Future Trends and Aging Analysis of Battery Energy Storage Systems for Electric Vehicles
Abstract
:1. Introduction
Related Works
- The paper provides an overview of the latest technologies and developments of electrochemical batteries used in EVs.
- The best performance was reported by LiNi1−x−yMnxCoyO2, whereas LiFePO4 is the greenest and safest battery.
- Capacity fading of 18.42%, between 25–65 °C, is studied as a function of the cycle number and cell temperature.
- The lithium-ion market will be increasing by 11% and 65%, between 2020–2025, for light-duty and heavy-duty EVs.
- The lithium-ion cell production mass will rise by 81% and 74% for both light- and heavy-duty EVs in the market between 2020–2025.
2. Systematic Review of Recent Development in BESSs
3. Aging Analysis Considering Cell Temperature for HEVs: A Case Study
3.1. Mathematical Equations
3.2. Battery Cell Modeling and Settings
3.3. Results and Discussion
4. Potential and Future Prospects: A Prediction-Based Study on BESS for EVs
5. Conclusions
- Within the EV application operating temperature, the lithium-ion family batteries are, i.e., LiMO2, LiMn2O4, and LiFePO4. They are currently the best candidates because of their performance features, such as higher energy density, specific power, battery efficiency, and life cycle. Despite the technical suitability, such batteries may result in being more expensive compared to their alternatives. Therefore, advancements in battery technology or manufacturing processes are required to reduce their cost. LiFePO4 is the greenest and safest type; for instance, it does not produce oxygen, even when completely decomposed due to heating. The proposed batteries in terms of performance are LiNi1-x-yMnxCoyO2 because they can combine LiCoO2 and LiNiO2 and use much less Cobalt, making them safer.
- Among all modern rechargeable electromechanical batteries, the impact of temperature on capacity degradation and aging is unavoidable within the operation. For this reason, NaNiCl2 batteries have shown a greater thermal runaway range compared to other batteries. There is a gap in the literature on the thermal runaway of emerging lithium-ion batteries such as LiNiCoAlO2, LiNixMnyCozO2, and LiCoO2 cathodes.
- While the life cycle plays an important role in BESS design requirements, e.g., the US-advanced battery consortium defines a life cycle of 1000 cycles as one of the design requirements. In this paper, the aging effects and capacity degradation of a lithium-ion battery pack were investigated. Considering the battery cell temperature, the simulation-based study considered the HEV to operate for five hours driving under the WLTP drive cycle. The recorded results reported capacity fading of 18.42% between 25–65 °C. The equivalent cycle number also rose by 19% for the same range of ambient temperature. Additionally, the impact of charging/discharging currents from the battery cell bus was presented using QMC simulations; the evaluations compared the increase of cycles required to finish the five-hour driving cycle. Higher temperatures resulted in a higher cycle number with consideration of the capacity fading.
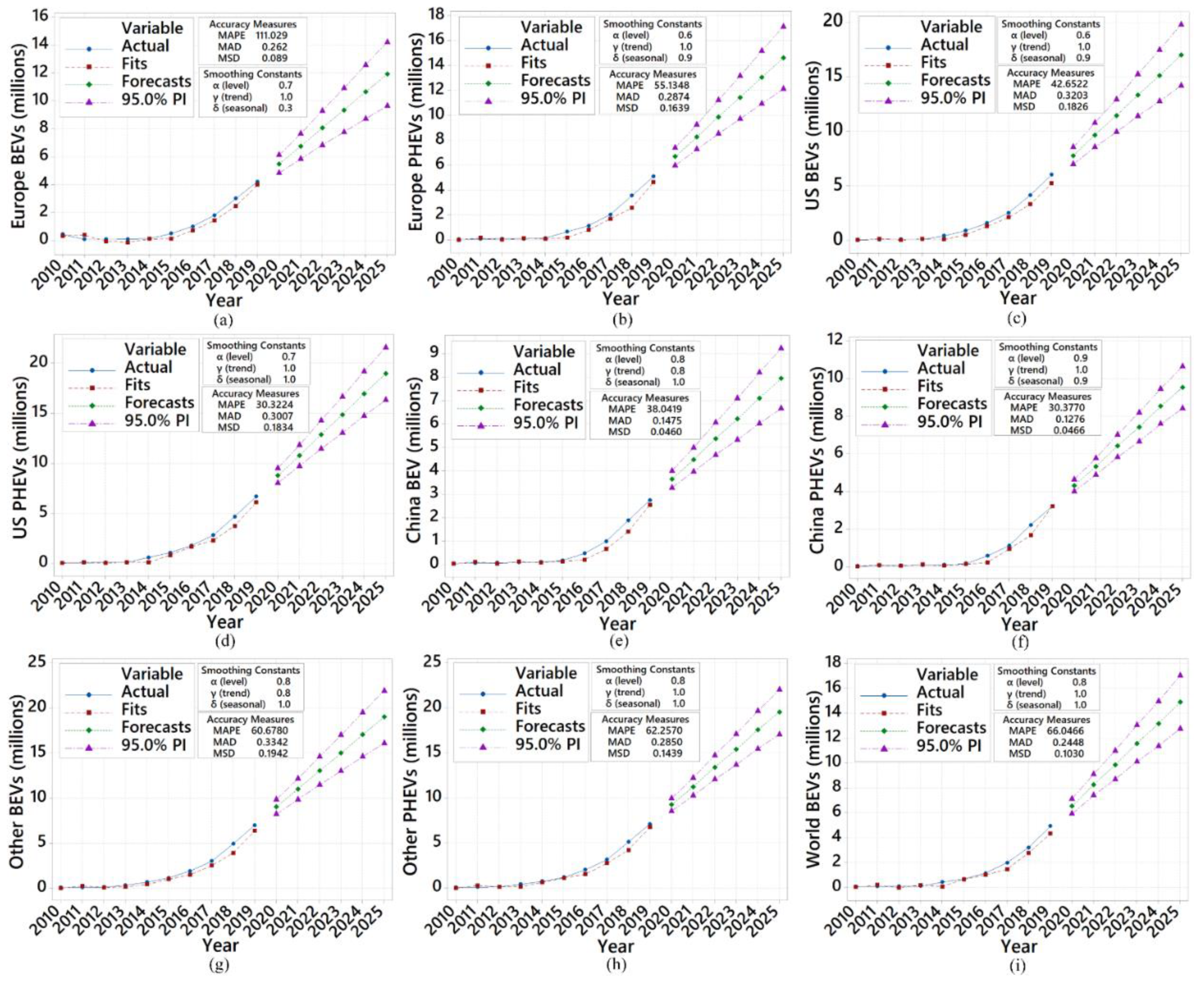
- Based on the predictions using additive Winter’s method, the growing global market of EVs will increase by 140% in 2025. The lithium-ion market will increase by 11% and 65%, between 2020–2025, for light-duty and heavy-duty EVs. The future short-term predictions also indicate that the lithium-ion cell production mass will rise by 81% and 74% for both light-duty and heavy-duty EVs in the market between 2020–2025.
- Based on the predictions in this study, the worldwide EV market will grow by approximately 140% up to 2025. Europe is likely to experience an increase of approximately 103% and 110% million in the sales for BEV and PHEV in the next five years. That implies that the number of vehicles sold in 2019 will more than double in five years. Similarly, the US demand will grow by approximately 135% for BEV and 114% for PHEV, almost tripling the 2019 recorded data. BEVs with a 110% increase and PHEVs with a 132% increase will significantly grow in China, regardless of population density.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| AC | Alternative current |
| BoL | Beginning of life |
| DoD | Depth of discharge |
| DC | Direct current |
| EV | Electric vehicle |
| EoL | End of life |
| ESS | Energy storage system |
| GHG | Greenhouse gas |
| HEV | Hybrid electric vehicle |
| PHEV | Plug-in hybrid electric vehicle |
| RUL | Remaining useful life |
| NiMH | Nickel-metal hybrid |
| SoC | State of charge |
| SoH | State of health |
| ICE | Internal combustion engine |
| BESS | Battery energy storage systems |
| ESS | Energy storage systems |
| BEV | Battery electric vehicle |
| LCA | Life cycle assessment |
| FB | Flow battery |
| SB | Secondary battery |
| LA | Lead-acid |
| SLI | Starting, lighting, and ignition |
| UPS | Uninterruptible power supply |
| VRLA | Valve regulated lead–acid |
| AGM | Absorbent glass mat |
| WLTP | Worldwide harmonized light vehicle test procedure |
| QMC | Quasi-Monte Carlo |
| ANOVA | Analysis of variance |
| BoL | Beginning of life |
| EoL | End of life |
| MAPE | Mean absolute percentage error |
| MAD | Mean absolute deviation |
| MSD | Mean squared displacement |
References
- Yasuoka, S.; Shuichi, D.; Imoto, Y.; Kai, T.; Yamazaki, T.; Ito, T.; Yano, T.; Takeno, K.; Yanagawa, H. Development of Highly Durable Ni-MH Batteries through Introduction of Highly Conductive Co Compound-Coated Ni(OH)2 Technology. ECS Trans. 2015, 66, 19–27. [Google Scholar] [CrossRef]
- Adams, S. Key Materials Challenges for Electrochemical Energy Storage Systems. Cosmos 2011, 7, 11–24. [Google Scholar] [CrossRef]
- Sobianowska-Turek, A.; Urbańska, W.; Banaszkiewicz, K.; Lewko, P.; Marcinkowski, T.; Pasiecznik, I. Recovery of Ni, Cd and Co from spent nickel-cadmium (Ni-Cd) and nickel-metal hydride (Ni-MH) batteries by solvent extraction. Chem. Ind. 2020, 99, 62–65. [Google Scholar]
- Wongrujipairoj, K.; Poolnapol, L.; Arpornwichanop, A.; Suren, S.; Kheawhom, S. Suppression of zinc anode corrosion for printed flexible zinc-air battery. Phys. Status Solidi 2016, 254, 1600442. [Google Scholar] [CrossRef]
- Ershad, N.F.; Mehrjardi, R.; Ehsani, M. High-Performance 4WD Electric Powertrain with Flywheel Kinetic Energy Recovery. IEEE Trans. Power Electron. 2020, 36, 772–784. [Google Scholar] [CrossRef]
- Islam, M.M.; Siffat, S.A.; Ahmad, I.; Liaquat, M.; Khan, S.A. Adaptive nonlinear control of unified model of fuel cell, battery, ultracapacitor and induction motor based hybrid electric vehicles. IEEE Access 2021, 9, 57486–57509. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Y.; Fu, M.; Wang, H. Multi-Objective Optimization-Based Real-Time Control Strategy for Battery/Ultracapacitor Hybrid Energy Management Systems. IEEE Access 2019, 7, 11640–11650. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Better thermal management options with heat storage systems for various applications: An Evaluation. Energy Storage 2019, 1, e47. [Google Scholar] [CrossRef] [Green Version]
- Tomoda, K.; Hoshi, N.; Haruna, J.; Cao, M.; Yoshizaki, A.; Hirata, K. Hydrolysis Rate Improvement in Hydrogen Generation System Fueled by Powdery Sodium Borohydride for Fuel-Cell Vehicle. IEEE Trans. Ind. Appl. 2013, 50, 2741–2748. [Google Scholar] [CrossRef]
- Muhsen, H.; Al-Muhtady, A. Optimized modeling of Ni-MH batteries primarily based on Taguchi approach and evaluation of used Ni-MH batteries. Turk. J. Electr. Eng. Comput. Sci. 2019, 27, 197–212. [Google Scholar] [CrossRef]
- Skvarenina, T. The Power Electronics Handbook; CRC Press: Boca Raton, FL, USA, 2002; p. 1664. ISBN 0849373360. [Google Scholar]
- Mohammed, S.A.Q.; Jung, J.-W. A Comprehensive State-of-the-Art Review of Wired/Wireless Charging Technologies for Battery Electric Vehicles: Classification/Common Topologies/Future Research Issues. IEEE Access 2021, 9, 19572–19585. [Google Scholar] [CrossRef]
- Naguib, M.; Kollmeyer, P.; Emadi, A. Lithium-Ion Battery Pack Robust State of Charge Estimation, Cell Inconsistency, and Balancing: Review. IEEE Access 2021, 9, 50570–50582. [Google Scholar] [CrossRef]
- Metwly, M.Y.; Abdel-Majeed, M.S.; Abdel-Khalik, A.S.; Hamdy, R.A.; Hamad, M.S.; Ahmed, S. A Review of In-tegrated On-Board EV Battery Chargers: Advanced Topologies, Recent Developments and Optimal Selection of FSCW Slot/Pole Combination. IEEE Access 2020, 8, 85216–85242. [Google Scholar] [CrossRef]
- Zhang, Y.; Nguyen, R.T.; Liaw, B. Status and Gap in Rechargeable Lithium Battery Supply Chain: Importance of Quantitative Failure Analysis. Proc. IEEE 2021, 109, 1029–1038. [Google Scholar] [CrossRef]
- Duru, K.K.; Karra, C.; Venkatachalam, P.; Betha, S.A.; Madhavan, A.A.; Kalluri, S. Critical Insights into Fast Charging Techniques for Lithium-Ion Batteries in Electric Vehicles. IEEE Trans. Device Mater. Reliab. 2021, 21, 137–152. [Google Scholar] [CrossRef]
- Vidal, C.; Malysz, P.; Kollmeyer, P.; Emadi, A. Machine Learning Applied to Electrified Vehicle Battery State of Charge and State of Health Estimation: State-of-the-Art. IEEE Access 2020, 8, 52796–52814. [Google Scholar] [CrossRef]
- Lehtola, T.A.; Zahedi, A. Electric Vehicle Battery Cell Cycle Aging in Vehicle to Grid Operations: A Review. IEEE J. Emerg. Sel. Top. Power Electron. 2021, 9, 423–437. [Google Scholar] [CrossRef]
- Pramanik, P.K.D.; Sinhababu, N.; Mukherjee, B.; Padmanaban, S.; Maity, A.; Upadhyaya, B.K.; Holm-Nielsen, J.B.; Choudhury, P. Power Consumption Analysis, Measurement, Management, and Issues: A State-of-the-Art Review of Smartphone Battery and Energy Usage. IEEE Access 2019, 7, 182113–182172. [Google Scholar] [CrossRef]
- Sundin, D.W.; Sponholtz, S. Thermal Management of Li-Ion Batteries with Single-Phase Liquid Immersion Cooling. IEEE Open J. Veh. Technol. 2020, 1, 82–92. [Google Scholar] [CrossRef]
- Karlsen, H.; Dong, T.; Yang, Z.; Carvalho, R. Temperature-Dependence in Battery Management Systems for Electric Vehicles: Challenges, Criteria, and Solutions. IEEE Access 2019, 7, 142203–142213. [Google Scholar] [CrossRef]
- Hannan, M.A.; Hoque, M.; Hussain, A.; Yusof, Y.; Ker, P.J. State-of-the-Art and Energy Management System of Lithium-Ion Batteries in Electric Vehicle Applications: Issues and Recommendations. IEEE Access 2018, 6, 19362–19378. [Google Scholar] [CrossRef]
