A Regional Review of Marine and Coastal Impacts of Climate Change on the ROPME Sea Area
Abstract
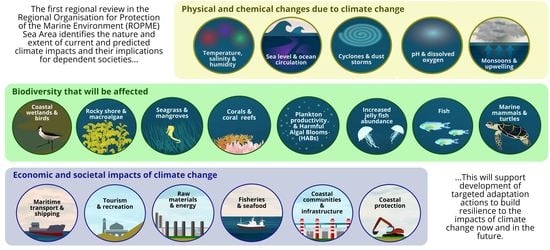
1. Introduction
2. Methods
3. Results
3.1. Temperature, Salinity, and Humidity
3.2. Sea Level and Ocean Circulation
3.3. pH and Dissolved Oxygen
3.4. Cyclones and Dust Storms
3.5. Monsoon Winds and Upwelling
3.6. Ecological Impacts on Coastal and Marine Ecosystems
3.6.1. Primary Productivity
3.6.2. Harmful Algal Blooms (HABs)
3.6.3. Jellyfish
3.6.4. Fish
3.6.5. Birds
3.6.6. Marine Mammals and Turtles
3.6.7. Corals and Coral Reefs
3.6.8. Salt Marshes, Mudflats, Sabkhas
3.6.9. Mangroves
3.6.10. Seagrasses
3.6.11. Rocky Shore and Macroalgal Communities
3.7. Impacts on Society and Economy
3.7.1. Provision of Seafood and Other Living Resources
Marine Wild Capture Fisheries
Aquaculture
Bait Fishing
Pearl Oyster Farming
3.7.2. Provision of Energy and Raw Materials
Fossil Oil and Gas
Power Generation Plants
Water and Desalination Plants
Renewable Energy
Marine Aggregates and Sand Mining
Wetland Resources
3.7.3. Provision of Space and Transport
Maritime Transport
Coastal Cities and Infrastructure
Waste and Sanitation
3.7.4. Provision of Leisure and Culture
Tourism, Recreation, and Wellbeing
Wildlife Watching
History and Heritage
3.7.5. Provision of Good Environmental Quality and Coastal Protection
Water Quality
Flood and Erosion Protection
Carbon Storage by Coastal and Marine Habitats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Special Report on the Ocean and Cryosphere in a Changing Climate; Chapter 1; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, A., Pirani, S.L., Connors, C., Péan, S., Berger, N., Caud, Y., Chen, L., Goldfarb, M.I., Gomis, M., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021; in press. [Google Scholar]
- Alosairi, Y.; Alsulaiman, N.; Rashed, A.; Al-Houti, D. World record extreme sea surface temperatures in the northwestern Arabian/Persian Gulf verified by in situ measurements. Mar. Pollut. Bull. 2020, 161, 111766. [Google Scholar] [CrossRef] [PubMed]
- Hoegh-Guldberg, O.; Cai, R.; Poloczanska, E.; Brewer, P.G.; Sundby, S.; Hilmi, K.; Fabry, V.J.; Jung, S. The Ocean. Climate Change 2014: Impacts, Adaptation, and Vulnerability. In Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 1655–1731. [Google Scholar]
- Lyons, B.P.; Cowie, W.J.; Maes, T.; Le Quesne, W.J.F. Marine plastic litter in the ROPME Sea Area: Current knowledge and recommendations. Ecol. Environ. Saf. 2020, 187, 109839. [Google Scholar] [CrossRef]
- Van Lavieren, H.; Burt, J.; Feary, D.; Cavalcante, G.; Marquis, E.; Benedetti, L.; Trick, C.; Kjerfve, B.; Sale, P. Managing the Growing Impacts of Development on Fragile Coastal and Marine Ecosystems: Lessons from the Gulf. A Policy Report; UNU-NWEH: Hamilton, ON, Canada, 2012. [Google Scholar]
- Vaughan, G.O.; Al-Mansoori, N.; Burt, J.A. The Arabian Gulf. In World Seas: An Environmental Evaluation; Elsevier BV: Amsterdam, The Netherlands, 2019; Volume 2, pp. 1–23. [Google Scholar]
- Devlin, M.; Massoud, M.; Hamid, S.; Al-Zaidan, A.; Al-Sarawi, H.; Al-Enezi, M.; Al-Ghofran, L.; Smith, A.; Barry, J.; Stentiford, G.; et al. Changes in the water quality conditions of Kuwait’s marine waters: Long term impacts of nutrient enrichment. Mar. Pollut. Bull. 2015, 100, 607–620. [Google Scholar] [CrossRef]
- Sheppard, C. Coral reefs in the Gulf are mostly dead now, but can we do anything about it? Mar. Pollut. Bull. 2016, 105, 593–598. [Google Scholar] [CrossRef]
- Sheppard, C.; Al-Husiani, M.; Al-Jamali, F.; Al-Yamani, F.; Baldwin, R.; Bishop, J.; Benzoni, F.; Dutrieux, E.; Dulvy, N.; Durvasula, S.R.V.; et al. The Gulf: A young sea in decline. Mar. Pollut. Bull. 2010, 60, 13–38. [Google Scholar] [CrossRef]
- ROPME. ROPME Marine Climate Change Impacts Evidence Report; Lincoln, S., Buckley, P., Howes, E.L., Maltby, K.M., Pinnegar, J.K., Le Quesne, W., Eds.; Cefas: Lowestoft, UK, 2020; 88p, Available online: http://ropme.org/430_Tech_Reports_Summary_EN.clx (accessed on 29 September 2020).
- Maltby, K.M.; Howes, E.L.; Lincoln, S.; Pinnegar, J.K.; Buckley, P.; Ali, T.S.; Al Balushi, B.; Al Ragum, A.; Al Shukaili, H.S.A.; Balmes, C.O.; et al. Identifying priority marine climate change risks to biodiversity and society in one of the world’s hottest subtropical regions, ROPME Sea Area. In press.
- ROPME. State of the Marine Environment Report-2013; ROPME/GC-16/1-ii Regional Organization for the Protection of the Marine Environment: Kuwait City, Kuwait, 2013; 225p. [Google Scholar]
- Noori, R.; Tian, F.; Berndtsson, R.; Abbasi, M.R.; Naseh, M.V.; Modabberi, A.; Soltani, A.; Kløve, B. Recent and future trends in sea surface temperature across the Persian Gulf and Gulf of Oman. PLoS ONE 2019, 14, e0212790. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashidi, T.B.; El-Gamily, H.I.; Amos, C.L.; Rakha, K.A. Sea surface temperature trends in Kuwait Bay, Arabian Gulf. Nat. Hazards 2008, 50, 73–82. [Google Scholar] [CrossRef]
- Lachkar, Z.; Mehari, M.; Al Azhar, M.; Lévy, M.; Smith, S. Fast local warming of sea-surface is the main factor of recent de-oxygenation in the Arabian Sea. Biogeosci. Discuss. 2020, 18, 5831–5849. [Google Scholar] [CrossRef]
- Piontkovski, S.; Al-Oufi, H. The Omani shelf hypoxia and the warming Arabian Sea. Int. J. Environ. Stud. 2014, 72, 256–264. [Google Scholar] [CrossRef]
- Johns, W.E.; Yao, F.; Olson, D.B.; Josey, S.A.; Grist, J.P.; Smeed, D.A. Observations of seasonal exchange through the Straits of Hormuz and the inferred heat and freshwater budgets of the Persian Gulf. J. Geophys. Res. Space Phys. 2003, 108, 3391. [Google Scholar] [CrossRef]
- Issa, I.E.; Al-Ansari, N.A.; Sherwani, G.; Knutsson, S. Expected Future of Water Resources within Tigris-Euphrates Rivers Basin, Iraq. J. Water Resour. Prot. 2014, 6, 421–432. [Google Scholar] [CrossRef]
- Le Quesne, W.; Fernand, L.; Ali, T.; Andres, O.; Antonpoulou, M.; Burt, J.; Dougherty, W.; Edson, P.; El Kharraz, J.; Glavan, J.; et al. Is the development of desalination compatible with sustainable development of the Arabian Gulf? Mar. Pollut. Bull. 2021, 173, 112940. [Google Scholar] [CrossRef]
- ROPME. State of the Marine Environment Report (SOMER-2003); Regional Organization for the Protection of the Marine Environment (ROPME): Kuwait City, Kuwait, 2003. [Google Scholar]
- Joseph, S.; Freeland, H.J. Salinity variability in the Arabian Sea. Geophys. Res. Lett. 2005, 32, L09607. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.-F.; et al. The representative concentration pathways: An overview. Clim. Chang. 2011, 109, 5–31. [Google Scholar] [CrossRef]
- AGEDI. Final Technical: Regional Desalination and Climate Change. LNRCCP. CCRG/IO. 2016. Available online: https://agedi.org/item/technical-report-regional-desalination-and-climate-change/ (accessed on 10 September 2020).
- Reynolds, M. Physical oceanography of the Gulf, Strait of Hormuz, and the Gulf of Oman: Results from the Mt Mitchell expedition. Mar. Pollut. Bull. 1993, 27, 35–39. [Google Scholar] [CrossRef]
- Sadrinasab, M. Three-dimensional flushing times of the Persian Gulf. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]
- UNEP. Regional Seas Reports and Studies No. 112 Rev. 10. Linden et al.: State of the Marine Environment in the ROPME Sea Area. 1990. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/11738/rsrs112.pdf?sequence=1&isAllowed=y (accessed on 29 September 2020).
- Dawoud, M.A.; Al Mulla, M.M. Environmental Impacts of Seawater Desalination: Arabian Gulf Case Study. Int. J. Environ. Sustain. 2012, 1, 22–37. [Google Scholar] [CrossRef]
- Hosseini, H.; Saadaoui, I.; Moheimani, N.; Al Saidi, M.; Al Jamali, F.; Al Jabri, H.; Ben Hamadou, R. Marine health of the Arabian Gulf: Drivers of pollution and assessment approaches focusing on desalination activities. Mar. Pollut. Bull. 2021, 164, 112085. [Google Scholar] [CrossRef] [PubMed]
- AGEDI. Regional Ocean Modelling for the Arabian Gulf Region-Future Scenarios and Capacity Building. LNRCCP. CCRG/USP. Abu Dhabi Global Environmental Data Initiative (AGEDI). 2015. Available online: https://www.researchgate.net/publication/299234015_Technical_Report_Regional_Downscaled_Climate_Change_Atmospheric_Modeling_Results?channel=doi&linkId=56efd96708ae01ae3e70df0a&showFulltext=true (accessed on 29 September 2020).
