supplement (radish, soybean or sunflower), the sex, the mass of each hamster and all two-way interactions between these variables were included as fixed effects. The identity of the individuals, the room and the date were included as random effects. We applied a model selection procedure based on the lowest AICc (Akaike information corrected for small samples) [47]. The complete model was the model including the additive effects of the fixed effects, as well as all 2-way interactions between them, plus the random effects. Model selection was performed on this complete model based on the lowest AICc, conserving the same random effect structure (i.e., only fixed effects varied). Nelder’s marginality principle was also applied, which implies that once a factor is included in an interaction, its simple effect will not be discussed [48]. Multiple comparisons were analyzed via a test of ordinal regression by pairs (R 3.4.3).
Food intake, food preferences and body mass loss: We investigated how the diet influenced both the average daily food intake (model 1), the daily intake of each seed type (food preferences; model 2) and Δbody mass during hibernation (model 3). The effect of the diet on these variables was respectively analyzed using linear mixed models (LMMs, daily food intake and daily intake of each seed type) and a linear model (LM, Δbody mass; IBM SPSS software for Windows, version 21.0. Armonk, NY, USA: IBM Corp.). Normality of the residuals was tested using the Kolmogorov–Smirnov test, and variance homogeneity was checked using the Levene test. The sex, the main food (maize or wheat), the supplement (soybean, radish or sunflower), and two-way interactions were included as fixed effects in both models 1 and 3. In model 2, we included the sex, the diet, the seed type and diet*seed type interaction as fixed effects. We included body mass at the onset of hibernation and the activity index as covariates in all three models. The identity of the individuals was included as a random effect for repeated measurements of the same individual in all three models.
Reproductive success: We used a generalized linear model to analyze the effect of the diet on the success of parturition (GLM, binomial, logit). We included the diet, the mother’s body mass upon emergence from hibernation and the interaction between these two variables as fixed effects. We then ran a second model to analyze the effect of the diet on the litter size using a generalized linear mixed model (GLMM; we used a quasi-Poisson distribution, as overdispersion was observed; log-link; R 3.6.1, MASS package). The full model included the diet, the body mass upon emergence from hibernation, the period (parturition or weaning) and the diet*period interaction as fixed effects. We included the identity of the mother as a random effect. Model selection was conducted on this full model and followed the same protocol as the one described above for the activity index. Multiple comparisons were conducted using the emmeans package (R 3.6.1).
The significance threshold was set at α < 0.05. Figures were prepared using GraphPad Prism software (version 5, La Jolla, CA, USA) or R 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria, 2019).
2.10. Ethics
The experimental protocol followed EU Directive 2010/63/EU guidelines for animal experiments and the care and use of laboratory animals. It was approved by the Ethical Committee (CREMEAS) under agreement numbers 00624–01 and 17484–2018103016124862.
3. Results
3.1. Macronutrient Content of the Diets
The seeds of all 5 types of crops contained less than 9% of water (from 4.1 ± 0.2% for sunflower to 8.8 ± 0.5% for wheat; Table 2). The main foods (wheat and maize) were essentially composed of carbohydrates (nearly 85%; Table 2). Supplemental seeds had greater contents in proteins, lipids and energy than the main food: soybean seeds had the highest protein content (41.4 ± 0.3%) while sunflower had the highest lipid and caloric contents (62.2 ± 0.3% and 31.4 ± 0.2 kJ per gram of DM), respectively, (Table 2). The lowest lipid and protein contents were found in wheat (3.1 ± 0.1%) and in maize (8.3 ± 0.1%), respectively, (Table 2).

Table 2.
Nutrient composition of seeds. Dry matter (DM) and water content are expressed in percentage of fresh matter (FM), ashes and macronutrients are expressed in percentage of DM, and energy is expressed in kilojoules per gram of DM. Values are given ± SEM.
3.2. Activity Index during Hibernation
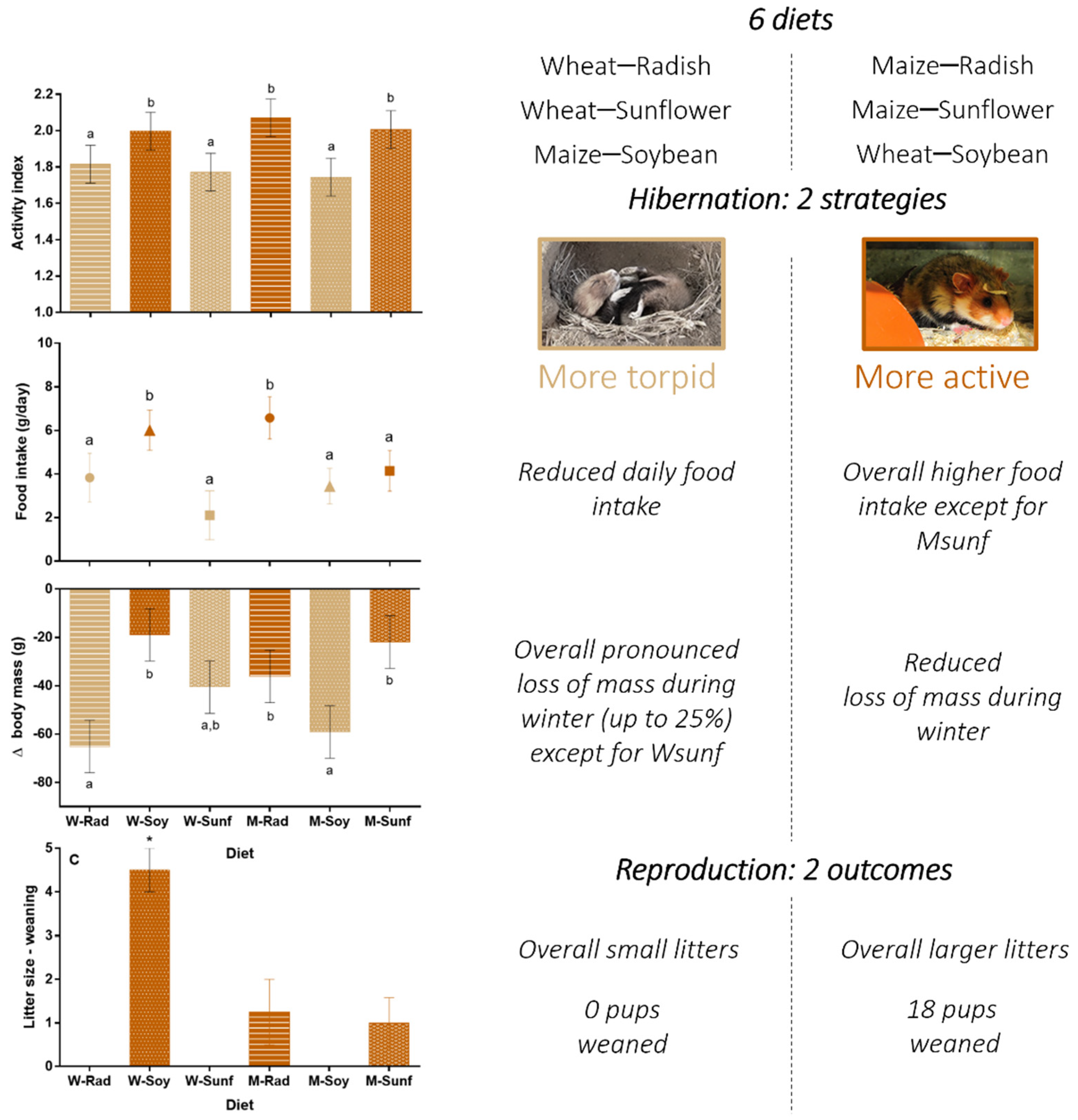
All hamsters displayed torpor (activity index of 1) during hibernation, except one female from the Mrad group that remained active throughout hibernation (proportions of each index per diet and sex are presented in Table S2). After model selection based on AICc and on the relative weight of each variable (see models and selection in Table S3), the best model to analyze the activity index was the model including the sex and the interaction between the main food (maize or wheat) and the supplement (soybean, sunflower or radish) as fixed effects. The activity index was significantly influenced by the sex (z = −2.79; p = 0.005), with males having a lower activity index than females (mean difference = 0.21 ± 0.02). It was also influenced by the main food*supplement interaction (Msoy, z = −2.38; p = 0.017, Mrad, z = 0.21, p = 0.83): hamsters in the Wsoy, Mrad and Msunf diet groups displayed a greater activity index than those in the Wrad, Wsunf and Msoy groups (Figure 1A, p < 0.001). In terms of proportions, hamsters in the Wrad, Wsunf and Msoy diet groups displayed a high proportion of index 1 (55–58.3%) and less than 40% of index 3, whereas hamsters in the three other diet groups (WSoy, Mrad and Msunf) displayed between 43.3–48.2% of index 1 and 46.9–50.3% of index 3 (see Table S2).