- Chemali, E.; Preindl, M.; Malysz, P.; Emadi, A. Electrochemical and Electrostatic Energy Storage and Management Systems for Electric Drive Vehicles: State-of-the-Art Review and Future Trends. IEEE J. Emerg. Sel. Top. Power Electron. 2016, 4, 1117–1134. [Google Scholar] [CrossRef]
- Hossain, E.; Murtaugh, D.; Mody, J.; Faruque, H.M.R.; Sunny, S.H.; Mohammad, N. A Comprehensive Review on Second-Life Batteries: Current State, Manufacturing Considerations, Applications, Impacts, Barriers & Potential Solutions, Business Strategies, and Policies. IEEE Access 2019, 7, 73215–73252. [Google Scholar] [CrossRef]
- Omariba, B.Z.; Zhang, L.; Sun, D. Review of Battery Cell Balancing Methodologies for Optimizing Battery Pack Performance in Electric Vehicles. IEEE Access 2019, 7, 129335–129352. [Google Scholar] [CrossRef]
- Xiong, R.; Cao, J.; Yu, Q.; He, H.; Sun, F. Critical Review on the Battery State of Charge Estimation Methods for Electric Vehicles. IEEE Access 2017, 6, 1832–1843. [Google Scholar] [CrossRef]
- How, D.N.T.; Hannan, M.A.; Lipu, M.S.H.; Ker, P.J. State of Charge Estimation for Lithium-Ion Batteries Using Model-Based and Data-Driven Methods: A Review. IEEE Access 2019, 7, 136116–136136. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Cheng, Q.; Guo, B.; Yang, J. Classification and Review of the Charging Strategies for Commercial Lithium-Ion Batteries. IEEE Access 2019, 7, 43511–43524. [Google Scholar] [CrossRef]
- Vidal, C.; Gross, O.; Gu, R.; Kollmeyer, P.; Emadi, A. xEV Li-Ion Battery Low-Temperature Effects—Review. IEEE Trans. Veh. Technol. 2019, 68, 4560–4572. [Google Scholar] [CrossRef]
- Dagnæs-Hansen, N.A.; Santos, F.I. Permanent magnet thrust bearings for flywheel energy storage systems: Analytical, numerical, and experimental comparisons. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2019, 233, 5280–5293. [Google Scholar] [CrossRef] [Green Version]
- El Mejdoubi, A.; Chaoui, H.; Gualous, H.; Bossche, P.V.D.; Omar, N.; Van Mierlo, J. Lithium-Ion Batteries Health Prognosis Considering Aging Conditions. IEEE Trans. Power Electron. 2019, 34, 6834–6844. [Google Scholar] [CrossRef]
- Jafari, M.; Gauchia, A.; Zhang, K.; Gauchia, L. Simulation and Analysis of the Effect of Real-World Driving Styles in an EV Battery Performance and Aging. IEEE Trans. Transp. Electrif. 2015, 1, 391–401. [Google Scholar] [CrossRef]
- Jafari, M.; Gauchia, A.; Zhao, S.; Zhang, K.; Gauchia, L. Electric Vehicle Battery Cycle Aging Evaluation in Real-World Daily Driving and Vehicle-to-Grid Services. IEEE Trans. Transp. Electrif. 2018, 4, 122–134. [Google Scholar] [CrossRef]
- Gao, B.; Guo, L.; Zheng, Q.; Huang, B.; Chen, H. Acceleration Speed Optimization of Intelligent EVs in Consideration of Battery Aging. IEEE Trans. Veh. Technol. 2018, 67, 8009–8018. [Google Scholar] [CrossRef]
- Liu, Z.; Onori, S.; Ivanco, A. Synthesis and Experimental Validation of Battery Aging Test Profiles Based on Real-World Duty Cycles for 48-V Mild Hybrid Vehicles. IEEE Trans. Veh. Technol. 2017, 66, 8702–8709. [Google Scholar] [CrossRef]
- Uno, M.; Kukita, A. Cycle Life Evaluation Based on Accelerated Aging Testing for Lithium-Ion Capacitors as Alternative to Rechargeable Batteries. IEEE Trans. Ind. Electron. 2016, 63, 1607–1617. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, H.M.; Shin, Y.-J. Aging Monitoring Method for Lithium-Ion Batteries Using Harmonic Analysis. IEEE Trans. Instrum. Meas. 2021, 70, 1–11. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.; Hu, X.; Lucu, M.; Widanage, W.D. Gaussian Process Regression with Automatic Relevance Determination Kernel for Calendar Aging Prediction of Lithium-Ion Batteries. IEEE Trans. Ind. Inform. 2020, 16, 3767–3777. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Liu, K.; Wang, X.; Gao, F.; Macro, J.; Widanage, W.D. Model Migration Neural Network for Predicting Battery Aging Trajectories. IEEE Trans. Transp. Electrif. 2020, 6, 363–374. [Google Scholar] [CrossRef]
- She, C.; Wang, Z.; Sun, F.; Liu, P.; Zhang, L. Battery Aging Assessment for Real-World Electric Buses Based on Incremental Capacity Analysis and Radial Basis Function Neural Network. IEEE Trans. Ind. Inform. 2020, 16, 3345–3354. [Google Scholar] [CrossRef]
- Vilsen, B.S.; Kær, K.S.; Stroe, I.D. Log-Linear Model for Predicting the Lithium-ion Battery Age Based on Resistance Extraction from Dynamic Aging Profiles. IEEE Trans. Ind. Appl. 2020, 56, 6937–6948. [Google Scholar] [CrossRef]
- Vilsen, B.S.; Kær, K.S.; Stroe, I.D. Lithium-Ion Battery Health Prognosis Based on a Real Battery Management System Used in Electric Vehicles. IEEE Trans. Veh. Technol. 2019, 68, 4110–4121. [Google Scholar]
- Corno, M.; Pozzato, G. Active Adaptive Battery Aging Management for Electric Vehicles. IEEE Trans. Veh. Technol. 2019, 69, 258–269. [Google Scholar] [CrossRef]
- Chang, F.; Roemer, F.; Lienkamp, M. Influence of Current Ripples in Cascaded Multilevel Topologies on the Aging of Lithium Batteries. IEEE Trans. Power Electron. 2020, 35, 11879–11890. [Google Scholar] [CrossRef]
- Sun, J.; Ma, Q.; Tang, C.; Wang, T.; Jiang, T.; Tang, Y. Research on Optimization of Charging Strategy Control for Aged Batteries. IEEE Trans. Veh. Technol. 2020, 69, 14141–14149. [Google Scholar] [CrossRef]
- Casals, L.C.; García, B.A.; Aguesse, F.; Iturrondobeitia, A. Second life of electric vehicle batteries: Relation between materials degradation and environmental impact. Int. J. Life Cycle Assess. 2017, 22, 82–93. [Google Scholar] [CrossRef]
- Faria, R.; Marques, P.; Moura, P.; Freire, F.; Delgado, J.; de Almeida, A.T. Impact of the electricity mix and use profile in the life-cycle assessment of electric vehicles. Renew. Sustain. Energy Rev. 2013, 24, 271–287. [Google Scholar] [CrossRef]
- Hawkins, R.T.; Singh, B.; Majeau-Bettez, H.G.; Strømman, A. Comparative Environmental Life Cycle Assessment of Conventional and Electric Vehicles. J. Ind. Ecol. 2013, 17, 53–64. [Google Scholar] [CrossRef]
- Marques, P.; Garcia, R.; Kulay, L.; Freire, F. Comparative life cycle assessment of lithium-ion batteries for electric vehicles addressing capacity fade. J. Clean. Prod. 2019, 229, 787–794. [Google Scholar] [CrossRef]
- Marques, P.; Garcia, R.; Freire, F. Life cycle assessment of electric and conventional cars in Portugal. In Proceedings of the Coference on Energy for Sustainability 2013, Sustainable Cities: Designing for People and the Planet, Coimbra, Portugal, 8–10 September 2013. [Google Scholar]
- Freire, F.; Marques, P. Electric Vehicles in Portugal: An integrated energy, greenhouse gas and cost life-cycle analysis. In Proceedings of the IEEE International Symposium on Sustainable Systems and Technology (ISSST), Cincinnati, OH, USA, 15–17 May 2013; pp. 1–6. [Google Scholar]
- Garcia, J.; Millet, D.; Tonnelier, P.; Richet, S.; Chenouard, R. A novel approach for global environmental performance evaluation of electric batteries for hybrid vehicles. J. Clean. Prod. 2017, 156, 406–417. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Ke, R.-Y.; Han, R.; Tang, B.-J. The analysis of the battery electric vehicle’s potentiality of environmental effect: A case study of Beijing from 2016 to 2020. J. Clean. Prod. 2017, 145, 395–406. [Google Scholar] [CrossRef]
- Frischknecht, R.; Flury, K. Life cycle assessment of electric mobility: Answers and challenges. Int. J. Life Cycle Assess. 2011, 16, 691–695. [Google Scholar] [CrossRef]
- Oliveira, L.; Messagie, M.; Rangaraju, S.; Sanfelix, J.; Rivas, M.H.; Van Mierlo, J. Key issues of lithium-ion batteries e from resource depletion to environmental performance indicators. J. Clean. Prod. 2015, 108, 354–362. [Google Scholar] [CrossRef]
- Kushnir, D.; Sandén, B. The time dimension and lithium resource constraints for electric vehicles. Resour. Policy 2012, 37, 93–103. [Google Scholar] [CrossRef]
- Lim, K.; Bastawrous, H.; Duong, V.-H.; See, K.; Zhang, P.; Dou, S.X. Fading Kalman filter-based real-time state of charge estimation in LiFePO4 battery-powered electric vehicles. Appl. Energy 2016, 169, 40–48. [Google Scholar] [CrossRef]
- Wei, Z.; Meng, S.; Xiong, B.; Ji, D.; Tseng, K.J. Enhanced online model identification and state of charge estimation for lithium-ion battery with a FBCRLS based observer. Appl. Energy 2016, 181, 332–341. [Google Scholar] [CrossRef]
- Yan, D.; Lu, L.; Li, Z.; Feng, X.; Ouyang, M.; Jiang, F. Durability comparison of four different types of high-power batteries in HEV and their degradation mechanism analysis. Appl. Energy 2016, 179, 1123–1130. [Google Scholar] [CrossRef]
- Gradin, K.T.; Poulikidou, S.; Björklund, A.; Luttropp, C. Scrutinising the electric vehicle material backpack. J. Clean. Prod. 2018, 172, 1699–1710. [Google Scholar] [CrossRef]
- Hasib, S.A.; Islam, S.; Chakrabortty, R.K.; Ryan, M.J.; Saha, D.K.; Ahamed, M.H.; Moyeen, S.I.; Das, S.K.; Ali, M.F.; Islam, M.R.; et al. A Comprehensive Review of Available Battery Datasets, RUL Prediction Approaches, and Advanced Battery Management. IEEE Access 2021, 9, 86166–86193. [Google Scholar] [CrossRef]
- Vikström, H.; Davidsson, S.; Höök, M. Lithium availability and future production outlooks. Appl. Energy 2013, 110, 252–266. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Salkuti, S.R. Optimal Energy Management of Railroad Electrical Systems with Renewable Energy and Energy Storage Systems. Sustainability 2019, 11, 6293. [Google Scholar] [CrossRef] [Green Version]
- Linden, D.; Reddy, T. Handbook of Batteries; McGraw-Hill Professional Publishing: New York, NY, USA, 2001. [Google Scholar]
- Wang, Z.-L.; Xu, D.; Xu, J.-J.; Zhang, X.-B. Oxygen electrocatalysts in metal–air batteries: From aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef]
- Reddy, B.T. Linden’s Handbook of Batteries, 4th ed.; McGrawHill: New York, NY, USA, 2011; ISBN 978-0-07-162419-0. [Google Scholar]
- Parvini, Y.; Vahidi, A.; Fayazi, S.A. Heuristic Versus Optimal Charging of Supercapacitors, Lithium-Ion, and Lead-Acid Batteries: An Efficiency Point of View. IEEE Trans. Control Syst. Technol. 2017, 26, 167–180. [Google Scholar] [CrossRef]
- Vulturescu, B.; Butterbach, S.; Forgez, C. Experimental Considerations on the Battery Lifetime of a Hybrid Power Source Made of Ultracapacitors and Lead-Acid Batteries. IEEE J. Emerg. Sel. Top. Power Electron. 2014, 2, 701–709. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, Z.; Zhao, Y.; Luo, B.; Li, D.; Zhao, T.; Sure, J.; Vishnu, S.M.; Abdelkader, A.; Harris, C.; et al. Effect of loading methods on the performance of hierarchical porous carbon/sulfur composites in lithium sulfur batteries. Electrochim. Acta 2021, 388, 138650. [Google Scholar] [CrossRef]