- Pal, J.S.; Eltahir, E.A.B. Future temperature in southwest Asia projected to exceed a threshold for human adaptability. Nat. Clim. Chang. 2016, 6, 197–200. [Google Scholar] [CrossRef]
- Sherwood, S.C.; Huber, M. An adaptability limit to climate change due to heat stress. Proc. Natl. Acad. Sci. USA 2010, 107, 9552–9555. [Google Scholar] [CrossRef] [PubMed]
- Alothman, A.; Bos, M.; Fernandes, R.; Ayhan, M. Sea level rise in the north-western part of the Arabian Gulf. J. Geodyn. 2014, 81, 105–110. [Google Scholar] [CrossRef]
- Ayhan, M.E.; Alothman, A. Sea level rise within the west of Arabian Gulf using tide gauge and continuous GPS measurements. Geophys. Res. Abstr. 2009, 11, EGU2009-6006-1. [Google Scholar]
- Lari, K.; Najafi Alamdari, M.; Abrehdary, M. Modelling of mean sea level in Persian Gulf and Oman Sea using T/P satellite altimetry data. Ecol. Environ. Conserv. 2012, 18, 21–27. [Google Scholar]
- Oppenheimer, M.; Glavovic, B.C.; Hinkel, J.; van de Wal, R.; Magnan, A.K.; Abd-Elgawad, A.; Cai, R.; Cifuentes-Jara, M.; DeConto, R.M.; Ghosh, T.; et al. (Eds.) IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Cambridge University Press: Cambridge, UK, 2019; In press. [Google Scholar]
- Unnikrishnan, A.; Shankar, D. Are sea-level-rise trends along the coasts of the north Indian Ocean consistent with global estimates? Glob. Planet. Chang. 2007, 57, 301–307. [Google Scholar] [CrossRef]
- Hoffmann, G.; Rupprechter, M.; Mayrhofer, C. Review of the long-term coastal evolution of North Oman-subsidence versus uplift [Review der langfristigen Küstenentwicklung Nordomans-Senkung und Hebung]. Z. Der Dtsch. Ges. Für Geowiss. 2013, 164, 237–252. [Google Scholar]
- Mattern, F.; Moraetis, D.; Abbasi, I.; Al Shukaili, B.; Scharf, A.; Claereboudt, M.; Looker, E.; Al Haddabi, N.; Pracejus, B. Coastal dynamics of uplifted and emerged late Pleistocene near-shore coral patch reefs at fins (eastern coastal Oman, Gulf of Oman). J. Afr. Earth Sci. 2018, 138, 192–200. [Google Scholar] [CrossRef]
- Sandeep, S.; Ajayamohan, R.S. Origin of cold bias over the Arabian Sea in Climate Models. Sci. Rep. 2014, 4, 6403. [Google Scholar] [CrossRef] [PubMed]
- Mezhoud, N.; Temimi, M.; Zhao, J.; Al Shehhi, M.R.; Ghedira, H. Analysis of the spatio-temporal variability of seawater quality in the southeastern Arabian Gulf. Mar. Pollut. Bull. 2016, 106, 127–138. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Karl, D.M.; Boyd, P.W.; Cheung, W.W.L.; Lluch-Cota, S.E.; Nojiri, Y.; Schmidt, D.N.; Zavialov, P.O. Ocean systems. Climate Change 2014: Impacts, Adaptation, and Vulnerability. In Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 411–484. [Google Scholar]
- Riegl, B.M.; Purkis, S.J. Coral Reefs of the Gulf: Adaptation to Climatic Extremes in the World’s Hottest Sea; Springer: Singapore, 2012; Volume 3, pp. 1–4. [Google Scholar]
- Saleh, A.; Samiei, J.V.; Amini-Yekta, F.; Hashtroudi, M.S.; Chen, C.-T.A.; Fumani, N.S. The carbonate system on the coral patches and rocky intertidal habitats of the northern Persian Gulf: Implications for ocean acidification studies. Mar. Pollut. Bull. 2020, 151, 110834. [Google Scholar] [CrossRef]
- Uddin, S.; Gevao, B.; Al-Ghadban, A.N.; Nithyanandan, M.; Al-Shamroukh, D. Acidification in Arabian Gulf – Insights from pH and temperature measurements. J. Environ. Monit. 2012, 14, 1479–1482. [Google Scholar] [CrossRef]
- Piontkovski, S.A.; Queste, B.Y. Decadal changes of the Western Arabian sea ecosystem. Int. Aquat. Res. 2016, 8, 49–64. [Google Scholar] [CrossRef]
- Pörtner, H.-O. Oxygen- and capacity-limitation of thermal tolerance: A matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 2010, 213, 881–893. [Google Scholar] [CrossRef]
- Helly, J.J.; Levin, L. Global distribution of naturally occurring marine hypoxia on continental margins. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2004, 51, 1159–1168. [Google Scholar] [CrossRef]
- Stramma, L.; Schmidtko, S.; Levin, L.A.; Johnson, G.C. Ocean oxygen minima expansions and their biological impacts. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2010, 57, 587–595. [Google Scholar] [CrossRef]
- Queste, B.Y.; Vic, C.; Heywood, K.J.; Piontkovski, S.A. Physical Controls on Oxygen Distribution and Denitrification Potential in the North West Arabian Sea. Geophys. Res. Lett. 2018, 45, 4143–4152. [Google Scholar] [CrossRef]
- Al-Ansari, E.M.; Rowe, G.; Abdel-Moati, M.; Yigiterhan, O.; Al-Maslamani, I.; Al-Yafei, M.; Al-Shaikh, I.; Upstill-Goddard, R. Hypoxia in the central Arabian Gulf Exclusive Economic Zone (EEZ) of Qatar during summer season. Estuar. Coast. Shelf Sci. 2015, 159, 60–68. [Google Scholar] [CrossRef]
- Saleh, A.; Abtahi, B.; Mirzaei, N.; Chen, C.-T.A.; Ershadifar, H.; Ghaemi, M.; Hamzehpour, A.; Abedi, E. Hypoxia in the Persian Gulf and the Strait of Hormuz. Mar. Pollut. Bull. 2021, 167, 112354. [Google Scholar] [CrossRef]
- Bopp, L.; Resplandy, L.; Orr, J.C.; Doney, S.C.; Dunne, J.P.; Gehlen, M.; Halloran, P.; Heinze, C.; Ilyina, T.; Séférian, R.; et al. Multiple stressors of ocean ecosystems in the 21st century: Projections with CMIP5 models. Biogeosciences 2013, 10, 6225–6245. [Google Scholar] [CrossRef]
- Lachkar, Z.; Lévy, M.; Smith, K.S.; Smith, S. Strong Intensification of the Arabian Sea Oxygen Minimum Zone in Response to Arabian Gulf Warming. Geophys. Res. Lett. 2019, 46, 5420–5429. [Google Scholar] [CrossRef]
- Long, M.C.; Deutsch, C.; Ito, T. Finding forced trends in oceanic oxygen. Glob. Biogeochem. Cycles 2016, 30, 381–397. [Google Scholar] [CrossRef]
- Rixen, T.; Cowie, G.; Gaye, B.; Goes, J.; Gomes, H.D.R.; Hood, R.R.; Lachkar, Z.; Schmidt, H.; Segschneider, J.; Singh, A. Reviews and syntheses: Present, past, and future of the oxygen minimum zone in the northern Indian Ocean. Biogeosciences 2020, 17, 6051–6080. [Google Scholar] [CrossRef]
- Rafiq, L.; Blaschke, T.; Tajbar, S. Arabian Sea cyclone: Structure analysis using satellite data. Adv. Space Res. 2015, 56, 2235–2247. [Google Scholar] [CrossRef]
- Bento, R.; Hoey, A.S.; Bauman, A.G.; Feary, D.A.; Burt, J.A. The implications of recurrent disturbances within the world’s hottest coral reef. Mar. Pollut. Bull. 2016, 105, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.; Foster, G.; Tourenq, C.; Shuriqi, M. Shifts in coral community structures following cyclone and red tide disturbances within the Gulf of Oman (UAE). Mar. Biol. 2011, 158, 955–968. [Google Scholar] [CrossRef]
- Haggag, M.; Badry, H. Hydrometeorological Modeling Study of Tropical Cyclone Phet in the Arabian Sea in 2010. Atmos. Clim. Sci. 2012, 2, 174–190. [Google Scholar] [CrossRef][Green Version]
- Murakami, H.; Sugi, M.; Kitoh, A. Future changes in tropical cyclone activity in the North Indian Ocean projected by high-resolution MRI-AGCMs. Clim. Dyn. 2013, 40, 1949–1968. [Google Scholar] [CrossRef]
- Lin, N.; Emanuel, K.A. Grey swan tropical cyclones. Nat. Clim. Chang. 2016, 6, 106–111. [Google Scholar] [CrossRef]
- El-Sabh, M.I.; Murty, T.S. Storm surges in the Arabian Gulf. Nat. Hazards 1989, 1, 371–385. [Google Scholar] [CrossRef]
- Paparella, F.; Xu, C.; Vaughan, G.O.; Burt, J.A. Coral Bleaching in the Persian/Arabian Gulf Is Modulated by Summer Winds. Front. Mar. Sci. 2019, 6, 1. [Google Scholar] [CrossRef]
- Alobaidi, M.; Almazroui, M.; Mashat, A.; Jones, P.D. Arabian Peninsula wet season dust storm distribution: Regionalization and trends analysis (1983–2013). Int. J. Climatol. 2017, 37, 1356–1373. [Google Scholar] [CrossRef]
- Chatziefthimiou, A.D.; Banack, S.A.; Metcalf, J.S. Harmful Algal and Cyanobacterial Harmful Algal Blooms in the Arabian Seas: Current Status, Implications, and Future Directions. In The Arabian Seas: Biodiversity, Environmental Challenges and Conservation Measures; Springer: Singapore, 2021; pp. 1083–1101. [Google Scholar]
- Harrison, P.; Piontkovski, S.; Al-Hashmi, K. Overview of decadal ecosystem changes in the Western Arabian Sea and the occurrence of algal blooms. J. Agric. Mar. Sci. 2018, 23, 11–23. [Google Scholar] [CrossRef]
- Thoppil, P.G.; Hogan, P.J. Persian Gulf response to a wintertime shamal wind event. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2010, 57, 946–955. [Google Scholar] [CrossRef]
- Kumar, K.R.; Attada, R.; Dasari, H.P.; Vellore, R.K.; Abualnaja, Y.O.; Ashok, K.; Hoteit, I. On the Recent Amplification of Dust Over the Arabian Peninsula During 2002–2012. J. Geophys. Res. Atmos. 2019, 124, 13220–13229. [Google Scholar] [CrossRef]
- Terradellas, E.; Nickovic, S.; Zhang, X.-Y. Airborne Dust: A Hazard to Human Health, Environment and Society. World Meteorol. Organ. Bull. 2015, 64. Available online: https://public.wmo.int/en/resources/bulletin/airborne-dust-hazard-human-health-environment-and-society (accessed on 2 November 2020).
- Sheppard, C.R. Physical environment of the Gulf relevant to marine pollution: An overview. Mar. Pollut. Bull. 1993, 27, 3–8. [Google Scholar] [CrossRef]
- Goes, J.I.; Thoppil, P.G.; Gomes, H.D.R.; Fasullo, J.T. Warming of the Eurasian Landmass Is Making the Arabian Sea More Productive. Science 2005, 308, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Piontkovski, S.A.; Claereboudt, M.R. Interannual changes of the Arabian Sea productivity. Mar. Biol. Res. 2012, 8, 189–194. [Google Scholar] [CrossRef]
- Roxy, M.K.; Modi, A.; Murtugudde, R.; Valsala, V.; Panickal, S.; Kumar, S.P.; Ravichandran, M.; Vichi, M.; Lévy, M. A reduction in marine primary productivity driven by rapid warming over the tropical Indian Ocean. Geophys. Res. Lett. 2016, 43, 826–833. [Google Scholar] [CrossRef]
- IPCC. The IPCC Scientific Assessment. Report Prepared for IPCC by Working Group 1. Houghton, J.T., Jenkins, G.J., Ephraums, J.J., Eds.; 1990. Available online: https://www.ipcc.ch/report/ar1/wg1/ (accessed on 17 September 2020).
- Burt, J.A.; Paparella, F.; Al-Mansoori, N.; Al-Mansoori, A.; Al-Jailani, H. Causes and consequences of the 2017 coral bleaching event in the southern Persian/Arabian Gulf. Coral Reefs 2019, 38, 567–589. [Google Scholar] [CrossRef]
- AGEDI. Technical Report: Regional Marine Biodiversity Vulnerability and Climate Change. LNRCCP. CCRG/UBC/Changing Ocean Research Unit/Sea Around Us. Abu Dhabi Global Environmental Data Initiative (AGEDI). 2015. Available online: https://agedi.org/item/technical-report-regional-marine-biodiversity-vulnerability-to-climate-change/ (accessed on 10 September 2020).
