Abstract
Cognitive failures represent everyday task failures that individuals are normally capable of completing. While cognitive failures measured with the Cognitive Failures Questionnaire can be considered a trait, the psychophysiological states associated with cognitive failures are yet to be fully understood. The aim of this paper was to investigate the extent to which the perception of experiencing cognitive failures in daily life is associated with both psychological (i.e., perceived emotional valence, emotional intensity, and stress), as well as physiological (i.e., vagally-mediated heart rate variability, vmHRV) variables. A total of 69 participants were involved in this study (47 male, 22 female; Mage = 22.4 years). Participants underwent a 5-min heart rate variability measurement and filled out the self-report psychological variables, before completing the Cognitive Failures Questionnaire, providing scores for Distractibility, Forgetfulness, and False Triggering. When combining the predictors together into a hierarchical regression analysis, only the model related to the Distractibility subscale was found to be significant (unique significant negative predictor: resting vmHRV). Further research should investigate whether influencing resting vmHRV, with interventions such as slow-paced breathing, may decrease the perception of cognitive failures related to distractibility.
1. Introduction
Forgetting why you went to another room in your house, having to read something again because you did not really pay attention the first time: some people may experience such kinds of cognitive failures every day. While the perception of experiencing cognitive failures in one’s daily life can be considered rather stable [1], the psychophysiological states associated with the perception of experiencing cognitive failures are still unknown. Consequently, the aim of this study was to address this gap, focusing on the self-reported affective experience and on the activity of the autonomic nervous system.
Cognitive failures, representing everyday-task failures that individuals are normally capable of completing, can be divided in terms of perception, memory, and motor functions [1,2,3] and are sometimes referred to as global absentmindedness [4]. Perceived cognitive failures can be measured with the Cognitive Failures Questionnaire (CFQ) [1]. The CFQ comprises three subscales: Distractibility, Forgetfulness, and False Triggering. Distractibility is related to everyday attention, and captures failures associated with faulty perception and failure to pay attention to relevant stimuli. Forgetfulness relates to everyday memory functioning for actions (e.g., forgetting directions, missing appointments, misplacing items). False Triggering involves slips of action or physical mishaps. Perceived cognitive failures measured by the CFQ are considered as a trait [5] associated with core self-evaluation [6], relatively stable across the lifespan [7], and with a large genetic underpinning [8]. Responses on the CFQ were found to be predicted by some personality traits [9,10,11], showing mostly negative relationships with conscientiousness and positive relationships with neuroticism.
Regarding psychological variables, some affective states may trigger cognitive failures. In particular, higher perceptions of anxiety and stress, which are typically correlated with less effective cognitive functioning, have also been associated with higher self-reported and observed cognitive failures [11,12,13]. In the general population, the proneness to perceiving cognitive failures may possibly contribute to the development or persistence of negative symptoms related to psychosis [14]. While causality cannot be inferred from previous research, overall, research points towards a positive association between negative emotional states and perceived stress with the perception of experiencing cognitive failures.
Regarding the physiological states associated with responses on the CFQ, some of the neural correlates of cognitive failures have already been investigated, such as electroencephalography and event-related potentials [15], or in terms of functional connectivity networks with fMRI [16]. However, less is known about the relationship between the CFQ and the autonomic nervous system, in particular with the parasympathetic branch.
Parasympathetic nervous activity can be indexed non-invasively via heart rate variability (HRV), and more specifically via vagally-mediated heart rate variability (vmHRV). HRV represents the time variation between peaks of the QRS complexes [17,18,19]. HRV represents the key outcome variable of the neurovisceral integration model [20,21], which assumes that there is a core set of neural structures providing the organism with the ability to adaptively regulate cognition and emotions. The neurovisceral integration model is based on the central autonomic network [22], and HRV is considered as the output of the central autonomic network. Further, HRV is suggested to index the degree to which the core integration system guided by the medial prefrontal cortex is integrated with the brainstem nuclei that directly regulate the heart, via the activity of the vagus nerve, the main nerve of the parasympathetic nervous system [23]. Consequently, the focus here is on vmHRV, which represents cardiac vagal activity, the activity of the vagus nerve regulating cardiac functioning.
