Diversity of the Seed Material of Selected Plant Species of Naturally Valuable Grassland Habitats in Terms of the Prognosis of Introduction Success
Abstract
:1. Introduction
2. Materials and Methods
2.1. Botanical Characteristics of Species
- 2 species of Cnidion meadows (habitat type code 6440; All. Cnidion dubii);
- 4 species of Molinia meadows (code 6410; All. Molinion caeruleae);
- 11 species of extensively used lowland hay meadows (code 6510; All. Arrhenatherion elatioris);
- 5 species of xerothermic calcareous grasslands (code 6210; Cl. Festuco-Brometea);
- 6 remaining species, belonging to other phytosociological units, but often found also in the above-mentioned types of meadows.
| Habitat—Code/Species | Family | Durability 1 | Life-Form 2 | Diaspore |
|---|---|---|---|---|
| Festuco-Brometea—6210 | ||||
| Artemisia campestris L. | Asteraceae | B, S | C | fruit |
| Centaurea stoebe L. | Asteraceae | D, B | H | fruit |
| Eryngium planum L. | Apiaceae | B | H | fruit |
| Scabiosa ochroleuca L. | Dipsacaceae | B, D | H | fruit |
| Verbascum thapsus L. | Scrophulariaceae | D | H | seed |
| Molinion caeruleae—6410 | ||||
| Galium boreale L. | Rubiaceae | B | H | fruit |
| Iris sibirica L. | Iridaceae | B | G | seed |
| Lychnis flos-cuculi L. | Caryophyllaceae | B | H | seed |
| Sanguisorba officinalis L. | Rosaceae | B | H | fruit |
| Cnidion dubii—6440 | ||||
| Allium angulosum L. | Amaryllidaceae | B | G | seed |
| Cnidium dubium (Schkuhr) Thell. | Apiaceae | B | H | fruit |
| Arrhenatherion elatioris—6510 | ||||
| Achillea millefolium L. | Asteraceae | B | H | fruit |
| Campanula patula L. | Campanulaceae | D, B | H | seed |
| Centaurea jacea L. | Asteraceae | B | H | fruit |
| Daucus carota L. | Apiaceae | D | H | fruit |