- Chowdhury, M.; Biswas, H.; Mitra, A.; Silori, S.; Sharma, D.; Bandyopadhyay, D.; Rahman Shaik, A.U.; Fernandes, V.; Narvekar, J. Southwest monsoon-driven changes in the phytoplankton community structure in the central Arabian Sea (2017–2018): After two decades of JGOFS. Prog. Oceanogr. 2021, 197, 102654. [Google Scholar] [CrossRef]
- Henson, S.A.; Cole, H.S.; Hopkins, J.; Martin, A.P.; Yool, A. Detection of climate change-driven trends in phytoplankton phenology. Glob. Chang. Biol. 2018, 24, e101–e111. [Google Scholar] [CrossRef]
- Rao, D.; Al-Yamani, F. Analysis of the relationship between phytoplankton biomass and the euphotic layer off Kuwait, Arabian Gulf. Ind. J. Mar. Sci. 1999, 28, 416–428. [Google Scholar]
- Sahay, A.; Ali, S.M.; Gupta, A.; Goes, J.I. Ocean color satellite determinations of phytoplankton size class in the Arabian Sea during the winter monsoon. Remote Sens. Environ. 2017, 198, 286–296. [Google Scholar] [CrossRef]
- Gregg, W.W.; Conkright, M.E.; Ginoux, P.; O’Reilly, J.E.; Casey, N.W. Ocean primary production and climate: Global decadal changes. Geophys. Res. Lett. 2003, 30, 1809. [Google Scholar] [CrossRef]
- Al-Said, T.; Al-Ghunaim, A.; Rao, D.S.; Al-Yamani, F.; Al-Rifaie, K.; Al-Baz, A. Salinity-driven decadal changes in phytoplankton community in the NW Arabian Gulf of Kuwait. Environ. Monit. Assess. 2017, 189, 2756. [Google Scholar] [CrossRef] [PubMed]
- Ben-Hasan, A.; Walters, C.; Christensen, V.; Al-Husaini, M.; Al-Foudari, H. Is reduced freshwater flow in Tigris-Euphrates rivers driving fish recruitment changes in the Northwestern Arabian Gulf? Mar. Pollut. Bull. 2018, 129, 1–7. [Google Scholar] [CrossRef]
- Al Shehhi, M.R.; Gherboudj, I.; Ghedira, H. An overview of historical harmful algae blooms outbreaks in the Arabian Seas. Mar. Pollut. Bull. 2014, 86, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Bauman, A.G.; Burt, J.; Feary, D.A.; Marquis, E.; Usseglio, P. Tropical harmful algal blooms: An emerging threat to coral reef communities? Mar. Pollut. Bull. 2010, 60, 2117–2122. [Google Scholar] [CrossRef]
- Harrison, P.J.; Piontkovski, S.; Al-Hashmi, K. Understanding How Physical-Biological Coupling Influences Harmful algal Blooms. Mar. Pollut. Bull. 2017, 114, 25–34. [Google Scholar] [CrossRef]
- Samimi-Namin, K.; Risk, M.J.; Hoeksema, B.W.; Zohari, Z.; Rezai, H. Coral mortality and serpulid infestations associated with red tide, in the Persian Gulf. Coral Reefs 2010, 29, 509. [Google Scholar] [CrossRef]
- Zhao, J.; Temimi, M.; Ghedira, H. Characterization of harmful algal blooms (HABs) in the Arabian Gulf and the Sea of Oman using MERIS fluorescence data. ISPRS J. Photogramm. Remote Sens. 2015, 101, 125–136. [Google Scholar] [CrossRef]
- Alosairi, Y.; Al-Ragum, A.; Al-Houti, D. Environmental mechanisms associated with fish kill in a semi-enclosed water body: An integrated numerical modeling approach. Ecotoxicol. Environ. Saf. 2021, 217, 112238. [Google Scholar] [CrossRef]
- Al-Gheilani, H.M.; Matsuoka, K.; AlKindi, A.Y.; Amer, S.; Waring, C. Fish kills incidents and harmful algal blooms in Omani waters. J. Agric. Mar. Sci. 2011, 16, 23–33. [Google Scholar] [CrossRef]
- Al-Azri, A.; Piontkovski, S.; Al-Hashmi, K.; Al-Gheilani, H.; Al-Habsi, H.; Al-Khusaibi, S.; Al-Azri, N. The occurrence of algal blooms in Omani coastal waters. Aquat. Ecosyst. Health Manag. 2012, 15, 56–63. [Google Scholar] [CrossRef]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.A.; Vilizzi, L.; Lee, L.; Wood, L.E.; Cowie, W.J.; Burt, J.; Mamiit, R.J.E.; Ali, H.; Davison, P.I.; Fenwick, G.; et al. Identifying potentially invasive non-native marine and brackish water species for the Arabian Gulf and Sea of Oman. Glob. Chang. Biol. 2020, 26, 2081–2092. [Google Scholar] [CrossRef] [PubMed]
- Purcell, J.E.; Uye, S.-I.; Lo, W.-T. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: A review. Mar. Ecol. Prog. Ser. 2007, 350, 153–174. [Google Scholar] [CrossRef]
- Purcell, J.E. Climate effects on formation of jellyfish and ctenophore blooms: A review. J. Mar. Biol. Assoc. UK 2005, 85, 461–476. [Google Scholar] [CrossRef]
- Purcell, J.E. Jellyfish and Ctenophore Blooms Coincide with Human Proliferations and Environmental Perturbations. Annu. Rev. Mar. Sci. 2012, 4, 209–235. [Google Scholar] [CrossRef] [PubMed]
- Hays, G.C.; Doyle, T.K.; Houghton, J.D. A Paradigm Shift in the Trophic Importance of Jellyfish? Trends Ecol. Evol. 2018, 33, 874–884. [Google Scholar] [CrossRef]
- Robinson, K.; Ruzicka, J.; Decker, M.B.; Brodeur, R.; Hernandez, F.; Quiñónes, J.; Acha, M.; Uye, S.-I.; Mianzán, H.; Graham, W.M. Jellyfish, Forage Fish, and the World’s Major Fisheries. Oceanography 2014, 27, 104–115. [Google Scholar] [CrossRef]
- Bouwmeester, J.; Riera, R.; Range, P.; Ben-Hamadou, R.; Samimi-Namin, K.; Burt, J.A. Coral and Reef Fish Communities in the Thermally Extreme Persian/Arabian Gulf: Insights into Potential Climate Change Effects. In Perspectives on the Marine Animal Forests of the World; Springer: Singapore, 2020; pp. 63–86. [Google Scholar]
- Shraim, R.; Dieng, M.M.; Vinu, M.; Vaughan, G.; McParland, D.; Idaghdour, Y.; Burt, J.A. Environmental Extremes Are Associated with Dietary Patterns in Arabian Gulf Reef Fishes. Front. Mar. Sci. 2017, 4, 1–14. [Google Scholar] [CrossRef]
- Vaughan, G.O.; Shiels, H.A.; Burt, J.A. Seasonal variation in reef fish assemblages in the environmentally extreme southern Persian/Arabian Gulf. Coral Reefs 2021, 40, 405–416. [Google Scholar] [CrossRef]
- Bargahi, H.R.; Shokri, M.R.; Kaymaram, F.; Fatemi, M.R. Changes in reef fish assemblages following multiple bleaching events in the world’s warmest sea (Kish Island, the Persian Gulf). Coral Reefs 2020, 39, 1–22. [Google Scholar] [CrossRef]
- Grandcourt, E. Reef fish and fisheries in the Gulf. In Coral Reefs of the Gulf: Adaptation to Climatic Extremes; Riegl, B.M., Dodge, R.E., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 127–161. [Google Scholar]
- Morgan, G. Country review: Bahrain. In Review of the State of World Marine Capture Fisheries Management: Indian Ocean; FAO Fisheries Technical Paper, 488; DeYoung, C., Ed.; Food and Agricultural Organization of the United Nations: Rome, Italy, 2006; pp. 187–194. [Google Scholar]
- Riegl, B. Effects of the 1996 and 1998 positive sea-surface temperature anomalies on corals, coral diseases and fish in the Arabian Gulf (Dubai, UAE). Mar. Biol. 2002, 140, 29–40. [Google Scholar] [CrossRef]
- Al Dhaheri, S.; Javed, S.; Grandcourt, E.; Cibahy, A.; Kulaib, R.; Al Mazrouei, S. Biodiversity. In Abu Dhabi State of the Environment Report 2017; Al Mubarak, R.K., Al Mazrouei, S., Launay, F., Perry, R., Eds.; Environment Agency: Abu Dhabi, United Arab Emirates, 2017. [Google Scholar]
- Al-Ansi, M.A.; Abdel-Moati, M.A.R.; Al-Ansari, I.S. Causes of Fish Mortality Along the Qatari Waters (Arabian Gulf). Int. J. Environ. Stud. 2002, 59, 59–71. [Google Scholar] [CrossRef]
- Burt, J.; Bartholomew, A.; Usseglio, P.; Bauman, A.; Sale, P.F. Are artificial reefs surrogates of natural habitats for corals and fish in Dubai, United Arab Emirates? Coral Reefs 2009, 28, 663–675. [Google Scholar] [CrossRef]
- Coles, S.L.; Tarr, B.A. Reef fish assemblages in the Western Arabian Gulf: A geographically isolated population in an extreme environment. Bull. Mar. Sci. 1990, 47, 696–720. [Google Scholar]
- Brandl, S.J.; Johansen, J.L.; Casey, J.M.; Tornabene, L.; Morais, R.A.; Burt, J.A. Extreme environmental conditions reduce coral reef fish biodiversity and productivity. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- D’Agostino, D.; Burt, J.A.; Reader, T.; Vaughan, G.O.; Chapman, B.B.; Santinelli, V.; Cavalcante, G.H.; Feary, D.A. The influence of thermal extremes on coral reef fish behaviour in the Arabian/Persian Gulf. Coral Reefs 2020, 39, 733–744. [Google Scholar] [CrossRef]
- D’Agostino, D.; Burt, J.A.; Santinelli, V.; Vaughan, G.O.; Fowler, A.M.; Reader, T.; Taylor, B.M.; Hoey, A.S.; Cavalcante, G.H.; Bauman, A.G.; et al. Growth impacts in a changing ocean: Insights from two coral reef fishes in an extreme environment. Coral Reefs 2021, 40, 433–446. [Google Scholar] [CrossRef]
- Buchanan, J.R.; Ralph, G.M.; Krupp, F.; Harwell, H.; Abdallah, M.; Abdulqader, E.; Al-Husaini, M.; Bishop, J.M.; Burt, J.A.; Choat, J.H.; et al. Regional extinction risks for marine bony fishes occurring in the Persian/Arabian Gulf. Biol. Conserv. 2019, 230, 10–19. [Google Scholar] [CrossRef]
- Wabnitz, C.C.C.; Lam, V.W.Y.; Reygondeau, G.; Teh, L.C.L.; Al-Abdulrazzak, D.; Khalfallah, M.; Pauly, D.; Palomares, M.L.D.; Zeller, D.; Cheung, W.W.L. Climate change impacts on marine biodiversity, fisheries and society in the Arabian Gulf. PLoS ONE 2018, 13, e0194537. [Google Scholar] [CrossRef]
- Jabado, R.W.; Kyne, P.M.; Pollom, R.A.; Ebert, D.A.; Simpfendorfer, C.A.; Ralph, G.M.; Dulvy, N.K. (Eds.) The Conservation Status of Sharks, Rays, and Chimaeras in the Arabian Sea and Adjacent Waters. Environment Agency—Abu Dhabi, UAE and IUCN Species Survival Commission Shark Specialist Group, Vancouver, Canada. 2017. Available online: https://portals.iucn.org/library/node/46906 (accessed on 29 September 2020).
- Al-Obaid, S.; Samraoui, B.; Thomas, J.; El-Serehy, H.A.; Alfarhan, A.H.; Schneider, W.; O’Connell, M. An overview of wetlands of Saudi Arabia: Values, threats, and perspectives. Ambio 2016, 46, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Zwarts, L.; Felemban, H.; Price, A.R.G. Wader counts along the Saudi Arabian Gulf coast suggest that the Gulf harbours millions of waders. Wader Study Group Bull. 1991, 63, 25–32. [Google Scholar]
- Daunt, F.; Mitchell, I.; Frederiksen, M. Seabirds. Marine Climate Change Impacts Partnership: Science Review MCCIP. Sci. Rev. 2017, 2017, 42–46. [Google Scholar] [CrossRef]
- MCCIP. Marine Climate Change Impacts: 10 Years’ Experience of Science to Policy Reporting; Summary Report, Marine Climate Change Impacts Partnership; Frost, M., Baxter, J., Buckley, P., Dye, S., Stoker, B., Eds.; Lowestoft, UK, 2017; 12p, Available online: http://www.mccip.org.uk/media/1770/mccip-report-card-2017-final-artwork-spreads.pdf (accessed on 17 September 2020).
- International Union for the Conservation of Nature (IUCN). The IUCN Red List of Threatened Species Version 2018-1. 2018. Available online: https://www.iucnredlist.org/ (accessed on 9 September 2020).