According to the neurovisceral integration model [20,21], higher cognitive effectiveness is linked to greater activity of the parasympathetic nervous system. This relationship originates from the common structures and networks at stake for cardiac control regulation and for cognitive regulation. Optimal functioning of the prefrontal cortex ensures that the flow of activity along neural pathways will establish adequate mappings and linkings between inputs, internal states, and the outputs needed to perform a given task [24], consequently enabling flexible responses to ever changing environments. To the best of our knowledge, the relationship between vmHRV and the CFQ has never been investigated. When considering perceived cognitive failures, based on the neurovisceral integration model we would predict that a higher vmHRV is associated with a lower perception of experiencing cognitive failures. Specifically, we would expect a stronger relationship with the Distractibility subscale, given distractibility is related to issues with sustained attention, the ability to inhibit irrelevant stimuli and focus on relevant ones, which was found to be predicted by vmHRV [21]. This would also be in line with previous research showing that vmHRV was negatively associated with attentional lapses [25,26]. Attentional lapses, defined as a greater proportion of rare but longer response time, and greater response time variability, can be considered as an indicator of greater distractibility. In this study, we focus on resting vmHRV, specifically when the individual is not engaged in any other activity or task [19,27], and on its association with the perception of cognitive failures happening outside the lab, providing a real-world context to our study.
To sum up, so far little is known about the psychophysiological states associated with the perception of experiencing cognitive failures. Additionally, research examining the relationship between vmHRV and cognitive failures has been mostly lab based, focusing on distractibility and investigating attentional lapses [25,26]. Consequently, the current study aimed to advance the literature by focusing on the perception of cognitive failures that happened outside the lab, in everyday life. Based on the neurovisceral integration model [20,21], we first assume that the subscales of the CFQ, and in particular Distractibility, will be negatively associated with vmHRV. Second, based on previous empirical research, we expect negative affective states to be positively related to all subscales of the CFQ.
2. Materials and Methods
2.1. Participants
In the absence of previous research related to this topic, we based our power calculation on theoretical considerations, and assumed a medium effect size for the relationship between vmHRV and cognitive failures. A G*Power (Faul, Erdfelder, Buchner, and Lang, 2009) a priori power calculation for regression analysis (one tail) to detect a medium effect size R2 = 0.25, power (1-β) = 0.80, provided an estimated sample size of 59. In order to anticipate for potential dropouts and technical issues, a sample size of 72 participants was recruited to participate in this study. Due to technical issues with the electrocardiography (ECG) measurement of three participants data was removed, and the data of 69 remaining participants were used for the analysis (47 male, 22 female; Mage = 22.4 years, age range = 19–36 years; BMI = 22.74, SD = 1.66). Exclusion criteria were smoking, any kind of self-reported cardiovascular, respiratory, or neurological diseases, any psychiatric disorders, and regular medication potentially affecting the cardiovascular or respiratory systems.
2.2. Material and Measures
2.2.1. vmHRV Indexed via Heart Rate Variability
vmHRV was indexed via the root mean square of successive differences (RMSSD), calculated from HRV, given it was found to be relatively free of respiratory influences compared to other vmHRV indicators, such as high-frequency (HF)-HRV [28]. HRV was measured via an ECG device (Faros 180°, Bittium, Kuopio, Finland), at a sampling rate of 500 Hz. We used two disposable ECG pre-gelled electrodes (Ambu L-00-S/25, Ambu GmbH, Bad Nauheim, Germany). The negative electrode was placed on the right infraclavicular fossa (just below the right clavicle) while the positive electrode was placed on the left side of the chest, below the pectoral muscle in the left anterior axillary line. The Kubios software (University of Eastern Finland, Kuopio, Finland) was used to extract RMSSD and the other HRV parameters. The ECG signal was visually inspected for artefacts and corrected manually if needed (<0.01% of the total heartbeats) [19]. Specifically, we used the manual artifact correction mode offered by Kubios, which identifies a R peak on the ECG signal by placing a red cross on it, in case the R peak has not been detected automatically. The RR time courses were detrended using the Smooth priors method, with 500 as a smoothing parameter, and a cutoff frequency of 0.035 Hz. In order to provide an overview of the different HRV parameters, following Laborde, Mosley, and Thayer [19], we also extracted heart rate and the standard deviation of the NN interval (SDNN) for the time-domain and frequency-domain (Fast Fourier Transform), low-frequency (LF: 0.04 to 0.15 Hz), HF (0.15 to 0.40 Hz), and the LF/HF ratio. For the spectral analysis, the following parameters were used: the Fast Fourier Transform spectrum was calculated using the Welch’s periodogram method, with a window width of 300s, and a window overlap of 50%. Finally, we also extracted the respiratory frequency from the ECG signal, based on the ECG-derived respiration algorithm of Kubios [29]. The Kubios respiratory rate estimate is computed by using information both from the ECG R-wave amplitude modulation as well as from the power spectral distribution of RR intervals data. This method has been found to be a valid estimate of the respiratory frequency measured directly with either an impedance-based measurement, a thoracic piezoresistive band, or a spirometer providing continuous ventilatory flow signal [30].
