Climate-Change Impacts on the Southernmost Mediterranean Arctic-Alpine Plant Populations
Abstract
:1. Introduction
- (a)
- How will climate-change influence the plant diversity patterns of the arctic-alpine taxa occurring in Greece?
- (b)
- Where are currently located the arctic-alpine species richness hotspots in Greece?
- (c)
- Will these hotspots shift in the future and have they shifted since the Last Glacial Maximum?
- (d)
- Which conservation measures need to be taken for the efficient protection and management of these glacial relicts?
2. Materials and Methods
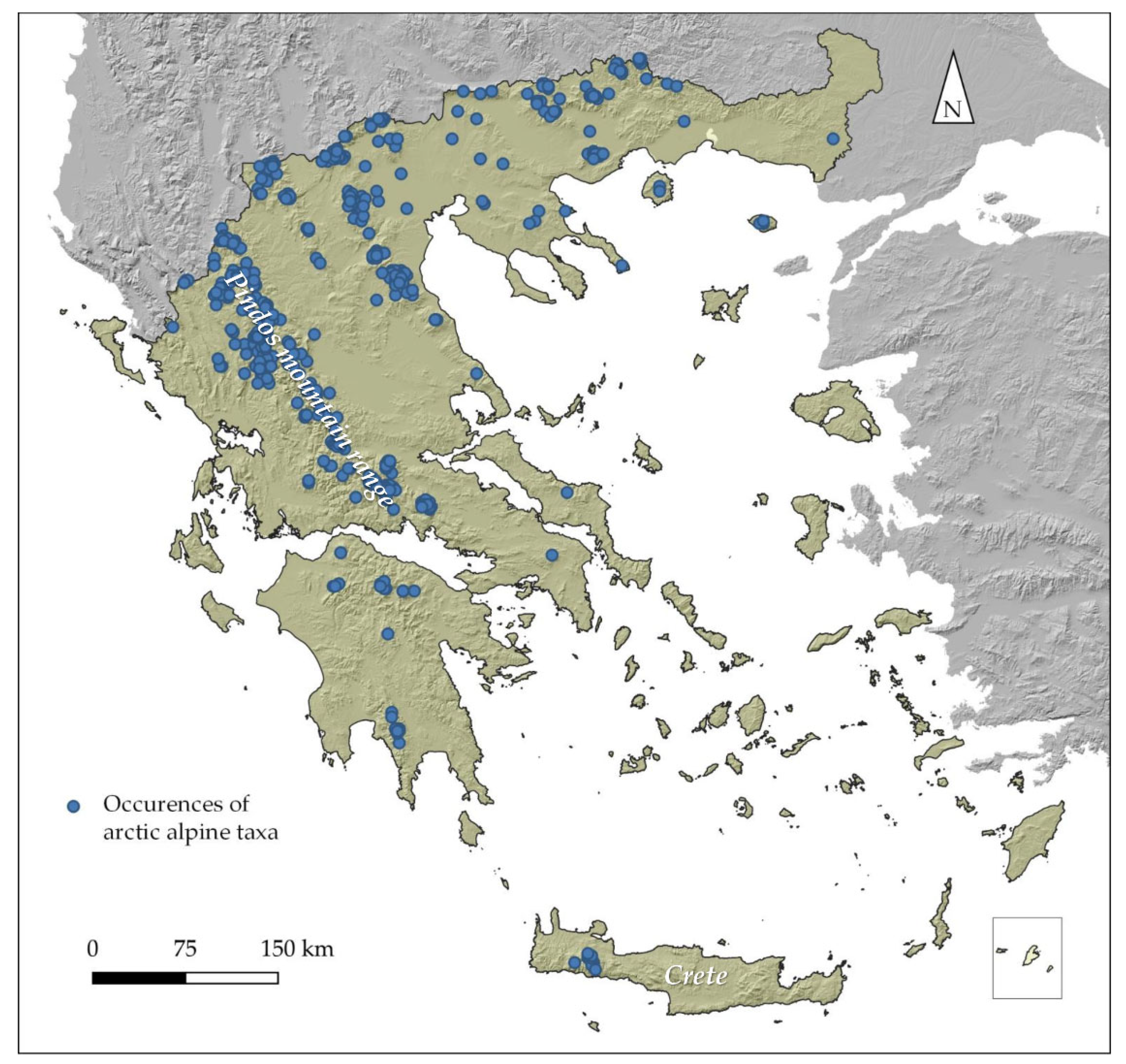
2.1. Species Occurrence Data
2.2. Environmental Data
2.3. Species Distribution Models
2.4. Biodiversity Hotspots Detection
2.5. Latitudinal and Altitudinal Shifts of the Biodiversity Hotspots
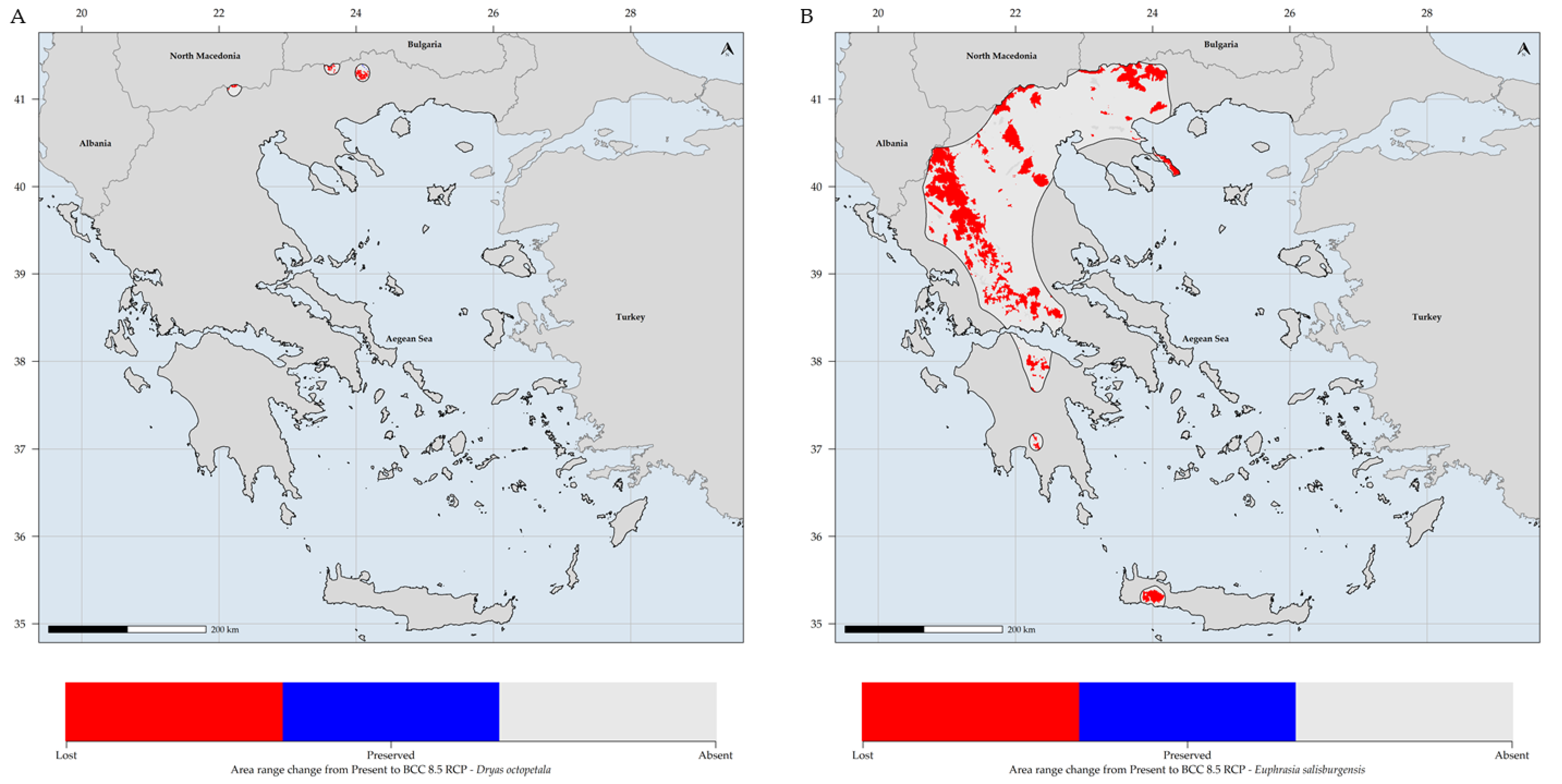
3. Results
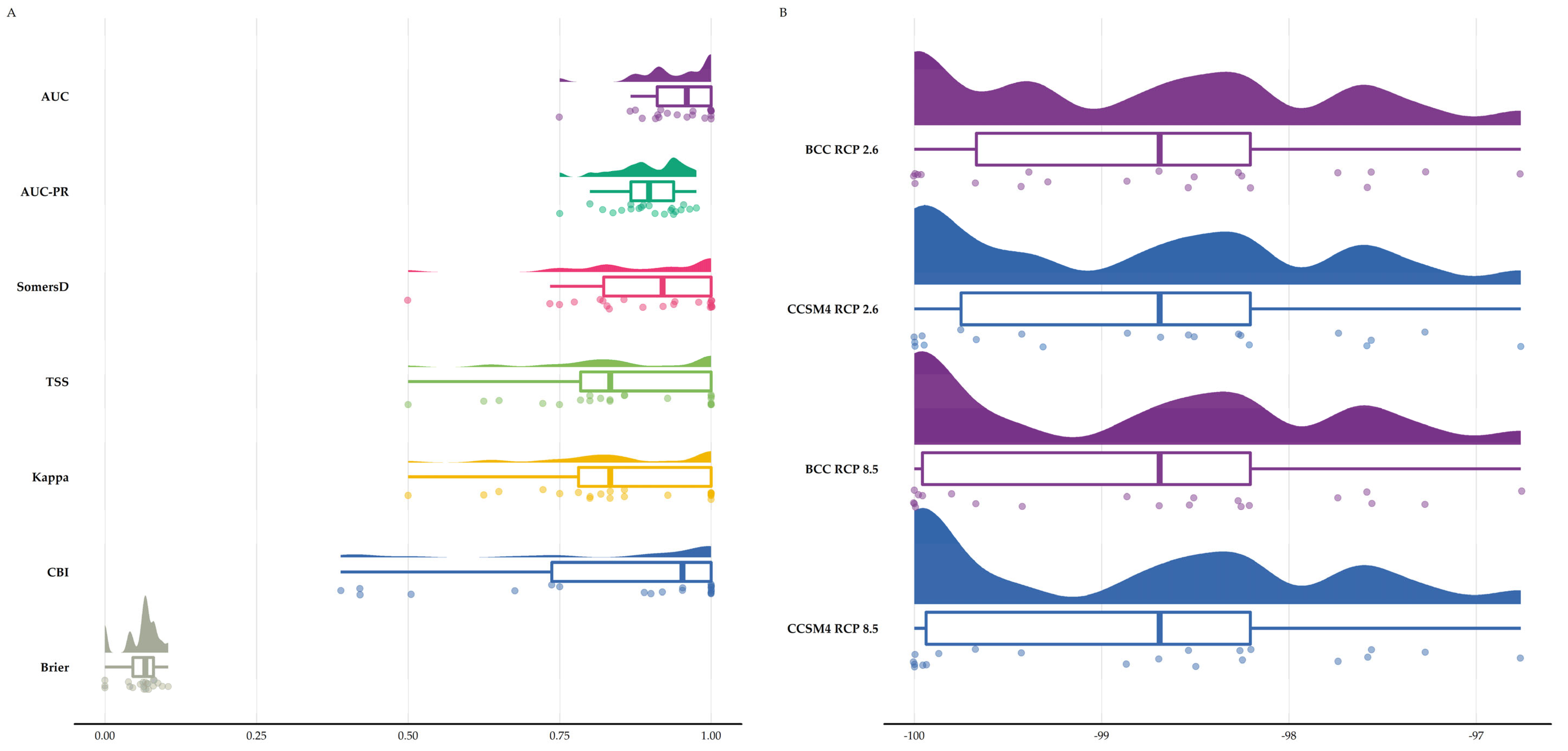
3.1. Species Distribution Models
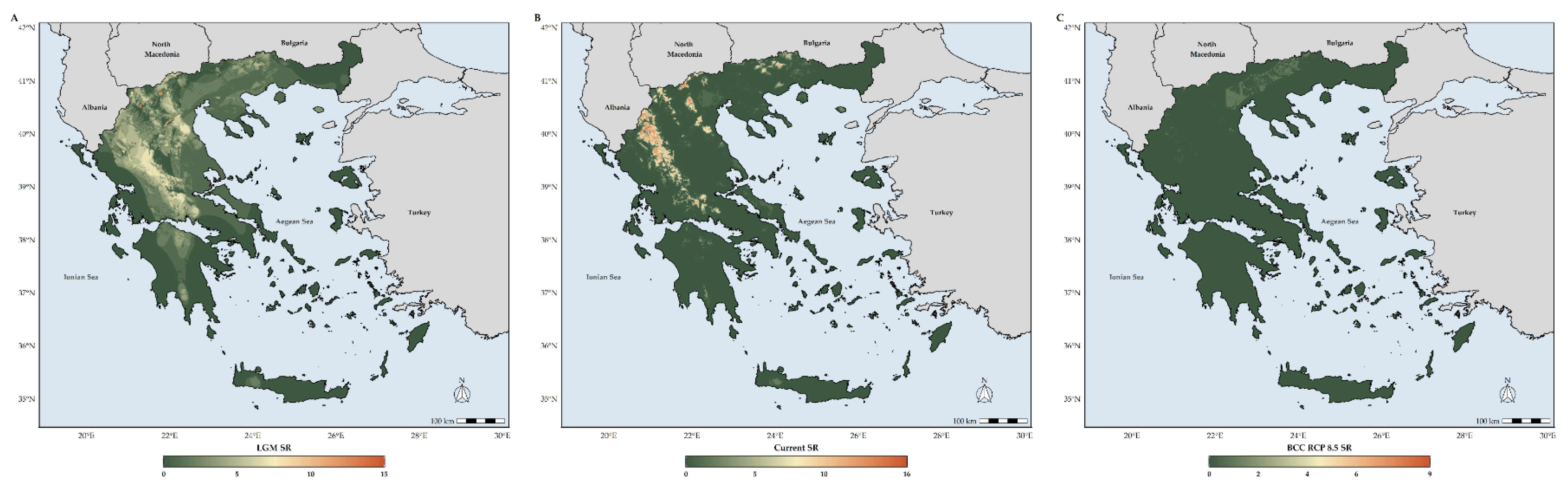
3.2. Biodiversity Hotspots Detection
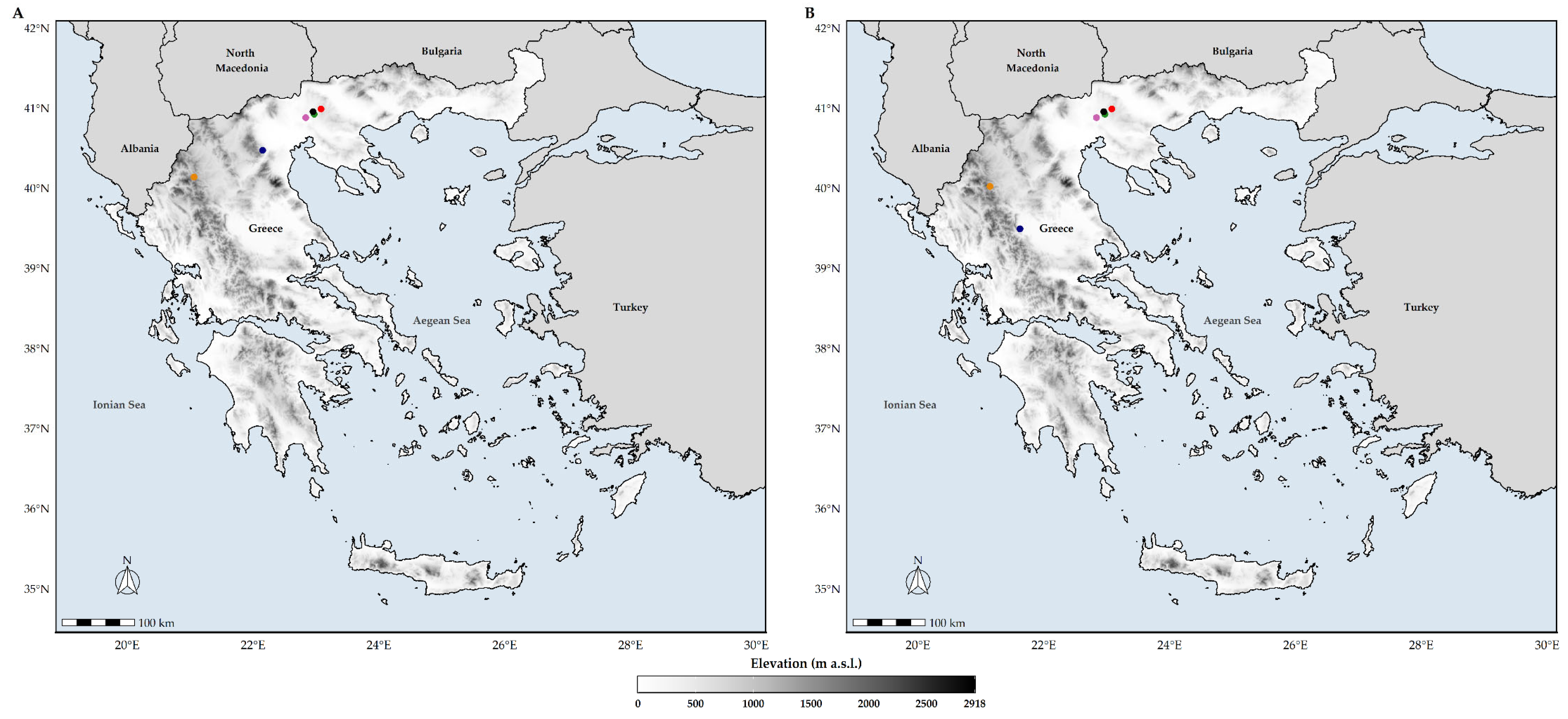
3.3. Latitudinal and Altitudinal Shifts of the Biodiversity Hotspots
4. Discussion
4.1. Climate-Change Impacts
4.2. Species Richness Hotspots
4.3. Conservation Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stevanović, V.; Vukojičić, S.; Šinžar-Sekulić, J.; Lazarević, M.; Tomović, G.; Tan, K. Distribution and diversity of Arctic-Alpine species in the Balkans. Plant Syst. Evol. 2009, 283, 219–235. [Google Scholar] [CrossRef]
- Dítě, D.; Hájek, M.; Svitková, I.; Košuthová, A.; Šoltés, R.; Kliment, J. Glacial-relict symptoms in the Western Carpathian flora. Folia Geobot. Phytotaxon. 2018, 53, 277–300. [Google Scholar] [CrossRef]
- Gentili, R.; Bacchetta, G.; Fenu, G.; Cogoni, D.; Abeli, T.; Rossi, G.; Salvatore, M.C.; Baroni, C.; Citterio, S. From cold to warm-stage refugia for boreo-alpine plants in southern European and Mediterranean mountains: The last chance to survive or an opportunity for speciation? Biodiversity 2015, 16, 247–261. [Google Scholar] [CrossRef]
- Birks, H.H. The Late-Quaternary history of arctic and alpine plants. Plant Ecol. Divers. 2008, 1, 135–146. [Google Scholar] [CrossRef]
- Abbott, R.J. History, evolution and future of arctic and alpine flora: Overview. Plant Ecol. Divers. 2008, 1, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Billings, W.D. Adaptations and Origins of Alpine Plants. Arct. Alp. Res. 1974, 6, 129. [Google Scholar] [CrossRef]
- Wasof, S.; Lenoir, J.; Aarrestad, P.A.; Alsos, I.G.; Armbruster, W.S.; Austrheim, G.; Bakkestuen, V.; Birks, H.J.B.; Bråthen, K.A.; Broennimann, O.; et al. Disjunct populations of European vascular plant species keep the same climatic niches. Glob. Ecol. Biogeogr. 2015, 24, 1401–1412. [Google Scholar] [CrossRef] [Green Version]
- Clarke, C.L.; Edwards, M.E.; Gielly, L.; Ehrich, D.; Hughes, P.D.M.; Morozova, L.M.; Haflidason, H.; Mangerud, J.; Svendsen, J.I.; Alsos, I.G. Persistence of arctic-alpine flora during 24,000 years of environmental change in the Polar Urals. Sci. Rep. 2019, 9, 19613. [Google Scholar] [CrossRef] [Green Version]
- Lesica, P.; McCune, B. Decline of arctic-alpine plants at the southern margin of their range following a decade of climatic warming. J. Veg. Sci. 2004, 15, 679. [Google Scholar] [CrossRef]