- Chatting, M.; Smyth, D.; Al-Maslamani, I.; Obbard, J.; Al-Ansi, M.; Hamza, S.; Al-Mohanady, S.F.; Al-Kuwari, A.J.; Marshall, C.D. Nesting ecology of hawksbill turtles, Eretmochelys imbricata, in an extreme environmental setting. PLoS ONE 2018, 13, e0203257. [Google Scholar] [CrossRef]
- Fuller, W.; Godley, B.; Hodgson, D.; Reece, S.; Witt, M.; Broderick, A. Importance of spatio-temporal data for predicting the effects of climate change on marine turtle sex ratios. Mar. Ecol. Prog. Ser. 2013, 488, 267–274. [Google Scholar] [CrossRef][Green Version]
- Maneja, R.H.; Miller, J.D.; Li, W.; Thomas, R.; El-Askary, H.; Perera, S.; Flandez, A.V.B.; Basali, A.U.; Alcaria, J.F.A.; Gopalan, J.; et al. Multidecadal analysis of beach loss at the major offshore sea turtle nesting islands in the northern Arabian Gulf. Ecol. Indic. 2021, 121, 107146. [Google Scholar] [CrossRef]
- Pilcher, N.J.; Antonopoulou, M.; Perry, L.; Abdel-Moati, M.A.; Al Abdessalaam, T.Z.; Albeldawi, M.; Al Ansi, M.; Al-Mohannadi, S.F.; Al Zahlawi, N.; Baldwin, R.; et al. Identification of Important Sea Turtle Areas (ITAs) for hawksbill turtles in the Arabian Region. J. Exp. Mar. Biol. Ecol. 2014, 460, 89–99. [Google Scholar] [CrossRef]
- Preen, A. Distribution, abundance and conservation status of dugongs and dolphins in the southern and western Arabian Gulf. Biol. Conserv. 2004, 118, 205–218. [Google Scholar] [CrossRef]
- Rabaoui, L.; Roa-Ureta, R.H.; Yacoubi, L.; Lin, Y.-J.; Maneja, R.; Joydas, T.V.; Panickan, P.; Gopalan, J.; Loughland, R.; Prihartato, P.K.; et al. Diversity, Distribution, and Density of Marine Mammals Along the Saudi Waters of the Arabian Gulf: Update From a Multi-Method Approach. Front. Mar. Sci 2021, 8, 687445. [Google Scholar] [CrossRef]
- Abdulqader, E.A.; Abdurahiman, P.; Mansour, L.; Harrath, A.H.; Qurban, M.A.; Rabaoui, L. By-catch and discards of shrimp trawling in the Saudi waters of the Arabian Gulf: Ecosystem impact assessment and implications for a sustainable fishery management. Fish. Res. 2020, 229, 105596. [Google Scholar] [CrossRef]
- Abdulqader, E.A.; Miller, J.; Al-Mansi, A.; Al-Abdulkader, K.; Fita, N.; Al-Nadhiri, H.; Rabaoui, L. Turtles and other marine megafauna bycatch in artisanal fisheries in the Saudi waters of the Arabian Gulf. Fish. Res. 2017, 196, 75–84. [Google Scholar] [CrossRef]
- Baldwin, R.; Gallagher, M.; Van Waerebeek, K. A Review of Cetaceans from Waters off the Arabian Peninsula. In The Natural History of Oman: A Festschrift for Michael Gallagher Chapter: A Review of Cetaceans from waters off the Arabian Peninsula; Fisher, M., Ghazanfar, S.A., Spalton, J.A., Eds.; Backhuys Publishers: Leiden, The Netherlands, 1999. [Google Scholar] [CrossRef]
- López, B.D.; Methion, S.; Das, H.; Bugla, I.; Al Hameli, M.; Al Ameri, H.; Al Hashmi, A.; Grandcourt, E. Vulnerability of a top marine predator in one of the world’s most impacted marine environments (Arabian Gulf). Mar. Biol. 2021, 168, 1–11. [Google Scholar] [CrossRef]
- Naser, H.A. Effects of reclamation on macrobenthic assemblages in the coastline of the Arabian Gulf: A microcosm experimental approach. Mar. Pollut. Bull. 2011, 62, 520–524. [Google Scholar] [CrossRef]
- Notarbartolo Di Sciara, G.N.; Baldwin, R.; Braulik, G.; Collins, T.; Natoli, A. Marine Mammals of the Arabian Seas. In The Arabian Seas: Biodiversity, Environmental Challenges and Conservation Measures; Springer: Singapore, 2021; pp. 637–678. [Google Scholar]
- ROPME. Status and Trends of Coral Reefs in the ROPME Sea Area. Past Present and Future; Regional Organization for the Protection of the Marine Environment (ROPME): Kuwait City, Kuwait, 2020; 120p, Available online: http://ropme.org/430_Tech_Reports_Summary_EN.clx (accessed on 2 November 2020).
- Claereboudt, M.R. Oman. In World Seas: An Environmental Evaluation; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 25–47. [Google Scholar]
- Maghsoudlou, A.; Araghi, P.E.; Wilson, S.; Taylor, O.; Medio, D. Status of the Coral Reefs in the ROPME Sea Area (The Persian Gulf, Gulf of Oman and Arabian Sea). In Status of Coral Reefs of the World: 2008; Wilkinson, C., Ed.; Global Coral Reef Monitoring Network and Reef and Rainforest Research Center: Townsville, Australia, 2008; pp. 79–90. [Google Scholar]
- Rezai, H.; Wilson, S.; Claereboudt, M.; Riegl, B. Coral Reef Status in the ROPME Sea Area: Arabian/Persian Gulf, Gulf of Oman and Arabian Sea. In Status of Coral Reefs of the World: 2004; Wilkinson, C., Ed.; Australian Institute of Marine Science: Townsville, Australia, 2004; Volume 1, 301p. [Google Scholar]
- Howells, E.J.; Abrego, D.; Meyer, E.; Kirk, N.L.; Burt, J. Host adaptation and unexpected symbiont partners enable reef-building corals to tolerate extreme temperatures. Glob. Chang. Biol. 2016, 22, 2702–2714. [Google Scholar] [CrossRef] [PubMed]
- Kirk, N.L.; Howells, E.J.; Abrego, D.; Burt, J.A.; Meyer, E. Genomic and transcriptomic signals of thermal tolerance in heat-tolerant corals (Platygyra daedalea) of the Arabian/Persian Gulf. Mol. Ecol. 2018, 27, 5180–5194. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, S.; Shokri, M.R.; Tohidfar, M. The enhanced expression of heat stress-related genes in scleractinian coral ‘Porites harrisoni’ during warm episodes as an intrinsic mechanism for adaptation in ‘the Persian Gulf’. Coral Reefs 2021, 40, 1013–1028. [Google Scholar] [CrossRef]
- Howells, E.J.; Bauman, A.G.; Vaughan, G.O.; Hume, B.C.C.; Voolstra, C.R.; Burt, J. Corals in the hottest reefs in the world exhibit symbiont fidelity not flexibility. Mol. Ecol. 2020, 29, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Hume, B.C.C.; D’Angelo, C.; Smith, E.G.; Stevens, J.R.; Burt, J.; Wiedenmann, J. Symbiodinium thermophilum sp. nov., a thermotolerant symbiotic alga prevalent in corals of the world’s hottest sea, the Persian/Arabian Gulf. Sci. Rep. 2015, 5, srep08562–8562. [Google Scholar] [CrossRef] [PubMed]
- Oladi, M.; Shokri, M.R.; Rajabi-Maham, H. Extremophile symbionts in extreme environments; a contribution to the diversity of Symbiodiniaceae across the northern Persian Gulf and Gulf of Oman. J. Sea Res. 2018, 144, 105–111. [Google Scholar] [CrossRef]
- Smith, E.G.; Vaughan, G.O.; Ketchum, R.N.; McParland, D.; Burt, J.A. Symbiont community stability through severe coral bleaching in a thermally extreme lagoon. Sci. Rep. 2017, 7, 2428. [Google Scholar] [CrossRef]
- Varasteh, T.; Shokri, M.R.; Rajabi-Maham, H.; Behzadi, S.; Hume, B.C.C. Symbiodinium thermophilum symbionts in Porites harrisoni and Cyphastrea microphthalma in the northern Persian Gulf, Iran. J. Mar. Biol. Assoc. UK 2018, 98, 2067–2073. [Google Scholar] [CrossRef]
- Howells, E.J.; Dunshea, G.; McParland, D.; Vaughan, G.O.; Heron, S.F.; Pratchett, M.S.; Burt, J.A.; Bauman, A.G. Species-Specific Coral Calcification Responses to the Extreme Environment of the Southern Persian Gulf. Front. Mar. Sci. 2018, 5, 56. [Google Scholar] [CrossRef]
- Kourandeh, M.B.; Nabavi, S.M.B.; Shokri, M.R.; Ghanemi, K. Variation in skeletal extension, density and calcification of the Scleractinian coral Porites lobate across the northern Persian Gulf. Reg. Stud. Mar. Sci. 2018, 24, 364–369. [Google Scholar] [CrossRef]
- Riegl, B.M.; Purkis, S.J.; Al-Cibahy, A.S.; Abdel-Moati, M.A.; Hoegh-Guldberg, O. Present Limits to Heat-Adaptability in Corals and Population-Level Responses to Climate Extremes. PLoS ONE 2011, 6, e24802. [Google Scholar] [CrossRef]
- Vajed-Samiei, J.; Dab, K.; Ghezellou, P.; Shirvani, A. Some scleractinian corals of Larak Island, Persian Gulf. Zootaxa 2013, 3636, 101–143. [Google Scholar] [CrossRef]
- Naser, H.A. Marine Ecosystem Diversity in the Arabian Gulf: Threats and Conservation. Chapter 12. In Biodiversity-The Dynamic Balance of the Planet; Grillo, O., Ed.; InTech: London, UK, 2014; pp. 297–328. [Google Scholar]
- Coles, S.L.; Riegl, B. Thermal tolerances of reef corals in the Gulf: A review of the potential for increasing coral survival and adaptation to climate change through assisted translocation. Mar. Pollut. Bull. 2013, 72, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, C.R. Status of Coral Reefs of the World: Summary of Threats and Remedial Action; Cambridge University Press (CUP): Cambridge, UK, 2012; pp. 3–39. [Google Scholar]
- Burt, J.A.; Bauman, A.G. Suppressed coral settlement following mass bleaching in the southern Persian/Arabian Gulf. Aquat. Ecosyst. Health Manag. 2020, 23, 166–174. [Google Scholar] [CrossRef]
- Burt, J.; Coles, S.; van Lavieren, H.; Taylor, O.; Looker, E.; Samimi-Namin, K. Oman’s coral reefs: A unique ecosystem challenged by natural and man-related stresses and in need of conservation. Mar. Pollut. Bull. 2016, 105, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Ben Lamine, E.; Mateos-Molina, D.; Antonopoulou, M.; Burt, J.; Das, H.S.; Javed, S.; Muzaffar, S.; Giakoumi, S. Identifying coastal and marine priority areas for conservation in the United Arab Emirates. Biodivers. Conserv. 2020, 29, 2967–2983. [Google Scholar] [CrossRef]
- Aeby, G.S.; Howells, E.; Work, T.; Abrego, D.; Williams, G.J.; Wedding, L.M.; Caldwell, J.M.; Moritsch, M.; Burt, J. Localized outbreaks of coral disease on Arabian reefs are linked to extreme temperatures and environmental stressors. Coral Reefs 2020, 39, 829–846. [Google Scholar] [CrossRef]
- Howells, E.J.; Vaughan, G.O.; Work, T.M.; Burt, J.; Abrego, D. Annual outbreaks of coral disease coincide with extreme seasonal warming. Coral Reefs 2020, 39, 771–781. [Google Scholar] [CrossRef]
- Riegl, B. Climate change and coral reefs: Different effects in two high-latitude areas (Arabian Gulf, South Africa). Coral Reefs 2003, 22, 433–446. [Google Scholar] [CrossRef]
- Riegl, B.M.; Bruckner, A.W.; Samimi-Namin, K.; Purkis, S.J. Diseases, Harmful Algae Blooms (HABs) and Their Effects on Gulf Coral Populations and Communities. In Coral Reefs of the World; Springer: Singapore, 2012; Volume 3, pp. 107–125. [Google Scholar]
- Burt, J.; Al-Harthi, S.; Al-Cibahy, A. Long-term impacts of coral bleaching events on the world’s warmest reefs. Mar. Environ. Res. 2011, 72, 225–229. [Google Scholar] [CrossRef]
- Wong, P.P.; Losada, I.J.; Gattuso, J.P.; Hinkel, J.; Khattabi, A.; McInnes, K.L.; Saito, Y.; Sallenger, A. Coastal systems and low-lying areas. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects; Contribution of Working Group II to the Fifth Assessment Report of the Inter-governmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 361–409. [Google Scholar]