- Fotouhi, A.; Auger, D.J.; Propp, K.; Longo, S.; Purkayastha, R.; O’Neill, L.; Walus, S. Lithium–Sulfur Cell Equivalent Circuit Network Model Parameterization and Sensitivity Analysis. IEEE Trans. Veh. Technol. 2017, 66, 7711–7721. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Yan, Z.; Shi, S.; Zhang, L.; Zhang, T.; Yang, M.; Bai, L.; Fu, H.; Yang, X.S.; Li, Z.; et al. Titanium oxide nanowire clots with two-phase composition as multi-effect sulfur reservoirs for lithium-sulfur batteries. Scr. Mater. 2021, 202, 113989. [Google Scholar] [CrossRef]
- Sun, W.; Li, Y.; Liu, S.; Liu, C.; Tan, X.; Xie, K. Mechanism investigation of iron selenide as polysulfide mediator for long-life lithium-sulfur batteries. Chem. Eng. J. 2021, 416, 129166. [Google Scholar] [CrossRef]
- Stroe, D.I.; Knap, V.; Swierczynski, M.; Schaltz, E. Electrochemical Impedance Spectroscopy-Based Electric Circuit Modeling of Lithium–Sulfur Batteries During a Discharging State. IEEE Trans. Ind. Appl. 2019, 55, 631–637. [Google Scholar] [CrossRef] [Green Version]
- Garapati, M.S.; Sundara, R. Enhancing polysulfide confinement and redox kinetics by electrocatalytic interlayer for highly stable lithium–sulfur batteries. Electrochim. Acta 2020, 362, 137035. [Google Scholar] [CrossRef]
- Fotouhi, A.; Auger, J.D.; Propp, K.; Longo, S. Lithium–Sulfur Battery State-of-Charge Observability Analysis and Estimation. IEEE Trans. Power Electron. 2018, 33, 5847–5859. [Google Scholar] [CrossRef]
- Gong, Z.; van de Ven, B.A.; Gupta, K.M.; da Silva, C.; Amon, C.H.; Bergveld, H.J.; Donkers, M.T.; Trescases, O. Distributed Control of Active Cell Balancing and Low-Voltage Bus Regulation in Electric Vehicles Using Hierarchical Model-Predictive Control. IEEE Trans. Ind. Electron. 2020, 67, 10464–10473. [Google Scholar] [CrossRef]
- Basic, H.; Pandzic, H.; Miletic, M.; Pavic, I. Experimental Testing and Evaluation of Lithium-Ion Battery Cells for a Special-Purpose Electric Vacuum Sweeper Vehicle. IEEE Access 2020, 8, 1–12. [Google Scholar]
- Soares dos Santos, G.; José Grandinetti, F.; Augusto Rocha Alves, R.; de Queiróz Lamas, W. Design and Simulation of an Energy Storage System with Batteries Lead Acid and Lithium-Ion for an Electric Vehicle: Battery vs. Conduction Cycle Efficiency Analysis. IEEE Lat. Am. Trans. 2020, 18, 1345–1352. [Google Scholar] [CrossRef]
- Xiong, R.; Tian, J.; Shen, W.; Sun, F. A Novel Fractional Order Model for State of Charge Estimation in Lithium-Ion Batteries. IEEE Trans. Veh. Technol. 2019, 68, 4130–4139. [Google Scholar] [CrossRef]
- Yu, J. State-of-Health Monitoring and Prediction of Lithium-Ion Battery Using Probabilistic Indication and State-Space Model. IEEE Trans. on Instrum. Meas. 2015, 64, 2937–2949. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Sun, F.; Wang, Z. An Overview on Thermal Safety Issues of Lithium-ion Batteries for Electric Vehicle Application. IEEE Access 2018, 6, 23848–23863. [Google Scholar] [CrossRef]
- Xia, Z.; Qahouq, J.A.A. Lithium-Ion Battery Ageing Behavior Pattern Characterization and State-of-Health Estimation Using Data-Driven Method. IEEE Access. 2021, 9, 98287–98304. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, H.; Wang, H.; Ouyang, M. Hybrid Lithium Iron Phosphate Battery and Lithium Titanate Battery Systems for Electric Buses. IEEE Trans. Veh. Technol. 2017, 67, 956–965. [Google Scholar] [CrossRef]
- Ai, S.; Mazumdar, S.; Li, H.; Cao, Y.; Li, T. Nano-silica doped Composite Polymer Chitosan/Poly(ethylene oxide)-Based Electrolyte with High Electrochemical Stability Suitable for Quasi Solid-state Lithium Metal Batteries. J. Electroanal. Chem. 2021, 895, 115464. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Chen, C.; Feng, X.; Zhang, Z. High-performance polymer electrolyte membrane modified with isocyanate-grafted Ti3+ doped TiO2 nanowires for lithium batteries. Appl. Surf. Sci. 2021, 563, 150248. [Google Scholar] [CrossRef]
- Lee, K.-P.; Gopalan, A.I.; Manesh, K.M.; Santhosh, P.; Kim, K.S. Influence of Finely Dispersed Carbon Nanotubes on the Performance Characteristics of Polymer Electrolytes for Lithium Batteries. IEEE Trans. Nanotechnol. 2007, 6, 362–367. [Google Scholar] [CrossRef]
- Yuan, X.; Razzaq, A.A.; Chen, Y.; Lian, Y.; Zhao, X.; Peng, Y.; Deng, Z. Polyacrylonitrile-based gel polymer electrolyte filled with Prussian blue forhigh-performance lithium polymer batteries. Chin. Chem. Lett. 2021, 32, 890–894. [Google Scholar] [CrossRef]
- Sun, M.; Zeng, Z.; Peng, L.; Han, Z.; Yu, C.; Cheng, S.; Xie, J. Ultrathin polymer electrolyte film prepared by in situ polymerization for lithium metal batteries. Mater. Today Energy 2021, 21, 100785. [Google Scholar] [CrossRef]
- Makeen, P.; Ghali, H.A.; Memon, S. Experimental and Theoretical Analysis of the Fast Charging Polymer Lithium-Ion Battery Based on Cuckoo Optimization Algorithm (COA). IEEE Access 2020, 8, 140486–140496. [Google Scholar] [CrossRef]
- Meng, J.; Luo, G.; Gao, F. Lithium Polymer Battery State-of-Charge Estimation Based on Adaptive Unscented Kalman Filter and Support Vector Machine. IEEE Trans. Power Electron. 2016, 31, 2226–2238. [Google Scholar] [CrossRef]
- Lee, K.-T.; Dai, M.-J.; Chuang, C.-C. Temperature-Compensated Model for Lithium-Ion Polymer Batteries with Extended Kalman Filter State-of-Charge Estimation for an Implantable Charger. IEEE Trans. Ind. Electron. 2017, 65, 589–596. [Google Scholar] [CrossRef]
- Antonucci, V.; Branchini, L.; Brunaccini, G.; De Pascale, A.; Ferraro, M.; Melino, F.; Orlandini, V.; Sergi, F. Thermal integration of a SOFC power generator and a Na–NiCl2 battery for CHP domestic application. Appl. Energy 2017, 185, 1256–1267. [Google Scholar] [CrossRef]
- Heinz, M.V.; Graeber, G.; Landmann, D.; Battaglia, C. Pressure management and cell design in solid-electrolyte batteries, at the example of a sodium-nickel chloride battery. J. Power Sources 2020, 465, 228268. [Google Scholar] [CrossRef]
- Braccoa, S.; Delfinoa, F.; Truccoa, A.; Zin, S. Electrical storage systems based on Sodium/Nickel chloride batteries: A mathematical model for the cell electrical parameter evaluation validated on a real smart microgrid application. J. Power Sources 2018, 399, 372–382. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, S.; Ao, X.; Wu, X.; Yang, J.; Wen, Z. Enhanced stability performance of nickel nanowire with 3D conducting network for planar sodium-nickel chloride batteries. J. Power Sources 2017, 360, 345–352. [Google Scholar] [CrossRef]
- Sessa, S.D.; Palone, F.; Necci, A.; Benato, R. Sodium-nickel chloride battery experimental transient modelling for energy stationary storage. J. Energy Storage 2017, 9, 40–46. [Google Scholar] [CrossRef]
- Sessa, S.D.; Crugnola, G.; Todeschini, M.; Zin, S.; Benato, R. Sodium nickel chloride battery steady-state regime model for stationary electrical energy storage. J. Energy Storage 2016, 6, 105–115. [Google Scholar] [CrossRef]
- Lu, X.; Li, G.; Kim, Y.J.; Lemmon, P.J.; Sprenkle, L.V.; Yang, Z. The effects of temperature on the electrochemical performance of sodiumenickel chloride batteries. J. Power Sources 2012, 215, 288–295. [Google Scholar] [CrossRef]
- Longo, S.; Antonucci, V.; Cellura, M.; Ferraro, M. Life cycle assessment of storage systems: The case study of a sodium/nickel chloride battery. J. Clean. Prod. 2014, 85, 337–346. [Google Scholar] [CrossRef]
- Capasso, C.; Lauria, D.; Veneri, O. Experimental evaluation of model-based control strategies of sodium-nickel chloride battery plus supercapacitor hybrid storage systems for urban electric vehicles. Appl. Energy 2018, 228, 2478–2489. [Google Scholar] [CrossRef]
- Shinde, N.M.; Shinde, P.V.; Yun, J.M.; Mane, R.S.; Kim, K.H. Room-temperature chemical synthesis of 3-D dandelion-type nickel chloride (NiCl2@NiF) supercapattery nanostructured materials. J. Colloid Interface Sci. 2020, 578, 547–554. [Google Scholar] [CrossRef]
- Chang, H.J.; Lu, X.; Bonnett, J.F.; Canfield, N.L.; Son, S.; Park, Y.-C.; Jung, K.; Sprenkle, V.L.; Li, G. Development of intermediate temperature sodium nickel chloride rechargeable batteries using conventional polymer sealing technologies. J. Power Sources 2017, 348, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Shi, L.; Gao, X.; Wang, J.; Hu, Y.; Wu, X.; Wen, Z. Constructing a charged-state Na-NiCl2 battery with NiCl2/graphene aerogel composite as cathode. Chem. Eng. J. 2021, 421, 127853. [Google Scholar] [CrossRef]
- Kumar, D.; Rajouria, S.K.; Kuhar, S.B.; Kanchan, D. Progress and prospects of sodium-sulfur batteries: A review. Solid State Ionics 2017, 312, 8–16. [Google Scholar] [CrossRef]
- Syali, M.S.; Kumar, D.; Mishra, K.; Kanchan, D. Recent advances in electrolytes for room-temperature sodium-sulfur batteries: A review. Energy Storage Mater. 2020, 31, 352–372. [Google Scholar] [CrossRef]
- Kumar, V.; Eng, A.Y.S.; Wang, Y.; Nguyen, D.-T.; Ng, M.-F.; Seh, Z.W. An artificial metal-alloy interphase for high-rate and long-life sodium–sulfur batteries. Energy Storage Mater. 2020, 29, 1–8. [Google Scholar] [CrossRef]
- Wang, H.; Deng, C.; Li, X.; Yan, D.; Xie, M.; Zhang, S.; Huang, B. Designing dual-defending system based on catalytic and kinetic iron Pyrite@C hybrid fibers for long-life room-temperature sodium-sulfur batteries. Chem. Eng. J. 2021, 420, 129681. [Google Scholar] [CrossRef]
- Wan, H.; Weng, W.; Han, F.; Cai, L.; Wang, C.; Yao, X. Bio-inspired Nanoscaled Electronic/Ionic Conduction Networks for Room-Temperature All-Solid-State Sodium-Sulfur Battery. Nano Today 2020, 33, 100860. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Han, H.-J.; Repich, H.; Zhi, R.-C.; Qu, C.-Z.; Kong, L.; Kaskel, S.; Wang, H.-Q.; Xu, F.; Li, H.-J. Recent progress on the design of hollow carbon spheres to host sulfur in room-temperature sodium–sulfur batteries. New Carbon Mater. 2020, 35, 630–645. [Google Scholar] [CrossRef]
- Galushkin, N.E.; Yazvinskaya, N.N.; Galushkin, D.N. Nickel-cadmium batteries with pocket electrodes as hydrogen energy storage units of high-capacity. J. Energy Storage 2021, 39, 102597. [Google Scholar] [CrossRef]
- Basant, N.; Singh, J.; Kumari, B.; Sinam, G.; Gautam, A.; Singh, G.; Mishra, K.; Mallick, S. Nickel and cadmium phytoextraction efficiencies of vetiver and lemongrass grown on Ni–Cd battery waste contaminated soil: A comparative study of linear and nonlinear models. J. Environ. Manag. 2021, 295, 113144. [Google Scholar] [CrossRef]