- Dirnböck, T.; Dullinger, S.; Grabherr, G. A regional impact assessment of climate and land-use change on alpine vegetation. J. Biogeogr. 2003, 30, 401–417. [Google Scholar] [CrossRef]
- Engler, R.; Randin, C.F.; Thuiller, W.; Dullinger, S.; Zimmermann, N.E.; Araújo, M.B.; Pearman, P.B.; Le Lay, G.; Piedallu, C.; Albert, C.H.; et al. 21st century climate change threatens mountain flora unequally across Europe. Glob. Chang. Biol. 2011, 17, 2330–2341. [Google Scholar] [CrossRef]
- Niskanen, A.K.J.; Niittynen, P.; Aalto, J.; Väre, H.; Luoto, M. Lost at high latitudes: Arctic and endemic plants under threat as climate warms. Divers. Distrib. 2019, 25, 809–821. [Google Scholar] [CrossRef] [Green Version]
- Svitková, I.; Svitok, M.; Petrík, A.; Bernátová, D.; Senko, D.; Šibík, J. The Fate of Endangered Rock Sedge (Carex rupestris) in the Western Carpathians—The Future Perspective of an Arctic-Alpine Species under Climate Change. Diversity 2019, 11, 172. [Google Scholar] [CrossRef] [Green Version]
- Lakušić, D.; Liber, Z.; Nikolić, T.; Surina, B.; Kovačić, S.; Bogdanović, S.; Stefanović, S. Molecular phylogeny of the Campanula pyramidalis species complex (Campanulaceae) inferred from chloroplast and nuclear non-coding sequences and its taxonomic implications. Taxon 2013, 62, 505–524. [Google Scholar] [CrossRef] [Green Version]
- Surina, B.; Schönswetter, P.; Schneeweiss, G.M. Quaternary range dynamics of ecologically divergent species (Edraianthus serpyllifolius and E. tenuifolius, Campanulaceae) within the Balkan refugium. J. Biogeogr. 2011, 38, 1381–1393. [Google Scholar] [CrossRef]
- Surina, B.; Schneeweiss, G.M.; Glasnović, P.; Schönswetter, P. Testing the efficiency of nested barriers to dispersal in the Mediterranean high mountain plant Edraianthus graminifolius (Campanulaceae). Mol. Ecol. 2014, 23, 2861–2875. [Google Scholar] [CrossRef] [PubMed]
- Kutnjak, D.; Kuttner, M.; Niketić, M.; Dullinger, S.; Schönswetter, P.; Frajman, B. Escaping to the summits: Phylogeography and predicted range dynamics of Cerastium dinaricum, an endangered high mountain plant endemic to the western Balkan Peninsula. Mol. Phylogenet. Evol. 2014, 78, 365–374. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular plants of Greece: An annotated checklist. Englera 2013, 31, 1–372. [Google Scholar]
- Kougioumoutzis, K.; Kokkoris, I.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction Risk Assessment of the Greek Endemic Flora. Biology 2021, 10, 195. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Kallimanis, A.; Strid, A.; Dimopoulos, P. Plant Endemism Centres and Biodiversity Hotspots in Greece. Biology 2021, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular plants of Greece: An annotated checklist. Supplement. Willdenowia 2016, 46, 301–347. [Google Scholar] [CrossRef] [Green Version]
- Gaston, K.J.; David, R. Hotspots Across Europe. Biodivers. Lett. 1994, 2, 108. [Google Scholar] [CrossRef]
- Médail, F.; Diadema, K. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J. Biogeogr. 2009, 36, 1333–1345. [Google Scholar] [CrossRef]
- Tzedakis, P. The Balkans as Prime Glacial Refugial Territory of European Temperate Trees. In Balkan Biodiversity; Springer: Berlin/Heidelberg, Geramny, 2004; pp. 49–68. [Google Scholar]