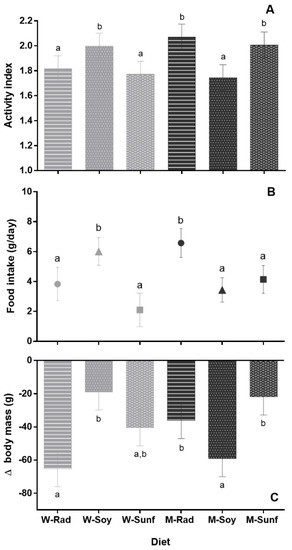
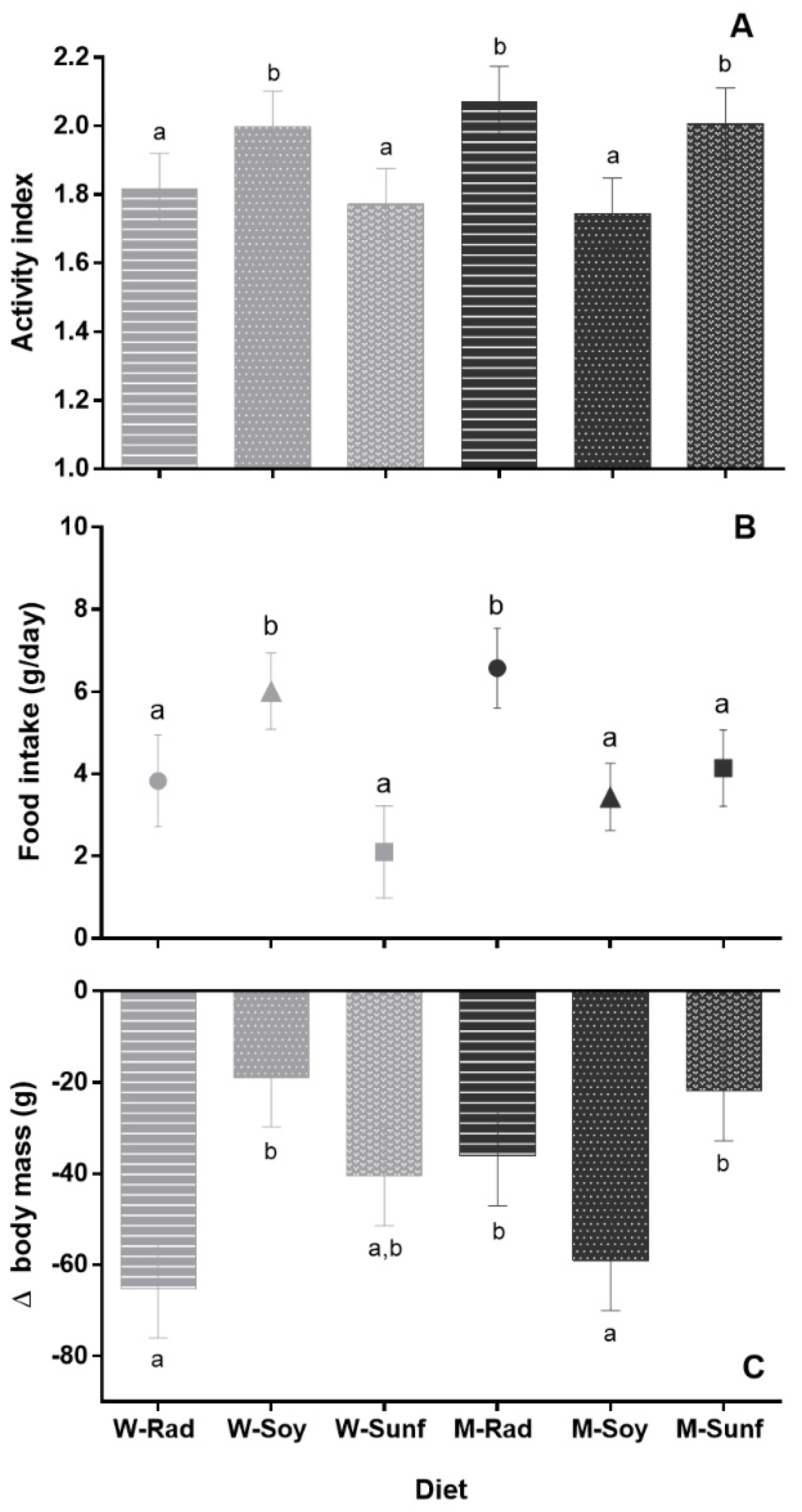
Figure 1.
Hamsters’ hibernation parameters according to crop associations. Each bar represents the mean value of all animals (males and females) for a given diet, +/− SEM. (A) Activity index, (B) food intake (in grams of dry matter per day) and (C) Δbody mass (average loss of mass during hibernation in grams) are represented. Different letters highlight significant differences between the diets, p < 0.05. W = wheat (light gray), M = maize (dark gray), Rad = fodder radish (circles), Soy = soybean triangles) and Sunf = sunflower (squares). (A) This graph should be considered only as a representation of the results, as the CLM approach is not based on averaging.
3.3. Food Intake and Body Mass Loss during Hibernation
Hamsters ingested between 2.1 ± 1.1 and 6.6 ± 1.0 g of food (dry matter) per day throughout hibernation (Figure 1B). Their total daily food intake was influenced by diet (see Figure 1B; F5,43 = 2.8, p = 0.029) and activity index (R2 = 0.69; F1;43 = 93.5, p < 0.001). However, we found no effect of sex (F1;43 = 0.47, p = 0.5) or sex*diet interaction (F5;43 = 0.70, p = 0.6). Overall, hamsters from the Mrad and Wsoy groups ingested significantly more food than hamsters from the other groups (see Figure 1B, p < 0.05).
Hamsters’ body mass loss during hibernation (Δbody mass) was significantly affected by sex (F1;70 = 27.5, p < 0.001), diet (F5;70 = 2.9, p = 0.021; Figure 1C), activity index (F1;70 = 69.5, p < 0.001, R2 = 0.34) and body mass at the onset of hibernation (F1;70 = 83.8, p < 0.001; R2 = 0.33) but not by sex*diet interaction (F5;70 = 1.2, p = 0.3; see Table S4 for means ± SEM at each period and for both sexes). Hamsters from the Msunf, Mrad and WSoy groups had a reduced Δbody mass compared to hamsters from the Wrad and Msoy groups during hibernation, whereas hamsters from the Wsunf group displayed an intermediate Δbody mass (Figure 1C).