| Geranium pratense L. | Geraniaceae | B | H | seed |
| Lathyrus pratensis L. | Fabaceae | B | H | seed |
| Leontodon autumnalis L. | Asteraceae | B | H | fruit |
| Plantago lanceolata L. | Plantaginaceae | B | H | seed |
| Rumex acetosa L. | Polygonaceae | B | H | fruit |
| Tragopogon orientalis L. | Asteraceae | D | H | fruit |
| Trifolium pratense L. | Fabaceae | B | H | seed |
| Others | ||||
| Armeria maritima (MILL.) WILLD | Plumbaginaceae | B | H | fruit |
| Hypericum perforatum L. | Hypericaceae | B | H | seed |
| Linaria vulgaris L. | Plantaginaceae | B | G, H | seed |
| Lysimachia vulgaris L. | Primulaceae | B | H | seed |
| Potentilla erecta (L.) RAEUSCH | Rosaceae | B | H | fruit |
| Veronica longifolia L. | Plantaginaceae | B | H | seed |
2.2. Seed Collecting
2.3. Morphometric Characteristic and Germination Capacity Analysis
2.4. Statistical Analyses
2.5. Weather Conditions
3. Results
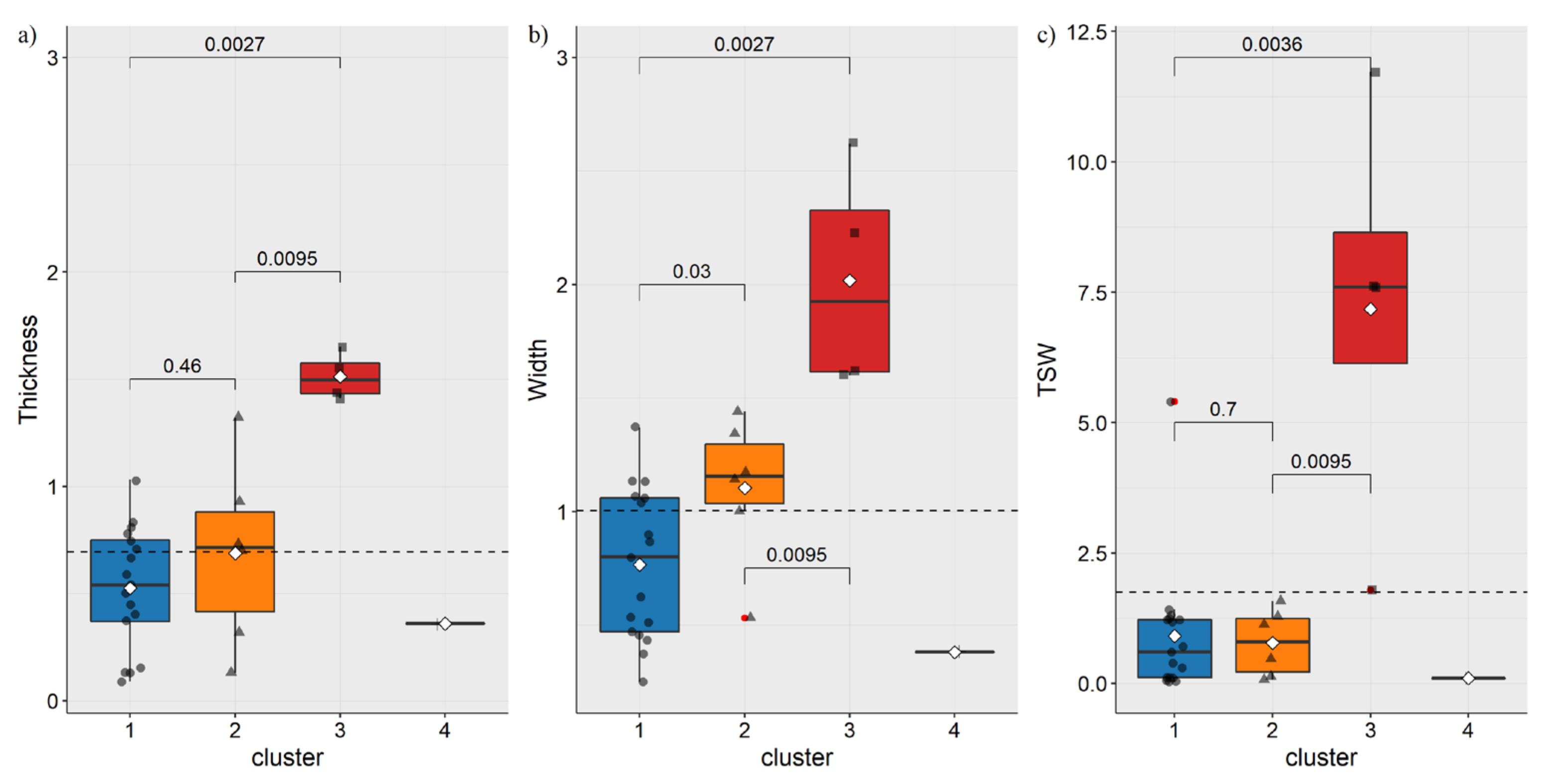
3.1. Morphometric Characteristic of Species
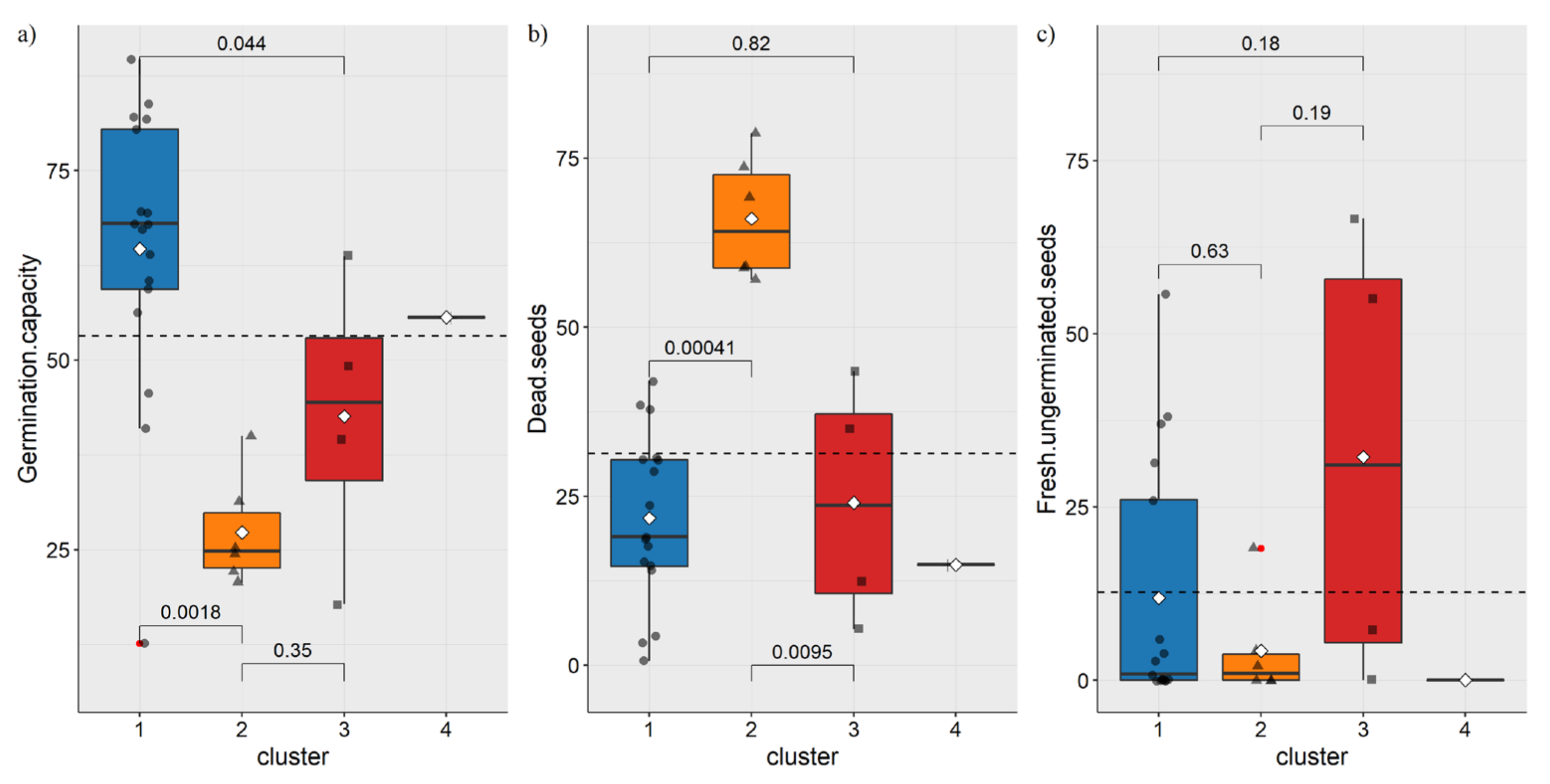
3.2. Germination Capacity Analysis
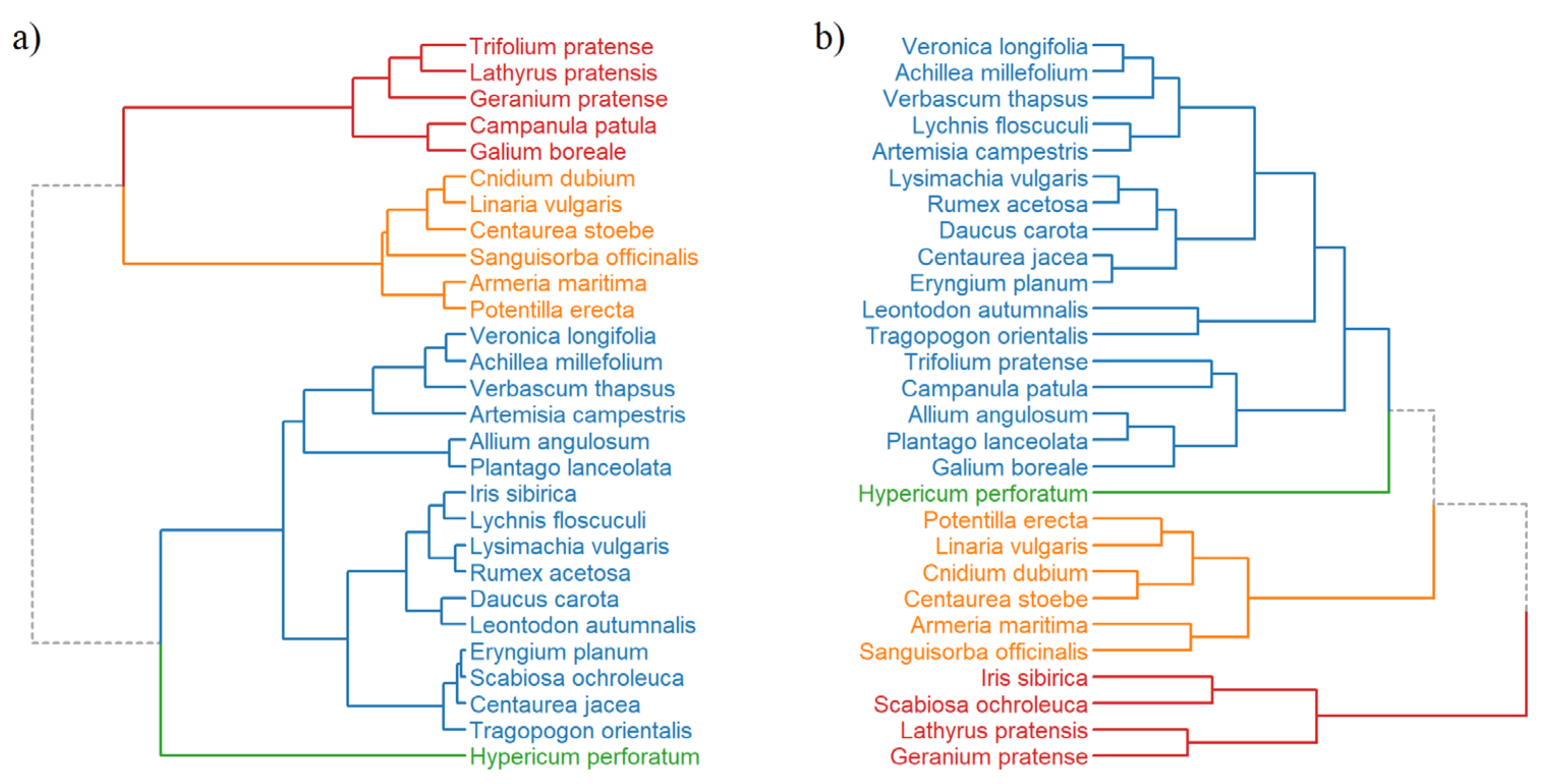
3.3. Hierarchical Clustering and PCA Analysis of Seed Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warda, M.; Kozłowski, S. Grassland—A Polish resource. In Grassland—A European Resource? Proceedings of the 24th General Meeting of the European Grassland Federation, Lublin, Poland, 3–7 June 2012, Grassland Science in Europe; Polish Grassland Society: Lublin, Poland, 2012; Volume 17, pp. 3–16. [Google Scholar]
- Wesche, K.; Krause, B.; Culmsee, H.; Leuschner, C. Fifty years of change in central European grassland vegetation: Large losses in species richness and animal-pollinated plants. Biol. Conserv. 2012, 150, 76–85. [Google Scholar] [CrossRef]