- Kokkoris, I.; Dimitrellos, G.; Kougioumoutzis, K.; Laliotis, I.; Georgiadis, T.; Tiniakou, A. The native flora of Mountain Panachaikon (Peloponnese, Greece): New records and diversity. J. Biol. Res. 2014, 21, 9. [Google Scholar] [CrossRef] [Green Version]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Trigas, P.; Strid, A.; Dimopoulos, P. Plant Diversity Patterns and Conservation Implications under Climate-Change Scenarios in the Mediterranean: The Case of Crete (Aegean, Greece). Diversity 2020, 12, 270. [Google Scholar] [CrossRef]
- Papageorgiou, A.C.; Tsiripidis, I.; Mouratidis, T.; Hatziskakis, S.; Gailing, O.; Eliades, N.-G.H.; Vidalis, A.; Drouzas, A.D.; Finkeldey, R. Complex fine-scale phylogeographical patterns in a putative refugial region forFagus sylvatica(Fagaceae). Bot. J. Linn. Soc. 2014, 174, 516–528. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.D. Plant Evolution in the Mediterranean; Oxford University Press: Oxford, UK, 2005; ISBN 0198515332. [Google Scholar]
- Urban, M.C. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef] [Green Version]
- Gray, A. The ecology of plant extinction: Rates, traits and island comparisons. Oryx 2019, 53, 424–428. [Google Scholar] [CrossRef] [Green Version]
- Thuiller, W.; Lavergne, S.; Roquet, C.; Boulangeat, I.; Lafourcade, B.; Araújo, M.B. Consequences of climate change on the tree of life in Europe. Nat. Cell Biol. 2011, 470, 531–534. [Google Scholar] [CrossRef]
- Le Roux, J.J.; Hui, C.; Castillo, M.L.; Iriondo, J.M.; Keet, J.-H.; Khapugin, A.A.; Médail, F.; Rejmánek, M.; Theron, G.; Yannelli, F.A.; et al. Recent Anthropogenic Plant Extinctions Differ in Biodiversity Hotspots and Coldspots. Curr. Biol. 2019, 29, 2912–2918.e2. [Google Scholar] [CrossRef]
- Vellend, M.; Baeten, L.; Becker-Scarpitta, A.; Boucher-Lalonde, V.; McCune, J.L.; Messier, J.; Myers-Smith, I.; Sax, D.F. Plant Biodiversity Change Across Scales During the Anthropocene. Annu. Rev. Plant Biol. 2017, 68, 563–586. [Google Scholar] [CrossRef] [Green Version]
- Enquist, B.J.; Feng, X.; Boyle, B.; Maitner, B.; Newman, E.A.; Jørgensen, P.M.; Roehrdanz, P.R.; Thiers, B.M.; Burger, J.R.; Corlett, R.T.; et al. The commonness of rarity: Global and future distribution of rarity across land plants. Sci. Adv. 2019, 5, eaaz0414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newbold, T.; Oppenheimer, P.; Etard, A.; Williams, J.J. Tropical and Mediterranean biodiversity is disproportionately sensitive to land-use and climate change. Nat. Ecol. Evol. 2020, 4, 1630–1638. [Google Scholar] [CrossRef]
- Newbold, T. Future effects of climate and land-use change on terrestrial vertebrate community diversity under different scenarios. Proc. Proc. R. Soc. B Biol. Sci. R. Soc. 2018, 285, 20180792. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Contu, S.; Hill, S.L.L.; Beck, J.; Liu, Y.; Meyer, C.; Phillips, H.R.P.; Scharlemann, J.P.W.; Purvis, A. Widespread winners and narrow-ranged losers: Land use homogenizes biodiversity in local assemblages worldwide. PLoS Biol. 2018, 16, e2006841. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Olden, J.D.; Lockwood, J.L.; Record, S.; McKinney, M.L.; Baiser, B. Changes in taxonomic and phylogenetic diversity in the Anthropocene. Proc. R. Soc. B Boil. Sci. 2020, 287, 20200777. [Google Scholar] [CrossRef] [PubMed]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Trigas, P.; Strid, A.; Dimopoulos, P. Spatial Phylogenetics, Biogeographical Patterns and Conservation Implications of the Endemic Flora of Crete (Aegean, Greece) under Climate Change Scenarios. Biology 2020, 9, 199. [Google Scholar] [CrossRef]