3.4. Food Selection and Macronutrient Intake
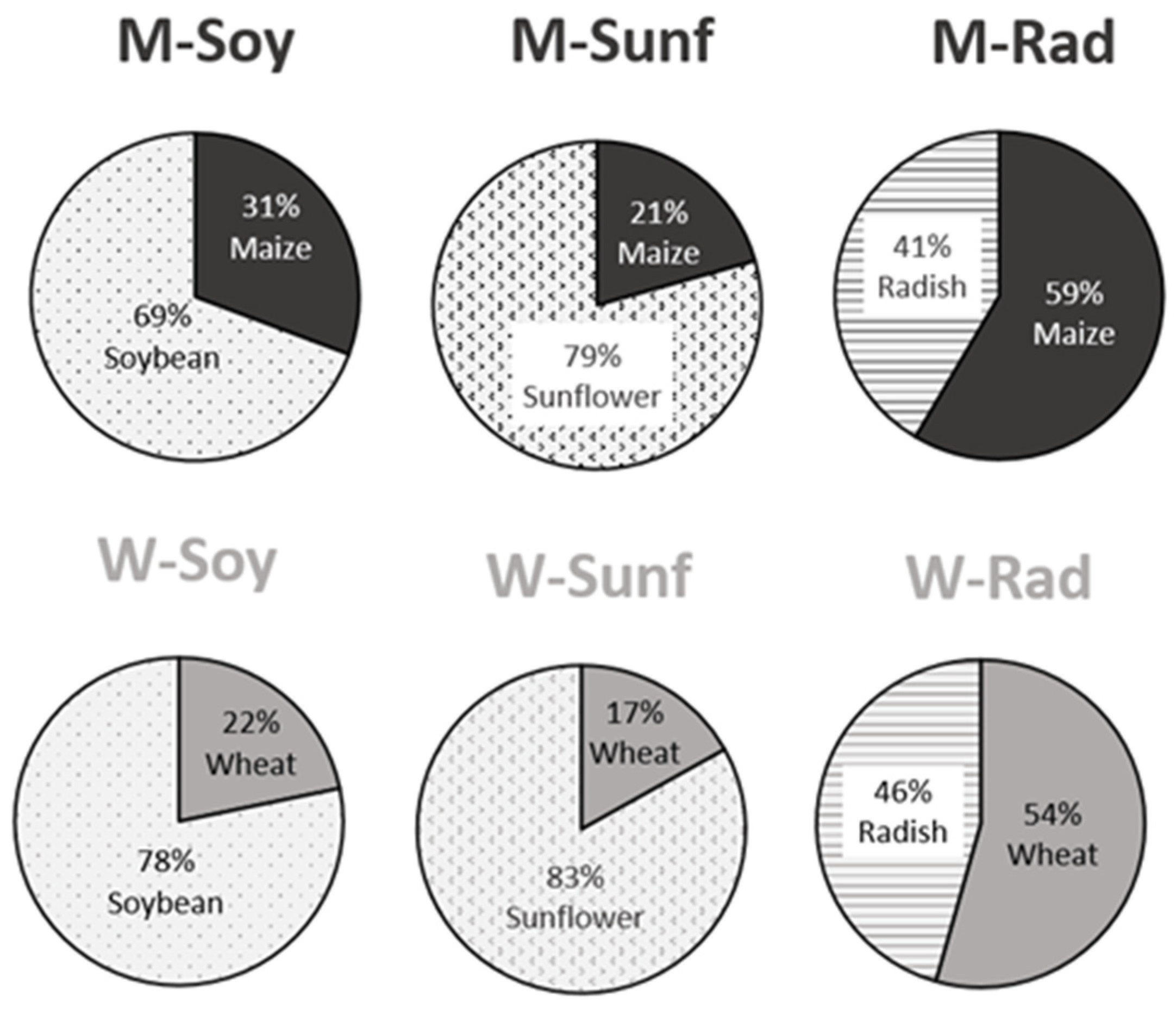
We found a significant effect of the two-way interaction on hamsters’ food intake during hibernation (diet*seed type, Figure 2; F1;97 = 6.3, p < 0.001). In four diet groups (Msoy, Msunf, Wsoy, Wsunf), hamsters ingested a larger proportion of the supplement (intake of 69–78% soybean and 79–83% sunflower, Figure 2) compared to the main food (21–31% maize and 17–22% wheat), whereas in the two radish-supplemented diets, they ingested similar amounts of the main food and the supplement (Figure 2).
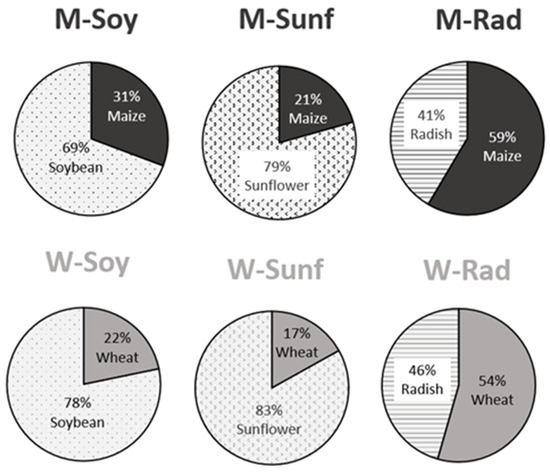
Figure 2.
Consumption of each type of seed during hibernation in the 6 diet groups. W = wheat, M = maize, Rad = fodder radish, Soy = soybean and Sunf = sunflower. See methodology and Table 2 for details on the diets.
The resulting macronutrient consumption for each individual can be plotted in tridimensional space (see Figure 3), where each dimension represents a single macronutrient (see methodology for details).
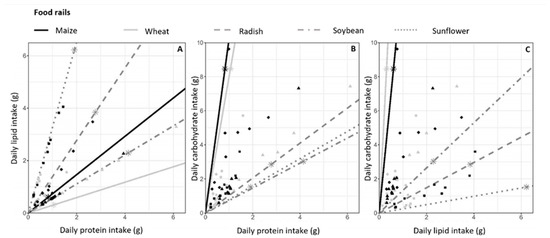
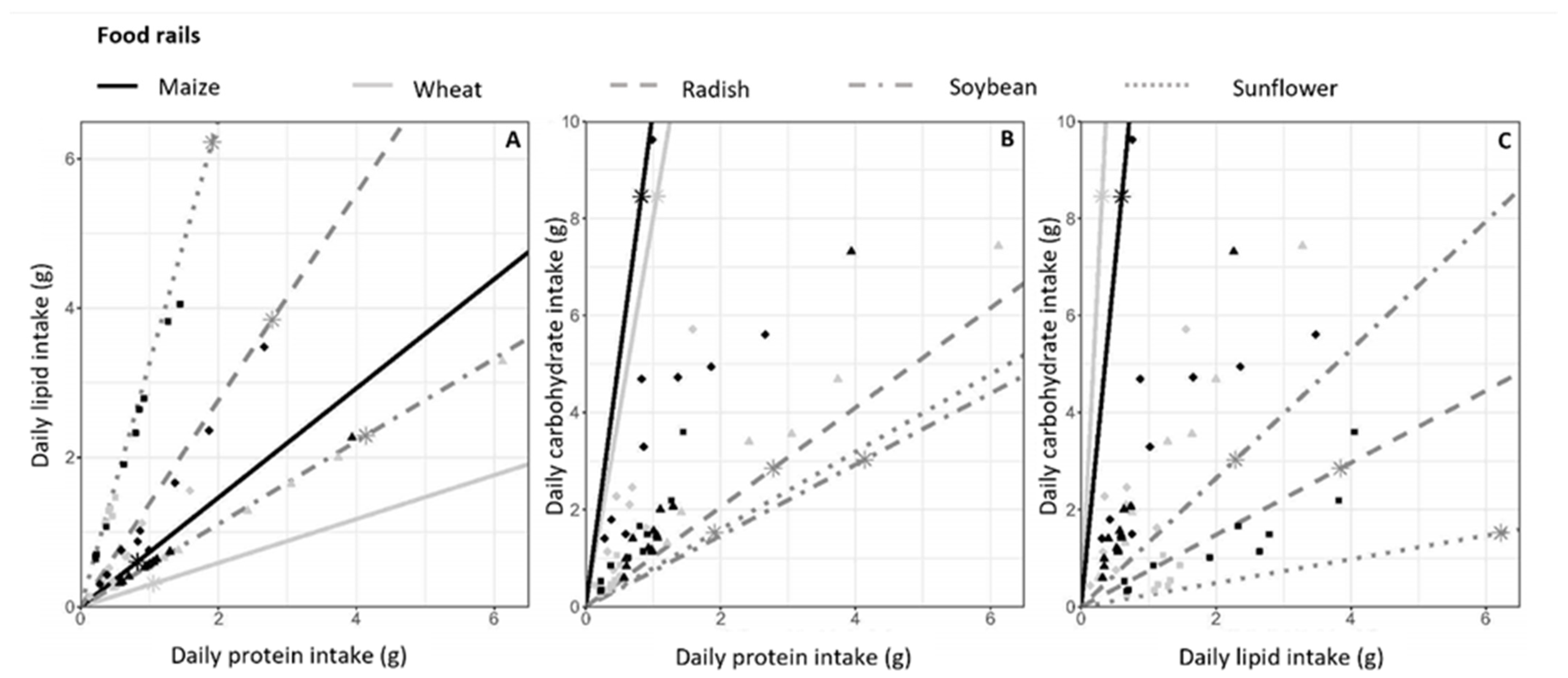
Figure 3.
Daily macronutrient intake. Mean daily macronutrient intake (lipid, protein and carbohydrate in grams) by each hamster during hibernation. (A) Lipid versus protein intake, (B) carbohydrate versus protein intake and (C) carbohydrate versus lipid intake. Food rails, i.e., the ratio of macronutrients contained in each seed type, are represented by lines. The asterisks on each food rail represent the macronutrient intake that would result from the consumption of 10g of the associated seeds. Symbols represent food consumption of each hamster according to their diet, as follows: ▴ Msoy, ▴ Wsoy, ⬥ Mrad, ⬥ Wrad, ■ Msunf, ■ Wsunf. If a hamster ate only 1 of the 2 foods in the diet, the macronutrient intake point would be on the food rail of the considered food. The further the point is from the origin of the graph, the higher the consumption.