- Burt, J.; Van Lavieren, H.; Feary, D. Persian Gulf reefs: An important asset for climate science in urgent need of protection. Ocean Chall. 2014, 20, 49–56. [Google Scholar]
- Burt, J.A.; Smith, E.G.; Warren, C.; Dupont, J. An assessment of Qatar’s coral communities in a regional context. Mar. Pollut. Bull. 2016, 105, 473–479. [Google Scholar] [CrossRef]
- Brandt, M.E.; McManus, J.W. Disease incidence is related to bleaching extent in reef-building corals. Ecology 2009, 90, 2859–2867. [Google Scholar] [CrossRef]
- Chan, N.C.S.; Connolly, S. Sensitivity of coral calcification to ocean acidification: A meta-analysis. Glob. Chang. Biol. 2012, 19, 282–290. [Google Scholar] [CrossRef]
- Mollica, N.R.; Guo, W.; Cohen, A.L.; Huang, K.-F.; Foster, G.; Donald, H.K.; Solow, A.R. Ocean acidification affects coral growth by reducing skeletal density. Proc. Natl. Acad. Sci. USA 2018, 115, 1754–1759. [Google Scholar] [CrossRef]
- Burke, L.; Reytar, K.; Spalding, M.; Perry, A. Reefs at Risk Revisited; World Resources Institute: Washington, DC, USA, 2011; ISBN 978-1-56973-762-00. [Google Scholar]
- Burt, J.A. The environmental costs of coastal urbanization in the Arabian Gulf. City 2014, 18, 760–770. [Google Scholar] [CrossRef]
- Petersen, K.L.; Paytan, A.; Rahav, E.; Levy, O.; Silverman, J.; Barzel, O.; Potts, D.; Bar-Zeev, E. Impact of brine and antiscalants on reef-building corals in the Gulf of Aqaba – Potential effects from desalination plants. Water Res. 2018, 144, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Shokri, M.R.; Mohammadi, M. Effects of recreational SCUBA diving on coral reefs with an emphasis on tourism suitability index and carrying capacity of reefs in Kish Island, the northern Persian Gulf. Reg. Stud. Mar. Sci. 2021, 45, 101813. [Google Scholar] [CrossRef]
- Howells, E.J.; Abrego, D.; Liew, Y.J.; Burt, J.A.; Meyer, E.; Aranda, M. Enhancing the heat tolerance of reef-building corals to future warming. Sci. Adv. 2021, 7, 6070. [Google Scholar] [CrossRef] [PubMed]
- Al-Farraj, A. An evolutionary model for sabkha development on the north coast of the UAE. J. Arid. Environ. 2005, 63, 740–755. [Google Scholar] [CrossRef]
- Lokier, S.W.; Court, W.M.; Onuma, T.; Paul, A. Implications of sea-level rise in a modern carbonate ramp setting. Geomorphology 2018, 304, 64–73. [Google Scholar] [CrossRef]
- Lutz, S.J. Blue Carbon-First Level Exploration of Natural Coastal Carbon in the Arabian Peninsula, with Special Focus on the UAE and Abu Dhabi. A Rapid Feasibility Study 2011; AGEDI/EAD; UNEP/GRID: Arendal, Norway, 2011; ISBN 978-82-7701-100-4. [Google Scholar]
- Wilson, M.A.; Shahid, S.A.; Abdelfattah, M.A.; Kelley, J.A.; Thomas, J.E. Anhydrite Formation on the Coastal Sabkha of Abu Dhabi, United Arab Emirates. In Developments in Soil Classification, Land Use Planning and Policy Implications: Innovative Thinking of Soil Inventory for Land Use Planning and Management of Land Resources; Shahid, S.A., Taha, F.K., Abdelfattah, M.A., Eds.; Springer Science + Business Media: Dordrecht, The Netherlands, 2013; pp. 175–201. [Google Scholar] [CrossRef]
- Cusack, M.; Saderne, V.; Arias-Ortiz, A.; Masque, P.; Krishnakumar, P.K.; Rabaoui, L.; A Qurban, M.; Qasem, A.M.; Prihartato, P.; A Loughland, R.; et al. Organic carbon sequestration and storage in vegetated coastal habitats along the western coast of the Arabian Gulf. Environ. Res. Lett. 2018, 13, 074007. [Google Scholar] [CrossRef]
- McLeod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Ellison, J.C. Vulnerability assessment of mangroves to climate change and sea-level rise impacts. Wetl. Ecol. Manag. 2015, 23, 115–137. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA,, 2013; 1535p. [Google Scholar]
- Lelieveld, J.; Hadjinicolaou, P.; Kostopoulou, E.; Chenoweth, J.; El Maayar, M.; Giannakopoulos, C.; Hannides, C.; Lange, M.A.; Tanarhte, M.; Tyrlis, E.; et al. Climate change and impacts in the Eastern Mediterranean and the Middle East. Clim. Chang. 2012, 114, 667–687. [Google Scholar] [CrossRef]
- Ward, R.D.; Friess, D.A.; Day, R.H.; MacKenzie, R.A. Impacts of climate change on mangrove ecosystems: A region by region overview. Ecosyst. Health Sustain. 2016, 2, e01211. [Google Scholar] [CrossRef]
- Dodd, R.S.; Blasco, F.; Rafii, Z.A.; Torquebiau, E. Mangroves of the United Arab Emirates: Ecotypic diversity in cuticular waxes at the bioclimatic extreme. Aquat. Bot. 1999, 63, 291–304. [Google Scholar] [CrossRef]
- Li, W.; El-Askary, H.; Qurban, M.A.; Li, J.; ManiKandan, K.P.; Piechota, T. Using multi-indices approach to quantify mangrove changes over the Western Arabian Gulf along Saudi Arabia coast. Ecol. Indicators 2019, 102, 734–745. [Google Scholar] [CrossRef]
- Clough, B.F. Primary productivity and growth of mangrove forests. 225-249. In Tropical Mangrove Ecosystems; Robertson, A.I., Alongi, D.M., Eds.; American Geophysical Union: Washington, DC, USA, 2013. [Google Scholar]
- Ranasinghe, R.; Duong, T.M.; Uhlenbrook, S.; Roelvink, D.; Stive, M. Climate-change impact assessment for inlet-interrupted coastlines. Nat. Clim. Chang. 2012, 3, 83–87. [Google Scholar] [CrossRef]
- Al-Kahtany, K.; El-Sorogy, A.; Al-Kahtany, F.; Youssef, M. Heavy metals in mangrove sediments of the central Arabian Gulf shoreline, Saudi Arabia. Arab. J. Geosci. 2018, 11, 155. [Google Scholar] [CrossRef]
- Almahasheer, H. High levels of heavy metals in Western Arabian Gulf mangrove soils. Mol. Biol. Rep. 2019, 46, 1585–1592. [Google Scholar] [CrossRef]
- Blankespoor, B.; Dasgupta, S.; Laplante, B. Sea-Level Rise and Coastal Wetlands. Ambio 2014, 43, 996–1005. [Google Scholar] [CrossRef]
- Almahasheer, H. Spatial coverage of mangrove communities in the Arabian Gulf. Environ. Monit. Assess. 2018, 190, 85. [Google Scholar] [CrossRef] [PubMed]
- Erftemeijer, P.L.; Cambridge, M.L.; Price, B.A.; Ito, S.; Yamamoto, H.; Agastian, T.; Burt, J.A. Enhancing growth of mangrove seedlings in the environmentally extreme Arabian Gulf using treated sewage sludge. Mar. Pollut. Bull. 2021, 170, 112595. [Google Scholar] [CrossRef]
- Friis, G.; Burt, J.A. Evolution of mangrove research in an extreme environment: Historical trends and future opportunities in Arabia. Ocean. Coast. Manag. 2020, 195, 105288. [Google Scholar] [CrossRef]
- Fouda, M.; Al-Muharrami, M. Significance of mangroves in the arid environment of the Sultanate of Oman. J. Sci. Res. 1996, 1, 41–49. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Crooks, S. Blue Carbon in the Northern and Eastern Emirates, UAE: Support of Blue Carbon at the National Level Extension. Abu Dhabi Global Environment Data Initiative (AGEDI), Ministry of Environment and Water (MOEW). Abu Dhabi, United Arab Emirates. 2015; 74p. Available online: https://www.moccae.gov.ae/assets/download/47ec3708/National%20Blue%20Carbon%20Project%20%E2%80%93%20Decision%20Maker%20Summary.pdf.aspx (accessed on 29 September 2021).
- Price, A.; Sheppard, C.; Roberts, C. The Gulf: Its biological setting. Mar. Pollut. Bull. 1993, 27, 9–15. [Google Scholar] [CrossRef]
- Asaf, S.; Khan, A.L.; Numan, M.; Al-Harrasi, A. Mangrove tree (Avicennia marina): Insight into chloroplast genome evolutionary divergence and its comparison with related species from family Acanthaceae. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Friis, G.; Vizueta, J.; Smith, E.G.; Nelson, D.R.; Khraiwesh, B.; Qudeimat, E.; Salehi-Ashtiani, K.; Ortega, A.; Marshell, A.; Duarte, C.M.; et al. A high-quality genome assembly and annotation of the gray mangrove, Avicennia marina. G3 Genes Genomes Genet 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Erftemeijer, P.; Shuail, D.A. Seagrass habitats in the Arabian Gulf: Distribution, tolerance thresholds and threats. Aquat. Ecosyst. Health Manag. 2012, 15, 73–83. [Google Scholar] [CrossRef]
- Phillips, R. The seagrasses of the Arabian Gulf and Arabian Region. In World Atlas of Seagrasses; Green, E., Short, F., Eds.; UNEP-WCMC, University of California Press: Berkeley, CA, USA, 2003; pp. 74–81. [Google Scholar]
- Vousden, D.H. The Bahrain Marine Habitat Survey: A Study of the Marine Habitats in the Waters of Bahrain and their Relationship to Physical, Chemical, Biological and Anthropogenic Influences; Environmental Protection Secretariat: Manama, Bahrain, 1986; Volume 1. [Google Scholar]
- Wilson, S.C. Northwestern Arabian Sea and Gulf of Oman. In Seas of the Millennium—An Environmental Evaluation; Chapter 54; Sheppard, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Duarte, C.M.; Middelburg, J.J.; Caraco, N. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2005, 2, 1–8. [Google Scholar] [CrossRef]
- Campbell, J.E.; Lacey, E.A.; Decker, R.A.; Crooks, S.; Fourqurean, J.W. Carbon Storage in Seagrass Beds of Abu Dhabi, United Arab Emirates. Chesap. Sci. 2014, 38, 242–251. [Google Scholar] [CrossRef]
- Duarte, C.M.; Kennedy, H.; Marbà, N.; Hendriks, I. Assessing the capacity of seagrass meadows for carbon burial: Current limitations and future strategies. Ocean. Coast. Manag. 2013, 83, 32–38. [Google Scholar] [CrossRef]
- Schile, L.M.; Kauffman, J.B.; Crooks, S.; Fourqurean, J.W.; Glavan, J.; Megonigal, P. Limits on carbon sequestration in arid blue carbon ecosystems. Ecol. Appl. 2017, 27, 859–874. [Google Scholar] [CrossRef]
- Björk, M.; Short, F.; Mcleod, E.; Beer, S. Managing Seagrasses for Resilience to Climate Change; IUCN: Gland, Switzerland, 2008; p. 56. [Google Scholar]
- Edwards, A.J. Chapter 10: Impact of climatic change on coral reefs, mangroves, and tropical seagrass ecosystems. In Climate Change: Impact on Coastal Habitation; Eisma, D., Ed.; Lewis Publishers: Amsterdam, The Netherlands, 1995; pp. 209–234. [Google Scholar]
- Green, E.P.; Short, F.T. (Eds.) World Atlas of Seagrasses. In Prepared by the UNEP World Conservation Monitoring Centre; University of California Press: Berkeley, CA, USA, 2003. [Google Scholar]
- Marbà, N.; Duarte, C. Growth response of the seagrass Cymodocea nodosa to experimental burial and erosion. Mar. Ecol. Prog. Ser. 1994, 107, 307–311. [Google Scholar] [CrossRef]