2.2.2. Cognitive Failure Questionnaire
We used the German version [31] of the CFQ [1]. The German version of the CFQ contains 32 items with three main dimensions: Distractibility (e.g., “Do you fail to hear people speaking to you when you are doing something else?”), Forgetfulness (e.g., “Do you read something and find you haven’t been thinking about it and must read it again?”), and False Triggering (e.g., “Do you drop things?”). Using a five-point Likert scale (ranging from “0 = never” to “4 = very often”), participants had to indicate how often each of the mentioned events happened to them in the past six months. Reliability in the current study was the following: Forgetfulness (α = 0.70), Distractibility (α = 0.65), and False Triggering (α = 0.66). Alphas between 0.60 and 0.70 are acceptable in smaller samples [32].
2.2.3. Visual Analogue Scale—Perceived Stress
A visual analogue scale (VAS), consisting of a 100-mm vertical line, was used to assess perceived stress intensity. The instruction was: “Please indicate on the line below how stressed you feel right now”. The line was anchored by the words “not stressed at all” at the extreme left of the line, and “extremely stressed” at the extreme right of the line. Participants were required to cross a point somewhere on the line, corresponding to their subjective stress intensity. The value of perceived stress intensity was represented by the value (in cm) from the extreme left of the line. Previous research has used this scale to assess perceived stress intensity [33,34,35].
2.2.4. Self-Assessment Manikin—Perceived Emotional Arousal, Perceived Emotional Valence, and Perceived Control
The self-assessment manikin [36] is a picture-oriented instrument containing five images for each of two affective dimensions (i.e., emotional valence and emotional intensity) that the participant rates on a 9-point scale (1 to 9). The main instruction for the two dimensions was: “Please make a cross corresponding to how you feel right now”. Valence is depicted on a negative (a frowning figure), neutral, and positive figure (a smiling figure). The scale was anchored with the words “unpleasant” and “pleasant”. Higher scores reflect a more positive valence. Arousal is depicted ranging from low arousal (eyes closed) to high arousal (eyes wide open). The scale was anchored with the words “calm” and “activated”. Higher scores consequently represent higher arousal.
2.3. Procedure
The study protocol was approved by a university research ethics committee (N° 08/2016). Participants were recruited via flyers at a local university campus and via posts on social network groups linked to the university. In line with recommendations for psychophysiological experiments involving HRV measurements [19], participants were instructed to follow their usual sleep routine the night before the experiment, not to consume alcohol or engage in strenuous physical activity in the previous 24 hours, nor to drink any beverage except water or eat 2 hours before taking part in the experiment. All participants gave written informed consent before participating, and were informed that they could withdraw from the study at any time without explanation, and without any consequences. The participants came once to the lab. The whole session lasted 20 min. The full protocol is depicted in Figure 1. After being welcomed to the lab, they were asked to fill out an informed consent form and a demographic questionnaire [19].
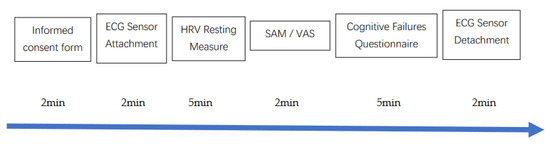
Figure 1.