- 4Olden, J.D.; Comte, L.; Giam, X. The Homogocene: A research prospectus for the study of biotic homogenisation. NeoBiota 2018, 37, 23–36. [Google Scholar] [CrossRef] [Green Version]
- Eidesen, P.B.; Ehrich, D.; Bakkestuen, V.; Alsos, I.G.; Gilg, O.; Taberlet, P.; Brochmann, C. Genetic roadmap of the Arctic: Plant dispersal highways, traffic barriers and capitals of diversity. New Phytol. 2013, 200, 898–910. [Google Scholar] [CrossRef] [Green Version]
- Paun, O.; Schönswetter, P.; Winkler, M.; Consortium, I.; Tribsch, A. Historical divergence vs. contemporary gene flow: Evolutionary history of the calcicoleRanunculus alpestrisgroup (Ranunculaceae) in the European Alps and the Carpathians. Mol. Ecol. 2008, 17, 4263–4275. [Google Scholar] [CrossRef]
- Ronikier, M.; Schneeweiss, G.M.; Schönswetter, P. The extreme disjunction between Beringia and Europe in Ranunculus glacialis sl (Ranunculaceae) does not coincide with the deepest genetic split–a story of the importance of temperate mountain ranges in arctic–alpine phylogeography. Mol. Ecol. 2012, 21, 5561–5578. [Google Scholar] [CrossRef]
- Stehlik, I.; Blattner, F.R.; Holderegger, R.; Bachmann, K. Nunatak survival of the high Alpine plant Eritrichium nanum (L.) Gaudin in the central Alps during the ice ages. Mol. Ecol. 2002, 11, 2027–2036. [Google Scholar] [CrossRef]
- Stehlik, I. Resistance or emigration? Response of alpine plants to the ice ages. Taxon 2003, 52, 499–510. [Google Scholar] [CrossRef] [Green Version]
- Winkler, M.; Tribsch, A.; Schneeweiss, G.M.; Brodbeck, S.; Gugerli, F.; Holderegger, R.; Abbott, R.J.; Schönswetter, P. Tales of the unexpected: Phylogeography of the arctic-alpine model plantSaxifraga oppositifolia(Saxifragaceae) revisited. Mol. Ecol. 2012, 21, 4618–4630. [Google Scholar] [CrossRef]
- Fragnière, Y.; Pittet, L.; Clément, B.; Bétrisey, S.; Gerber, E.; Ronikier, M.; Parisod, C.; Kozlowski, G. Climate Change and Alpine Screes: No Future for Glacial Relict Papaver occidentale (Papaveraceae) in Western Prealps. Diversity 2020, 12, 346. [Google Scholar] [CrossRef]
- Theodoridis, S.; Patsiou, T.S.; Randin, C.; Conti, E. Forecasting range shifts of a cold-adapted species under climate change: Are genomic and ecological diversity within species crucial for future resilience? Ecography 2018, 41, 1357–1369. [Google Scholar] [CrossRef] [Green Version]
- Theodoridis, S.; Randin, C.F.; Szövényi, P.; Boucher, F.C.; Patsiou, T.-S.; Conti, E. How Do Cold-Adapted Plants Respond to Climatic Cycles? Interglacial Expansion Explains Current Distribution and Genomic Diversity in Primula farinosa L. Syst. Biol. 2016, 66, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Guerrina, M.; Theodoridis, S.; Minuto, L.; Conti, E.; Casazza, G. First evidence of post-glacial contraction of Alpine endemics: Insights from Berardia subacaulis in the European Alps. J. Biogeogr. 2021. [Google Scholar] [CrossRef]
- Corlett, R.T. Safeguarding our future by protecting biodiversity. Plant Divers. 2020, 42, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Abeli, T.; Vamosi, J.C.; Orsenigo, S. The importance of marginal population hotspots of cold-adapted species for research on climate change and conservation. J. Biogeogr. 2018, 45, 977–985. [Google Scholar] [CrossRef]