- Dillon, P.G. The Evolution of Grassland in the European Union in Terms of Utilisation, Productivity, Food Security and the Importance of Adoption of Technical Innovations in Increasing Sustainability of Pasture-Based Ruminant Production Systems. Sustainable Meat and Milk Production from Grasslands. Proceedings of the 27th General Meeting of the European Grassland Federation, Cork, Ireland, 17–21 June 2018; Teagasc: Dublin, Ireland, 2018; Volume 23, pp. 3–16. [Google Scholar]
- Kucharski, L. Vegetation of oat-grass meadows in central Poland. Steciana 2014, 18, 119–125. [Google Scholar] [CrossRef]
- Klarzyńska, A.A.; Kryszak, A. Floristic diversity of extensively used fresh meadows (6510) in the Wielki Łęg Obrzański Complex. Acta Agrobot. 2015, 68, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Warda, M.; Stamirowska-Krzaczek, E.; Kulik, M.; Tatarczak, M.; Bochniak, A. Vegetation changes and rare plant species in grasslands in the middle Wieprz Valley (PLH060005). Rocz. Ochr. Sr. 2018, 20, 481–494. [Google Scholar]
- Dengler, J.; Janišová, M.; Török, P.; Wellstein, C. Biodiversity of palaearctic grasslands: A synthesis. Agric. Ecosyst. Environ. 2014, 182, 1–14. [Google Scholar] [CrossRef] [Green Version]
- GIOŚ (Główny Inspektorat Ochrony Środowiska). Monitoring Gatunków i Siedlisk Przyrodniczych Ze Szczególnym Uwzględnieniem Specjalnych Obszarów Ochrony Siedlisk Natura 2000. Wyniki Monitoringu w Latach 2013–2014 (Siedlisko 6210) oraz 2016–2018 (Siedliska 6410, 6440, 6510); Państwowy Monitoring Środowiska. Available online: http://Www.Siedliska.Gios.Gov.Pl/Pl/ (accessed on 10 November 2021).
- Kiehl, K.; Kirmer, A.; Donath, T.W.; Rasran, L.; Hölzel, N. Species introduction in restoration projects—Evaluation of different techniques for the establishment of semi-natural grasslands in central and northwestern Europe. Basic Appl. Ecol. 2010, 11, 285–299. [Google Scholar] [CrossRef]