As shown in Figure 3, wheat and maize food rails were close (Figure 3A) or very close (Figure 3B,C) in each diet, indicating that these two seed types had a similar ratio of the three macronutrients, that is, carbohydrates, lipids and proteins. The consumption of 10g of supplements (soybean, sunflower or radish) provided more lipids and proteins, whereas more carbohydrates were provided by maize and wheat. As seen in Figure 3A, the lipid/protein intake ratio of hamsters followed the lipid/protein ratio of their respective food supplement. For ratios including carbohydrates (Figure 3B,C), the macronutrient intake of each hamster did not match the food rails. This is due to the high carbohydrate content of wheat and maize, driving the macronutrient ratio toward their respective food rails. Hamsters had the possibility of diversified nutrient consumption, represented by the area between the two food rails for each diet. However, macronutrient intake was highly variable between individuals in terms of quantity in all diets, but the proportion of fat, proteins and carbohydrates was very similar between individuals on the same diet. This shows a food selection based on macronutrients by hamsters, which was consistent across individuals.
3.5. Female Reproductive Success
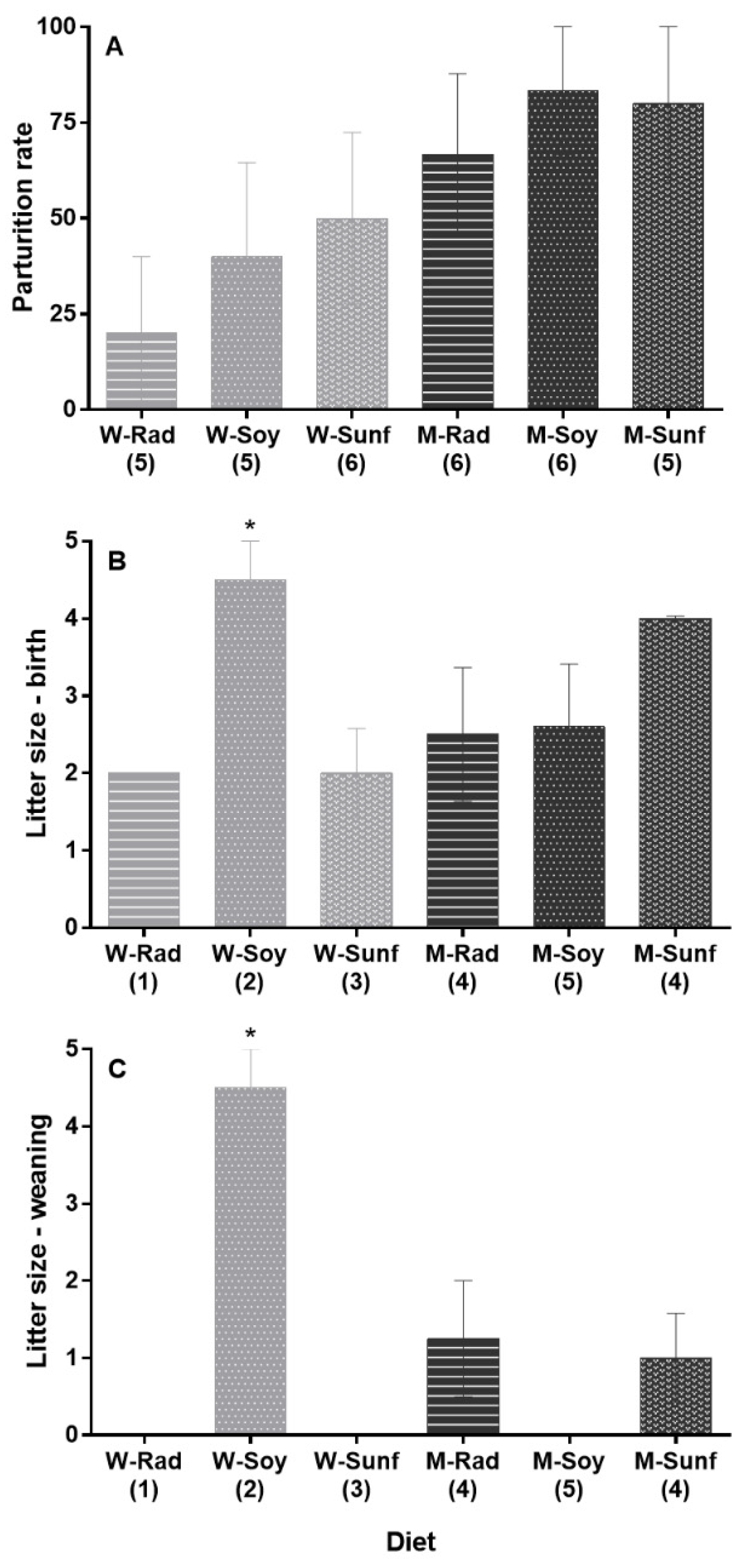
Not all females successfully bred, as in some cases, major attacks on males, or from males, occurred, leading to significant injuries, which reduced the sample size from N = 7 females/diet group to N = 5–6 females/diet group, as shown in Figure 4A. Births were, however, recorded in all six groups, and the parturition rate ranged from 20% (Wrad diet) to 83% (Msoy and Msunf diets, Figure 4A). We found no effect of the diet (Χ2 = 6.91, p = 0.23, Figure 4A), the body mass after hibernation (Χ2 = 0.78, p = 0.38) or the diet*body mass interaction (Χ2 = 7.81, p = 0.17) on the parturition rate (considering only successful parturitions, thus females that gave birth to at least one living pup; model and output are presented in Table S5).
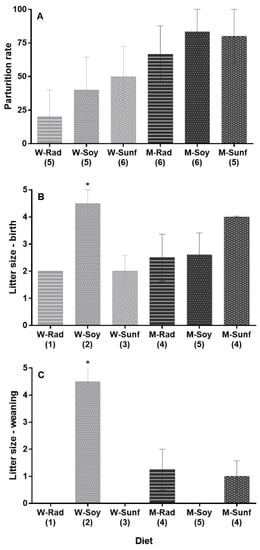
Figure 4.
Female reproductive traits according to their diet. (A) Parturition success (representing females that successfully delivered; no significant diet effect, p > 0.05). Mean litter size is presented (B) at birth and (C) at weaning. Sample sizes (N females) are indicated in brackets. Mean litter size is shown separately at birth and weaning for representative purposes only, as we found no diet*period interaction effect but identified an overall diet effect on this trait (p < 0.05): litters of females from the Wsoy group were significantly larger on average than those from the Wsunf, Msoy and Mrad groups (p = 0.011, p = 0.008 and p = 0.011, respectively, as represented by *), but not larger than litters from the Msunf group (p > 0.05). Multiple comparisons were not possible with the Wrad group, as only one female gave birth, and none of her pups survived. W = wheat, M = maize, Rad = fodder radish, Soy = soybean and Sunf = sunflower. See methodology and Table 2 for details. Maize-based diets are represented in light gray, whereas wheat-based diets are represented in dark gray (Means ± SEM).
Regarding the litter size, model selection revealed three models with equivalent AICc (ΔAICc < 0.6) and weight (GLMM, quasi-Poisson, model 1: period + body mass as fixed effects; model 2: period; model 3: period + diet). Hereafter, we discuss model 3, which included both the period (birth and weaning) and the diet as fixed effects (other models can be found in Table S6). Model 3 revealed an overall effect of the diet (Χ2 = 19.39, p = 0.002) and the period (Χ2 = 18.39, p < 0.001) on the litter size (model 3, Table S6). On average, females had more pups at birth than at weaning (estimate: 1.13 ± 0.29, t = 3.87, Figure 4B,C). Regarding the diet, litters of females from the Wsoy group were larger than those from the Wsunf, Msoy and Mrad groups (estimates for Wsoy–Wsunf: 1.50 ± 0.51, t = 2.95, p = 0.011; Wsoy–Msoy: 1.24 ± 0.39, t = 3.16, p = 0.008 and Wsoy–Mrad: 1.50 ± 0.51, t = 2.95, p = 0.011; see Table S7), but not larger than litters from the Msunf group (p > 0.05; Figure 4B,C). Multiple comparisons were not possible with the Wrad group, as only one female gave birth, and none of her pups survived. The diet*period interaction was excluded from the three best models (Table S6). However, although no pups survived in the Msoy, Wrad and Wsunf diet groups, a total of nine, five and four pups were weaned in the Wsoy, Mrad and Msunf groups, respectively (Figure 4C).
