Impact of Microplastics on Oil Dispersion Efficiency in the Marine Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
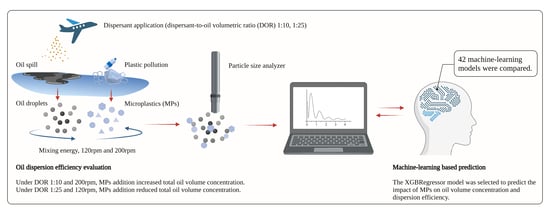
2.2. Experimental Design
2.3. Sample Analysis
2.4. Machine Learning Aided Modeling
3. Results and Discussion
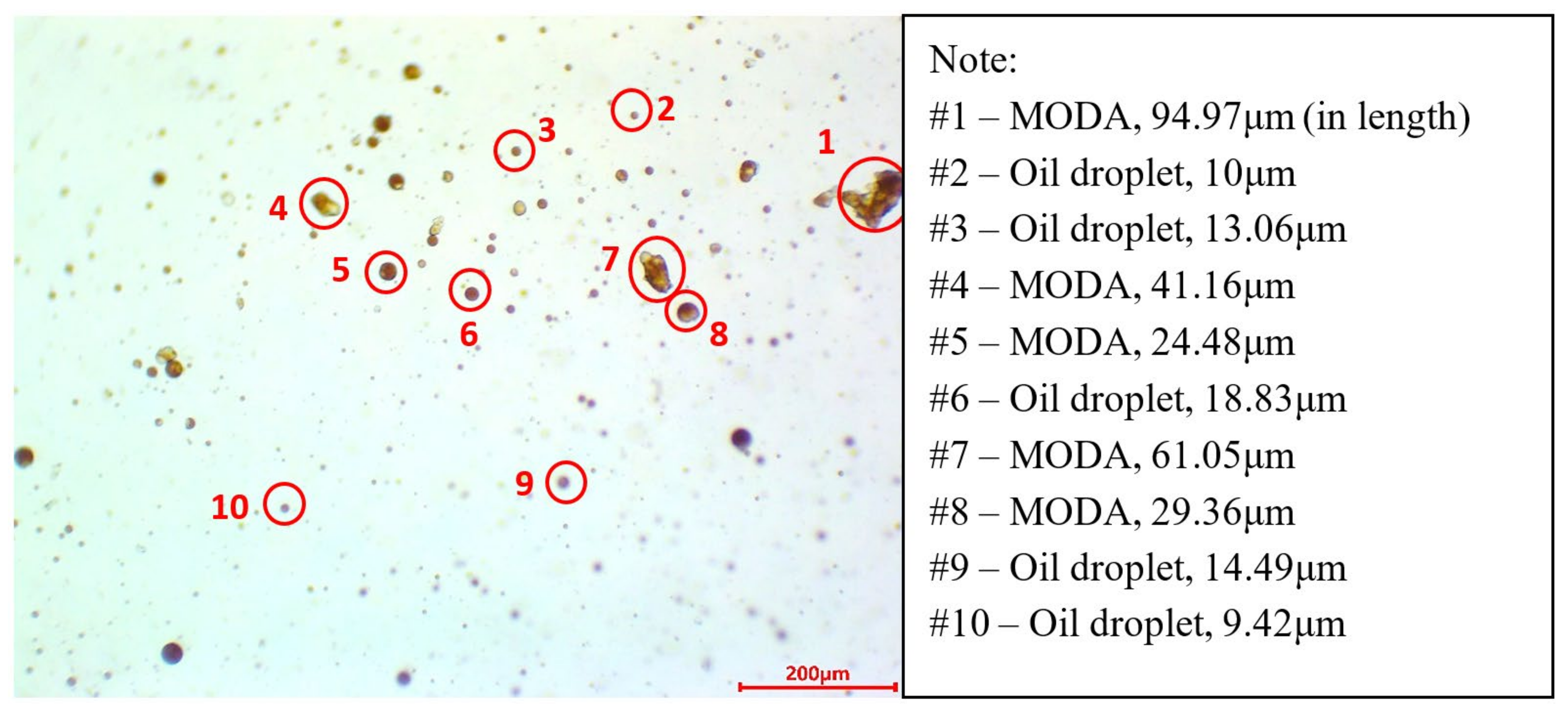
3.1. Characterization of MPs and MODA
3.2. Impact of MPs on Oil Droplet Size
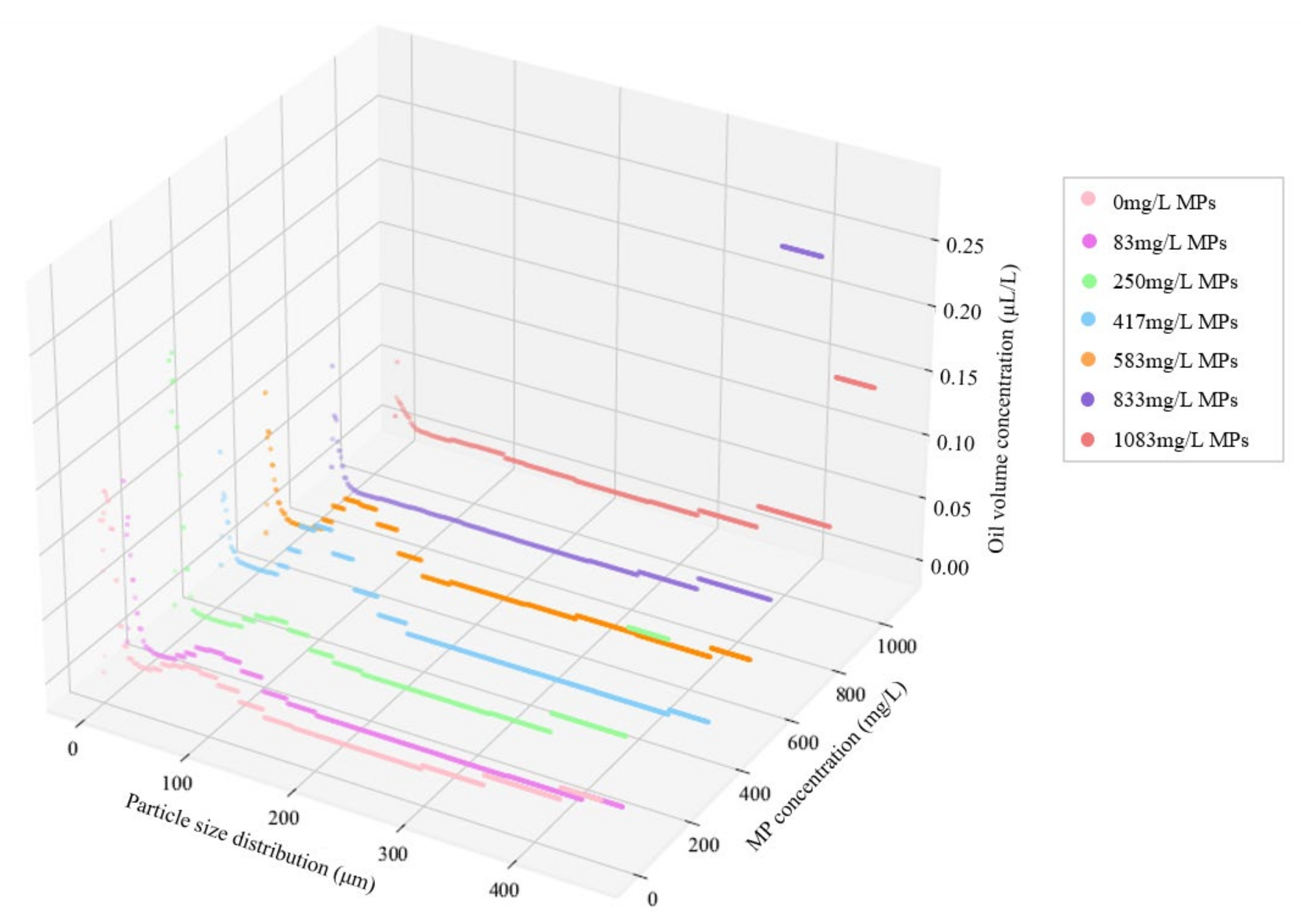
3.3. Impact of MPs on Oil Volume Concentration
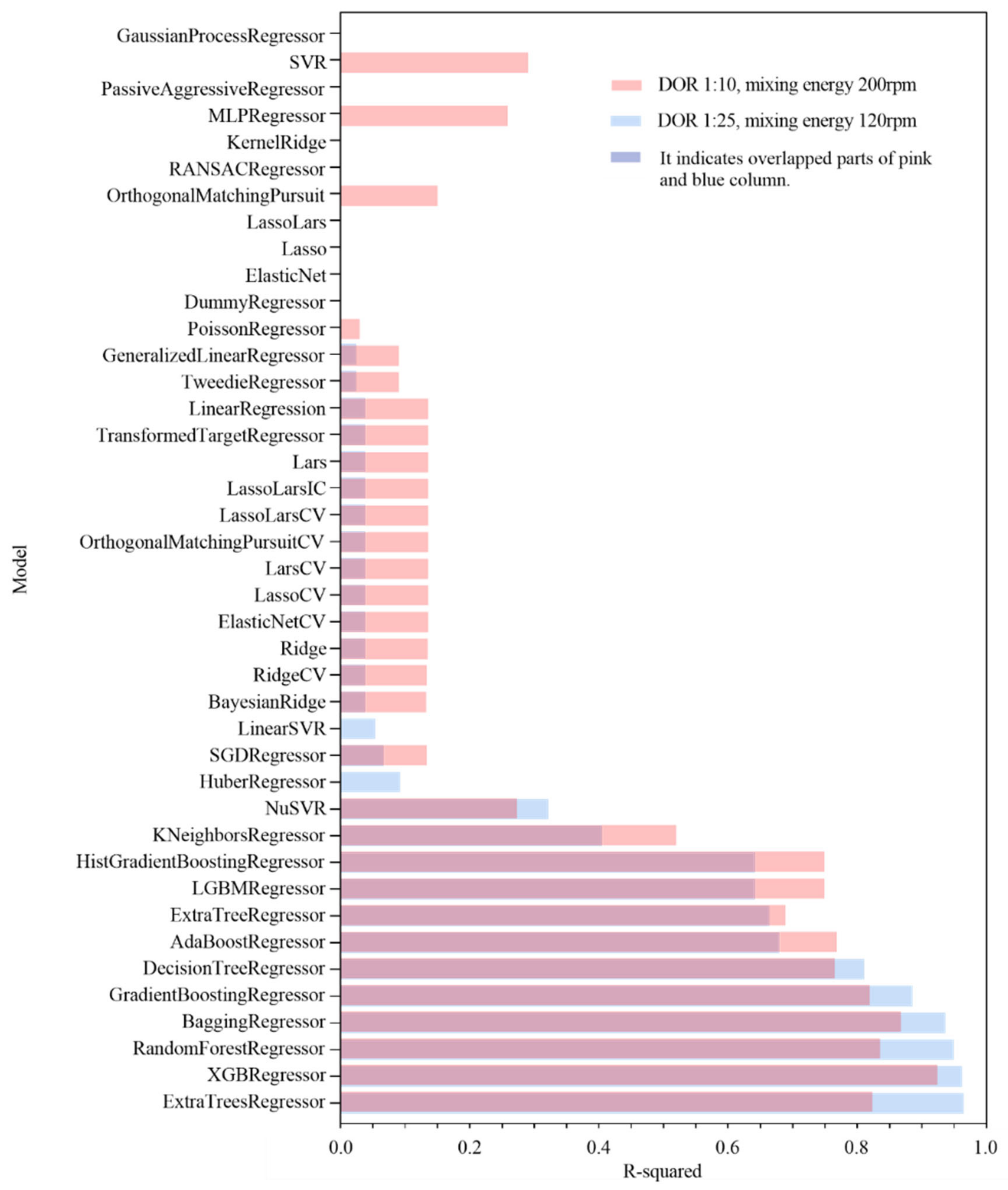
3.4. Regression Models for Oil Volume Concentration Prediction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Lin, W.; Li, X.; Yang, M.; Lee, K.; Chen, B.; Zhang, B.H. Brominated Flame Retardants, Microplastics, and Biocides in the Marine Environment: Recent Updates of Occurrence, Analysis, and Impacts. Adv. Mar. Biol. 2018, 81, 167–211. [Google Scholar]
- Uddin, S.; Fowler, S.W.; Uddin, M.F.; Behbehani, M.; Naji, A. A review of microplastic distribution in sediment profiles. Mar. Pollut. Bull. 2021, 163, 111973. [Google Scholar] [CrossRef] [PubMed]
- Kane, I.A.; Clare, M.A. Dispersion, accumulation, and the ultimate fate of microplastics in deep-marine environments: A review and future directions. Front. Earth Sci. 2019, 7, 80. [Google Scholar]
- Choy, C.A.; Robison, B.H.; Gagne, T.O.; Erwin, B.; Firl, E.; Halden, R.U.; Hamilton, J.A.; Katija, K.; Lisin, S.E.; Rolsky, C. The vertical distribution and biological transport of marine microplastics across the epipelagic and mesopelagic water column. Sci. Rep. 2019, 9, 7843. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Chen, H.; Zhou, X.; Tian, Y.; Lin, C.; Wang, W.; Zhou, K.; Zhang, Y.; Lin, H. Microplastic abundance, distribution and composition in the mid-west Pacific Ocean. Environ. Pollut. 2020, 264, 114125. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.; Chase, Z.; Zhang, J.; Holl, M.M.B.; Willis, K.; Williams, A.; Hardesty, B.D.; Wilcox, C. Microplastic pollution in deep-sea sediments from the Great Australian Bight. Front. Mar. Sci. 2020, 7, 808. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Chen, Q.; Kalogerakis, N.; Ji, R.; Ma, Y. Interactions between microplastics and organic pollutants: Effects on toxicity, bioaccumulation, degradation, and transport. Sci. Total Environ. 2020, 748, 142427. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Liu, G.; Zhang, Z.; Wu, H.; Cui, B.; Bai, J.; Zhang, W. Size effect of polystyrene microplastics on sorption of phenanthrene and nitrobenzene. Ecotoxicol. Environ. Saf. 2019, 173, 331–338. [Google Scholar] [CrossRef]
- Wardrop, P.; Shimeta, J.; Nugegoda, D.; Morrison, P.D.; Miranda, A.; Tang, M.; Clarke, B.O. Chemical pollutants sorbed to ingested microbeads from personal care products accumulate in fish. Environ. Sci. Technol. 2016, 50, 4037–4044. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-J.; Xu, J.-K.; Yan, Z.-H.; Ren, H.-Q.; Zhang, Y. Microplastics enhance the developmental toxicity of synthetic phenolic antioxidants by disturbing the thyroid function and metabolism in developing zebrafish. Environ. Int. 2020, 140, 105750. [Google Scholar] [CrossRef] [PubMed]
- Granby, K.; Rainieri, S.; Rasmussen, R.R.; Kotterman, M.J.; Sloth, J.J.; Cederberg, T.L.; Barranco, A.; Marques, A.; Larsen, B.K. The influence of microplastics and halogenated contaminants in feed on toxicokinetics and gene expression in European seabass (Dicentrarchus labrax). Environ. Res. 2018, 164, 430–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Zhu, Z.; Merlin, F.; Yang, M.; Chen, B.; Lee, K.; Zhang, B. Ecological impact analysis of dispersants and dispersed oil: An overview. JEIL 2021, 5, 120–133. [Google Scholar] [CrossRef]