- Sengl, P.; Magnes, M.; Weitenthaler, K.; Wagner, V.; Erdős, L.; Berg, C. Restoration of lowland meadows in Austria: A comparison of five techniques. Basic Appl. Ecol. 2017, 24, 19–29. [Google Scholar] [CrossRef]
- UN (United Nation). Resolution Adopted by the General Assembly on 1 March 2019, 73/284. United Nations Decade on Ecosystem Restoration (2021–2030), A/RES/73/284. 2019. Available online: https://Undocs.Org/A/RES/73/284 (accessed on 10 November 2021).
- Walker, K.J.; Stevens, P.A.; Stevens, D.P.; Mountford, J.O.; Manchester, S.J.; Pywell, R.F. The restoration and re-creation of Species-rich lowland grassland on land formerly managed for intensive agriculture in the UK. Biol. Conserv. 2004, 119, 1–18. [Google Scholar] [CrossRef]
- Bossuyt, B.; Honnay, O. Can the seed bank be used for ecological restoration? An overview of seed bank characteristics in European communities. J. Veg. Sci. 2008, 19, 875–884. [Google Scholar] [CrossRef]
- Janicka, M. The evaluation of soil seed bank in two arrhenatherion meadow habitats in central Poland. Acta Sci. Pol. Agric. 2016, 15, 25–38. [Google Scholar]
- Hölzel, N.; Buisson, E.; Dutoit, T. Species introduction—A major topic in vegetation restoration. Appl. Veg. Sci. 2012, 15, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Scotton, M.; Kirmer, A.; Krautzer, B. Practical Handbook for Seed Harvest and Ecological Restoration of Species-Rich Grasslands; Cooperativa Liberia Editrice Università di Padova: Padova, Italy, 2012; ISBN 978-88-6129-800-2. [Google Scholar]