- Sun, M.; Su, T.; Zhang, S.-B.; Li, S.-F.; Anberree-Lebreton, J.; Zhou, Z.-K. Variations in Leaf Morphological Traits of Quercus guyavifolia (Fagaceae) were Mainly Influenced by Water and Ultraviolet Irradiation at High Elevations on the Qinghai-Tibet Plateau, China. Int. J. Agric. Biol. 2016, 18, 266–273. [Google Scholar] [CrossRef]
- Rixen, C.; Wipf, S.; Frei, E.R.; Stöckli, V. Faster, higher, more? Past, present and future dynamics of alpine and arctic flora under climate change. Alp. Bot. 2014, 124, 77–79. [Google Scholar] [CrossRef] [Green Version]
- Fassou, G.; Kougioumoutzis, K.; Iatrou, G.; Trigas, P.; Papasotiropoulos, V. Genetic Diversity and Range Dynamics of Helleborus odorus subsp. cyclophyllus under Different Climate Change Scenarios. Forests 2020, 11, 620. [Google Scholar] [CrossRef]
- Stathi, E.; Kougioumoutzis, K.; Abraham, E.M.; Trigas, P.; Ganopoulos, I.; Avramidou, E.V.; Tani, E. Population genetic variability and distribution of the endangered Greek endemic Cicer graecum under climate change scenarios. AoB Plants 2020, 12, plaa007. [Google Scholar] [CrossRef]
- Charitonidou, M.; Kougioumoutzis, K.; Halley, J.M. An Orchid in Retrograde: Climate-Driven Range Shift Patterns of Ophrys helenae in Greece. Plants 2021, 10, 470. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kotsakiozi, P.; Stathi, E.; Trigas, P.; Parmakelis, A. Conservation Genetics of Four Critically Endangered Greek Endemic Plants: A Preliminary Assessment. Diversity 2021, 13, 152. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Djordjević, V. Modelling sexually deceptive orchid species distributions under future climates: The importance of plant–pollinator interactions. Sci. Rep. 2020, 10, 10623. [Google Scholar] [CrossRef]
- Bai, Y.; Wei, X.; Li, X. Distributional dynamics of a vulnerable species in response to past and future climate change: A window for conservation prospects. PeerJ 2018, 6, e4287. [Google Scholar] [CrossRef]
- Robertson, M.P.; Visser, V.; Hui, C. Biogeo: An R package for assessing and improving data quality of occurrence record datasets. Ecography 2016, 39, 394–401. [Google Scholar] [CrossRef] [Green Version]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Van Proosdij, A.S.J.; Sosef, M.S.M.; Wieringa, J.; Raes, N. Minimum required number of specimen records to develop accurate species distribution models. Ecography 2016, 39, 542–552. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Boehner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef] [Green Version]
- Title, P.O.; Bemmels, J.B. ENVIREM: An expanded set of bioclimatic and topographic variables increases flexibility and improves performance of ecological niche modeling. Ecography 2017, 41, 291–307. [Google Scholar] [CrossRef] [Green Version]
- Hengl, T.; de Jesus, J.M.; Heuvelink, G.B.M.; Gonzalez, M.R.; Kilibarda, M.; Blagotić, A.; Shangguan, W.; Wright, M.N.; Geng, X.; Bauer-Marschallinger, B.; et al. SoilGrids250m: Global gridded soil information based on machine learning. PLoS ONE 2017, 12, e0169748. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, A.; Reuter, H.I.; Nelson, A.; Guevara, E. Hole-Filled SRTM for the Globe Version 4. CGIAR-CSI SRTM 90m Database. Available online: http//srtm.csi.cgiar.org (accessed on 8 December 2021).