- Yang, M.; Chen, B.; Xin, X.; Song, X.; Liu, J.; Dong, G.; Lee, K.; Zhang, B. Interactions between microplastics and oil dispersion in the marine environment. J. Hazard. Mater. 2021, 403, 123944. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Zeferino, J.C.; Ojeda-Benítez, S.; Cruz-Salas, A.A.; Martínez-Salvador, C.; Vázquez-Morillas, A. Microplastics in Mexican beaches. Resour. Conserv. Recycl. 2020, 155, 104633. [Google Scholar] [CrossRef]
- Aliabad, M.K.; Nassiri, M.; Kor, K. Microplastics in the surface seawaters of Chabahar Bay, Gulf of Oman (Makran coasts). Mar. Pollut. Bull. 2019, 143, 125–133. [Google Scholar] [CrossRef]
- Al-Lihaibi, S.; Al-Mehmadi, A.; Alarif, W.M.; Bawakid, N.O.; Kallenborn, R.; Ali, A.M. Microplastics in sediments and fish from the Red Sea coast at Jeddah (Saudi Arabia). Environ. Chem. 2019, 16, 641–650. [Google Scholar] [CrossRef]
- Al-Salem, S.; Uddin, S.; Al-Yamani, F. An assessment of microplastics threat to the marine environment: A short review in context of the Arabian/Persian Gulf. Mar. Environ. Res. 2020, 159, 104961. [Google Scholar] [CrossRef]
- Aslam, H.; Ali, T.; Mortula, M.M.; Attaelmanan, A.G. Evaluation of microplastics in beach sediments along the coast of Dubai, UAE. Mar. Pollut. Bull. 2020, 150, 110739. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.B.; Al-Maslamani, I.; Obbard, J.P. Prevalence of microplastics in the marine waters of Qatar. Mar. Pollut. Bull. 2016, 111, 260–267. [Google Scholar] [CrossRef]
- Naji, A.; Azadkhah, S.; Farahani, H.; Uddin, S.; Khan, F.R. Microplastics in wastewater outlets of Bandar Abbas city (Iran): A potential point source of microplastics into the Persian Gulf. Chemosphere 2021, 262, 128039. [Google Scholar] [CrossRef] [PubMed]