Experimental protocol. Notes: ECG: Electrocardiography: HRV: Heart Rate Variability; SAM: Self-Assessment Manikin; VAS: Visual Analogue Scale.
Participants were seated on a chair during the entire experiment, with the upper body and the arms being supported. The ECG Faros 180° device for HRV measurement was attached. All measurements were collected with eyes opened, knees at 90°, hands on thighs, and lasted 5 min, following HRV recommendations [17,19]. At the end of the 5-min period, participants completed the self-report measures (SAM and VAS) and the CFQ. At the end of the experiment, the ECG device was detached, and participants were thanked and debriefed.
2.4. Data Analysis
The HRV data were obtained with the Kubios software. Data were checked for normality and outliers. Regarding outliers, 0.001% of the cases were found to be univariate outliers (>2 SD, z-scores higher than 2.58). Running the analyses with outliers removed did not change the pattern of results, thus we report findings with potential outliers included in analyses. As the RMSSD data were non-normally distributed, a log-transformation was applied, as is often recommended for HRV research [19]. Heart rate [37] and respiratory frequency [19] were also considered as control variables in the analysis (log-transformed). The self-report variables were also mostly non-normally distributed, and similar to RMSSD we applied a log-transformation. Hierarchical regression was used to identify the predictors of each of the CFQ subscales (i.e., Distractibility, Forgetfulness, False Triggering), controlling for age, sex, and BMI at Step 1 [38], and entering the following dependent variables at Step 2: emotional valence, emotional arousal, perceived stress intensity, RMSSD, heart rate, and respiratory frequency. For both steps 1 and 2, variables were entered as a block in a single step with the simultaneous enter method.
3. Results
Descriptive statistics can be found in Table 1.

Table 1.
Descriptive statistics.
We performed the multiple regression analyses. For the subscale Distractibility, Step 1 was not significant (adjusted R2 = 0.04, p = 0.887); however, the model became significant at Step 2 (adjusted R2 = 0.16, p = 0.020). At Step 2, the only significant predictor was RMSSD (β = –0.397, p = 0.025), see Table 2 for detailed results. The models for the other two subscales were not significant: Forgetfulness (p = 0.733, detailed results in Table 3) and False Triggering (p = 0.170, detailed results in Table 4).

Table 2.
Hierarchical regression analysis for the Distractibility subscale.

Table 3.
Hierarchical regression analysis for the Forgetfulness subscale.

Table 4.
Hierarchical regression analysis for the False Triggering subscale.
4. Discussion
The aim of this study was to investigate to which extent cognitive failures experienced in daily life were related to psychophysiological states. Our hypothesis was partially supported. When considering the reciprocal influence of psychophysiological states within a hierarchical regression model, the model predicting the Distractibility subscale was found to be significant, with resting vmHRV a significant predictor, while the self-report psychological variables emotional valence, emotional arousal, and perceived stress intensity did not contribute significantly to the model. The models for Forgetfulness and False Triggering were found to be non-significant.
Our findings of vmHRV negatively predicting perceived distractibility are in line with the neurovisceral integration model [20,21,22]. Specifically, this model suggests that vmHRV, being an outcome of the central autonomic network [22], represents the effectiveness of executive functioning. This stems from vmHRV reflecting the ability of the organism to adequately adjust sufficient mappings between input, internal states, and outputs needed to perform a given task [24]. This theoretical rationale provides a basis for higher resting vmHRV being a protective factor against distraction, and hence related to lower perceived distractibility. In lab research, vmHRV was shown to be a marker of selective attention and to be a protective factor against distractors [39,40,41]. VmHRV was also negatively related to attentional lapses [25,26], as measured with intraindividual response time variability. The current research extends lab-based findings linking vmHRV to perceived distractibility in a real-life context. The finding that vmHRV was associated with Distractibility but not to the other subscales may point toward the fact that the neurophysiological mechanisms underlying cognitive failures differ based on the nature of cognitive failures. Further research is therefore encouraged to investigate the perception of cognitive failures using physiological measurements other than vmHRV, such as with electroencephalography [42,43].