4. Discussion
Results of this study emphasized that there was no single crops meeting the nutritional requirements of hamsters during hibernation and reproduction, but rather favorable crop associations. Wsoy, Msunf and Mrad associations were overall more adapted than the three other associations tested. We also found that common hamsters used two different strategies during hibernation. They either (1) remained mainly active and relied on their food hoards or (2) spared energy using torpor and relied less on their hoards. The composition of these hoards influenced the adoption of one or the other strategy, given that hamsters were significantly more active and ingested overall more food in the Wsoy, Mrad and Msunf groups compared to the maize–soybean, Wsunf and Wrad groups (see Figure 5 in Section 4.4 below for a synthesis of the results). In terms of body mass, the first strategy appeared to be associated with a reduced loss of mass throughout hibernation compared to the second strategy, a parameter that was positively associated with subsequent reproductive success. In all cases, females from the diet groups with the greatest activity and lowest loss of mass during hibernation (Wsoy, Msunf and Mrad) displayed higher success of reproduction, with the greatest reproductive success being recorded in the Wsoy diet group.
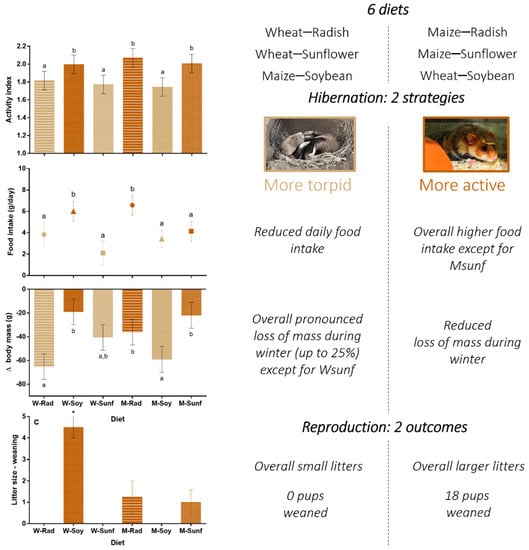
Figure 5.
Summary figure regrouping the main results of our study under the two strategies of hibernation (more torpid or more active). This figure aims to provide a bigger picture to assess the effects of each diet and both strategies on hamsters’ hibernation and reproductive traits and can be used to inform conservation actions. Details about statistics can be found in the Results section of the manuscript, in Figure 1, Figure 3 and Figure 4 and in Table S3.
4.1. Activity Index and ΔBody Mass: Sex and Diet Effects
We found sex differences in both the activity index and Δbody mass during hibernation: on average, females were more active and lost more mass than males. Similar sex differences have been recorded in other species [49], including the common hamster [50,51]. Females usually hibernate for shorter periods than males [50]. When supplemented with food, however, the trend is reversed, and males hibernate for shorter periods and display fewer torpor events during hibernation than females [51]. Thus, while males’ hibernation patterns are influenced by the presence and the size of their hoards, females do not seem to respond to additional food [51]. Our results, however, highlight that, beyond the size of the hoards [33], their composition seems to be of major significance, even for females. We indeed found that the diet had a strong effect on hamsters’ activity index and Δbody mass, emphasizing that hamsters on the Wsoy, Msunf and Mrad diets were more active and lost less mass than hamsters on the Msoy and Wrad diets, independent of sex. Interestingly, the Msunf group responded differently from the five others during hibernation: hamsters displayed a reduced loss of mass (small Δbody mass) despite a lower food intake than in the other diet groups. This could be explained by the high energy value of sunflower or its high PUFA content [27]. In the two latter diet groups, hamsters lost approximately 25% of their initial body mass (i.e., body mass at the onset of hibernation). Such loss of mass is severe and could have important consequences for hamsters’ survival and reproduction in the wild [15,27,50,51]. As such, the Msoy and Wrad diets seem unsuitable to slow down the decline in body mass upon emergence from hibernation, which has been recorded in farmlands since 1937 [15], while Msunf, Mrad and especially Wsoy seem more appropriate. Nonetheless, when looking at Δbody mass compared to the amount of food ingested (Figure 3B,C), the Mrad diet seems to be less “efficient” than the Wsoy and Msunf diets. Hamsters from this diet group ingested the largest amounts of food but did not reduce their Δbody mass compared to hamsters from Wsoy and Msunf; they tended to lose more mass than individuals from these two diet groups, although the difference was not significant. In addition, signs of cartilage atrophy were observed in the ears of hamsters on the Mrad diet (see Figure S1), suggesting the presence of a nutritional deficiency in this association. Taken together and considering that hamsters would not necessarily have access to ad libitum food in the wild, the Wsoy and Msunf associations seem to be the most appropriate.
4.2. Diet Effect on Reproduction
Regarding hamsters’ reproductive success, the three crop associations that were favorable for hibernation (Wsoy, Msunf and Mrad) were also the most favorable for reproduction, considering that pups were weaned only in these three diet groups. In the Wsoy group, females had litters of 4.5 ± 0.5 pups, which is within the average recorded in other studies [18,19,26], but only 40% of females gave birth. We did not find significant differences in the parturition rate between the diets, potentially due to a lack of statistical power associated with our low sample size at reproduction. However, in the Msunf and Mrad groups, 80% and 67% of females gave birth, respectively. These females had smaller litters at birth (4.0 ± 0.0 and 2.5 ± 0.9, respectively) than females of the Wsoy group (4.5 ± 0.5). At weaning, this difference in litter size increased: it became 4 to 5 times higher in the Wsoy than in Msunf and Mrad groups (Figure 4B,C). This resulted in nine, five and four pups being weaned in the Wsoy, Mrad and Msunf diet groups, respectively, and no pups in the three other diet groups (Msoy, Wsunf and Wrad; see summary Figure 5 in Section 4.4 below). This success of reproduction remains relatively low compared to other studies conducted in captive or semi-captive conditions [18,19,26]. However, it echoes the recorded decline in reproductive success over the distribution range of the species [11]. The reduced reproductive success recorded in some diet groups could be due to seeds lacking proteins [26] or other nutrients essential for reproduction [26]. The protein intake on the Wsoy diet was, for instance, higher than that on Msunf and Mrad, which may have contributed to the 100% pup survival in this diet group.
Interestingly, diet effects on hamsters’ hibernation and reproduction are unlikely to be solely explained by the macronutrient or protein contents of the diets. For example, the two protein-rich diets (Msoy and Wsoy), from which hamsters ingested approximately the same proportion of soybean (see Figure 2 and Figure 3), had very different outcomes regarding both hibernation and reproduction. Furthermore, in the Msoy diet group, we recorded a 100% maternal infanticide rate (similarly to what was previously observed and related to a vitamin B3 deficiency in maize-based diets [18]), suggesting an imbalance leading to vitamin B3-like deficiencies in this association. Soybean thus does not appear to be an appropriate crop to counteract the nutrient deficiencies in maize. In the wild, hamsters will likely supplement their diet with invertebrates and weeds [16,19]. However, when emerging from hibernation in early spring, such food items remain relatively rare, especially in intensively managed farming landscapes, and hamsters thus largely rely on their hoards for their first reproduction [15,16,52].
Taken together, our results thus suggest that the quantity of proteins or lipids per se were not the sole predictor of successful hibernation and reproduction in this species, which would also be modulated by the quality of proteins (e.g., representativeness and ratio between all essential amino acids [53]) and fatty acids (e.g., representativeness and ratio between different fatty acids [34,54,55]) or the content of vitamins/antioxidants [18,56].
4.3. Favorable Crop Associations and Recommendations
Our results highlight that no crop alone can be considered as a favorable food that meets hamsters’ nutritional requirements during hibernation and subsequent reproduction. Soybean was beneficial when associated with wheat but not when associated with maize. On the contrary, sunflower and radish were unfavorable when associated with wheat but not with maize. It is thus important to consider the complementarity of crops when suggesting associations beneficial for the species, as monoculture-based diets are not suitable for hamsters’ hibernation and reproduction [18,19]. However, associating wheat with soybean, or maize with sunflower and radish, seems to provide a promising avenue to increase diversity and heterogeneity in agroecosystems where hamsters are found. A study conducted in exclosures highlighted that associating maize, wheat, sunflower and a fodder crop (alfalfa) provided the appropriate diversity of food for hamsters’ reproduction compared to maize and wheat alone [19]. It thus seems that a way to improve hamsters’ habitats in Western Europe would be to include an oilseed crop such as sunflower and a fodder crop such as radish or alfalfa within agroecosystems while ensuring extensive management of crops [14,57,58]. Taken together, these plants seem to provide food with the required nutrients to support hamsters’ hibernation and reproduction. However, achieving such crop diversity in intensive farmlands may be a long and complex process, and crop associations such as wheat–soybean and maize–sunflower may offer solutions that are easier to implement and more adapted to farmers’ constraints. Other crop associations that meet those two parameters are likely to be beneficial for hamsters and should be the focus of future studies.