- Paul, S.; Shakya, A.K.; Ghosh, P.K. Bacterially-assisted recovery of cadmium and nickel as their metal sulfide nanoparticles from spent Ni–Cd battery via hydrometallurgical route. J. Environ. Manag. 2020, 261, 110113. [Google Scholar] [CrossRef]
- Rahangdale, D.; Kumar, A. Acrylamide grafted chitosan based ion imprinted polymer for the recovery of cadmium from nickel-cadmium battery waste. J. Environ. Chem. Eng. 2018, 6, 1828–1839. [Google Scholar] [CrossRef]
- Pourabdollah, K. Development of electrolyte inhibitors in nickel cadmium batteries. Chem. Eng. Sci. 2017, 160, 304–312. [Google Scholar] [CrossRef]
- Espinosa, D.C.R.; Tenório, J.A.S. Recycling of nickel–cadmium batteries using coal as reducing agent. J. Power Sources 2006, 157, 600–604. [Google Scholar] [CrossRef]
- Norian, K. Equivalent circuit components of nickel–cadmium battery at different states of charge. J. Power Sources 2011, 196, 5205–5208. [Google Scholar] [CrossRef]
- Tang, H.; Sun, Z.; Chang, K.; Hou, Y.; Li, B.; Hou, Y.; Chang, Z. Uniform carbon coating drastically enhances the electro-chemical performance of a Fe3O4 electrode for alkaline nickel-iron rechargeable batteries. Int. J. Hydrogen Energy 2019, 44, 24895–24904. [Google Scholar] [CrossRef]
- Pramanik, A.; Maiti, S.; Chattopadhyay, S.; De, G.; Mahanty, S. ‘Cotton-ball’ shaped porous iron-nickel sulfide: A high-rate cathode for long-life aqueous rechargeable battery. Mater. Res. Bull. 2021, 140, 111307. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, C.; Chang, K.; Shangguan, E.; Li, B.; Chang, Z. Synthesis of NiS coated Fe3O4 nanoparticles as high-performance positive materials for alkaline nickel-iron rechargeable batteries. Int. J. Hydrogen Energy 2017, 42, 24939–24947. [Google Scholar] [CrossRef]
- Guo, C.; Li, C.M. Molecule-confined FeOx nanocrystals mounted on carbon as stable anode material for high energy density nickel-iron batteries. Nano Energy 2017, 42, 166–172. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Chen, X.; Xiao, T.; Tan, X.; Xiang, P.; Jiang, L. Enhancing electrochemical performance of Fe2O3 via in situ sulfurization and carbon coating modification for nickel-iron rechargeable batteries. Electrochim. Acta 2018, 290, 332–338. [Google Scholar] [CrossRef]
- Zhi, H.; Ni, S.; Su, X.; Xie, W.; Zhang, H.; Sun, X. Separation and recovery of rare earth from waste nickel-metal hydride batteries by phosphate based extraction-precipitation. J. Rare Earths 2021, 1–26. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Zhang, L.; Zhang, S.; Guo, W.; Zhao, Y.; Zhang, H.; Li, Y.; Han, S. The electrochemical characteristics of AB4-type rare earth–Mg–Ni-based superlattice structure hydrogen storage alloys for nickel metal hydride battery. J. Magnes. Alloys 2021, 1–10. [Google Scholar] [CrossRef]
- Odegbemi, F.; Idowu, G.A.; Adebayo, A.O. Nickel recovery from spent nickel-metal hydride batteries using LIX-84I-impregnated activated charcoal. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100452. [Google Scholar] [CrossRef]
- Li, M.; Wang, C.; Yang, C. Development of high-performance hydrogen storage alloys for applications in nickel-metal hydride batteries at ultra-low temperature. J. Power Sources 2021, 491, 229585. [Google Scholar] [CrossRef]
- Vargas, S.J.; Schaeffer, N.; Souza, J.C.; da Silva, L.H.; Hespanhol, M.C. Green separation of lanthanum, cerium and nickel from waste nickel metal hydride battery. Waste Manag. 2021, 125, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, G.; Zhang, L.; Ma, C.; Zhao, Y.; Zhang, H.; Ding, Z.; Fu, Y.; Li, Y.; Han, S. Electrochemical features of Ce2Ni7-type La0.65Nd0.15Mg0.25Ni3.20M0.10 (M = Ni, Mn and Al) hydrogen storage alloys for rechargeable nickel metal hydride battery. J. Alloys Compd. 2021, 861, 158469. [Google Scholar] [CrossRef]
- Wang, W.; Qin, R.; Wu, R.; Tao, X.; Zhang, H.; Ding, Z.; Fu, Y.; Zhang, L.; Wu, L.; Li, Y.; et al. A promising anode candidate for rechargeable nickel metal hydride power battery: An A5B19-type La–Sm–Nd–Mg–Ni–Al-based hydrogen storage alloy. J. Power Sources 2020, 465, 228236. [Google Scholar] [CrossRef]
- Ding, J.; Zheng, H.; Gao, H.; Wang, S.; Wu, S.; Fang, S.; Cheng, F. Operando non-topological conversion constructing the high-performance nickel-zinc battery anode. Chem. Eng. J. 2021, 414, 128716. [Google Scholar] [CrossRef]
- Cheng, Y.; Guo, H. Interface modification of electrodes through polyethylene glycol in rechargeable zinc-nickel batteries. Chem. Eng. Sci. 2020, 232, 116372. [Google Scholar] [CrossRef]
- Yuan, L.; Yang, Z.; Cui, F.; Rong, Y.; Su, Q.; Chen, H.; Wu, J.; Deng, L. Flower-like Zn-Al-In layered double oxides synthesized by a facile hydrothermal method as ultra-high cycle stability anodic for zinc-nickel battery. J. Alloys Compd. 2021, 863, 158574. [Google Scholar] [CrossRef]
- Wei, J.S.; Zhu, Z.Y.; Zhao, X.; Song, T.B.; Huang, J.H.; Zhang, Y.X.; Liu, X.; Chen, L.; Niu, X.Q.; Wang, Y.G.; et al. Self-assembled ZnO-carbon dots anode materials for high performance nickel-zinc alkaline batteries. Chem. Eng. J. 2021, 425, 130660. [Google Scholar] [CrossRef]
- Cui, F.; Yang, Z.; Chen, L.; Zeng, X.; Meng, J.; Jiang, Y. Preparation of spherical carbonated foam/Zn–Al layered double oxides composite anode and its superior cycling stability in Zinc–Nickel secondary batteries. J. Power Sources 2021, 500, 229957. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, Y.; Lu, Y.; Sun, Y.; Wu, Q.; Pang, Y.; Shen, Z.; Chen, H. Aluminum-doping-based method for the improvement of the cycle life of cobalt–nickel hydroxides for nickel–zinc batteries. J. Colloid Interface Sci. 2021, 587, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, K.; Xiao, E.; Zhang, J.; Zheng, M. Real-time peak power prediction for zinc nickel single flow batteries. J. Power Sources 2020, 448, 227346. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Zhou, Z.; Pan, Y.; Yu, Z.; Pei, Z.; Zhao, S.; Wei, L.; Chen, Y. Rechargeable zinc-air batteries with neutral electrolytes: Recent advances, challenges, and prospects. EnergyChem 2021, 3, 100055. [Google Scholar] [CrossRef]
- Hosseini, S.; Soltani, S.M.; Li, Y.-Y. Current status and technical challenges of electrolytes in zinc–air batteries: An in-depth review. Chem. Eng. J. 2021, 408, 127241. [Google Scholar] [CrossRef]
- Wei, H.-L.; Tan, A.-D.; Hu, S.-Z.; Piao, J.-H.; Fu, Z.-Y. Efficient spinel iron-cobalt oxide/nitrogen-doped ordered mesoporous carbon catalyst for rechargeable zinc-air batteries. Chin. J. Catal. 2021, 42, 1451–1458. [Google Scholar] [CrossRef]
- Mainar, A.R.; Iruin, E.; Blázquez, J.A. High performance secondary zinc-air/silver hybrid battery. J. Energy Storage 2021, 33, 102103. [Google Scholar] [CrossRef]
- Xu, L.; Wu, S.; Deng, D.; Wang, C.; Qian, J.; Lu, G.; Li, H. Fabricating highly active and stable tungsten carbide electrocatalyst for rechargeable zinc–air batteries: An approach of dual metal Co-adjusted the electronic structure. J. Alloys Compd. 2021, 868, 159236. [Google Scholar] [CrossRef]
- Yu, W.; Shang, W.; Xiao, X.; Ma, Y.; Chen, Z.; Chen, B.; Xu, H.; Ni, M.; Tan, P. Elucidating the mechanism of discharge performance improvement in zinc-air flow batteries: A combination of experimental and modeling investigations. J. Energy Storage 2021, 40, 102779. [Google Scholar] [CrossRef]
- Logeshwaran, N.; Ramakrishnan, S.; Chandrasekaran, S.S.; Vinothkannan, M.; Kim, A.R.; Sengodan, S.; Velusamy, D.B.; Varadhan, P.; He, J.-H.; Yoo, D.J. An efficient and durable trifunctional electrocatalyst for zinc–air batteries driven overall water splitting. Appl. Catal. B: Environ. 2021, 297, 120405. [Google Scholar] [CrossRef]
- Pan, L.; Chen, D.; Pei, P.; Huang, S.; Ren, P.; Song, X. A novel structural design of air cathodes expanding three-phase reaction interfaces for zinc-air batteries. Appl. Energy 2021, 290, 116777. [Google Scholar] [CrossRef]
- Gao, L.; Li, Z.; Zou, Y.; Yin, S.; Peng, P.; Shao, Y.; Liang, X. A High-Performance Aqueous Zinc-Bromine Static Battery. iScience 2020, 23, 101348. [Google Scholar] [CrossRef]
- Xu, P.; Li, T.; Zheng, Q.; Zhang, H.; Yin, Y.; Li, X. A low-cost bromine-fixed additive enables a high capacity retention zinc-bromine batteries. J. Energy Chem. 2021, 65, 89–93. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, Q.; Li, Y.; Wang, J.; Lund, P.D. Review of zinc dendrite formation in zinc bromine redox flow battery. Renew. Sustain. Energy Rev. 2020, 127, 109838. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, R.; Liu, K.; Sun, J.; Chan, K.; Zhao, T. Mesoporous carbon derived from pomelo peel as a high-performance electrode material for zinc-bromine flow batteries. J. Power Sources 2019, 442, 227255. [Google Scholar] [CrossRef]
- Archana, K.S.; Naresh, R.P.; Enale, H.; Rajendran, V.; Mohan, A.M.V.; Bhaskar, A.; Ragupathy, P.; Dixon, D. Effect of positive electrode modification on the performance of zinc-bromine redox flow batteries. J. Energy Storage 2020, 29, 101462. [Google Scholar] [CrossRef]
- Jin, C.X.; Lei, H.Y.; Liu, M.Y.; Tan, A.D.; Piao, J.H.; Fu, Z.Y.; Liang, Z.X.; Wang, H.H. Low-dimensional nitrogen-doped carbon for Br2/Br− redox reaction in zinc-bromine flow battery. Chem. Eng. J. 2020, 380, 122606. [Google Scholar] [CrossRef]
- Wu, M.C.; Zhao, T.S.; Wei, L.; Jiang, H.R.; Zhang, R.H. Improved electrolyte for zinc-bromine flow batteries. J. Power Sources 2018, 384, 232–239. [Google Scholar] [CrossRef]
- Yu, F.; Pang, L.; Wang, X.; Waclawik, E.R.; Wang, F.; Ostrikov, K.; Wang, H. Aqueous alkaline–acid hybrid electrolyte for zinc-bromine battery with 3V voltage window. Energy Storage Mater. 2019, 19, 56–61. [Google Scholar] [CrossRef]
- Lai, Q.; Zhang, H.; Li, X.; Zhang, L.; Cheng, Y. A novel single flow zinc–bromine battery with improved energy density. J. Power Sources 2013, 235, 1–4. [Google Scholar] [CrossRef]
- Shimin, Z. Investigation of the charge/discharge characteristics of aqueous zinc–ferric chloride batteries. J. Power Sources 2006, 160, 1442–1446. [Google Scholar] [CrossRef]
- Deyab, A.M. Hydroxyethyl cellulose as efficient organic inhibitor of zincecarbon battery corrosion in ammonium chloride solution: Electrochemical and surface morphology studies. J. Power Sources 2015, 280, 190–194. [Google Scholar] [CrossRef]