Contrary to our hypothesis, the subjective psychological variables did not predict the CFQ subscales. The finding that, when associated with physiological variables in the hierarchical regression analysis, and in particular to vmHRV, the psychological variables do not contribute significantly to the subscale Distractibility, may be related to the state/trait nature of the variables. Given the CFQ is supposed to reflect stable dispositions of the individual, thus the stronger association with vmHRV in comparison to state psychological variables may be due to the trait nature of vmHRV [44]. After considering potential influential factors [45,46], vmHRV was deemed to be relatively stable [44]. On a methodological level, it would be interesting to test the relationship between psychological affective states and cognitive failures in real time, either observed during experiments or self-reported.
Our study had some strengths, such as being the first novel empirical test of the relationship between cognitive failures and vmHRV. Nonetheless, our study also had some limitations. First, other variables that may influence cognitive failures, such as personality traits [9,10], have not been controlled for. Second, our sample was unbalanced regarding sex, with 22 female and 47 male participants. Despite of the fact sex was controlled for in the statistical analyses, further studies should endeavor to recruit more balanced samples in terms of sex, given sex is known to influence HRV [38]. Third, self-report retrospective measures of cognitive failures may be biased, and future research should consider including objective measures of cognitive failures and cognitive performance. Fourth, our sample of young adults may not experience many cognitive failure symptoms, and future research should consider investigating the relationship between vmHRV and perceived cognitive failures in older adults. Fifth, we likely overestimated the strength of relationship between vmHRV and perceived cognitive failure in our a priori power calculation. Computing the achieved power of our study with G*Power returns a value of power (1 – β) = 0.62. Future research should consequently test a larger sample size when investigating the links between vmHRV and perceived cognitive failures. Finally, we only considered here the relationship between the CFQ and resting vmHRV. In line with recent theoretical and methodological recommendations and the “3 Rs” (i.e., resting, reactivity, recovery) of vmHRV functioning advocated by the vagal tank theory [19,27], future research should investigate the relationship of the CFQ together with vmHRV measured during (i.e., reactivity) and after (i.e., recovery) for example cognitive or emotional tasks.
5. Conclusions
The potential negative consequences of cognitive failures, which go from potentially rather harmless daily small distractions to more serious consequences with accidents [47,48,49], triggered the interest of researchers to develop interventions to face cognitive failures. Those interventions considered different time scales, and were realized either on an acute basis manipulating the perceptual load [50], or on a more chronic basic, with long-term interventions based on mindfulness [51] or targeting stress management [52].
Taking into account the findings of the current study that resting vmHRV was negatively associated with the perception of experiencing cognitive failures (Distractibility) in daily life may pave the road for the development of a new line of interventions to help people cope with cognitive failures. Specifically, interventions aiming to increase vmHRV [46,53], such as those based on slow-paced breathing, may provide both support for a quick fix [54,55] or a long-term benefit [56]. Specifically, slow-paced breathing has been shown to improve executive functioning [57,58] and in particular inhibition, which would play an important role into decreasing the tendency to distractibility. Consequently, future research should aim to further understand the psychophysiological correlates of cognitive failures, in order to help high scorers better face everyday challenges with less cognitive failures.