- Vermaat, J.E.; Agawin, N.S.R.; Fortes, M.D.; Uri, J.S. The capacity of seagrass to survive increased turbidity and siltation: The significance of growth form and light use. Ambio 1997, 25, 499–504. [Google Scholar]
- Manzello, D.P.; Enochs, I.C.; Melo, N.; Gledhill, D.K.; Johns, E.M. Ocean acidification refugia of the Florida Reef Tract. PLoS ONE 2012, 7, e41715. [Google Scholar] [CrossRef]
- Vinagre, C.; Leal, I.; Mendonça, V.; Madeira, D.; Narciso, L.; Diniz, M.; Flores, A. Vulnerability to climate warming and acclimation capacity of tropical and temperate coastal organisms. Ecol. Indic. 2016, 62, 317–327. [Google Scholar] [CrossRef]
- Coles, S.L.; Looker, E.; Burt, J. Twenty-year changes in coral near Muscat, Oman estimated from manta board tow observations. Mar. Environ. Res. 2015, 103, 66–73. [Google Scholar] [CrossRef]
- Schils, T.; Wilson, S.C. Temperature threshold as a biogeographic barrier in northern Indian Ocean macroalgae. J. Phycol. 2006, 42, 749–756. [Google Scholar] [CrossRef]
- Sheppard, C.R.C.; Price, A.R.G.; Roberts, C.M. Marine Ecology of the Arabian Region: Patterns and Processes in Extreme Tropical Environments; Academic Press: London, UK, 1992. [Google Scholar]
- De Waal, S.; Khoom, S.; Al-Ghassani, S. Southwest monsoon effects on the growth of juvenile abalone (Haliotis mariae Wood, 1828) along the western Dhofar coast, Arabian Sea, Sultanate of Oman. Reg. Stud. Mar. Sci. 2016, 3, 189–193. [Google Scholar] [CrossRef]
- Hattam, C.; Atkins, J.P.; Beaumont, N.; Börger, T.; Böhnke-Henrichs, A.; Burdon, D.; De Groot, R.; Hoefnagel, E.; Nunes, P.A.; Piwowarczyk, J.; et al. Marine ecosystem services: Linking indicators to their classification. Ecol. Indic. 2015, 49, 61–75. [Google Scholar] [CrossRef]
- Hooper, T.; Cooper, P.; Hunt, A.; Austen, M. A methodology for the assessment of local-scale changes in marine environmental benefits and its application. Ecosyst. Serv. 2014, 8, 65–74. [Google Scholar] [CrossRef][Green Version]
- Ben-Hasan, A.; Christensen, V. Vulnerability of the marine ecosystem to climate change impacts in the Arabian Gulf—an urgent need for more research. Glob. Ecol. Conserv. 2019, 17, e00556. [Google Scholar] [CrossRef]
- RECOFI. Trends and Emerging Issues of the Gulf Fisheries: A Regional Perspective. Regional Commission for Fisheries (RECOFI). In Proceedings of the Fourth meeting of the Working Group on Fisheries Management, Muscat, Oman, 3–5 October 2010. [Google Scholar]
- Cheung, W.W.; Lam, V.W.; Sarmiento, J.L.; Kearney, K.; Watson, R.; Pauly, D. Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish. 2009, 10, 235–251. [Google Scholar] [CrossRef]
- Deutsch, C.; Ferrel, A.; Seibel, B.; Pörtner, H.-O.; Huey, R.B. Climate change tightens a metabolic constraint on marine habitats. Science 2015, 348, 1132–1135. [Google Scholar] [CrossRef]
- Pörtner, H.-O.; Peck, M. Climate change effects on fishes and fisheries: Towards a cause-and-effect understanding. J. Fish Biol. 2010, 77, 1745–1779. [Google Scholar] [CrossRef] [PubMed]
- Al-Maktoumi, A.; Zekri, S.; El-Rawy, M.; Abdalla, O.; Al-Wardy, M.; Al-Rawas, G.; Charabi, Y. Assessment of the impact of climate change on coastal aquifers in Oman. Arab. J. Geosci. 2018, 11, 501. [Google Scholar] [CrossRef]
- ICES. Report of the ICES-IOC Working Group on Harmful Algal Bloom Dynamics (WGHABD). 24–27 April 2012. Available online: https://www.ices.dk/sites/pub/Publication%20Reports/Expert%20Group%20Report/SSGHIE/2012/WGHABD12.pdf (accessed on 7 April 2021).
- Muthian, T.; Al-Aisery, A.; Al-Kharusi, L. Harmful algal blooms and their impacts in the middle and outer ROPME sea area. Int. J. Ocean. Oceanogr. 2007, 1, 85–98. [Google Scholar]
- Pinnegar, J.K.; Howes, E.L.; Engelhard, G.H.; Le Quesne, W.J.F. Omani Fisheries and Aquaculture Climate Change Risk Assessment. Centre for Environment, Fisheries and Aquaculture Science (Cefas), Lowestoft, United Kingdom. 2020. Available online: https://www.sustainablefinance.hsbc.com/carbon-transition/climate-change-risk-assessment (accessed on 21 October 2020).
- Richlen, M.L.; Morton, S.L.; Jamali, E.A.; Rajan, A.; Anderson, D.M. The catastrophic 2008–2009 red tide in the Arabian gulf region, with observations on the identification and phylogeny of the fish-killing dinoflagellate Cochlodinium polykrikoides. Harmful Algae 2010, 9, 163–172. [Google Scholar] [CrossRef]
- Townhill, B.L.; Redford, Z.; Pecl, G.; van Putten, I.; Pinnegar, J.K.; Hyder, K. Climate change influences on marine recreational fishers and their catches. Fish Fish. 2019, 20, 977–992. [Google Scholar] [CrossRef]
- De Silva, S.S.; Soto, D. Climate change and aquaculture: Potential impacts, adaptation and mitigation. In Climate Change Implications for Fisheries and Aquaculture: Overview of Current Scientific Knowledge; FAO Fisheries and Aquaculture Technical Paper. No., 530; Cochrane, K., De Young, C., Soto, D., Bahri, T., Eds.; FAO: Rome, Italy, 2009; pp. 151–212. Available online: https://www.scribd.com/document/23975867/FAO-Climate-Change-Implications-for-Fisheries-and-Aquaculture-TP-530 (accessed on 21 October 2020).
- Risk, M.J.; Haghshenas, S.A.; Arab, A.R. Cage aquaculture in the Persian Gulf: A cautionary tale for Iran and the world. Mar. Pollut. Bull. 2021, 166, 112079. [Google Scholar] [CrossRef] [PubMed]
- Doubleday, Z.A.; Prowse, T.A.; Arkhipkin, A.; Pierce, G.J.; Semmens, J.; Steer, M.; Leporati, S.C.; Lourenço, S.; Quetglas, A.; Sauer, W.; et al. Global proliferation of cephalopods. Curr. Biol. 2016, 26, R406–R407. [Google Scholar] [CrossRef]
- Monahan, P. World octopus and squid populations are booming. Science News. 2016. Available online: https://www.science.org/content/article/world-octopus-and-squid-populations-are-booming (accessed on 29 March 2021). [CrossRef]
- The National. The Qatar Pearl Legacy Aims to Revive the Gulf’s Pearl-Farming Tradition. UAE. 2015. Available online: https://www.thenationalnews.com/arts-culture/the-qatar-pearl-legacy-aims-to-revive-the-gulf-s-pearl-farming-tradition-1.96570 (accessed on 29 September 2020).
- Liu, W.; Yu, Z.; Huang, X.; Shi, Y.; Lin, J.; Zhang, H.; Yi, X.; He, M. Effect of ocean acidification on growth, calcification, and gene expression in the pearl oyster, Pinctada fucata. Mar. Environ. Res. 2017, 130, 174–180. [Google Scholar] [CrossRef]
- IPIECA. Addressing adaptation in the oil and gas industry. International Petroleum Industry Environmental Conservation Association IPIECA: London, UK. 2013. Available online: http://www.ipieca.org/publication/addressing-adaptation-oil-and-gas-industry (accessed on 17 September 2020).
- Dell, J. Petroleum Industry: Adaptation to Projected Impacts of Climate Change. Presentation at IPIECA workshop “Addressing Adaptation in the Oil and Gas Industry”. 9 October 2012, London, UK. Available online: https://www.ipieca.org/resources/awareness-briefing/addressing-adaptation-in-the-oil-and-gas-industry/ (accessed on 26 September 2020).
- Fencl, A.; Swartz, C.; Yates, D. Climate Change Impacts, Vulnerability, and Adaptation. Stockholm Environment Institute. Prepared for the Environ-ment Agency of Abu Dhabi. 2009. Available online: http://seius.org/Publications_PDF/SEI-EAD-ImpactVulnerabilityAdaptation-09.pdf (accessed on 17 September 2020).
- De Sa, A.; Al Zubaidy, S. Gas turbine performance at varying ambient temperature. Appl. Therm. Eng. 2011, 31, 2735–2739. [Google Scholar] [CrossRef]
- Mokhatab, S.; Mak, J.Y.; Valappil, J.V.; Wood, D.A. Handbook of Liquefied Natural Gas; Gulf Professional Publishing: Houston, TX, USA, 2014; p. 589. ISBN 9780124045859. [Google Scholar] [CrossRef]
- Bartos, M.; Chester, M.; Johnson, N.; Gorman, B.; Eisenberg, D.; Linkov, I.; Bates, M. Impacts of rising air temperatures on electric transmission ampacity and peak electricity load in the United States. Environ. Res. Lett. 2016, 11, 114008. [Google Scholar] [CrossRef]
- INMR. Impact of Climate Change on Power Systems & Electrical Insulation: Experience in Italy. Independent T&D Information Resources, World’s Leading Transmission and Distribution Technical Journal. 2019. Available online: https://www.inmr.com/impact-climate-change-power-systems-electrical-insulation-experience-italy/ (accessed on 29 September 2020).
- AlFarra, H.J.; Abu-Hijleh, B. The potential role of nuclear energy in mitigating CO2 emissions in the UAE. Energy Policy 2012, 42, 272–285. Available online: https://www.sciencedirect.com/science/article/pii/S0301421511009827 (accessed on 29 September 2020). [CrossRef]
- El-Katiri, L. The GCC and the Nuclear Question. Oxford Energy Comment. The Oxford Institute for Energy Studies, University of Oxford. December 2012. Available online: https://eprints.soas.ac.uk/14651/1/The-GCC-and-the-Nuclear-Question.pdf (accessed on 20 October 2020).
- Kim, B.K.; Jeong, Y.H. High cooling water temperature effects on design and operational safety of npps in the gulf region. Nucl. Eng. Technol. 2013, 45, 961–968. [Google Scholar] [CrossRef]
- Mideksa, T.K.; Kallbekken, S. The impact of climate change on the electricity market: A review. Energy Policy 2010, 38, 3579–3585. [Google Scholar] [CrossRef]
- Abdulaziz, P.; Al-Tisan, I.; Al-Daili, M.; Green, T.; Ghani, A.; Javeed, M. Effects of environment on source water for desalination plants on the eastern coast of Saudi Arabia. Desalination 2000, 132, 29–40. [Google Scholar] [CrossRef]
- UAE State of Energy Report. United Arab Emirates Ministry of Energy and Industry. 2019. Available online: https://www.moei.gov.ae/en/open-data.aspx (accessed on 17 September 2020).
- Al-Senafy, M.; Al-Fahad, K.; Hadi, K. Water management strategies in the Arabian Gulf countries. Dev. Water Sci. 2003, 50, 221–224. [Google Scholar] [CrossRef]
- Ulrichsen, K.C. Internal and External Security in the Arab Gulf States. Middle East Policy 2009, 16, 39–58. [Google Scholar] [CrossRef]
- Verner, D. Adaptation to a Changing Climate in the Arab Countries: A Case for Adaptation Governance and Leadership in Building Climate Resilience; MENA Development Report; The World Bank: Washington, DC, USA, 2012; p. 402. ISBN 978-0-8213-9458-8. [Google Scholar] [CrossRef]
- WWAP. The United Nations World Water Development Report 2015: Water for a Sustainable Word; United Nations World Water Assessment Programme: Paris, France, 2015; Available online: https://unesdoc.unesco.org/ark:/48223/pf0000231823/PDF/231823eng.pdf.multi (accessed on 10 September 2020).