4.4. From Knowledge to Action
We used different approaches to disseminate these results and new knowledge to farmers, conservation practitioners, researchers and policymakers as part of the LIFE+ ALISTER project. These included summary presentations of our results to general assemblies of farmers and distribution of synthesized information in general and specialized media and newsletters to conservation practitioners and policymakers (see Figure 5).
Before any recommendation was officially made following the dissemination of these synthesized results (Figure 5), the best innovation came from farmers. Some of them started to spread sunflower within maize fields (Figure 6A) or integrated sunflower and fodder crops within summer intercrop covers sown after wheat harvest (Figure 6D) [59]. Another immediate response came from the French Office for Biodiversity (OFB), which diversified parcels where captive-bred hamsters are released every year as part of a conservation effort to sustain wild populations [59,60,61]. These parcels were previously composed of unharvested wheat [57]. Since 2017, they have been composed of unharvested wheat diversified with strips of flowers, including a fodder crop and sunflower (Figure 6B) [59]. Other farmers developed double-cropping systems such as rye–pea (Figure 6C) or wheat–pea (Figure 6E); such associations were implemented because they are similar to the wheat–soybean association and naturally provide nitrogen to the soil. While these innovations and responses are not proof of success (their durability and benefits for hamster populations remain to be determined), they offer opportunities for real-time monitoring, allowing the quick adaptation of strategies through policymaking [62]. Indeed, once knowledge on an endangered species is acquired, it can take years for measures to be implemented through policymaking, if implemented at all [63]. However, species facing risks of extinction often require urgent measures to be taken [64,65]. Collaboration and communication with all stakeholders from the initiation of research programs thus offer interesting avenues to implement science-based solutions more quickly.

Figure 6.
Examples of innovative approaches from farmers, conservation practitioners and researchers to implement sunflower, protein crops and fodder crops to diversify agroecosystems dominated by maize and wheat. (A) Sunflower sown by a farmer within a maize field in 2018 (broadcast seeding) © Charlotte Kourkgy (OFB), (B) strips of fodder crops and wildflowers, including sunflower, sown on parcels where captive-bred hamsters are released every year, with fields of maize and wheat © Charlotte Kourkgy (OFB), (C) association between a spring cereal (rye) and a protein crop (pea) © Florian Kletty, (D) intercrop cover including fodder crops and sunflower, sown after wheat harvest in July 2018 © Caroline Habold, (E) wheat–pea association implemented in the area where the common hamster is found in Alsace, France © Germain Schmidt.
Wildflower strips, vegetables and fodder crops are known to have a positive impact on hamster populations, increasing burrow density [58,66,67]. However, the benefits of fodder crops depend on their management [14,57]. The benefits of sunflower for hamster survival and reproduction when associated with wheat, maize and a fodder crop were previously confirmed [19]. Thus, in 2021, sunflower, fodder crops and the wheat–soybean association were included as favorable crops for hamsters as part of the new collective agri-environmental measures for the species in France. Such agri-environmental measures for hamsters are likely to benefit other species, as similar agri-environmental measures implemented for pollinators appeared to be beneficial for hamsters [66]. By enhancing the diversity and heterogeneity of agroecosystems, it is expected that measures implemented for hamsters will be suitable for other farmland species or will even provide extended services in such systems [9]. Sunflower has, for instance, many benefits for biodiversity and agriculture [68,69,70]. It attracts birds, some of which reduce pest populations [68,69]. It also provides key benefits for pollinators, considering its major medicinal values for bees [70,71]. Its implementation in maize-dominated landscapes thus appears to be a simple way to improve habitat heterogeneity. Crop associations with complementary nutritional values for common hamsters are also likely beneficial for agroecosystems as a whole; for instance, double-cropping systems such as the wheat–soybean association, which is largely generalized in the US and in Asia, seem to provide benefits in terms of resistance and resilience to climate change compared to single cropping [72].
5. Conclusions
The main conclusion of this study is that there is not one “favorable” crop for hamsters, but rather favorable crop associations. Indeed, sunflower and radish were overall (i.e., when considering hibernation and reproduction) beneficial when associated with maize, whereas they were unfavorable when associated with wheat. In contrast, soybean was beneficial when associated with wheat but not when associated with maize. The implementation of such crop associations will only be possible with the implementation of innovative agricultural practices that should integrate farmers from the onset of the initiation of research projects. Such conservation measures implemented for the common hamster may help to transition toward more biodiversity-friendly and sustainable agricultural practices in areas where intensive conventional farming prevails.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/su132413521/s1, Figure S1. Signs of cartilage atrophy in the ears of common hamsters on the Mrad diet after hibernation; Table S1. Macronutrient and micronutrient composition of the crops selected for the experiment; Table S2. Proportions (%) of indexes 1, 2 and 3 recorded for each sex on each diet throughout winter; Table S3. Model selection looking at the effect of diet (base and complement), sex and body mass on activity index; Table S4. Hamsters’ average body mass (g) according to the diet, sex and period; Table S5. Output of the model looking at the effect of the diet and the body mass on females’ parturition rate; Table S6. Model selection for the effect of the diet, the body mass and the period (birth or weaning) on litter size; Table S7. Multiple comparisons of model 3 from Table S6 on diet effect on litter size.
Author Contributions
Conceptualization, M.L.T. and C.H.; methodology, M.L.T.; validation, C.H. and J.-P.R.; formal analysis, M.L.T. and F.K.; investigation, M.L.T.; resources, C.H.; data curation, M.L.T. and F.K.; writing—original draft preparation, M.L.T.; writing—review and editing, F.K., C.H. and J.-P.R.; visualization, M.L.T. and F.K.; supervision, C.H.; project administration, M.L.T. and C.H.; funding acquisition, C.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the LIFE+ Biodiversity grant no. LIFE12 BIO/FR/000979 and the Ministère de l’Écologie, du Développement Durable et de l’Énergie (MEDDE).
Institutional Review Board Statement
The study followed the European Directive 2010/63/EU on the protection of animals used for scientific purposes, and was approved by the Ethical Committee (CREMEAS) and the Ministère de l’Enseignement Supérieur et de la Recherche (MESR) under agreement number 00624-01 on 29 November 2013 and renewed on 2 April 2019, under APAFIS# agreement 17484-2018103016124862 v3.
Data Availability Statement
Original data are available on OSF. Tissier, Mathilde. 2021. “Dataset—Sustainable Agriculture: Nutritional Benefits of Wheat–Soybean and Maize–Sunflower Associations for Hibernation and Reproduction of Endangered Common Hamsters.” OSF. 1 December. osf.io/e8bdj.
Acknowledgments
Many thanks to the farmers who participated in the consultation, namely, those of the AFSAL and CUMA de la Plaine, and to our partners from the OFB and the CAA for their valuable feedback. We thank Emilio Rojas from Wildstats for his help on statistical analysis of the activity index. Many thanks to the three anonymous reviewers for their feedback and suggestions, which helped us improve the manuscript. We are also grateful to animal caretakers and animal housing manager for their work and help, which made this work possible. Thanks to Timothée Gerard for his comments on the introduction and to Rita Fragueira and Thibaut Barra for their help with analyses of food consumption and food composition.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [PubMed]
- Stoate, C.; Boatman, N.; Borralho, R.; Carvalhob, C.R.; Snoo, G.; Eden, P. Ecological impacts of arable intensification in Europe. J. Environ. Manag. 2001, 63, 337–365. [Google Scholar] [CrossRef]
- Benton, T.G.; Bryant, D.M.; Cole, L.; Crick, H.Q.P. Linking agricultural practice to insect and bird populations: A historical study over three decades. J. Appl. Ecol. 2002, 39, 673–687. [Google Scholar] [CrossRef]
- Geiger, F.; Bengtsson, J.; Berendse, F.; Weisser, W.W.; Emmerson, M.; Morales, M.B.; Ceryngier, P.; Liira, J.; Tscharntke, T.; Winqvist, C.; et al. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Wickramasinghe, L.P.; Harris, S.; Jones, G.; Jennings, N.V. Abundance and Species Richness of Nocturnal Insects on Organic and Conventional Farms: Effects of Agricultural Intensification on Bat Foraging. Conserv. Biol. 2004, 18, 1283–1292. [Google Scholar] [CrossRef]
- Didham, R.K.; Basset, Y.; Collins, C.M.; Leather, S.R.; Littlewood, N.A.; Menz, M.H.M.; Müller, J.; Packer, L.; Saunders, M.E.; Schönrogge, K.; et al. Interpreting insect declines: Seven challenges and a way forward. Insect Conserv. Divers. 2020, 13, 103–114. [Google Scholar] [CrossRef]
- Guzman, L.M.; Johnson, S.A.; Mooers, A.O.; M’Gonigle, L.K. Using historical data to estimate bumble bee occurrence: Variable trends across species provide little support for community-level declines. Biol. Conserv. 2021, 257, 109141. [Google Scholar] [CrossRef]
- Hędrzak, M.; Badach, E.; Kornaś, S. Preliminary Assumptions for Identification of the Common Hamster (Cricetus cricetus) as a Service Provider in the Agricultural Ecosystem. Sustainability 2021, 13, 6793. [Google Scholar] [CrossRef]
- Banaszek, A.; Bogomolov, P.; Feoktistova, N.; La Haye, M.; Monecke, S.; Reiners, T.E.; Rusin, M.; Surov, A.; Weinhold, U.; Ziomek, J. Cricetus cricetus. IUCN Red List of Threaten Species. Available online: https://dx.doi.org/10.2305/IUCN.UK.2020-2.RLTS.T5529A111875852.en (accessed on 14 September 2021).