Author Contributions
Conceptualization, M.Y. and S.L.; methodology, S.L. and F.D.; formal analysis, S.L. and M.Y.; investigation, S.L. and M.Y.; resources, S.L.; data curation, S.L.; writing—original draft preparation, M.Y., S.L., U.B., R.S.V., and F.D.; writing—review and editing, M.Y., S.L., U.B., R.S.V., and F.D.; visualization, U.B. and R.S.V.; supervision, S.L. and F.D.; project administration, S.L. and F.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The experimental protocol was approved by the Ethics Committee of the German University Cologne (Project Identification Code N° 08/2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data can be shared by the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Broadbent, D.E.; Cooper, P.F.; FitzGerald, P.; Parkes, K.R. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br. J. Clin. Psychol. 1982, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.C. Confirmatory factor analysis of the cognitive failures questionnaire: Evidence for dimensionality and construct validity. Personal. Individ. Differ. 2004, 37, 307–324. [Google Scholar] [CrossRef]
- Wallace, J.C.; Kass, S.J.; Stanny, C.J. The cognitive failures questionnaire revisited: Dimensions and correlates. J. Gen. Psychol. 2002, 129, 238–256. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, Y.; Klein, R.M. Are Individual Differences in Absentmindedness Correlated with Individual Differences in Attention? J. Individ. Differ. 2009, 30, 220–237. [Google Scholar] [CrossRef]
- Bridger, R.S.; Johnsen, S.A.; Brasher, K. Psychometric properties of the Cognitive Failures Questionnaire. Ergonomics 2013, 56, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, R.R.A.; Lang, J.W.B.; Weijters, T. Self-reported cognitive failures: A core self-evaluation? Personal. Individ. Differ. 2010, 49, 717–722. [Google Scholar] [CrossRef]
- Rast, P.; Zimprich, D.; Van Boxtel, M.; Jolles, J. Factor structure and measurement invariance of the cognitive failures questionnaire across the adult life span. Assessment 2009, 16, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Markett, S.; Montag, C.; Diekmann, C.; Reuter, M. Dazed and confused: A molecular genetic approach to everyday cognitive failure. Neurosci. Lett. 2014, 566, 216–220. [Google Scholar] [CrossRef]
- Tirre, W.C. Dimensionality and Determinants of Self-Reported Cognitive Failures. Int. J. Psychol. Res. 2018, 11, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Konen, T.; Karbach, J. Self-Reported Cognitive Failures in Everyday Life: A Closer Look at Their Relation to Personality and Cognitive Performance. Assessment 2020, 27, 982–995. [Google Scholar] [CrossRef]
- Mecacci, L.; Righi, S.; Rocchetti, G. Cognitive failures and circadian typology. Personal. Individ. Differ. 2004, 37, 107–113. [Google Scholar] [CrossRef]
- Mahone, A.M. Cognitive Failures and Stress. Psychol. Rep. 1998, 82, 1432–1434. [Google Scholar] [CrossRef]
- Schrier, E.; Geertzen, J.H.; Dijkstra, P.U. Subjective cognitive dysfunction in rehabilitation outpatients with musculoskeletal disorders or chronic pain. Eur. J. Phys. Rehabil. Med. 2017, 53, 582–589. [Google Scholar] [CrossRef]
- Pfeifer, S.; van Os, J.; Hanssen, M.; Delespaul, P.; Krabbendam, L. Subjective experience of cognitive failures as possible risk factor for negative symptoms of psychosis in the general population. Schizophr. Bull. 2009, 35, 766–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roche, R.A.; Garavan, H.; Foxe, J.J.; O’Mara, S.M. Individual differences discriminate event-related potentials but not performance during response inhibition. Exp. Brain Res. 2005, 160, 60–70. [Google Scholar] [CrossRef]
- Bey, K.; Montag, C.; Reuter, M.; Weber, B.; Markett, S. Susceptibility to everyday cognitive failure is reflected in functional network interactions in the resting brain. Neuroimage 2015, 121, 1–9. [Google Scholar] [CrossRef]
- Malik, M. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef] [Green Version]