- Hijmans, R.J. Package ‘Raster’—Geographic Data Analysis and Modeling. CRAN Repos; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Evans, J.S. spatialEco-R Package Version 1.2-0; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- McSweeney, C.F.; Jones, R.G.; Lee, R.W.; Rowell, D.P. Selecting CMIP5 GCMs for downscaling over multiple regions. Clim. Dyn. 2014, 44, 3237–3260. [Google Scholar] [CrossRef] [Green Version]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2013, 37, 191–203. [Google Scholar] [CrossRef]
- Breiner, F.T.; Nobis, M.P.; Bergamini, A.; Guisan, A. Optimizing ensembles of small models for predicting the distribution of species with few occurrences. Methods Ecol. Evol. 2017, 9, 802–808. [Google Scholar] [CrossRef] [Green Version]
- Breiner, F.T.; Guisan, A.; Nobis, M.P.; Bergamini, A. Including environmental niche information to improve IUCN Red List assessments. Divers. Distrib. 2017, 23, 484–495. [Google Scholar] [CrossRef] [Green Version]
- Breiner, F.T.; Guisan, A.; Bergamini, A.; Nobis, M.P. Overcoming limitations of modelling rare species by using ensembles of small models. Methods Ecol. Evol. 2015, 6, 1210–1218. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Di Cola, V.; Brönnimann, O.; Petitpierre, B.; Breiner, F.T.; D’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A.; et al. ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Carlson, C.J. Embarcadero: Species distribution modelling with Bayesian additive regression trees in r. Methods Ecol. Evol. 2020, 11, 850–858. [Google Scholar] [CrossRef]
- Carlson, C.J.; Bevins, S.N.; Schmid, B.V. Plague risk in the western United States over seven decades of environmental change. bioRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Dauby, G.; Stévart, T.; Droissart, V.; Cosiaux, A.; Deblauwe, V.; Simo-Droissart, M.; Sosef, M.S.M.; Lowry, P.P.; Schatz, G.E.; Gereau, R.E.; et al. ConR: An R package to assist large-scale multispecies preliminary conservation assessments using distribution data. Ecol. Evol. 2017, 7, 11292–11303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, L.; Diniz-Filho, J.A.F.; Lohmann, L.G. A comparison of hull methods for estimating species ranges and richness maps. Plant Ecol. Divers. 2017, 10, 389–401. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. The effect of sample size on the accuracy of species distribution models: Considering both presences and pseudo-absences or background sites. Ecography 2019, 42, 535–548. [Google Scholar] [CrossRef] [Green Version]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the ability of habitat suitability models to predict species presences. Ecol. Model. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Measuring and comparing the accuracy of species distribution models with presence-absence data. Ecography 2010, 34, 232–243. [Google Scholar] [CrossRef]
- Sofaer, H.R.; Hoeting, J.A.; Jarnevich, C.S. The area under the precision-recall curve as a performance metric for rare binary events. Methods Ecol. Evol. 2019, 10, 565–577. [Google Scholar] [CrossRef]
- Broennimann, O.; Di Cola, V.; Guisan, A. Ecospat: Spatial Ecology Miscellaneous Methods. R Package Version 3.2; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Hammer, B.; Frasco, M. Metrics: Evaluation Metrics for Machine Learning. R Package Version 0.1.4; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Barbosa, A.M.; Real, R.; Brown, J.A.; Muñoz, A.-R. New measures for assessing model equilibrium and prediction mismatch in species distribution models. Divers. Distrib. 2013, 19, 1333–1338. [Google Scholar] [CrossRef]
- Schwarz, J.; Heider, D. GUESS: Projecting machine learning scores to well-calibrated probability estimates for clinical decision-making. Bioinformatics 2018, 35, 2458–2465. [Google Scholar] [CrossRef]
- Signorell, A.; Aho, K.; Anderegg, N.; Aragon, T.; Arppe, A.; Baddeley, A.; Bolker, B.; Caeiro, F.; Champely, S.; Chessel, D. DescTools: Tools for Descriptive Statistics. R Package Version 0.99-40; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Smith, A.B. enmSdm: Tools for Modeling Species Niches and Distributions. R Package Version 0.5.1.5; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Yan, Y. MLmetrics: Machine Learning Evaluation Metrics. R Package Version 1.1.1.; R Core Team: Vienna, Austria, 2016. [Google Scholar]
- Raes, N.; ter Steege, H. A null-model for significance testing of presence-only species distribution models. Ecography (Cop.) 2007, 30, 727–736. [Google Scholar] [CrossRef]
- Araújo, M.B.; Anderson, R.P.; Barbosa, A.M.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B.; et al. Standards for distribution models in biodiversity assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting thresholds of occurrence in the prediction of species distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD-a platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Alsos, I.G.; Eidesen, P.B.; Ehrich, D.; Skrede, I.; Westergaard, K.; Jacobsen, G.H.; Landvik, J.Y.; Taberlet, P.; Brochmann, C. Frequent Long-Distance Plant Colonization in the Changing Arctic. Science 2007, 316, 1606–1609. [Google Scholar] [CrossRef]
- Dullinger, S.; Gattringer, A.; Thuiller, W.; Moser, D.; Zimmermann, N.; Guisan, A.; Willner, W.; Plutzar, C.; Leitner, M.; Mang, T.; et al. Extinction debt of high-mountain plants under twenty-first-century climate change. Nat. Clim. Chang. 2012, 2, 619–622. [Google Scholar] [CrossRef]
- Engler, R.; Randin, C.F.; Vittoz, P.; Czáka, T.; Beniston, M.; Zimmermann, N.; Guisan, A. Predicting future distributions of mountain plants under climate change: Does dispersal capacity matter? Ecography 2009, 32, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Crisp, M.D.; Laffan, S.; Linder, H.P.; Monro, A. Endemism in the Australian flora. J. Biogeogr. 2001, 28, 183–198. [Google Scholar] [CrossRef]