- Jugovic, Z.B.; Trisovi, L.T.; Stevanovic, J.S.; Maksimovic, M.D.; Grgur, N.B. Comparative studies of chloride and chloride/citrate-based electrolytes for zinc–polyaniline batteries. Electrochim. Acta 2006, 51, 6268–6274. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, X. Generation of activated carbon nanofibers from electrospun polyacrylonitrile-zinc chloride composites for use as anodes in lithium-ion batteries. Electrochem. Commun. 2009, 11, 684–687. [Google Scholar] [CrossRef]
- Borchers, N.; Clark, S.; Horstmann, B.; Jayasayee, K.; Juel, M.; Stevens, P. Innovative zinc-based batteries. J. Power Sources 2021, 484, 229309. [Google Scholar] [CrossRef]
- Kar, M.; Gonzalo, C.P. Emergence of nonaqueous electrolytes for rechargeable zinc batteries. Curr. Opin. Green Sustain. Chem. 2021, 28, 100426. [Google Scholar] [CrossRef]
- Zhao, S.; An, H.; Chen, S. A study of a high-power, ammonium chloride zinc/manganese dioxide dry battery. J. Power Sources 1998, 76, 218–220. [Google Scholar] [CrossRef]
- Hu, L.; Xiao, P.; Xue, L.; Li, H.; Zhai, T. The rising zinc anodes for high-energy aqueous batteries. EnergyChem 2021, 3, 100052. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, C.; Chang, N.; Song, Y.; Zhang, H.; Yin, Y.; Li, X. Act in contravention: A non-planar coupled electrode design utilizing “tip effect” for ultra-high areal capacity, long cycle life zinc-based batteries. Sci. Bull. 2021, 66, 889–896. [Google Scholar] [CrossRef]
- Samadani, E.S.; Fraser, A.R.; Fowler, M. A Review Study of Methods for Lithium-Ion Battery Health Monitoring and Remaining Life Estimation in Hybrid Electric Vehicles; SAE Int.: Warrendale, PA, USA, 2012. [Google Scholar]
- Pattipati, B.; Sankavaram, C.; Pattipati, K. System Identification and Estimation Framework for Pivotal Automotive Battery Management System Characteristics. IEEE Trans. Syst. Man Cybern. Part C Appl. Rev. 2011, 41, 869–884. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Lin, X.; Kim, Y.; Mohan, S.; Siegel, J.B.; Stefanopoulou, A.G. Modeling and Estimation for Advanced Battery Management. Annu. Rev. Control Robot. Auton. Syst. 2019, 2, 393–426. [Google Scholar] [CrossRef]
- Ahmed, R.; El Sayed, M.; Arasaratnam, I.; Tjong, J.; Habibi, S. Reduced-Order Electrochemical Model Parameters Identification and State of Charge Estimation for Healthy and Aged Li-Ion Batteries—Part II: Aged Battery Model and State of Charge Estimation. IEEE J. Emerg. Sel. Top. Power Electron. 2014, 2, 678–690. [Google Scholar] [CrossRef]
- Abdelbaky, M.; Peeters, J.R.; Dewulf, W. On the influence of second use, future battery technologies, and battery lifetime on the maximum recycled content of future electric vehicle batteries in Europe. Waste Manag. 2021, 125, 1–9. [Google Scholar] [CrossRef]
- Arai, J.; Yamaki, T.; Yamauchi, S.; Yuasa, T.; Maeshima, T.; Sakai, T.; Koseki, M.; Horiba, T. Development of a high power lithium secondary battery for hybrid electric vehicles. J. Power Sources 2005, 146, 788–792. [Google Scholar] [CrossRef]
- Vora, P.A. Modeling the impact of battery degradation within lifecycle cost-based design optimization of heavy-duty hybrid electric vehicles. Ann. Arbor: ProQuest Diss. Theses 2016, 875. Available online: https://docs.lib.purdue.edu/open_access_dissertations/875 (accessed on 1 August 2021).
- Warner, J.T. Lithium-Ion Battery Chemistries; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–353. [Google Scholar]
- Gianfranco, P.; Boryann, L. Behaviour of Lithium-Ion Batteries in Electric Vehicles; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–344. ISBN 978-3-319-69950-9. [Google Scholar]
- Schismenos, S.; Chalaris, M.; Stevens, G. Battery hazards and safety: A scoping review for lead acid and silver-zinc batteries. Saf. Sci. 2021, 140, 105290. [Google Scholar] [CrossRef]
- Moseley, P.; Bonnet, B.; Cooper, A.; Kellaway, M. Lead–acid battery chemistry adapted for hybrid electric vehicle duty. J. Power Sources 2007, 174, 49–53. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Chakraborty, B. Substrate materials and novel designs for bipolar lead-acid batteries: A review. J. Energy Storage 2020, 32, 101764. [Google Scholar] [CrossRef]
- Tian, S.; Hong, M.; Ouyang, M. An Experimental Study and Nonlinear Modeling of Discharge I–V Behavior of Valve-Regulated Lead–Acid Batteries. IEEE Trans. Energy Convers. 2009, 24, 452–458. [Google Scholar] [CrossRef]
- Bressanini, G.L.; Busarello, T.D.C.; Peres, A. Design and implementation of lead-acid battery state-of-health and state-of-charge measurements. In Proceedings of the 2017 Brazilian Power Electronics Conference (COBEP), Juiz de Fora, Brazil, 19–22 November 2017; pp. 1–6. [Google Scholar]
- Moseley, P.; Rand, D.; Garche, J. Lead–acid batteries for future automobiles. In Lead-Acid Batteries for Future Automobiles; Elsevier: Amsterdam, The Netherlands, 2017; pp. 601–618. [Google Scholar]
- Saiju, R.; Heier, S. Performance analysis of lead acid battery model for hybrid power system. In Proceedings of the 2008 IEEE/PES Transmission and Distribution Conference and Exposition, Chicago, IL, USA, 21–24 April 2008; pp. 1–6. [Google Scholar]
- Zhu, T.; Wills, R.G.; Lot, R.; Kong, X.; Yan, X. Optimal sizing and sensitivity analysis of a battery-supercapacitor energy storage system for electric vehicles. Energy 2021, 221, 119851. [Google Scholar] [CrossRef]
- Gong, J.; Wang, Y.; Zhao, A.; Zhao, Z. Optimal sizing of portable modular batteries for electric vehicles. J. Clean. Prod. 2020, 277, 123868. [Google Scholar] [CrossRef]
- Hussain, A.; Bui, V.-H.; Kim, H.-M. Optimal Sizing of Battery Energy Storage System in a Fast EV Charging Station Considering Power Outages. IEEE Trans. Transp. Electrif. 2020, 6, 453–463. [Google Scholar] [CrossRef]
- Bullock, K.R. Carbon reactions and effects on valve-regulated lead-acid (VRLA) battery cycle life in high-rate, partial state-of-charge cycling. J. Power Sources 2010, 195, 4513–4519. [Google Scholar] [CrossRef]
- Sawai, K.; Ohmae, T.; Suwaki, H.; Shiomi, M.; Osumi, S. Idling-stop vehicle road tests of advanced valve-regulated lead-acid (VRLA) battery. J. Power Sources 2007, 174, 54–60. [Google Scholar] [CrossRef]
- Ohmae, T.; Sawai, K.; Shiomi, M.; Osumi, S. Advanced technologies in VRLA batteries for automotive applications. J. Power Sources 2006, 154, 523–529. [Google Scholar] [CrossRef]
- Li, G.; Lu, X.; Kim, Y.J.; Viswanathan, V.; Meinhardt, K.; Sprenkle, V. A Planar Zebra Battery Based on Low-Cost Intermediate-Temperature Na-FeCl2 Redox Chemistry. Electrochem. Soc. Abstr. 2015, 17, MA2015-026. [Google Scholar]
- Ruiz, V.; Pfrang, A.; Kriston, A.; Omar, N.; van den Bossche, P.; Boon-Brett, L. A review of international abuse testing standards and regulations for lithium ion batteries in electric and hybrid electric vehicles. Renew. Sustain. Energy Rev. 2018, 81, 1427–1452. [Google Scholar] [CrossRef]
- Balaji, J.; Sethuraman, G.M.; Roh, S.H.; Jung, H.Y. Recent developments in sol-gel based polymer electrolyte membranes for vanadium redox flow batteries—A review. Polym. Test. 2020, 89, 106567. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Wan, C. Review of gel-type polymer electrolytes for lithium-ion batteries. J. Power Sources 1999, 77, 183–197. [Google Scholar] [CrossRef]
- Stephan, A.M. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- Ren, W.; Ding, C.; Fu, X.; Huang, Y. Advanced gel polymer electrolytes for safe and durable lithium metal batteries: Challenges, strategies, and perspectives. Energy Storage Mater. 2021, 34, 515–535. [Google Scholar] [CrossRef]
- Baazizi, M.; Dahbi, M.; Aqil, M.; Ghamouss, F.; Saadoune, I. A Ni-rich Cathode Material for Lithium-ion Batteries with Improved Safety and Cost. In Proceedings of the 2019 7th International Renewable and Sustainable Energy Conference (IRSEC), Agadir, Morocco, 27–30 November 2019; pp. 1–4. [Google Scholar]
- Lande, L.; Kallitsis, E.; Hales, A.; Edge, S.J.; Korre, A.; Offer, G. Cost and carbon footprint reduction of electric vehicle lithium-ion batteries through efficient thermal management. Appl. Energy 2021, 289, 116737. [Google Scholar] [CrossRef]
- Saxena, S.; Ning, Y.; Thompson, R.; Pecht, M. Role of the rest period in capacity fade of Graphite/LiCoO2 batteries. J. Power Sources 2021, 484, 229246. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Du, L.; Xu, X.; Ma, Y.; Qu, B.; Fan, P.; Yin, G.; Yang, F.; Zhu, L. Identifying the aging mechanism in multiple overdischarged LiCoO2/ mesocarbon microbeads batteries. Ceram. Int. 2021, 47, 21253–21262. [Google Scholar] [CrossRef]
- Li, D.; Zhang, B.; Ou, X.; Zhang, J.; Meng, K.; Ji, G.; Li, P.; Xu, J. Ammonia leaching mechanism and kinetics of LiCoO2 material from spent lithium-ion batteries. Chin. Chem. Lett. 2021, 32, 2333–2337. [Google Scholar] [CrossRef]
- Prasad, K.H.; Julakanti, V.R.; Rangaraju, G.; Sumithra, M.; Sundaraganesan, N. Structural and ImpedanceStudies of Nanocrystalline LiCoO2 Particle. In Proceedings of the 2020 International Conference on System, Computation, Automation and Net-working (ICSCAN), Puducherry, India, 27–28 March 2020; pp. 1–4. [Google Scholar]
- Chen, S.; Zhang, X.; Xia, M.; Wei, K.; Zhang, L.; Zhang, X.; Cui, Y.; Shu, J. Issues and challenges of layered lithium nickel cobalt manganese oxides for lithium-ion batteries. J. Electroanal. Chem. 2021, 895, 115412. [Google Scholar] [CrossRef]
- Bharathraj, S.; Adiga, S.; Mayya, K.; Song, T.; Kim, J.; Sung, Y. Degradation-guided optimization of charging protocol for cycle life enhancement of Li-ion batteries with Lithium Manganese Oxide-based cathodes. J. Power Sources 2020, 474, 228659. [Google Scholar] [CrossRef]
- Horesh, N.; Quinn, C.; Wang, H.; Zane, R.; Ferry, M.; Tong, S.; Quinn, C.J. Driving to the future of energy storage: Techno-economic analysis of a novel method to recondition second life electric vehicle batteries. Appl. Energy 2021, 295, 117007. [Google Scholar] [CrossRef]
- Wu, W.; Lin, B.; Xie, C.; Elliott, R.J.; Radcliffe, J. Does energy storage provide a profitable second life for electric vehicle batteries? Energy Econ. 2020, 92, 105010. [Google Scholar] [CrossRef]
- Haram, M.H.S.M.; Lee, J.W.; Ramasamy, G.; Ngu, E.E.; Thiagarajah, S.P.; Lee, Y.H. Feasibility of utilising second life EV batteries: Applications, lifespan, economics, environmental impact, assessment, and challenges. Alex. Eng. J. 2021, 60, 4517–4536. [Google Scholar] [CrossRef]