- Saeed, T.; Al-Jandal, N.; Al-Mutairi, A.; Taqi, H. Microplastics in Kuwait marine environment: Results of first survey. Mar. Pollut. Bull. 2020, 152, 110880. [Google Scholar] [CrossRef] [PubMed]
- Abayomi, O.A.; Range, P.; Al-Ghouti, M.A.; Obbard, J.P.; Ben-Hamadou, R. Microplastics in coastal environments of the Arabian Gulf. Mar. Pollut. Bull. 2017, 124, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Fowler, S.W.; Saeed, T. Microplastic particles in the Persian/Arabian Gulf–a review on sampling and identification. Mar. Pollut. Bull. 2020, 154, 111100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Matchinski, E.; Chen, B.; Ye, X.; Jing, L.; Lee, K. Marine oil spills—Oil pollution, sources and effects. In World Seas: An Environmental Evaluation, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 391–406. [Google Scholar]
- Cai, Q.; Zhu, Z.; Chen, B.; Zhang, B. Oil-in-water emulsion breaking marine bacteria for demulsifying oily wastewater. Water Res. 2019, 149, 292–301. [Google Scholar] [CrossRef]
- Brakstad, O.G.; Nordtug, T.; Throne-Holst, M. Biodegradation of dispersed Macondo oil in seawater at low temperature and different oil droplet sizes. Mar. Pollut. Bull. 2015, 93, 144–152. [Google Scholar] [CrossRef]
- Merlin, F.; Zhu, Z.; Yang, M.; Chen, B.; Lee, K.; Boufadel, M.C.; Isaacman, L.; Zhang, B. Dispersants as marine oil spill treating agents: A review on mesoscale tests and field trials. Environ. Syst. Res. 2021, 10, 37. [Google Scholar] [CrossRef]
- Brandvik, P.J.; Storey, C.; Davies, E.J.; Leirvik, F. Quantification of oil droplets under high pressure laboratory experiments simulating deep water oil releases and subsea dispersants injection (SSDI). Mar. Pollut. Bull. 2019, 138, 520–525. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. The Use of Dispersants in Marine Oil Spill Response; The National Academies Press: Washington, DC, USA, 2020. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.; Zhao, L.; Boufadel, M.C.; King, T.; Robinson, B.; Conmy, R.; Lee, K. Impact of mixing time and energy on the dispersion effectiveness and droplets size of oil. Chemosphere 2017, 166, 246–254. [Google Scholar] [CrossRef]
- Li, C.; Miller, J.; Wang, J.; Koley, S.; Katz, J. Size distribution and dispersion of droplets generated by impingement of breaking waves on oil slicks. J. Geophys. Res. Oceans 2017, 122, 7938–7957. [Google Scholar] [CrossRef]
- Feng, Q.; An, C.; Chen, Z.; Yin, J.; Zhang, B.; Lee, K.; Wang, Z. Investigation into the impact of aged microplastics on oil behavior in shoreline environments. J. Hazard. Mater. 2021, 421, 126711. [Google Scholar] [CrossRef]
- Shan, J.; Wang, J.; Zhan, J.; Liu, L.; Wu, F.; Wang, X. Sorption behaviors of crude oil on polyethylene microplastics in seawater and digestive tract under simulated real-world conditions. Chemosphere 2020, 257, 127225. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, B.; Xin, X.; Liu, B.; Zhu, Z.; Dong, G.; Zhao, Y.; Lee, K.; Chen, B. Microplastic-oil-dispersant agglomerates in the marine environment: Formation mechanism and impact on oil dispersion. J. Hazard. Mater. 2021, 127825. [Google Scholar] [CrossRef]
- Sequoia Scientific, Inc. LISST-200X Measures Particle Size Distribution and Concentration, and Optical VSF. Available online: http://www.sequoiasci.com/product/lisst-200x/ (accessed on 20 March 2021).
- Sequoia Scientific, Inc. LISST-200X Particle Size Analyzer User’s Manual. Available online: https://www.sequoiasci.com/wp-content/uploads/2016/02/LISST-200X_Users_Manual_v1_3B.pdf (accessed on 20 March 2021).