- Golińska, B.; Czerwiński, M.; Goliński, P. Harvesting Seeds of an Arrhenatherion Meadow as a Source of Propagation Material for Grassland Restoration. Grassland Resources for Extensive Farming Systems in Marginal Lands: Major Drivers and Future Scenarios. Proceedings of the 19th Symposium of the European Grassland Federation, Alghero, Italy, 7–10 May 2017; CNR ISPAAM: Portici, Italy, 2017; Volume 22, pp. 485–487. [Google Scholar]
- Pywell, R.F.; Bullock, J.M.; Roy, D.B.; Warman, L.; Walker, K.J.; Rothery, P. Plant traits as predictors of performance in ecological restoration. J. Appl. Ecol. 2003, 40, 65–77. [Google Scholar] [CrossRef]
- Díaz, S.; Purvis, A.; Cornelissen, J.H.C.; Mace, G.M.; Donoghue, M.J.; Ewers, R.M.; Jordano, P.; Pearse, W.D. Functional traits, the phylogeny of function, and ecosystem service vulnerability. Ecol. Evol. 2013, 3, 2958–2975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-416677-6. [Google Scholar]
- Fischer, L.K.; von der Lippe, M.; Rillig, M.C.; Kowarik, I. Creating novel urban grasslands by reintroducing native species in wasteland vegetation. Biol. Conserv. 2013, 159, 119–126. [Google Scholar] [CrossRef]
- Leishman, M.R. Does the seed size/number trade-off model determine plant community structure? An assessment of the model mechanisms and their generality. Oikos 2001, 93, 294–302. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, Z.; Krebs, C.J. Seed size and number make contrasting predictions on seed survival and dispersal dynamics: A case study from oil tea Camellia oleifera. For. Ecol. Manag. 2015, 343, 1–8. [Google Scholar] [CrossRef]
- Engst, K.; Baasch, A.; Bruelheide, H. Predicting the establishment success of introduced target species in grassland restoration by functional traits. Ecol. Evol. 2017, 7, 7442–7453. [Google Scholar] [CrossRef] [Green Version]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. Flowering Plants and Pteridophytes of Poland. A Checklist; W. Szafer Institute of Botany, Polish Academy of Science: Kraków, Poland, 2002; ISBN 978-83-85444-83-1. [Google Scholar]