- Linder, H.P. Plant diversity and endemism in sub-Saharan tropical Africa. J. Biogeogr. 2001, 28, 169–182. [Google Scholar] [CrossRef]
- Linder, H.P. On Areas of Endemism, with an Example from the African Restionaceae. Syst. Biol. 2001, 50, 892–912. [Google Scholar] [CrossRef]
- Guerin, G.R. biomapME: Biodiversity Mapping and Macroecology. R Package v2.0; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Guerin, G.R.; Ruokolainen, L.; Lowe, A.J. A georeferenced implementation of weighted endemism. Methods Ecol. Evol. 2015, 6, 845–852. [Google Scholar] [CrossRef]
- Guerin, G.R.; Lowe, A.J. ‘Sum of inverse range-sizes’ (SIR), a biodiversity metric with many names and interpretations. Biodivers. Conserv. 2015, 24, 2877–2882. [Google Scholar] [CrossRef]
- González-Orozco, C.E.; Laffan, S.W.; Miller, J.T. Spatial distribution of species richness and endemism of the genus Acacia in Australia. Aust. J. Bot. 2011, 59, 601–609. [Google Scholar] [CrossRef]
- Daru, B.H.; Elliott, T.L.; Park, D.; Davies, T.J. Understanding the Processes Underpinning Patterns of Phylogenetic Regionalization. Trends Ecol. Evol. 2017, 32, 845–860. [Google Scholar] [CrossRef]
- Daru, B.H.; Karunarathne, P.; Schliep, K. phyloregion: R package for biogeographical regionalization and macroecology. Methods Ecol. Evol. 2020, 11, 1483–1491. [Google Scholar] [CrossRef]
- Daru, B.H.; Farooq, H.; Antonelli, A.; Faurby, S. Endemism patterns are scale dependent. Nat. Commun. 2020, 11, 2115. [Google Scholar] [CrossRef] [PubMed]
- Cañadas, E.M.; Fenu, G.; Peñas, J.; Lorite, J.; Mattana, E.; Bacchetta, G. Hotspots within hotspots: Endemic plant richness, environmental drivers, and implications for conservation. Biol. Conserv. 2014, 170, 282–291. [Google Scholar] [CrossRef]
- Pebesma, E. Simple Features for R: Standardized Support for Spatial Vector Data. R J. 2018, 10, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Allen, M.; Poggiali, D.; Whitaker, K.; Marshall, T.R.; Kievit, R.A. Raincloud plots: A multi-platform tool for robust data visualization. Wellcome Open Res. 2019, 4, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taberlet, P.; Fumagalli, L.; Wust-Saucy, A.; Cosson, J.F. Comparative phylogeography and postglacial colonization routes in Europe. Mol. Ecol. 1998, 7, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, G.M. Mediterranean Peninsulas: The Evolution of Hotspots. In Biodiversity Hotspots; Springer: Berlin/Heidelberg, Geramny, 2011; pp. 123–147. [Google Scholar]
- UN. The Sustainable Development Goals Report 2020; UN: New York, NY, USA, 2020. [Google Scholar]
- Schickhoff, U.; Singh, R.B.; Mal, S. Mountain Landscapes in Transition; Schickhoff, U., Singh, R.B., Mal, S., Eds.; Sustainable Development Goals Series; Springer International Publishing: Basel, Switzerland, 2021; ISBN 978-3-030-70237-3. [Google Scholar]
- Geppert, C.; Perazza, G.; Wilson, R.J.; Bertolli, A.; Prosser, F.; Melchiori, G.; Marini, L. Consistent population declines but idiosyncratic range shifts in Alpine orchids under global change. Nat. Commun. 2020, 11, 5835. [Google Scholar] [CrossRef] [PubMed]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsos, I.G.; Ehrich, D.; Thuiller, W.; Eidesen, P.B.; Tribsch, A.; Schönswetter, P.; Lagaye, C.; Taberlet, P.; Brochmann, C. Genetic consequences of climate change for northern plants. Proc. R. Soc. B Boil. Sci. 2012, 279, 2042–2051. [Google Scholar] [CrossRef] [Green Version]
- Augustinos, A.; Sotirakis, K.; Trigas, P.; Kalpoutzakis, E.; Papasotiropoulos, V.; Avgoustinos, A. Genetic Variation in Three Closely Related Minuartia (Caryophyllaceae) Species Endemic to Greece: Implications for Conservation Management. Folia Geobot. Phytotaxon. 2014, 49, 603–621. [Google Scholar] [CrossRef]
- López-Vinyallonga, S.; López-Pujol, J.; Constantinidis, T.; Susanna, A.; Garcia-Jacas, N. Mountains and refuges: Genetic structure and evolutionary history in closely related, endemic Centaurea in continental Greece. Mol. Phylogenet. Evol. 2015, 92, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Valli, A.-T.; Koumandou, V.L.; Iatrou, G.; Andreou, M.; Papasotiropoulos, V.; Trigas, P. Conservation biology of threatened Mediterranean chasmophytes: The case of Asperula naufraga endemic to Zakynthos island (Ionian islands, Greece). PLoS ONE 2021, 16, e0246706. [Google Scholar] [CrossRef] [PubMed]
- Varsamis, G.; Merou, T.; Karapatzak, E.; Papageorgiou, A.C.; Fotiadis, G.; Tsiftsis, S. Genetic diversity of alpine Dryas octopetala populations at their southern distribution limit in Europe. Nord. J. Bot. 2021, 39, 1–8. [Google Scholar] [CrossRef]
- Hirao, A.S.; Watanabe, M.; Tsuyuzaki, S.; Shimono, A.; Li, X.; Masuzawa, T.; Wada, N. Genetic diversity within populations of an arctic-alpine species declines with decreasing latitude across the Northern Hemisphere. J. Biogeogr. 2017, 44, 2740–2751. [Google Scholar] [CrossRef]
- Crawford, R.M. Cold climate plants in a warmer world. Plant Ecol. Divers. 2008, 1, 285–297. [Google Scholar] [CrossRef]
- Bjorkman, A.; Vellend, M.; Frei, E.R.; Henry, G.H.R. Climate adaptation is not enough: Warming does not facilitate success of southern tundra plant populations in the high Arctic. Glob. Chang. Biol. 2016, 23, 1540–1551. [Google Scholar] [CrossRef]
- Cotto, O.; Wessely, J.; Georges, D.; Klonner, G.; Schmid, M.; Dullinger, S.; Thuiller, W.; Guillaume, F. A dynamic eco-evolutionary model predicts slow response of alpine plants to climate warming. Nat. Commun. 2017, 8, 15399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, J.M.; Chalmandrier, L.; Lenoir, J.; Burgess, T.I.; Essl, F.; Haider, S.; Kueffer, C.; McDougall, K.; Milbau, A.; Nuñez, M.A.; et al. Lags in the response of mountain plant communities to climate change. Glob. Chang. Biol. 2018, 24, 563–579. [Google Scholar] [CrossRef]