- Berntson, G.G.; Bigger, J.T.; Eckberg, D.L.; Grossman, P.; Kaufmann, P.G.; Malik, M.; Nagaraja, H.N.; Porges, S.W.; Saul, J.P.; Stone, P.H.; et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Physiol. 2017, 8, 213. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.; Thayer, J.F.; Khalsa, S.S.; Lane, R.D. The hierarchical basis of neurovisceral integration. Neurosci. Biobehav. Rev. 2017, 75, 274–296. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Hansen, A.L.; Saus-Rose, E.; Johnsen, B.H. Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. Med. 2009, 37, 141–153. [Google Scholar] [CrossRef]
- Benarroch, E.E. The central autonomic network. In Clinical Autonomic Disorders, Low, P.A., Ed.; Lippincott-Raven: Philadelphia, PA, USA, 1997; pp. 17–23. [Google Scholar]
- Brodal, P. The Central Nervous System—Structure and Function, 5th ed.; Oxford University Press: New York, NY, USA, 2016. [Google Scholar]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Ann. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.P.; Thayer, J.F.; Koenig, J. Resting cardiac vagal tone predicts intraindividual reaction time variability during an attention task in a sample of young and healthy adults. Psychophysiology 2016, 53, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Spangler, D.P.; Williams, D.P.; Speller, L.F.; Brooks, J.R.; Thayer, J.F. Resting heart rate variability is associated with ex-Gaussian metrics of intra-individual reaction time variability. Int. J. Psychophysiol. 2018, 125, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Mosley, E.; Mertgen, A. Vagal Tank Theory: The Three Rs of Cardiac Vagal Control Functioning—Resting, Reactivity, and Recovery. Front. Neurosci. 2018, 12, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penttila, J.; Helminen, A.; Jartti, T.; Kuusela, T.; Huikuri, H.V.; Tulppo, M.P.; Coffeng, R.; Scheinin, H. Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: Effects of various respiratory patterns. Clin. Physiol. 2001, 21, 365–376. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Niskanen, J.P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Kubios HRV--heart rate variability analysis software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef]
- Lipponen, J.A.; Tarvainen, M. Accuracy of Kubios HRV software respiratory rate estimation algorithms. White Pap. 2021. [Google Scholar]
- Klumb, P.L. Cognitive failures and performance differences: Validation studies of a German version of the cognitive failures questionnaire. Ergonomics 1995, 38, 1456–1467. [Google Scholar] [CrossRef] [PubMed]
- Pallant, J. SPSS Survival Manual—A Step by Step Guide to Data Analysis Using SPSS for Windows, 10th ed.; Open University Press: Buckingham, UK, 2001. [Google Scholar]
- Lesage, F.-X.; Berjot, S. Validity of occupational stress assessment using a visual analogue scale. Occup. Med. 2011, 61, 434–436. [Google Scholar] [CrossRef] [Green Version]
- Lesage, F.-X.; Berjot, S.; Deschamps, F. Clinical stress assessment using a visual analogue scale. Occup. Med. 2012, 62, 600–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laborde, S.; Lautenbach, F.; Allen, M.S. The contribution of coping-related variables and heart rate variability to visual search performance under pressure. Physiol. Behav. 2015, 139, 532–540. [Google Scholar] [CrossRef]
- Bradley, M.; Lang, P.J. Measuring emotion: The self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry 1994, 25, 49–59. [Google Scholar] [CrossRef]
- De Geus, E.J.C.; Gianaros, P.J.; Brindle, R.C.; Jennings, J.R.; Berntson, G.G. Should heart rate variability be „corrected” for heart rate? Biological, quantitative, and interpretive considerations. Psychophysiology 2019, 56, e13287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antelmi, I.; de Paula, R.S.; Shinzato, A.R.; Peres, C.A.; Mansur, A.J.; Grupi, C.J. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am. J. Cardiol. 2004, 93, 381–385. [Google Scholar] [CrossRef]
- Park, G.; Vasey, M.W.; Van Bavel, J.J.; Thayer, J.F. Cardiac vagal tone is correlated with selective attention to neutral distractors under load. Psychophysiology 2013, 50, 398–406. [Google Scholar] [CrossRef]
- Spangler, D.P.; Friedman, B.H. A Little Goes a Long Way: Low Working Memory Load Is Associated with Optimal Distractor Inhibition and Increased Vagal Control under Anxiety. Front. Hum. Neurosci. 2017, 11, 43. [Google Scholar] [CrossRef] [Green Version]