- Vabalas, A.; Gowen, E.; Poliakoff, E.; Casson, A.J. Machine learning algorithm validation with a limited sample size. PLoS ONE 2019, 14, e0224365. [Google Scholar] [CrossRef] [PubMed]
- Sheykhmousa, M.; Mahdianpari, M.; Ghanbari, H.; Mohammadimanesh, F.; Ghamisi, P.; Homayouni, S. Support vector machine vs. random forest for remote sensing image classification: A meta-analysis and systematic review. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 6308–6325. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, B.; Song, X.; Kang, Q.; Ye, X.; Zhang, B. A data-driven binary-classification framework for oil fingerprinting analysis. Environ. Res. 2021, 201, 111454. [Google Scholar] [CrossRef] [PubMed]
- Venosa, A.D.; Holder, E. Laboratory-Scale Testing of Dispersant Effectiveness of 20 Oils Using the Baffled Flask Test; US Environmental Protection Agency: Washington, DC, USA, 2011; pp. 600–699.
- Hossain, M.R.; Jiang, M.; Wei, Q.; Leff, L.G. Microplastic surface properties affect bacterial colonization in freshwater. J. Basic Microbiol. 2019, 59, 54–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebedeva, E.V.; Fogden, A. Adhesion of oil to kaolinite in water. Environ. Sci. Technol. 2010, 44, 9470–9475. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, G.; Verma, A.K.; Radhika, S. K-nearest neighbors and grid search cv based real time fault monitoring system for industries. In Proceedings of the IEEE 5th International Conference for Convergence in Technology (I2CT), Pune, India, 29–31 March 2019; pp. 1–5. [Google Scholar]
- Förch, R.; Schönherr, H.; Jenkins, A.T.A. Surface Design: Applications in Bioscience and Nanotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 978-3-527-40789-7. [Google Scholar]
- Thielmann, F.; Naderi, M.; Ansari, M.A.; Stepanek, F. The effect of primary particle surface energy on agglomeration rate in fluidised bed wet granulation. Powder Technol. 2008, 181, 160–168. [Google Scholar] [CrossRef]
- Li, Z.; Lee, K.; King, T.; Boufadel, M.C.; Venosa, A.D. Evaluating chemical dispersant efficacy in an experimental wave tank: 2—Significant factors determining in situ oil droplet size distribution. Environ. Eng. Sci. 2009, 26, 1407–1418. [Google Scholar] [CrossRef]
- Song, X.; Jing, L.; Chen, B.; Zhu, Z.; Cai, Q.; Ye, X.; Zheng, X.; Hill, S.J.; Zhang, B. The effect of pressure variation on droplet size distribution of dispersed oil under simulated deep-water conditions. Heliyon 2021, 7, e06291. [Google Scholar] [CrossRef] [PubMed]
- Buribayev, Z.; Joldasbayev, S.; Yerkosova, A.; Zhassuzak, M. Building a system for predicting the yield of grain crops based on machine learning using the XGBRegressor algorithm. In Proceedings of the IEEE International Conference on Smart Information Systems and Technologies (SIST), Nur-Sultan, Kazakhstan, 28–30 April 2021; pp. 1–5. [Google Scholar]





| MPs Type | Size | Water Contact Angle (°) | Density (g/cm3) |
|---|---|---|---|
| PE MPs | 6.00–8.50 μm | 106.60 | 1.07 |
| Models | Hyperparameters | Values |
|---|---|---|
| XGBRegressor | colsample_bytree | 1 |
| importance_type | gain | |
| learning_rate | 0.3 | |
| max_depth | 6 | |
| n_estimators | 100 | |
| reg_lambda | 1 | |
| tree_method | Exact |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Zhang, B.; Chen, Y.; Xin, X.; Lee, K.; Chen, B. Impact of Microplastics on Oil Dispersion Efficiency in the Marine Environment. Sustainability 2021, 13, 13752. https://doi.org/10.3390/su132413752
Yang M, Zhang B, Chen Y, Xin X, Lee K, Chen B. Impact of Microplastics on Oil Dispersion Efficiency in the Marine Environment. Sustainability. 2021; 13(24):13752. https://doi.org/10.3390/su132413752
Chicago/Turabian StyleYang, Min, Baiyu Zhang, Yifu Chen, Xiaying Xin, Kenneth Lee, and Bing Chen. 2021. "Impact of Microplastics on Oil Dispersion Efficiency in the Marine Environment" Sustainability 13, no. 24: 13752. https://doi.org/10.3390/su132413752