- Sun, S.I.; Chipperfield, A.J.; Kiaee, M.; Wills, R. Effects of market dynamics on the time-evolving price of second-life electric vehicle batteries. J. Energy Storage 2018, 19, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Behi, H.; Karimi, D.; Behi, M.; Ghanbarpour, M.; Jaguemont, J.; Sokkeh, M.A.; Gandoman, F.H.; Berecibar, M.; Van Mierlo, J. A new concept of thermal management system in Li-ion battery using air cooling and heat pipe for electric vehicles. Appl. Therm. Eng. 2020, 174, 115280. [Google Scholar] [CrossRef]
- Akinlabi, A.H.; Solyali, D. Configuration, design, and optimization of air-cooled battery thermal management system for electric vehicles: A review. Renew. Sustain. Energy Rev. 2020, 125, 109815. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Negnevitsky, M.; Zhang, H. A review of air-cooling battery thermal management systems for electric and hybrid electric vehicles. J. Power Sources 2021, 501, 230001. [Google Scholar] [CrossRef]
- Cheng, L.; Garg, A.; Jishnu, A.; Gao, L. Surrogate based multi-objective design optimization of lithium-ion battery air-cooled system in electric vehicles. J. Energy Storage 2020, 31, 101645. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, F. A novel electric vehicle thermal management system based on cooling and heating of batteries by refrigerant. Energy Convers. Manag. 2021, 237, 114145. [Google Scholar] [CrossRef]
- Tang, X.; Guo, Q.; Li, M.; Wei, C.; Pan, Z.; Wang, Y. Performance analysis on liquid-cooled battery thermal management for electric vehicles based on machine learning. J. Power Sources 2021, 494, 229727. [Google Scholar] [CrossRef]
- Akbarzadeh, M.; Jaguemont, J.; Kalogiannis, T.; Karimi, D.; He, J.; Jin, L.; Xie, P.; Van Mierlo, J.; Berecibar, M. A novel liquid cooling plate concept for thermal management of lithium-ion batteries in electric vehicles. Energy Convers. Manag. 2021, 231, 113862. [Google Scholar] [CrossRef]
- Monika, K.; Chakraborty, C.; Roy, S.; Dinda, S.; Singh, S.A.; Datta, S.P. An improved mini-channel based liquid cooling strategy of prismatic LiFePO4 batteries for electric or hybrid vehicles. J. Energy Storage 2021, 35, 102301. [Google Scholar] [CrossRef]
- Chung, Y.; Kim, M.S. Thermal analysis and pack level design of battery thermal management system with liquid cooling for electric vehicles. Energy Convers. Manag. 2019, 196, 105–116. [Google Scholar] [CrossRef]
- Tan, X.; Lyu, P.; Fan, Y.; Rao, J.; Ouyang, K. Numerical investigation of the direct liquid cooling of a fast-charging lithium-ion battery pack in hydrofluoroether. Appl. Therm. Eng. 2021, 196, 117279. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, X.; Xiang, G.; Li, H. Optimization of liquid cooling and heat dissipation system of lithium-ion battery packs of automobile. Case Stud. Therm. Eng. 2021, 26, 101012. [Google Scholar] [CrossRef]
- Li, Y.; Guo, H.; Qi, F.; Guo, Z.; Li, M.; Tjernberg, L.B. Investigation on liquid cold plate thermal management system with heat pipes for LiFePO4 battery pack in electric vehicles. Appl. Therm. Eng. 2021, 185, 116382. [Google Scholar] [CrossRef]
- Alaoui, C. Solid-State Thermal Management for Lithium-Ion EV Batteries. IEEE Trans. Veh. Technol. 2013, 62, 98–107. [Google Scholar] [CrossRef]
- Hong, H.S.; Jang, S.D.; Park, S.; Yun, S.; Kim, Y. Thermal performance of direct two-phase refrigerant cooling for lithium-ion batteries in electric vehicles. Appl. Therm. Eng. 2020, 173, 115213. [Google Scholar] [CrossRef]
- Shen, M.; Gao, Q. System simulation on refrigerant-based battery thermal management technology for electric vehicles. Energy Convers. Manag. 2020, 203, 112176. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, Y.; Wang, G.; Deng, F.; Zhu, J. An experimental investigation of refrigerant emergency spray on cooling and oxygen suppression for overheating power battery. J. Power Sources 2019, 415, 33–43. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, G.; Wang, M. A comprehensive assessment of refrigerants for cabin heating and cooling on electric vehicles. Appl. Therm. Eng. 2020, 174, 115258. [Google Scholar] [CrossRef]
- Yun, S.; Hong, S.H.; Song, K.S.; Kwon, J.; Kim, Y. Experimental and numerical analyses of quenching performance of hot stamping blanks by two-phase refrigerant cooling using R1234yf. Int. J. Heat Mass Transf. 2021, 173, 121231. [Google Scholar] [CrossRef]
- Putra, N.; Sandi, A.F.; Ariantara, B.; Abdullah, N.; Mahlia, T.M.I. Performance of beeswax phase change material (PCM) and heat pipe as passive battery cooling system for electric vehicles. Case Stud. Therm. Eng. 2020, 21, 100655. [Google Scholar] [CrossRef]
- Koyama, R.; Arai, Y.; Yamauchi, Y.; Takeya, S.; Endo, F.; Hotta, A.; Ohmura, R. Thermophysical properties of trimethylolethane (TME) hydrate as phase change material for cooling lithium-ion battery in electric vehicle. J. Power Sources 2019, 427, 70–76. [Google Scholar] [CrossRef]
- Shen, Z.-G.; Chen, S.; Liu, X.; Chen, B. A review on thermal management performance enhancement of phase change materials for vehicle lithium-ion batteries. Renew. Sustain. Energy Rev. 2021, 148, 111301. [Google Scholar] [CrossRef]
- Niu, J.; Xie, N.; Zhong, Y.; Gao, X.; Fang, Y.; Zhang, Z. Numerical analysis of battery thermal management system coupling with low-thermal-conductive phase change material and liquid cooling. J. Energy Storage 2021, 39, 102605. [Google Scholar] [CrossRef]
- Ping, P.; Zhang, Y.; Kong, D.; Du, J. Investigation on battery thermal management system combining phase changed material and liquid cooling considering non-uniform heat generation of battery. J. Energy Storage 2021, 36, 102448. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H. Thermal characteristics of power battery module with composite phase change material and external liquid cooling. Int. J. Heat Mass Transf. 2020, 156, 119820. [Google Scholar] [CrossRef]
- Luo, M.; Song, J.; Ling, Z.; Zhang, Z.; Fang, X. Phase change material coat for battery thermal management with integrated rapid heating and cooling functions from −40 °C to 50 °C. Mater. Today Energy 2021, 20, 100652. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, J.; Cao, M.; Jiang, G.; Yan, Q.; Hu, J. Experimental study on the thermal management of batteries based on the coupling of composite phase change materials and liquid cooling. Appl. Therm. Eng. 2021, 185, 116415. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, L.; Yang, L.; Du, X. Numerical study of combined air and phase change cooling for lithium-ion battery during dynamic cycles. Int. J. Therm. Sci. 2021, 165, 106968. [Google Scholar] [CrossRef]
- Lyu, Y.; Siddique, A.; Majid, S.; Biglarbegian, M.; Gadsden, S.; Mahmud, S. Electric vehicle battery thermal management system with thermoelectric cooling. Energy Rep. 2019, 5, 822–827. [Google Scholar] [CrossRef]
- Lyu, Y.; Siddique, A.R.M.; Gadsden, S.A.; Mahmud, S. Experimental investigation of thermoelectric cooling for a new battery pack design in a copper holder. Results Eng. 2021, 10, 100214. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, H.; Li, J.; Xia, P. Thermal performance of a cylindrical battery module impregnated with PCM composite based on thermoelectric cooling. Energy 2019, 188, 116048. [Google Scholar] [CrossRef]
- Siddique, A.R.M.; Mahmud, S.; Van Heyst, B. A comprehensive review on a passive (phase change materials) and an active (thermoelectric cooler) battery thermal management system and their limitations. J. Power Sources 2018, 401, 224–237. [Google Scholar] [CrossRef]
- Suh, I.-S.; Cho, H.; Lee, M. Feasibility study on thermoelectric device to energy storage system of an electric vehicle. Energy 2014, 76, 436–444. [Google Scholar] [CrossRef]
- Yin, T.; Zhu, H.Z. Analytical model-based optimization of the thermoelectric cooler with temperature-dependent materials under different operating conditions. Appl. Energy 2021, 299, 117340. [Google Scholar] [CrossRef]
- Zhou, Z.; Lv, Y.; Qu, J.; Sun, Q.; Grachev, D. Performance evaluation of hybrid oscillating heat pipe with carbon nanotube nanofluids for electric vehicle battery cooling. Appl. Therm. Eng. 2021, 196, 117300. [Google Scholar] [CrossRef]
- Alaoui, C. Passive/Active BTMS For EV Lithium-Ion Batteries. IEEE Trans. Veh. Technol. 2018, 67, 3709–3719. [Google Scholar] [CrossRef]
- Liu, F.; Lan, F.; Chen, J. Dynamic thermal characteristics of heat pipe via segmented thermal resistance model for electric vehicle battery cooling. J. Power Sources 2016, 321, 57–70. [Google Scholar] [CrossRef]
- Tran, T.-H.; Harmand, S.; Sahut, B. Experimental investigation on heat pipe cooling for Hybrid Electric Vehicle and Electric Vehicle lithium-ion battery. J. Power Sources 2014, 265, 262–272. [Google Scholar] [CrossRef]
- Bernagozzi, M.; Georgoulas, A.; Miché, N.; Rouaud, C.; Marengo, M. Novel battery thermal management system for electric vehicles with a loop heat pipe and graphite sheet inserts. Appl. Therm. Eng. 2021, 194, 117061. [Google Scholar] [CrossRef]
- Behi, H.; Karimi, D.; Behi, M.; Jaguemont, J.; Ghanbarpourd, M.; Behniae, M.; Berecibar, M.; Van Mierlo, J. Thermal management analysis using heat pipe in the high current discharging of lithium-ion battery in electric vehicles. J. Energy Storage 2020, 32, 101893. [Google Scholar] [CrossRef]
- Yuan, X.; Tang, A.; Shan, C.; Liu, Z.; Li, J. Experimental investigation on thermal performance of a battery liquid cooling structure coupled with heat pipe. J. Energy Storage 2020, 32, 101984. [Google Scholar] [CrossRef]
- Yao, M.; Gan, Y.; Liang, J.; Dong, D.; Ma, L.; Liu, J.; Luo, Q.; Li, Y. Performance simulation of a heat pipe and refrigerant-based lithium-ion battery thermal management system coupled with electric vehicle air-conditioning. Appl. Therm. Eng. 2021, 191, 116878. [Google Scholar] [CrossRef]
- Kleiner, J.; Singh, R.; Schmid, M.; Komsiyska, L.; Elger, G.; Endisch, C. Influence of heat pipe assisted terminal cooling on the thermal behavior of a large prismatic lithium-ion cell during fast charging in electric vehicles. Appl. Therm. Eng. 2021, 188, 116328. [Google Scholar] [CrossRef]
- Behi, H.; Behi, M.; Karimi, D.; Jaguemont, J.; Ghanbarpour, M.; Behnia, M.; Berecibar, M.; Van Mierlo, J. Heat pipe air-cooled thermal management system for lithium-ion batteries: High power applications. Appl. Therm. Eng. 2021, 183, 116240. [Google Scholar] [CrossRef]
- Alihosseini, A.; Shafaee, M. Experimental study and numerical simulation of a Lithium-ion battery thermal management system using a heat pipe. J. Energy Storage 2021, 39, 102616. [Google Scholar] [CrossRef]
- IEA. Global EV Outlook 2020. Technology Report. Available online: https://www.iea.org/reports/global-ev-outlook-2020 (accessed on 11 August 2021).