- Szafer, W.; Kulczyński, S.; Pawłowski, B. Rośliny Polskie Część I i II. (Polish Plants); PWN: Warszawa, Poland, 1986; Volume 1/2, ISBN 83-01-05287-2. [Google Scholar]
- Ellenberg, H.; Leuschner, C. Vegetation Mitteleuropas mit den Alpen: In Ökologischer, Dynamischer und Historischer Sicht, 6th ed.; UTB: Stuttgart, Germany, 2010; ISBN 3-8252-8104-3. [Google Scholar]
- Cappers, R.T.J.; Bekker, R.M.; Jans, J.E.A. Digitale Zadenatlas van Nederland/Digital Seed Atlas of the Netherlands, 2nd ed.; Barkhuis Publishing: Groningen, The Netherlands, 2006; ISBN 9789077922958. [Google Scholar]
- ENSCONET. Seed Collecting Manual for Wild Species, 1st ed.; Royal Botanic Gardens: Kew, UK; Universidad Politécnica de Madrid: Madrid, Spain, 2009; ISBN 978-84-692-3926-1. [Google Scholar]
- ISTA (International Seed Testing Association). International Rules for Seed Testing; IHAR-PIB Radzików: Błonie, Poland, 2018. (In Polish) [Google Scholar]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. Handbook of Seed Technology for Genebanks: Volume II: Compendium of Specific Germination Information and Test Recommendation; International Board for Plant Genetic Resources: Rome, Italy, 1985; ISBN 978-92-9043-119-0. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing, Foundation for Statistical Computing; European Environment Agency: Vienna, Austria, 2021.
- John, A.; Fuentes, H.R.; George, F. Characterization of the water retention curves of Everglades wetland soils. Geoderma 2021, 381, 114724. [Google Scholar] [CrossRef]
- STATGRAPHICS Plus—Statistical Graphics System, version 4.1; STSC-Inc.: Rockvile, MD, USA, 1999.