- Anderson, D.M.; Boerlage, S.F.E.; Dixon, M.B. (Eds.) Harmful Algal Blooms (HABs) and Desalination: A Guide to Impacts, Monitoring and Management. Paris, Intergovernmental Oceanographic Commission of UNESCO. 539p. IOC Manuals and Guides No.78. (IOC/2017/MG/78). 2017. Available online: https://medrc.org/wp-content/uploads/2018/05/259512E.pdf (accessed on 29 September 2020).
- Al-Saidi, M. Coastal Development and Climate Risk Reduction in the Persian/Arabian Gulf. Clim. Chang. Ocean Gov. 2019, 4, 60–74. [Google Scholar] [CrossRef]
- UNFCCC. NDC Registry (Interim). 2021. Available online: https://www4.unfccc.int/sites/NDCStaging/Pages/All.aspx (accessed on 29 September 2020).
- Touati, F.; Alam Chowdhury, N.; Benhmed, K.; Gonzales, A.J.S.P.; Al-Hitmi, M.A.; Benammar, M.; Gastli, A.; Ben-Brahim, L. Long-term performance analysis and power prediction of PV technology in the State of Qatar. Renew. Energy 2017, 113, 952–965. [Google Scholar] [CrossRef]
- Reguero, B.G.; Losada, I.J.; Méndez, F.J. A recent increase in global wave power as a consequence of oceanic warming. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Pryor, S.; Barthelmie, R.J. Climate change impacts on wind energy: A review. Renew. Sustain. Energy Rev. 2010, 14, 430–437. [Google Scholar] [CrossRef]
- Rahman, S.A.; Varma, R.K.; Vanderheide, T. Generalised model of a photovoltaic panel. IET Renew. Power Gener. 2014, 8, 217–229. [Google Scholar] [CrossRef]
- AFED. Report of the Arab Forum for Environment and Development. Arab Environment Climate Change. Impact of Climate Change on Arab Countries. In Arab Forum for Environment and Development (AFED); Tolba, M.K., Saab, N.W., Eds.; Technical Publications, Environment and Development: Beirut, Lebanon, 2009; Available online: http://www.afedonline.org/afedreport09/Full%20English%20Report.pdf (accessed on 27 October 2020)ISBN 9953-437-28-9.
- Nadim, F.; Bagtzoglou, A.C.; Iranmahboob, J. Coastal management in the Persian Gulf region within the framework of the ROPME programme of action. Ocean Coast. Manag. 2008, 51, 556–565. [Google Scholar] [CrossRef]
- Esmaela, A.A.; Abbasb, F.S.; Hamzah, M.K. The Strategic Importance of the Strait of Hormuz and Its Impact on the Iranian—American Conflict. Int. J. Innov. Creat. Chang. 2020, 12, 335–345. [Google Scholar]
- Al Blooshi, A.; Al Dhaheri, S.; Grandcourt, E.; Al Meri, H.; Al Ameri, M.; Al Baharna, R.; Cowie, W. Fisheries. In Abu Dhabi State of the Environment Report 2017; Al Mubarak, R.K., Al Mazrouei, S., Launay, F., Perry, R., Eds.; Environment Agency: Abu Dhabi, United Arab Emirates, 2017. [Google Scholar]
- Asariotis, R.; Benamara, H.; Mohos-Naray, V. Port Industry Survey on Climate Change Impacts and Adaptation. UNCTAD Research Paper No. 18. UNCTAD/SER.RP/2017/18. United Nations Conference on Trade and Development. 2017. Available online: https://unctad.org/en/pages/PublicationWebflyer.aspx?publicationid=1964 (accessed on 17 September 2020).
- UNCTAD. Climate Change Impacts on Coastal Transportation Infrastructure in the Caribbean: Enhancing the Adaptive Capacity of Small Island Developing States (SIDS). SAINT LUCIA: A Case study. UNDA 1415O. 2017. Available online: https://sidsport-climateadapt.unctad.org/wp-content/uploads/2018/06/overview-of-project-and-key-results.pdf (accessed on 5 April 2021).
- Mentaschi, L.; Vousdoukas, M.I.; Voukouvalas, E.; Dosio, A.; Feyen, L. Global changes of extreme coastal wave energy fluxes triggered by intensified teleconnection patterns. Geophys. Res. Lett. 2017, 44, 2416–2426. [Google Scholar] [CrossRef]
- Rossouw, M.; Theron, A. Investigation of potential climate change impacts on ports and maritime operations around the S. African coast. In Maritime Transport and the Climate Change Challenge; Asariotis, R., Benamara, H., Eds.; Routledge: London, UK, 2012; pp. 286–304. [Google Scholar]
- IPCC SREX. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation. In A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change IPCC; Field, C.B., Barros, V., Stocker, T.F., Qin, D., Dokken, D.J., Ebi, K.L., Mastrandrea, M.D., Mach, K.J., Plattner, G.K., Allen, S.K., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012; 582p. [Google Scholar]
- Wamsley, T.V.; Cialone, M.A.; Smith, J.M.; Atkinson, J.H.; Rosati, J.D. The potential of wetlands in reducing storm surge. Ocean Eng. 2010, 37, 59–68. [Google Scholar] [CrossRef]
- Izaguirre, C.; Losada, I.J.; Camus, P.; Vigh, J.L.; Stenek, V. Climate change risk to global port operations. Nat. Climate Chang. 2021, 11, 14–20. [Google Scholar] [CrossRef]
- Rahmstorf, S. Climate Change: State of Science. In Maritime Transport and the Climate Change Challenge; Asariotis, R., Benamara, H., Eds.; Earthscan, UN Digital Library: Geneva, Switzerland, 2012; pp. 3–11. [Google Scholar]
- UNCTAD. Ad Hoc Expert Meeting on Climate Change Impacts and Adaptation: A Challenge for Global Ports. Information note by the UNCTAD secretariat. UNCTAD/DTL/TLB/2011/2. 2011. Available online: http://unctad.org/en/Docs/dtltlb2011d2_en.pdf (accessed on 15 September 2020).
- UNECE. Climate Change Impacts and Adaptation for International Transport Networks. Expert Group Report, ITC, UN Economic Commission for Europe ECE/TRANS/238. 2013. Available online: http://www.unece.org/fileadmin/DAM/trans/main/wp5/publications/climate_change_2014.pdf (accessed on 10 September 2020).
- ADCED. Transportation Infrastructure: Ensuring that Abu Dhabi’s Economy Remains on Track. The Economic Review. Abu Dhabi Council for Economic Development, Abu Dhabi. Econo. Rev. 2014, 17, 54. Available online: https://issuu.com/adced_the_economic_review/docs/ter_17_eng_arabic_combined (accessed on 27 October 2020).
- Valdor, P.F.; Gómez, A.G.; Juanes, J.A.; Kerléguer, C.; Steinberg, P.; Tanner, E.; MacLeod, C.; Knights, A.M.; Seitz, R.D.; Airoldi, L.; et al. A global atlas of the environmental risk of marinas on water quality. Mar. Pollut. Bull. 2019, 149, 110661. [Google Scholar] [CrossRef]
- Vogel, M.M.; Orth, R.; Cheruy, F.; Hagemann, S.; Lorenz, R.; Hurk, B.J.J.M.V.D.; Seneviratne, S.I. Regional amplification of projected changes in extreme temperatures strongly controlled by soil moisture-temperature feedbacks. Geophys. Res. Lett. 2017, 44, 1511–1519. [Google Scholar] [CrossRef]
- Al-Buloshi, A.; Al-Hatrushi, S.; Charabi, Y. GIS-based framework for the simulation of the impacts of sea level rise and coastal flooding on Oman. J. Earth Sci. Clim. Chang. 2014, 5, 1. [Google Scholar]
- Al-Jeneid, S.; Bahnassy, M.; Nasr, S.; El Raey, M. Vulnerability assessment and adaptation to the impacts of sea level rise on the Kingdom of Bahrain. Mitig. Adapt. Strat. Glob. Chang. 2007, 13, 87–104. [Google Scholar] [CrossRef]
- Dasgupta, S.; Laplante, B.; Meisner, C.; Wheeler, D.; Yan, J. The Impact Of Sea Level Rise On Developing Countries: A Comparative Analysis. Clim. Chang. 2009, 93, 379–388. [Google Scholar] [CrossRef]
- Melville-Rea, H.; Eayrs, C.; Anwahi, N.; Burt, J.A.; Holland, D.; Samara, F.; Paparella, F.; Al Murshidi, A.H.; Al-Shehhi, M.R.; Holland, D.M. A Roadmap for Policy-Relevant Sea level Rise Research in the United Arab Emirates. Front. Mar. Sci. 2021, 8, 670089. [Google Scholar] [CrossRef]
- Jevrejeva, S.; Jackson, L.P.; Grinsted, A.; Lincke, D.; Marzeion, B. Flood damage costs under the sea level rise with warming of 1.5 °C and 2 °C. Environ. Res. Lett. 2018, 13, 074014. [Google Scholar] [CrossRef]
- Nicholls, R.J.; Hanson, S.; Herweijer, C.; Patmore, N.; Hallegatte, S.; Corfee-Morlot, J.; Chateau, J.; Muir-Wood, R. Ranking Port Cities with High Exposure and Vulnerability to Climate Extremes: Exposure Estimates; OECD ENV/WKP 2007-1; OECD: Paris, France, 2008; 62p. [Google Scholar]
- Adamo, N.; Al-Ansari, N.; Sissakian, V.K.; Knutsson, S.; Laue, J. Climate change: Consequences on Iraq’s environment. J. Earth Sci. Geotech. Eng. 2018, 8, 43–58. [Google Scholar]
- USDOT. Impacts of Climate Change and Variability on Transportation Systems and Infrastructure: The Gulf Coast Study, Phase II. In A report by the US Department of Transportation, Center for Climate Change and Environmental Forecasting; Choate, A., Jaglom, W., Miller, R., Rodehorst, B., Schultz, P., Snow, C., Eds.; Department of Transportation: Washington, DC, USA, 2012; 470p. [Google Scholar]
- ADB. Guidelines for Climate Proofing Investment in the Water Sector: Water Supply and Sanitation; Asian Development Bank: Mandaluyong City, Philippines, 2017. [Google Scholar]
- Al Braiki, S.; Alseiari, F.; Sahel, M.; Mosa, M.; Salem, M.; Suleiman, W.; Othman, Y. Abu Dhabi State of the Environment Report 2017-Waste. Abu Dhabi: Environment Agency-Abu Dhabi. 2017. Available online: https://www.soe.ae/wp-content/uploads/2017/11/Waste_English.pdf (accessed on 27 October 2020).
- Environment Agency: Abu Dhabi. Waste and Environment Annual Report 2016; EAD Abu Dhabi: Abu Dhabi, United Arab Emirates, 2016; Available online: https://www.ead.ae/Publications/Waste%20and%20Environment%20Annual%20Report%202016/Waste%20and%20Environemnt%20Annual%20Report%202016.pdf (accessed on 8 June 2018).
- Winne, S.; Horrocks, L.; Kent, N.; Miller, K.; Hoy, C.; Benzie, M.; Power, R. Increasing the Climate Resilience of Waste Infrastructure. Oxford: AEA. 2012. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/183933/climate-resilience-full.pdf (accessed on 10 September 2020).
- Gladstone, W.; Curley, B.; Shokri, M.R. Environmental impacts of tourism in the Gulf and the Red Sea. Mar. Pollut. Bull. 2013, 72, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Perch-Nielsen, S.L.; Amelung, B.; Knutti, R. Future climate resources for tourism in Europe based on the daily tourism climate index. Clim. Chang. 2010, 103, 363–381. [Google Scholar] [CrossRef]
- Hassan, E.M.; Varshosaz, K.; Eisakhani, N. Analysis and Estimation of Tourism Climatic Index (TCI) and Temperature-Humidity Index (THI) in Dezfoul. In In Proceedings of the 4th International Conference on Environment, Energy and Biotechnology (ICEEB 2015), Madrid, Spain, 15–16 June 2015; Volume 85, pp. 35–39. [Google Scholar] [CrossRef]
- Blignaut, J.; Mander, M.; Inglesi-Lotz, R.; Glavan, J.; Parr, S. Introduction Economic value of the Abu Dhabi coastal and marine ecosystem services—Estimate and management applications. In Sustainability in the Gulf, Challenges and Opportunities; Routledge: London, UK, 2017; pp. 210–227. [Google Scholar]
- Mfarrej, M.B. Climate change patterns in the UAE: A qualitative research and review. Nat. Environ. Pollut. Technol. 2019, 18, 261–268. [Google Scholar]
- Roshan, G.; Yousefi, R.; Fitchett, J. Long-term trends in tourism climate index scores for 40 stations across Iran: The role of climate change and influence on tourism sustainability. Int. J. Biometeorol. 2016, 60, 33–52. [Google Scholar] [CrossRef]
- Agnew, M.D.; Viner, D. Potential Impacts of Climate Change on International Tourism. Tour. Hosp. Res. 2001, 3, 37–60. [Google Scholar] [CrossRef]
- Ancient Ports—Ports Antiques. Ancient Ports in the Indian Ocean & Gulf. 2021. Available online: https://www.ancientportsantiques.com/contacts/contributors-to-this-catalogue/ (accessed on 14 September 2020).