- Jaguemont, J.; Karimi, D.; Van Mierlo, J. Investigation of a Passive Thermal Management System for Lithium-Ion Capacitors. IEEE Trans. Veh. Techno 2019, 68, 10518–10524. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, X.; Ji, J.; Xu, X.; Zhang, Y. Research progress on power battery cooling technology for electric vehicles. J. Energy Storage 2020, 27, 101155. [Google Scholar] [CrossRef]
- Tete, P.R.; Gupta, M.M.; Joshi, S.S. Developments in battery thermal management systems for electric vehicles: A technical review. J. Energy Storage 2021, 35, 102255. [Google Scholar] [CrossRef]
- Goli, P.; Balandin, A.A. Graphene-enhanced phase change materials for thermal management of battery packs. In Proceedings of the Fourteenth Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (ITherm), Orlando, FL, USA, 27–30 May 2014. [Google Scholar] [CrossRef]
- Jaguemont, J.; Van Mierlo, J. A comprehensive review of future thermal management systems for battery electrified vehicles. J. Energy Storage 2020, 31, 101551. [Google Scholar] [CrossRef]
- Kim, J.; Oh, J.; Lee, H. Review on battery thermal management system for electric vehicles. Appl. Therm. Eng. 2019, 149, 192–212. [Google Scholar] [CrossRef]
- Arora, S. Selection of thermal management system for modular battery packs of electric vehicles: A review of existing and emerging technologies. J. Power Sources 2018, 400, 621–640. [Google Scholar] [CrossRef]
- Hentunen, A.; Lehmuspelto, T.; Suomela, J. Time-Domain Parameter Extraction Method for Thévenin-Equivalent Circuit Battery Models. IEEE Trans. Energy Convers. 2014, 29, 558–566. [Google Scholar] [CrossRef]
- Vatanparvar, K.; Faezi, S.; Burago, I.; Levorato, M.; Al Faruque, M.A. Extended Range Electric Vehicle With Driving Behavior Estimation in Energy Management. IEEE Trans. Smart Grid 2019, 10, 2959–2968. [Google Scholar] [CrossRef]
- Zhu, C.; Li, X.; Song, L.; Xiang, L. Development of a theoretically based thermal model for lithium ion battery pack. J. Power Sources 2013, 223, 155–164. [Google Scholar] [CrossRef]
- Worldwide Harmonised Light Vehicle Test Procedure (WLTP) Laboratory. WLTP Drive Cycle Data. Available online: https://www.wltpfacts.eu/ (accessed on 1 May 2021).
- Ökten, G.; Liu, Y. Randomized quasi-Monte Carlo methods in global sensitivity analysis. Reliab. Eng. Syst. Saf. 2021, 210, 107520. [Google Scholar] [CrossRef]
- Lamboni, M.; Iooss, B.; Popelin, A.-L.; Gamboa, F. Derivative-based global sensitivity measures: General links with Sobol’ indices and numerical tests. Math. Comput. Simul. 2013, 87, 45–54. [Google Scholar] [CrossRef] [Green Version]
- The Lithium-Ion Battery Life Cycle Report 2021. Circular Energy Storage. Available online: https://static1.squarespace.com/static/587657ddbe659497fb46664c/t/5fdaa991dc2ddb6396c30fa6/1608165783527/The+lithium-ion+battery+life+cycle+report+sample.pdf (accessed on 10 May 2021).
- Richa, K.; Babbitt, C.W.; Gaustad, G.; Wang, X. A future perspective on lithium-ion battery waste flows from electric vehicles. Resour. Conserv. Recycl. 2014, 83, 63–76. [Google Scholar] [CrossRef]
- Earl, J.; Fell, M. Electric vehicle manufacturers’ perceptions of the market potential for demand-side flexibility using electric vehicles in the United Kingdom. Energy Policy 2019, 129, 646–652. [Google Scholar] [CrossRef]
- Shafique, M.; Rafiq, M.; Azam, A.; Luo, X. Material flow analysis for end-of-life lithium-ion batteries from battery electric vehicles in the USA and China. Resour. Conserv. Recycl 2021, 178, 1–13. [Google Scholar] [CrossRef]
- Dehghani-Sanij, A.; Tharumalingam, E.; Dusseault, M.; Fraser, R. Study of energy storage systems and environmental challenges of batteries. Renew. Sustain. Energy Rev. 2019, 104, 192–208. [Google Scholar] [CrossRef]
- Zubi, G.; Lópeza, R.D.; Carvalhob, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
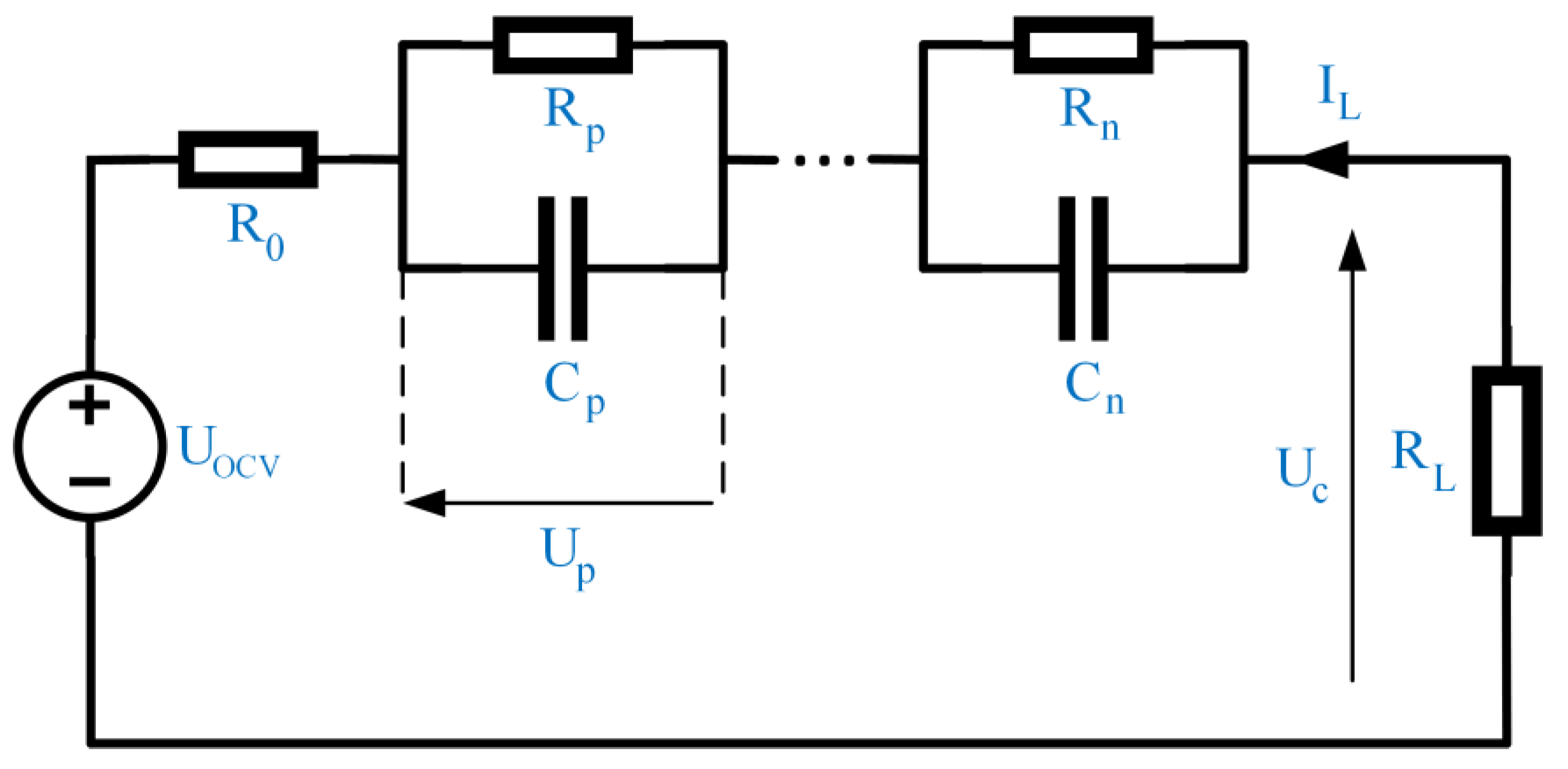
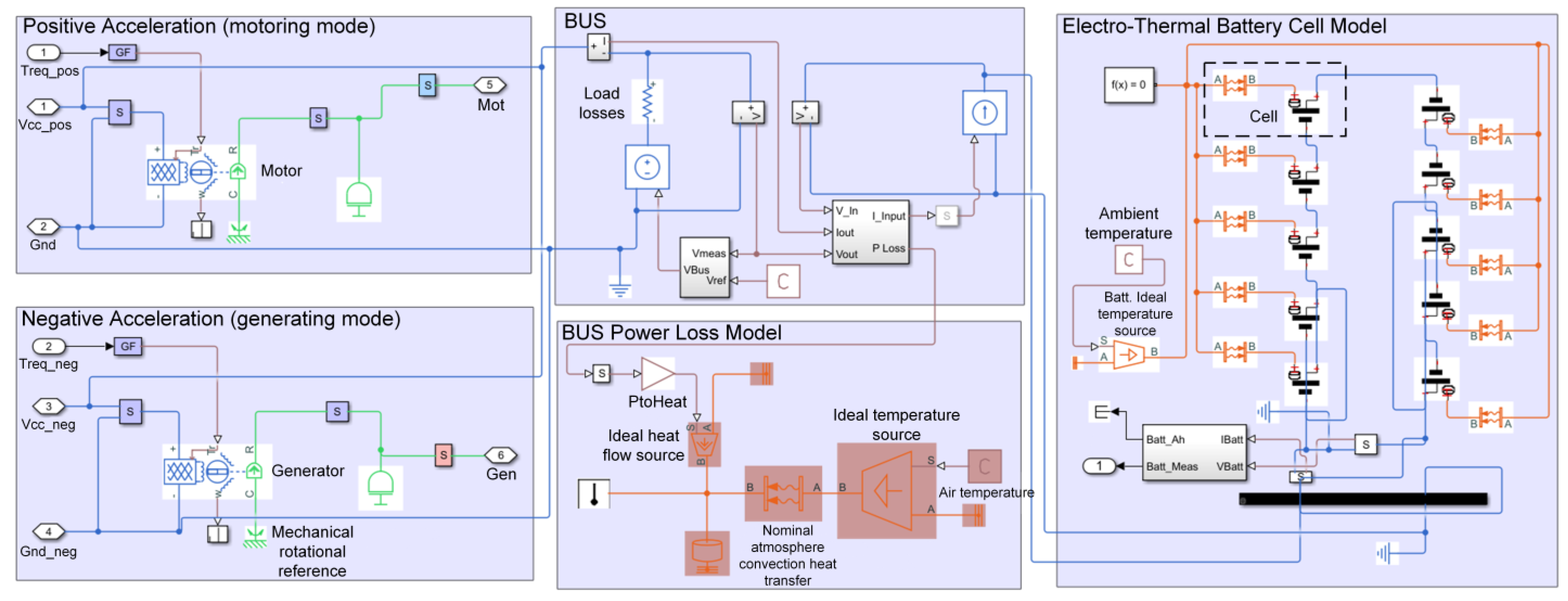
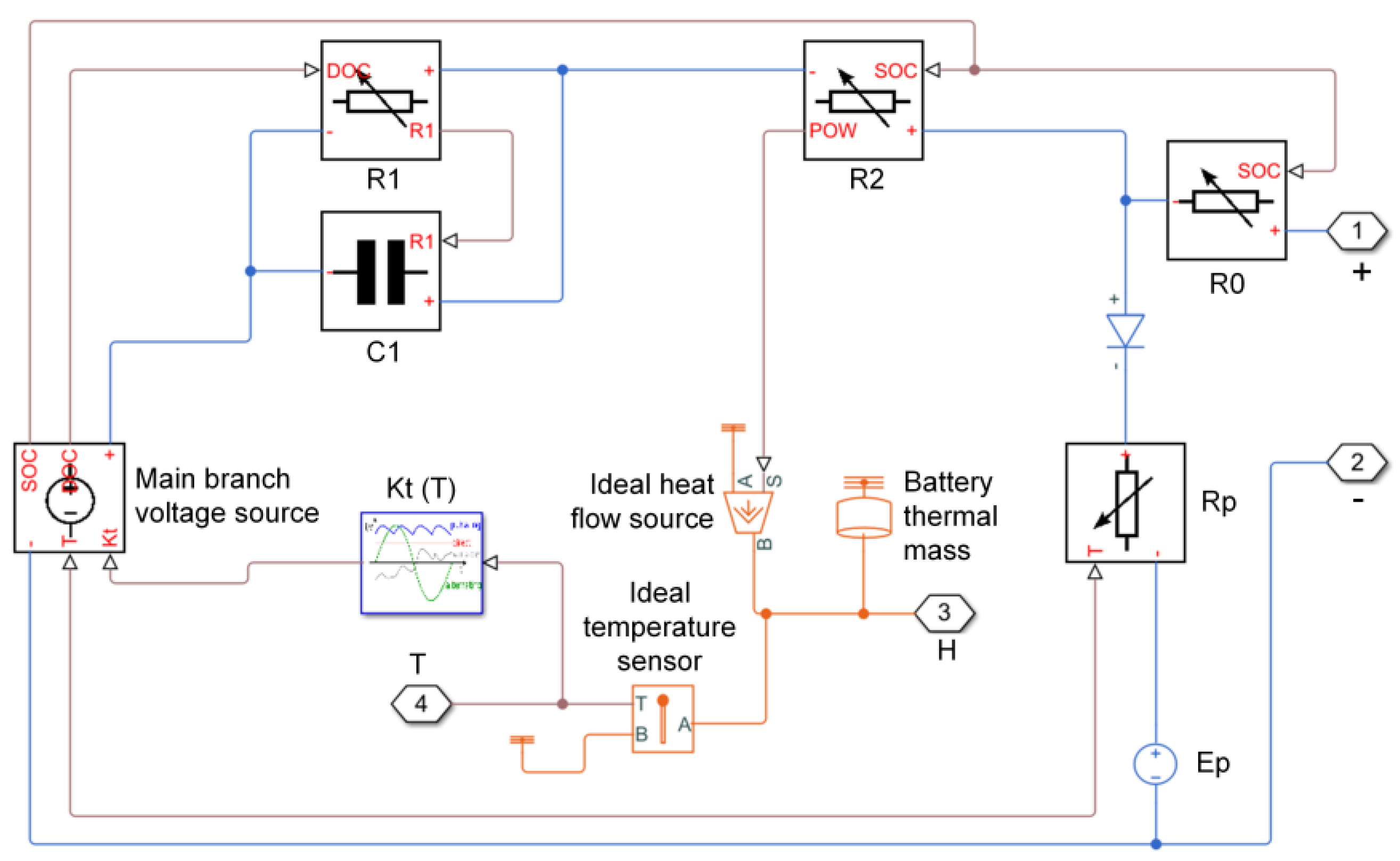
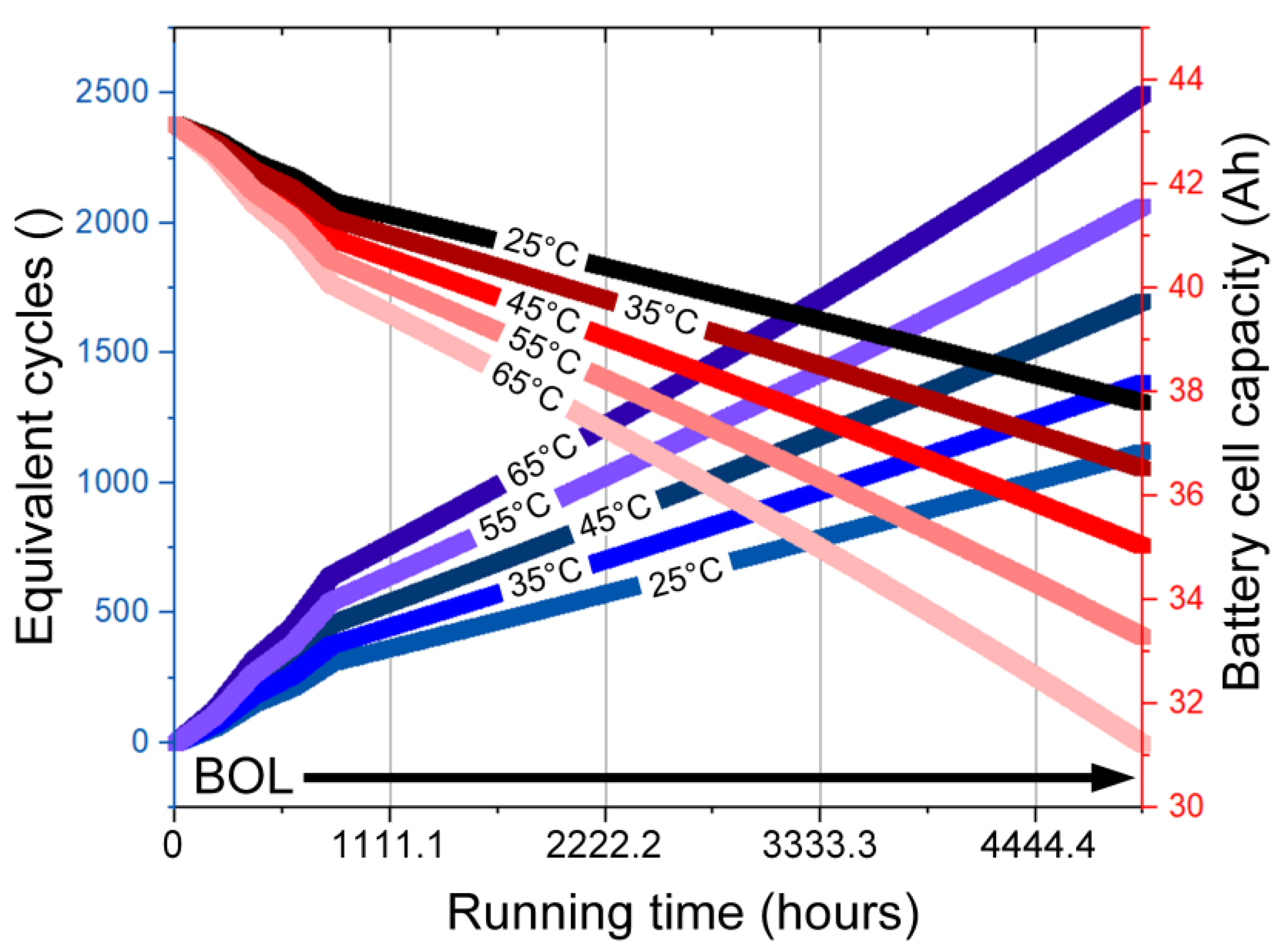



| Li-ion Battery Type | Specific Capacity (mAh/g) | Discharge Midpoint (V/Li/Li) |
|---|---|---|
| LiCoO2 (LCO) | 155 | 3.9 |
| LiFePO4 (LFP) | 160 | 3.45 |
| LiMn2O4 (LMO) | 120 | 4.05 |