- Vinczeffy, I. The Effect of Some Ecological Factors on Grass Yield. Proceedings of the 10. General Meeting of the European Grassland Federation, Ǻs, Norway, 26–30 June 1984; Norwegian State Agricultural Research Stations: Særheim, Norway, 1984; pp. 76–79. [Google Scholar]
- Török, P.; Miglécz, T.; Valkó, O.; Tóth, K.; Kelemen, A.; Albert, Á.J.; Matus, G.; Molnár, V.A.; Ruprecht, E.; Papp, L.; et al. New thousand-seed weight records of the Pannonian flora and their application in analysing social behaviour types. Acta Bot. Hung. 2013, 55, 429–472. [Google Scholar] [CrossRef] [Green Version]
- Borawska-Jarmułowicz, B.; Mastalerczuk, G.; Małuszyńska, E.; Borucki, W. Morphological diversity of seeds of Polish Festulolium cultivars depending on weather conditions. Biol. Plant. 2020, 64, 814–820. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Elwinger, G.F. Emergence and seedling structure of temperate grasses at different planting depths. Agron. J. 2004, 96, 685–691. [Google Scholar] [CrossRef] [Green Version]
- Carrington, M.E. Seed size and recruitment limitation influence seedling establishment in three tallgrass prairie species. Plant Ecol. 2014, 215, 1163–1172. [Google Scholar] [CrossRef]
- Milberg, P.; Andersson, L.; Thompson, K. Large-seeded spices are less dependent on light for germination than small-seeded ones. Seed Sci. Res. 2000, 10, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Cordazzo, C.V. Effect of seed mass on germination and growth in three dominant species in southern Brazilian coastal dunes. Braz. J. Biol. 2002, 62, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Souza, M.L.; Fagundes, M. Seed size as key factor in germination and seedling development of Copaifera langsdorffii (Fabaceae). Am. J. Plant Sci. 2014, 5, 2566–2573. [Google Scholar] [CrossRef] [Green Version]
- Janicka, M.; Pawluśkiewicz, B. The increasing in the floristic diversity of the abandoned Arrhenatherion elatioris meadows by Dicotyledonous species oversowing. J. Ecol. Eng. 2020, 21, 168–179. [Google Scholar] [CrossRef]
- Jensen, K. Dormancy patterns, germination ecology, and seed-bank types of twenty temperate fen grassland species. Wetlands 2004, 24, 152–166. [Google Scholar] [CrossRef]
- Pawluśkiewicz, B.; Janicka, M.; Piekut, K. Effects of different introduction methods on plant species establishment success in wet grassland restoration. Pol. J. Environ. Stud. 2019, 28, 1857–1867. [Google Scholar] [CrossRef]
- Moles, A.T.; Westoby, M. Seedling survival and seed size: A synthesis of the literature. J. Ecol. 2004, 92, 372–383. [Google Scholar] [CrossRef]
- Hay, F.R.; Probert, R.J. Advances in seed conservation of wild plant species: A review of recent research. Conserv. Physiol. 2013, 1, cot030. [Google Scholar] [CrossRef]
- Lehtilä, K.; Ehrlén, J. Seed size as an indicator of seed quality: A case study of Primula veris. Acta Oecol. 2005, 28, 207–212. [Google Scholar] [CrossRef]
- Segura, F.; Vicente, M.J.; Franco, J.A.; Martínez-Sánchez, J.J. Effects of maternal environmental factors on physical dormancy of Astragalus nitidiflorus seeds (Fabaceae), a critically endangered species of SE Spain. Flora 2015, 216, 71–76. [Google Scholar] [CrossRef]
- Eslami, S.V.; Gill, G.S.; McDonald, G. Effect of water stress during seed development on morphometric characteristics and dormancy of wild radish (Raphanus raphanistrum L.) seeds. Int. J. Plant Prod. 2012, 4, 159–168. [Google Scholar] [CrossRef]