- Giuliano, R.J.; Karns, C.M.; Bell, T.A.; Petersen, S.; Skowron, E.A.; Neville, H.J.; Pakulak, E. Parasympathetic and sympathetic activity are associated with individual differences in neural indices of selective attention in adults. Psychophysiology 2018, 55, e13079. [Google Scholar] [CrossRef]
- Burra, N.; Kerzel, D. The distractor positivity (Pd) signals lowering of attentional priority: Evidence from event-related potentials and individual differences. Psychophysiology 2014, 51, 685–696. [Google Scholar] [CrossRef]
- Sanger, J.; Bechtold, L.; Schoofs, D.; Blaszkewicz, M.; Wascher, E. The influence of acute stress on attention mechanisms and its electrophysiological correlates. Front. Behav. Neurosci. 2014, 8, 353. [Google Scholar] [CrossRef] [Green Version]
- Bertsch, K.; Hagemann, D.; Naumann, E.; Schachinger, H.; Schulz, A. Stability of heart rate variability indices reflecting parasympathetic activity. Psychophysiology 2012, 49, 672–682. [Google Scholar] [CrossRef]
- Fatisson, J.; Oswald, V.; Lalonde, F. Influence diagram of physiological and environmental factors affecting heart rate variability: An extended literature overview. Heart Int. 2016, 11, e32–e40. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Mertgen, A. A unifying conceptual framework of factors associated to cardiac vagal control. Heliyon 2018, 4, e01002. [Google Scholar] [CrossRef] [Green Version]
- Wallace, J.C.; Vodanovich, S. Can accidents and industrial mishaps be predicted? Further investigation into the relationship between cognitive failure and reports of accidents. J. Bus. Psychol. 2003, 17, 503–514. [Google Scholar] [CrossRef]
- Larson, G.E.; Alderton, D.L.; Neideffer, M.; Underhill, E. Further evidence on dimensionality and correlates of the Cognitive Failures Questionnaire. Br. J. Psychol. 1997, 88, 29–38. [Google Scholar] [CrossRef]
- Hassanzadeh-Rangi, N.; Farshad, A.A.; Khosravi, Y.; Zare, G.; Mirkazemi, R. Occupational cognitive failure and its relationship with unsafe behaviors and accidents. Int. J. Occup. Saf. Erg. 2014, 20, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Forster, S.; Lavie, N. High perceptual load makes everybody equal: Eliminating individual differences in distractibility with load. Psychol. Sci. 2007, 18, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Van der Gucht, K.; Melis, M.; Ahmadoun, S.; Gebruers, A.; Smeets, A.; Vandenbulcke, M.; Wildiers, H.; Neven, P.; Kuppens, P.; Raes, F.; et al. A mindfulness-based intervention for breast cancer patients with cognitive impairment after chemotherapy: Study protocol of a three-group randomized controlled trial. Trials 2020, 21, 290. [Google Scholar] [CrossRef]
- Willert, M.V.; Thulstrup, A.M.; Hertz, J.; Bonde, J.P. Sleep and cognitive failures improved by a three-month stress management intervention. Int. J. Stress Manag. 2010, 17, 193–213. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Ueberholz, L. Enhancing cardiac vagal activity: Factors of interest for sport psychology. Prog Brain Res. 2018, 240, 71–92. [Google Scholar] [CrossRef] [Green Version]
- Laborde, S.; Iskra, M.; Zammit, N.; Borges, U.; You, M.; Sevoz-Couche, C.; Dosseville, F. Slow-Paced Breathing: Influence of Inhalation/Exhalation Ratio and of Respiratory Pauses on Cardiac Vagal Activity. Sustainability 2021, 13, 7775. [Google Scholar] [CrossRef]
- Laborde, S.; Allen, M.S.; Borges, U.; Iskra, M.; Zammit, N.; You, M.; Hosang, T.; Mosley, E.; Dosseville, F. Psychophysiological effects of slow-paced breathing at six cycles per minute with or without heart rate variability biofeedback. Psychophysiology 2021, 59, e13952. [Google Scholar] [CrossRef]
- Laborde, S.; Hosang, T.; Mosley, E.; Dosseville, F. Influence of a 30-day slow paced breathing intervention compared to social media use on subjective sleep quality and cardiac vagal activity. J. Clin. Med. 2019, 8, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laborde, S.; Allen, M.S.; Borges, U.; Hosang, T.J.; Furley, P.; Mosley, E.; Dosseville, F. The Influence of Slow-Paced Breathing on Executive Function. J. Psychophysiol. 2021, 1–15. [Google Scholar] [CrossRef]
- Laborde, S.; Lentes, T.; Hosang, T.J.; Borges, U.; Mosley, E.; Dosseville, F. Influence of slow-paced breathing on inhibition after physical exertion. Front. Psychol. 2019, 10, 1923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).