| LiNi1−x−yMnxCoyO2 (NMC) | 180 | 3.8 |
| LiNi0.8 Co0.15Alx0.05O2 (NCA) | 200 | 3.73 |
| LiNi0.4Mn1.6O2 (LNMO) | 134 | 4.65 |
| Batt. Type | The Energy Density (Wh/kg) | Life Cycle | Internal Resistance (mΩ) | Cell Voltage (V) | Charging Temperature (°C) |
|---|---|---|---|---|---|
| Pb-O2 | 40 | 250 | <100 (12 V pack) | 2 | −20 to 50 |
| Ni-Cd | 62 | 1000 | 150 (6 V pack) | 1.2 | 0 to 45 |
| Li-Ion-PO₄3− | 115 | 1500 | 25–502 | 3.3 | 0 to 4510 |
| Li-Ion-Mn | 117 | 750 | 25–752 | 3.8 | 0 to 4510 |
| Li-Ion-Co | 170 | 750 | 17 | 3.6 | 0 to 4510 |
| Li4Ti5O12LTO | 90 | 7000 | 2 (per cell) | 2.4 | 0 to 45 |
| LSD-NiMH | 95 | 900 | 250 (6 V pack) | 1.2 | 0 to 45 |
| Ni-MH | 90 | 400 | 250 (6 V pack) | 1.2 | 0 to 45 |
| Chemistry Description | Lithium Cobalt Oxide | Lithium Manganese Oxide | Sodium-Nickel Chloride | Nickel-Metal Hydride | Zinc-Air |
|---|---|---|---|---|---|
| Reaction formula | LiCoO2 | LiMn2O4 | NaNiCl2 | NiMH | ZnOH42− |
| Nominal tension (V) | 3.60 | 3.70 | 2.85 | 1.20 | 1.4 |
| Specific energy (Wh/kg) | 150–200 | 100–150 | 94–130 | 300–400 | 350–500 |
| Charge (C-rate) | 0.7–1 | 0.7–1 | 0.3 | 0.1 | 0.8 |
| Discharge (C-rate) | 1 | 1 | 1 | 1 | 0.1 |
| Thermal runway (°C) | 150 | 250 | 270–350 | 40–70 | 280–320 |
| Brand | Model | Year | Top Speed (mph)/Range (mi) | Battery Capacity (kWh)/Fast Charging Time (h) | Normal and Maximum Battery Charging Power (kW) | Energy Consumption (Wh/mi) |
|---|---|---|---|---|---|---|
| Audi | e-tron 55 quattro | 2019 | 124/225 | 86.5/0.46 | 11 AC/155 DC | 315 |
| BMW | i3 | 2019 | 93/219 | 42.2/0.5 | 11 AC/49 DC | 195 |
| Audi | e-tron 50 quattro | 2020 | 118/175 | 64.7/0.45 | 11 AC/120 DC | 365 |
| Vauxhall * | Vivaro-e Life Elite L | 2020 | 81/110 | 50/0.52 | 7.4 AC/99 DC | 310 |
| Fiat | 500e Cabrio | 2020 | 93/135 | 42/0.45 | 11 AC/85 DC | 185 |
| Jaguar | I-Pace EV400 | 2020 | 124/225 | 90/0.32 | 11 AC/262 DC | 290 |
| Tesla | 3 long range | 2021 | 91/145 | 77/0.54 | 11 AC/190 DC | 190 |
| Citroën | e-C4 | 2021 | 81/115 | 45/0.52 | 7.4 AC/99 DC | 205 |
| Mercedes | EQA 250 | 2021 | 99/220 | 66.5/0.55 | 11 AC/100 DC | 250 |
| Ford | Mustang Mach-E ER | 2021 | 120/335 | 88/0.72 | 11 AC/150 DC | 260 |
| Tesla | Y long range | 2021 | 112/260 | 72.5/0.31 | 11 AC/250 DC | 240 |
| Lexus | UX 300e | 2021 | 99/160 | 54.3/1.15 | 6.6 AC/35 DC | 260 |
| Peugeot * | e-Traveller Long | 2021 | 81/115 | 50/0.52 | 7.4 AC/99 DC | 325 |
| BMW | iX3 | 2021 | 112/225 | 74/0.52 | 11 AC/155 DC | 255 |
| Thermal Cooling Methods | Efficiency (%) | Operating Temperature (°C) | Citations |
|---|---|---|---|
| Air | 40–60 | 50–200 | [203,204,205,206,207] |
| Liquid | 45–75 | 50–300 | [208,209,210,211,212,213,214] |
| Direct refrigerant | 55–80 | 25–600 | [215,216,217,218,219,220] |
| Phase change | 50–65 | −20–45 | [221,222,223,224,225,226,227,228,229] |
| Thermoelectric | 65–80 | 0–150 | [230,231,232,233,234,235] |
| Heat pipe | 50–75 | 25–300 | [236,237,238,239,240,241,242,243,244,245,246] |
| Parameters | Value | Unit |
|---|---|---|
| Rated capacity | 40 | Ah |
| Rated voltage | 12.8 | V |
| Internal resistance at BoL | 0.0151 | Ω |
| Internal resistance at EoL | 0.0154 | Ω |
| Cut-off voltage | 10 | V |
| Rated discharge current | 20 | A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asef, P.; Milan, M.; Lapthorn, A.; Padmanaban, S. Future Trends and Aging Analysis of Battery Energy Storage Systems for Electric Vehicles. Sustainability 2021, 13, 13779. https://doi.org/10.3390/su132413779
Asef P, Milan M, Lapthorn A, Padmanaban S. Future Trends and Aging Analysis of Battery Energy Storage Systems for Electric Vehicles. Sustainability. 2021; 13(24):13779. https://doi.org/10.3390/su132413779
Chicago/Turabian StyleAsef, Pedram, Marzia Milan, Andrew Lapthorn, and Sanjeevikumar Padmanaban. 2021. "Future Trends and Aging Analysis of Battery Energy Storage Systems for Electric Vehicles" Sustainability 13, no. 24: 13779. https://doi.org/10.3390/su132413779
APA StyleAsef, P., Milan, M., Lapthorn, A., & Padmanaban, S. (2021). Future Trends and Aging Analysis of Battery Energy Storage Systems for Electric Vehicles. Sustainability, 13(24), 13779. https://doi.org/10.3390/su132413779