- Paquette, A.; Hargreaves, A.L. Biotic interactions are more often important at species’ warm versus cool range edges. Ecol. Lett. 2021, 24, 2427–2438. [Google Scholar] [CrossRef]
- Panitsa, M.; Kokkoris, I.; Kougioumoutzis, K.; Kontopanou, A.; Bazos, I.; Strid, A.; Dimopoulos, P. Linking Taxonomic, Phylogenetic and Functional Plant Diversity with Ecosystem Services of Cliffs and Screes in Greece. Plants 2021, 10, 992. [Google Scholar] [CrossRef] [PubMed]
- Birks, H.J.B. Contributions of Quaternary botany to modern ecology and biogeography. Plant Ecol. Divers. 2019, 12, 189–385. [Google Scholar] [CrossRef]
- Ruiz-Labourdette, D.; Schmitz, M.F.; Pineda, F.D. Changes in tree species composition in Mediterranean mountains under climate change: Indicators for conservation planning. Ecol. Indic. 2013, 24, 310–323. [Google Scholar] [CrossRef]
- Minachilis, K.; Kougioumoutzis, K.; Petanidou, T. Climate change effects on multi-taxa pollinator diversity and distribution along the elevation gradient of Mount Olympus, Greece. Ecol. Indic. 2021, 132, 108335. [Google Scholar] [CrossRef]
- Padilla-García, N.; Machon, N.; Segarra-Moragues, J.G.; Martínez-Ortega, M.M. Surviving in southern refugia: The case of Veronica aragonensis, a rare endemic from the Iberian Peninsula. Alp. Bot. 2021, 131, 161–175. [Google Scholar] [CrossRef]
- Gómez, J.M.; Megias, A.G.; Lorite, J.; Abdelaziz, M.; Perfectti, F. The silent extinction: Climate change and the potential hybridization-mediated extinction of endemic high-mountain plants. Biodivers. Conserv. 2015, 24, 1843–1857. [Google Scholar] [CrossRef]
- Guerrina, M.; Conti, E.; Minuto, L.; Casazza, G. Knowing the past to forecast the future: A case study on a relictual, endemic species of the SW Alps, Berardia subacaulis. Reg. Environ. Chang. 2016, 16, 1035–1045. [Google Scholar] [CrossRef]
- Koo, K.A.; Kong, W.-S.; Park, S.U.; Lee, J.H.; Kim, J.; Jung, H. Sensitivity of Korean fir (Abies koreana Wils.), a threatened climate relict species, to increasing temperature at an island subalpine area. Ecol. Model. 2017, 353, 5–16. [Google Scholar] [CrossRef]
- Ramírez-Amezcua, Y.; Steinmann, V.W.; Ruiz-Sanchez, E.; Rojas-Soto, O.R. Mexican alpine plants in the face of global warming: Potential extinction within a specialized assemblage of narrow endemics. Biodivers. Conserv. 2016, 25, 865–885. [Google Scholar] [CrossRef]
- Seastedt, T.; Oldfather, M. Climate Change, Ecosystem Processes and Biological Diversity Responses in High Elevation Communities. Climate 2021, 9, 87. [Google Scholar] [CrossRef]
- Jiménez-Alfaro, B.; García-Calvo, L.; García, P.G.; Acebes, J.-L. Anticipating extinctions of glacial relict populations in mountain refugia. Biol. Conserv. 2016, 201, 243–251. [Google Scholar] [CrossRef]
- Hawkes, J.G.; Maxted, N.; Ford-Lloyd, B.V. The Ex Situ Conservation of Plant Genetic Resources; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 9401141363. [Google Scholar]
- Minterr, B.A.; Collins, J.P. Guidelines for Reintroductions and Other Conservation Translocations IUCN, V1.0; IUCN Species Survival Commission: Gland, Switzerland, 2013; Volume 20, ISBN 978-2-8317-1609-1. [Google Scholar]
- Haynes, K.R.; Friedman, J.; Stella, J.C.; Leopold, D.J. Assessing climate change tolerance and the niche breadth-range size hypothesis in rare and widespread alpine plants. Oecologia 2021, 196, 1233–1245. [Google Scholar] [CrossRef]
- Doxa, A.; Albert, C.; Leriche, A.; Saatkamp, A. Prioritizing conservation areas for coastal plant diversity under increasing urbanization. J. Environ. Manag. 2017, 201, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rumpf, S.B.; Hülber, K.; Klonner, G.; Moser, D.; Schütz, M.; Wessely, J.; Willner, W.; Zimmermann, N.E.; Dullinger, S. Range dynamics of mountain plants decrease with elevation. Proc. Natl. Acad. Sci. USA 2018, 115, 1848–1853. [Google Scholar] [CrossRef] [Green Version]
- Körner, C. Alpine Plant Life: Functional Ecology of High Mountain Ecosystems. Springer: New York, NY, USA, 1999. [Google Scholar]
- Fernández-Pascual, E.; Carta, A.; Mondoni, A.; Cavieres, L.A.; Rosbakh, S.; Venn, S.; Satyanti, A.; Guja, L.; Briceño, V.F.; Vandelook, F.; et al. The seed germination spectrum of alpine plants: A global meta-analysis. New Phytol. 2021, 229, 3573–3586. [Google Scholar] [CrossRef] [PubMed]
- Barredo, J.I.; Mauri, A.; Caudullo, G.; Dosio, A. Assessing Shifts of Mediterranean and Arid Climates Under RCP4.5 and RCP8.5 Climate Projections in Europe. Pure Appl. Geophys. Pageoph 2018, 175, 3955–3971. [Google Scholar] [CrossRef]
- Chevin, L.-M.; Lande, R.; Mace, G. Adaptation, Plasticity, and Extinction in a Changing Environment: Towards a Predictive Theory. PLoS Biol. 2010, 8, e1000357. [Google Scholar] [CrossRef] [Green Version]
- Nicotra, A.; Atkin, O.; Bonser, S.; Davidson, A.; Finnegan, E.; Mathesius, U.; Poot, P.; Purugganan, M.; Richards, C.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kougioumoutzis, K.; Kokkoris, I.P.; Strid, A.; Raus, T.; Dimopoulos, P. Climate-Change Impacts on the Southernmost Mediterranean Arctic-Alpine Plant Populations. Sustainability 2021, 13, 13778. https://doi.org/10.3390/su132413778
Kougioumoutzis K, Kokkoris IP, Strid A, Raus T, Dimopoulos P. Climate-Change Impacts on the Southernmost Mediterranean Arctic-Alpine Plant Populations. Sustainability. 2021; 13(24):13778. https://doi.org/10.3390/su132413778
Chicago/Turabian StyleKougioumoutzis, Konstantinos, Ioannis P. Kokkoris, Arne Strid, Thomas Raus, and Panayotis Dimopoulos. 2021. "Climate-Change Impacts on the Southernmost Mediterranean Arctic-Alpine Plant Populations" Sustainability 13, no. 24: 13778. https://doi.org/10.3390/su132413778