- Kumar, A. Environmental degradation along the Persian/Arabian Gulf coast of Saudi Arabia. Earth Science India. Popul. Issue 2017, 10, 1–13. Available online: https://www.researchgate.net/profile/Arun_Kumar605/publication/316693473_Environmental_degradation_along_the_PersianArabian_Gulf_Coast_of_Saudi_Arabia/links/590cfec8a6fdccad7b0e37fd/Environmental-degradation-along-the-Persian-Arabian-Gulf-Coast-of-Saudi-Arabia.pdf (accessed on 21 October 2020).
- UNESCO. Case Studies on Climate Change and World Heritage; UNESCO: Paris, France, 2007; Available online: https://portals.iucn.org/library/sites/library/files/documents/Bios-Cons-Nat-Pro-127.pdf (accessed on 15 September 2020).
- Von Schorlemer, S.; Maus, S. Climate Change as a Threat to Peace; Peter Lang Verlag: Berlin, Germany, 2015; Available online: https://www.peterlang.com/document/1067605 (accessed on 13 September 2021).
- Waycott, M.; McKenzie, L.J.; Mellors, J.E.; Ellison, J.C.; Sheaves, M.T.; Collier, C.; Schwarz, A.M.; Webb, A.; Johnson, J.E.; Payri, C.E. Vulnerability of mangroves, seagrasses and intertidal flats in the tropical Pacific to climate change. In Vulnerability of Tropical Pacific Fisheries and Aquaculture to Climate Change; Bell, J.D., Johnson, J.E., Hobday, A.J., Eds.; Secretariat of the Pacific Community: Noumea, New Caledonia, 2011; pp. 297–368. [Google Scholar]
- Paul, M. The protection of sandy shores – Can we afford to ignore the contribution of seagrass? Mar. Pollut. Bull. 2018, 134, 152–159. [Google Scholar] [CrossRef]
- Potouroglou, M.; Bull, J.; Krauss, K.W.; Kennedy, H.A.; Fusi, M.; Daffonchio, D.; Mangora, M.; Githaiga, M.N.; Diele, K.; Huxham, M. Measuring the role of seagrasses in regulating sediment surface elevation. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Beck, M.W.; Losada, I.J.; Menéndez, P.; Reguero, B.G.; Díaz-Simal, P.; Fernández, F. The global flood protection savings provided by coral reefs. Nat. Commun. 2018, 9, 2186. [Google Scholar] [CrossRef]
- AGEDI. Technical Report, Coastal Vulnerability Index. LNRCCP. CCRG. 2016. Available online: https://agedi.org/complete-resource-library/download-info/technical-report-uae-coastal-vulnerability-index-project/ (accessed on 29 September 2020).
- Alongi, D.M. The Impact of Climate Change on Mangrove Forests. Curr. Clim. Chang. Rep. 2015, 1, 30–39. [Google Scholar] [CrossRef]
- Al-Yamani, F.; Saburova, M. Illustrated Guide on the Flagellates of Kuwait’s Intertidal Soft Sediments; Kuwait Institute for Scientific Research: Kuwait City, Kuwait, 2010; ISBN 978-99906-95-04-5. [Google Scholar]
- Al-Yamani, F.; Skryabin, V.; Boltachova, N.; Revkov, N.; Makarov, M.; Grinstov, V.; Kolesnikova, E. Illustrated Atlas on the Zoobenthos of Kuwait. (KISR); Kuwait Institute for Scientific Research: Kuwait City, Kuwait, 2012; ISBN 99906-41-40-4. [Google Scholar]
- Saburova, M.; Al-Yamani, F.; Polikarpov, I. Biodiversity of free-living flagellates in Kuwait’s intertidal sediments. BioRisk 2009, 3, 97–110. [Google Scholar] [CrossRef][Green Version]
- NOAA Climate Change Portal. CMIP5. 2020. Available online: https://psl.noaa.gov/ipcc/ocn/timeseries.html (accessed on 29 September 2020).
- Burt, J.A.; Bartholomew, A. Towards more sustainable coastal development in the Arabian Gulf: Opportunities for ecological engineering in an urbanized seascape. Mar. Pollut. Bull. 2019, 142, 93–102. [Google Scholar] [CrossRef]
- Campos, E.J.D.; Vieira, F.; Cavalcante, G.; Kjerfve, B.; Abouleish, M.; Shahriar, S.; Mohamed, R.; Gordon, A.L. Impacts of brine disposal from water desalination plants on the physical environment in the Persian/Arabian Gulf. Environ. Res. Commun. 2020, 2, 125003. [Google Scholar] [CrossRef]
- Boero, F.; Brotz, L.; Gibbons, M.J.; Piraino, S.; Zampardi, S. Impacts and effects of ocean warming on jellyfish. In Explaining Ocean Warming: Causes, Scale, Effects and Consequences; Laffoley, D., Baxter, J.M., Eds.; IUCN: Gland, Switzerland, 2016; pp. 213–237. ISBN 978-2-8317-1806-4. [Google Scholar] [CrossRef]
- Duarte, C.M.; Pitt, K.; Lucas, C.; E Purcell, J.; Uye, S.-I.; Robinson, K.; Brotz, L.; Decker, M.B.; Sutherland, K.; Malej, A.; et al. Is global ocean sprawl a cause of jellyfish blooms? Front. Ecol. Environ. 2013, 11, 91–97. [Google Scholar] [CrossRef]

| Physical and Chemical Changes | Selected Key Impacts |
|---|---|
| Temperature, salinity, and humidity | Average sea temperature increases of one degree centigrade over past 30 years, with shallow areas warming faster than deeper areas; this trend will continue. The highest ever sea temperature was recorded in Kuwait. In the I-RSA, salinity increase observed and future increases in humidity projected. |
| Sea level and ocean circulation | Few sea level gauges available provide sea-level rise estimates of 2.2 mm/yr in northwest I-RSA, potentially higher than in wider Indian Ocean. |
| pH and dissolved oxygen | Few measurements available show a decrease in pH in the O-RSA of 0.1 (shallow) to 0.2 (deep) units in recent decades. Oxygen minima in M-RSA and O-RSA, and seasonal low oxygen zones in I-RSA, projected to worsen. |
| Cyclones and dust storms | More intense storms and cyclones expected to occur in the O-RSA and M-RSA, may even reach the I-RSA. Apparent increase in Shamal winds and dust storms in the I-RSA this century. |
| Monsoon winds and upwelling | Changes anticipated in monsoon strength and timing affecting the M-RSA and O-RSA, including influence on upwelling system, but complexity makes impacts hard to predict. |
| Current and future impacts on biodiversity | |
| Phytoplankton productivity | Large-scale changes in primary productivity would have important repercussions for the marine food chain, including fisheries, but high natural variability and a lack of high-resolution data mean that projections have low confidence. |
| Harmful Algal Blooms (HABs) | HABs appear to be increasing across the RSA, but links to climate change are not fully established. |
| Jellyfish | Outbreaks of jellyfish blooms and aggregations are becoming more frequent. This has been partly attributed to warming temperatures, as well as other factors such as loss of natural predators, thermal pollution from industrial sites and eutrophication. |
| Fish | Up to 10% of fish species occurring in the I-RSA may become regionally extinct by the end of the century, partly due to temperature and salinity changes. Pelagic fish species such as tuna and sardines in the M-RSA and O-RSA may decline due to the expansion of the OMZ. |
| Birds | The RSA coastline and especially its wetland areas are highly vulnerable to climate change impacts as well as human activities, and potential degradation and loss of habitat has serious implications for dependent bird populations. |
| Marine mammals and turtles | Indirect climate change effects on food availability for some species may be important. Temperature change may affect turtle hatchlings, and flooding and erosion could damage turtle nesting areas. |
| Corals and coral reefs | There has been a rapid decline in the RSA’s coral reefs in the last two decades; this will continue and intensify as temperatures increase, and is linked to a wide range of climatic drivers as well as other human pressures. |
| Saltmarshes, mudflats, sabkhas | Coastal wetlands are highly vulnerable to sea-level rise, storm and cyclone damage and increasingly arid conditions, as well as over-exploitation, coastal development and changes to drainage. |
| Mangroves | Changing rainfall patterns, increased evaporation and storm damage are likely to negatively affect mangroves, including the availability of sediment to keep pace with sea-level rise. |
| Seagrass | Seagrasses in the RSA can withstand elevated temperatures but are adversely affected by prolonged exposure. Turbidity, excess sediment loads and scouring during and following extreme weather can also damage seagrasses. However, increased carbon dioxide availability may stimulate growth. |
| Rocky shores and macroalgal communities | Rocky shore habitats could be negatively impacted by climate change, especially where species are already close to their thermal tolerance. Wave action and scouring from storms and cyclones could have serious impacts on their benthic communities. |
| Current and future impacts on economy and society | |
| Provision of seafood and other living resources | Warming sea temperatures, oxygen depletion and changes in salinity are likely to have significant adverse effects on fisheries in the RSA. |
| Provision of energy, raw materials, space and transport | Coastal industries could be physically affected by long-term sea-level rise, cyclone risk, rising temperature and salinity affecting cooling systems and desalination and mass outbreaks of HABs and jellyfish. Offshore infrastructure and shipping could be more exposed to the physical impact of wind and waves. The health and wellbeing of coastal communities could be impacted by recurrent inundation, storms and intense heat and humidity. |
| Provision of leisure and culture | Some areas that are currently the focus of tourism developments may become less favourable in future due to loss of climate comfort. Sea-level rise and changes in storms will affect coastal and marine habitats and accelerate beach erosion. |
| Provision of good environmental quality and coastal protection | The natural coastal protection afforded by mangroves, salt marshes, seagrass beds and coral reefs could be limited or lost through increased flooding and erosion. Their roles in improving water quality as well as climate mitigation through carbon storage could be negatively affected. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lincoln, S.; Buckley, P.; Howes, E.L.; Maltby, K.M.; Pinnegar, J.K.; Ali, T.S.; Alosairi, Y.; Al-Ragum, A.; Baglee, A.; Balmes, C.O.; et al. A Regional Review of Marine and Coastal Impacts of Climate Change on the ROPME Sea Area. Sustainability 2021, 13, 13810. https://doi.org/10.3390/su132413810
Lincoln S, Buckley P, Howes EL, Maltby KM, Pinnegar JK, Ali TS, Alosairi Y, Al-Ragum A, Baglee A, Balmes CO, et al. A Regional Review of Marine and Coastal Impacts of Climate Change on the ROPME Sea Area. Sustainability. 2021; 13(24):13810. https://doi.org/10.3390/su132413810
Chicago/Turabian StyleLincoln, Susana, Paul Buckley, Ella L. Howes, Katherine M. Maltby, John K. Pinnegar, Thamer S. Ali, Yousef Alosairi, Alanoud Al-Ragum, Alastair Baglee, Chiden Oseo Balmes, and et al. 2021. "A Regional Review of Marine and Coastal Impacts of Climate Change on the ROPME Sea Area" Sustainability 13, no. 24: 13810. https://doi.org/10.3390/su132413810
APA StyleLincoln, S., Buckley, P., Howes, E. L., Maltby, K. M., Pinnegar, J. K., Ali, T. S., Alosairi, Y., Al-Ragum, A., Baglee, A., Balmes, C. O., Hamadou, R. B., Burt, J. A., Claereboudt, M., Glavan, J., Mamiit, R. J., Naser, H. A., Sedighi, O., Shokri, M. R., Shuhaibar, B., ... Le Quesne, W. J. F. (2021). A Regional Review of Marine and Coastal Impacts of Climate Change on the ROPME Sea Area. Sustainability, 13(24), 13810. https://doi.org/10.3390/su132413810