- Surov, A.; Banaszek, A.; Bogomolov, P.; Feoktistova, N.; Monecke, S. Dramatic global decrease in the range and reproduction rate of the European hamster Cricetus cricetus. Endanger. Species Res. 2016, 31, 119–145. [Google Scholar] [CrossRef]
- Chaigne, A.; Tissier, M.L.; Habold, C.; Eidenschenck, J.; Uhlrich, B. Le Grand hamster (Cricetus cricetus) en Alsace, quel devenir? In Bourgogne Nature—Les Mammifères Sauvages, Recolonisation et Réémergence; Actes du 37ème colloque de la SFEPM: St-Brisson, France, 2014; pp. 312–322. [Google Scholar]
- Weinhold, U. Draft European action plan for the conservation of the common hamster (Cricetus cricetus L., 1758). In Proceedings of the Convention on the Conservation of European Wildlife and Natural Habitats (28th Meeting Standing Committee), Strasbourg, France, 24–27 November 2008. [Google Scholar]
- La Haye, M.; Swinnen, K.; Kuiters, A.; Leirs, H.; Siepel, H. Modelling population dynamics of the Common hamster (Cricetus cricetus): Timing of harvest as a critical aspect in the conservation of a highly endangered rodent. Biol. Conserv. 2014, 180, 53–61. [Google Scholar] [CrossRef]
- Tissier, M.L.; Handrich, Y.; Robin, J.-P.; Weitten, M.; Pevet, P.; Kourkgy, C.; Habold, C. How maize monoculture and increasing winter rainfall have brought the hibernating European hamster to the verge of extinction. Sci. Rep. 2016, 6, 25531. [Google Scholar] [CrossRef] [PubMed]
- Tissier, M.L.; Marchandeau, S.; Habold, C.; Handrich, Y.; Eidenschenck, J.; Kourkgy, C. Weeds as a predominant food source: A review of the diet of common hamsters Cricetus cricetus in farmlands and urban habitats. Mammal Rev. 2018, 49, 152–170. [Google Scholar] [CrossRef]
- Agreste Statistique Agricole 2014, 2017, 2018. Available online: https://agreste.agriculture.gouv.fr/agreste-web/ (accessed on 18 May 2021).
- Tissier, M.L.; Handrich, Y.; Dallongeville, O.; Robin, J.-P.; Habold, C. Diets derived from maize monoculture cause maternal infanticides in the endangered European hamster due to a vitamin B3 deficiency. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162168. [Google Scholar] [CrossRef]
- Tissier, M.L.; Kletty, F.; Handrich, Y.; Habold, C. Monocultural sowing in mesocosms decreases the species richness of weeds and invertebrates and critically reduces the fitness of the endangered European hamster. Oecologia 2018, 186, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Humphries, M.M.; Thomas, D.W.; Kramer, D.L. The Role of Energy Availability in Mammalian Hibernation: A Cost-Benefit Approach. Physiol. Biochem. Zool. 2003, 76, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Franceschini-Zink, C.; Millesi, E. Population Development and Life Expectancy in Common Hamsters. 2008. Available online: https://ucris.univie.ac.at/portal/en/publications/population-development-and-life-expectancy-in-common-hamsters(2302eda9-2118-47ce-869d-e2ced0930227).html (accessed on 12 March 2016).
- Millesi, E.; Strijkstra, A.M.; Hoffmann, I.E.; Dittami, J.P.; Daan, S. Sex and Age Differences in Mass, Morphology, and Annual Cycle in European Ground Squirrels, Spermophilus citellus. J. Mammal. 1999, 80, 218–231. [Google Scholar] [CrossRef][Green Version]
- Hoogland, J.L. The Black-Tailed Prairie Dog: Social Life of a Burrowing Mammal; University of Chicago Press: Chicago, IL, USA, 1995. [Google Scholar]
- Dark, J. Annual lipid cycles in hibernators: Integration of physiology and behavior. Annu. Rev. Nutr. 2005, 25, 469–497. [Google Scholar]
- Humphries, M.M.; Thomas, D.W.; Kramer, D.L. Torpor and Digestion in Food-Storing Hibernators. Physiol. Biochem. Zool. 2001, 74, 283–292. [Google Scholar] [CrossRef]
- Weitten, M.; Tissier, M.L.; Robin, J.-P.; Habold, C. Dietary proteins improve hibernation and subsequent reproduction in the European hamster, Cricetus cricetus. Am. J. Physiol. Integr. Comp. Physiol. 2018, 315, R848–R855. [Google Scholar] [CrossRef]
- Siutz, C.; Nemeth, M.; Wagner, K.-H.; Quint, R.; Ruf, T.; Millesi, E. Effects of food store quality on hibernation performance in common hamsters. PLoS ONE 2017, 12, e0185913. [Google Scholar] [CrossRef]
- Arnold, W.; Giroud, S.; Valencak, T.G.; Ruf, T. Ecophysiology of Omega Fatty Acids: A Lid for Every Jar. Physiology 2015, 30, 232–240. [Google Scholar] [CrossRef]
- Munro, D.; Thomas, D.W. The role of polyunsaturated fatty acids in the expression of torpor by mammals: A review. Zoology 2004, 107, 29–48. [Google Scholar] [CrossRef]
- Jastroch, M.; Giroud, S.; Barrett, P.; Geiser, F.; Heldmaier, G.; Herwig, A. Seasonal Control of Mammalian Energy Balance: Recent Advances in the Understanding of Daily Torpor and Hibernation. J. Neuroendocr. 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Munro, D.; Thomas, D.W.; Humphries, M.M. Torpor patterns of hibernating eastern chipmunks Tamias striatus vary in response to the size and fatty acid composition of food hoards. J. Anim. Ecol. 2005, 74, 692–700. [Google Scholar] [CrossRef]
- Humphries, M.M.; Thomas, D.W.; Hall, C.L.; Speakman, J.R.; Kramer, D.L. The energetics of autumn mast hoarding in eastern chipmunks. Oecologia 2002, 133, 30–37. [Google Scholar] [CrossRef]
- Siutz, C.; Millesi, E. Torpor patterns in common hamsters with and without access to food stores. J. Comp. Physiol. B 2017, 187, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Tarnoczi, T.J.; Berkes, F. Sources of information for farmers’ adaptation practices in Canada’s Prairie agro-ecosystem. Clim. Chang. 2009, 98, 299. [Google Scholar] [CrossRef]
- Marr, E.J.; Howley, P. The accidental environmentalists: Factors affecting farmers’ adoption of pro-environmental activities in England and Ontario. J. Rural. Stud. 2019, 68, 100–111. [Google Scholar] [CrossRef]
- AFZ, I. CIRAD, FAO Feedipedia. Anim Feed Ressources Inf Syst. Available online: http://www.feedipedia.org/ (accessed on 24 July 2020).
- USDA, Nutrition Data. In USDA SR-21, Nutrition Data. Available online: http://nutritiondata.self.com/ (accessed on 1 September 2016).
- HeckenBenner, B.; de PontBriand, S. CIPAN: Quand L’outil Réglementaire Devient un Atout Agronomique et Faunistique. Available online: https://docplayer.fr/21149047-Cipan-quand-l-outil-reglementaire-devient-un-atout-agronomique-et-faunistique.html (accessed on 24 July 2020).
- Chen, G.; Weil, R.R. Penetration of cover crop roots through compacted soils. Plant Soil 2010, 331, 31–43. [Google Scholar] [CrossRef]
- Mandal, S.; Yadav, S.; Singh, R.; Begum, G.; Suneja, P.; Singh, M. Correlation studies on oil content and fatty acid profile of some Cruciferous species. Genet. Resour. Crop. Evol. 2002, 49, 551–556. [Google Scholar] [CrossRef]
- Kjeldahl, C. A new method for the determination of nitrogen in organic matter. Z. Anal. Chem. 1883, 22, 366. [Google Scholar] [CrossRef]
- Campbell, R.R.; Leatherland, J.F. Estimating Body Protein and Fat from Water Content in Lesser Snow Geese. J. Wildl. Manag. 1980, 44, 438. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Raubenheimer, D.; Simpson, S.J. Integrative models of nutrient balancing: Application to insects and vertebrates. Nutr. Res. Rev. 1997, 10, 151–179. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Raubenheimer, D. A multi-level analysis of feeding behaviour: The geometry of nutritional decisions. Philos. Trans. R. Soc. B Biol. Sci. 1993, 342, 381–402. [Google Scholar] [CrossRef]
- Concannon, P.W.; Fullam, L.A.; Baldwin, B.H.; Tennant, B.C. Effects of Induction Versus Prevention of Hibernation on Reproduction in Captive Male and Female Woodchucks (Marmota Monax)1. Biol. Reprod. 1989, 41, 255–261. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 978-0-387-95364-9. [Google Scholar]
- Zar, J.H. Biostatistical Analysis; Pearson Prentice Hall./Pearson Education. Inc.: Upper Saddle River, NJ, USA, 1999; 255p. [Google Scholar]
- Michener, G.R. Sexual differences in over-winter torpor patterns of Richardson’s ground squirrels in natural hibernacula. Oecologia 1992, 89, 397–406. [Google Scholar] [CrossRef]
- Siutz, C.; Franceschini, C.; Millesi, E. Sex and age differences in hibernation patterns of common hamsters: Adult females hibernate for shorter periods than males. J. Comp. Physiol. B 2016, 186, 801–811. [Google Scholar] [CrossRef]
- Siutz, C.; Valent, M.; Ammann, V.; Niebauer, A.; Millesi, E. Sex-specific effects of food supplementation on hibernation performance and reproductive timing in free-ranging common hamsters. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Nechay, G.; Hamar, M.; Grulich, I. The Common Hamster (Cricetus cricetus [L.]); A Review. EPPO Bull. 1977, 7, 255–276. [Google Scholar] [CrossRef]
- Al-Badry, K.S.; Taha, H.M. Hibernation hypothermia and metabolism in hedgehogs—Changes in free amino acids and related compounds. Comp. Biochem. Physiol. Part A Physiol. 1982, 72, 541–547. [Google Scholar] [CrossRef]
- Giroud, S.; Stalder, G.; Gerritsmann, H.; Kübber-Heiss, A.; Kwak, J.; Arnold, W.; Ruf, T. Dietary Lipids Affect the Onset of Hibernation in the Garden Dormouse (Eliomys quercinus): Implications for Cardiac Function. Front. Physiol. 2018, 9, 1235. [Google Scholar] [CrossRef] [PubMed]
- Geiser, F. Influence of polyunsaturated and saturated dietary lipids on adipose tissue, brain and mitochondrial membrane fatty acid composition of a mammalian hibernator. Biochim. Et Biophys. Acta (BBA) Lipids Lipid Metab. 1990, 1046, 159–166. [Google Scholar] [CrossRef]
- Carey, H.V.; Andrews, M.T.; Martin, S.L. Mammalian Hibernation: Cellular and Molecular Responses to Depressed Metabolism and Low Temperature. Physiol. Rev. 2003, 83, 1153–1181. [Google Scholar] [CrossRef]
- Villemey, A.; Besnard, A.; Grandadam, J.; Eidenschenck, J. Testing restocking methods for an endangered species: Effects of predator exclusion and vegetation cover on common hamster (Cricetus cricetus) survival and reproduction. Biol. Conserv. 2013, 158, 147–154. [Google Scholar] [CrossRef]
- Wencel, M.-C.; Migot, P. Le grand hamster en France en 1998. In Proceedings of the International Hamster Workgroup Meeting, Muttersholtz, France, 31 October–1 November 1998; pp. 32–37. [Google Scholar]
- Tissier, M.L.; Habold, C.; Kletty, F.; Eidenschenck, J.; Marchandeau, S.; Handrich, Y.; Oswald, P.; Revel-Mouroz, A.; Kourkgy, C. Concilier agriculture et préservation de la faune de plaine: Le cas du grand hamster en Alsace. Faune Sauvage 2019, 322, 31–39. [Google Scholar]
- Amand, B.; Duponteil, A.; Strosser, P.; Boos, M. National Actions Plan for the Common Hamster Cricetus Cricetus; MEDDE: Paris, France, 2012; p. 175. [Google Scholar]
- Virion, M.-C. Plan National D’actions en Faveur du Hamster Commun Cricetus cricetus et de la Biodiversité de la Plaine d’Alsace 2019–2028. Direction Régionale de l’Environnement, de l’Aménagement et du Logement Grand Est. 130p. Ministère de la Transition Écologique et Solidaire. Available online: https://www.ecologie.gouv.fr/sites/default/files/pna-Hamster-final_compressed.pdf (accessed on 1 December 2021).
- Glavan, M.; Železnikar, Š.; Velthof, G.; Boekhold, S.; Langaas, S.; Pintar, M. How to Enhance the Role of Science in European Union Policy Making and Implementation: The Case of Agricultural Impacts on Drinking Water Quality. Water 2019, 11, 492. [Google Scholar] [CrossRef]
- Arlettaz, R.; Schaub, M.; Fournier, J.; Reichlin, T.S.; Sierro, A.; Watson, J.; Braunisch, V. From Publications to Public Actions: When Conservation Biologists Bridge the Gap between Research and Implementation. BioScience 2010, 60, 835–842. [Google Scholar] [CrossRef]
- Gregory, R.; Arvai, J.; Gerber, L.R. Structuring Decisions for Managing Threatened and Endangered Species in a Changing Climate. Conserv. Biol. 2013, 27, 1212–1221. [Google Scholar] [CrossRef]
- Naujokaitis-Lewis, I.; Pomara, L.Y.; Zuckerberg, B. Delaying conservation actions matters for species vulnerable to climate change. J. Appl. Ecol. 2018, 55, 2843–2853. [Google Scholar] [CrossRef]
- Fischer, C.; Wagner, C. Can agri-environmental schemes enhance non-target species? Effects of sown wildflower fields on the common hamster (Cricetus cricetus) at local and landscape scales. Biol. Conserv. 2016, 194, 168–175. [Google Scholar] [CrossRef]
- Bald, V.; Boetzl, F.A.; Krauss, J. Where do hamsters go after cereal harvest? A case study. Basic Appl. Ecol. 2021, 54, 98–107. [Google Scholar] [CrossRef]
- Hagy, H.M.; Linz, G.M.; Bleier, W.J. Wildlife Conservation Sunflower Plots and Croplands as Fall Habitat for Migratory Birds. Am. Midl. Nat. 2010, 164, 119–135. [Google Scholar] [CrossRef]
- Jones, G.A.; Sieving, K. Intercropping sunflower in organic vegetables to augment bird predators of arthropods. Agric. Ecosyst. Environ. 2006, 117, 171–177. [Google Scholar] [CrossRef]
- LoCascio, G.M.; Aguirre, L.; Irwin, R.E.; Adler, L.S. Pollen from multiple sunflower cultivars and species reduces a common bumblebee gut pathogen. R. Soc. Open Sci. 2019, 6, 190279. [Google Scholar] [CrossRef]
- Giacomini, J.J.; Leslie, J.; Tarpy, D.; Palmer-Young, E.C.; Irwin, R.E.; Adler, L.S. Medicinal value of sunflower pollen against bee pathogens. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Ramesh, K.; Ashok, K.P.; Biswas, A.K. Best Management Practices for Soybean under Soybean-Wheat System to Minimize the Impact of Climate Change. Indian J. Fertil. 2017, 13, 42–55. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).