- Fenner, M. Seeds: The Ecology of Regeneration in Plant Communities; CABI: Wallingford, UK, 2000; ISBN 978-0-85199-947-0. [Google Scholar]
- Steadman, K.J.; Ellery, A.J.; Chapman, R.; Moore, A.; Turner, N.C.; Steadman, K.J.; Ellery, A.J.; Chapman, R.; Moore, A.; Turner, N.C. Maturation temperature and rainfall influence seed dormancy characteristics of annual ryegrass (Lolium rigidum). Aust. J. Agric. Res. 2004, 55, 1047–1057. [Google Scholar] [CrossRef]
- Kiss, R.; Sonkoly, J.; Török, P.; Tóthmérész, B.; Deák, B.; Tóth, K.; Lukács, K.; Godó, L.; Kelemen, A.; Miglécz, T.; et al. Germination capacity of 75 herbaceous species of the Pannonian flora and implications for restoration. Acta Bot. Hung. 2018, 60, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Derakhshan, A.; Gherekhloo, J.; Vidal, R.A.; Prado, R.D. Quantitative description of the germination of littleseed canarygrass (Phalaris minor) in response to temperature. Weed Sci. 2014, 62, 250–257. [Google Scholar] [CrossRef]
- Eslami, S.V. Comparative germination and emergence ecology of two populations of common lambsquarters (Chenopodium album) from Iran and Denmark. Weed Sci. 2011, 59, 90–97. [Google Scholar] [CrossRef]
- Commander, L.E.; Merritt, D.J.; Rokich, D.P.; Dixon, K.W. Seed biology of Australian arid zone species: Germination of 18 species used for rehabilitation. J. Arid Environ. 2009, 73, 617–625. [Google Scholar] [CrossRef]
- Haslgrübler, P.; Krautzer, B.; Blaschka, A.; Graiss, W.; Pötsch, E.M. Quality and germination capacity of seed material harvested from an Arrhenatherion meadow. Grass Forage Sci. 2014, 69, 454–461. [Google Scholar] [CrossRef]
- Larson, J.E.; Sheley, R.L.; Hardegree, S.P.; Doescher, P.S.; James, J.J. Seed and seedling traits affecting critical life stage transitions and recruitment outcomes in dryland grasses. J. Appl. Ecol. 2015, 52, 199–209. [Google Scholar] [CrossRef]
- Kövendi-Jakó, A.; Csecserits, A.; Halassy, M.; Halász, K.; Szitár, K.; Török, K. Relationship of germination and establishment for twelve plant species in restored dry grassland. Appl. Ecol. Environ. Res. 2017, 15, 227–239. [Google Scholar] [CrossRef]
- Jones, K.D.; Kaye, T.N. Factors influencing germination of a functionally important grassland plant, Iris tenax. PLoS ONE 2014, 9, e90084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, G.; Nunes, A.; Clemente, A.; Correia, O. Testing germination of species for hydroseeding degraded Mediterranean areas. Restor. Ecol. 2012, 20, 623–630. [Google Scholar] [CrossRef]

| Year | Month | Growing Season | |||||
|---|---|---|---|---|---|---|---|
| IV | V | VI | VII | VIII | IX | IV–IX | |
| Mean temperature (°C) | |||||||
| 2015 | 9.1 | 13.7 | 18.1 | 20.5 | 23.0 | 15.6 | 16.7 |
| 2016 | 10.0 | 16.3 | 19.8 | 20.3 | 19.3 | 16.5 | 17.0 |
| 2017 | 8.0 | 14.9 | 18.9 | 19.2 | 20.0 | 14.1 | 15.8 |
| Sum of precipitation (mm) | |||||||
| 2015 | 31.4 | 57.7 | 37.5 | 66.2 | 11.3 | 73.1 | 277.2 |
| 2016 | 34.2 | 23.3 | 56.9 | 116.5 | 71.7 | 9.8 | 312.4 |
| 2017 | 62.1 | 68.3 | 106.2 | 111.0 | 68.5 | 146.8 | 562.9 |
| Characteristics of growing seasons | Hydrothermal index of Vinczeffy (mm·°C–1) | ||||||
| 2015 | extremely dry | 0.091 | |||||
| 2016 | very dry | 0.100 | |||||
| 2017 | wet | 0.194 | |||||
| Species | Length | Width | Thickness | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Year of Harvest (Y) | |||||||||
| 2016 | 2017 | Mean | 2016 | 2017 | Mean | 2016 | 2017 | Mean | |
| Artemisia campestris L. | 0.79 | 0.66 | 0.72 | 0.38 | 0.36 | 0.37 | 0.17 | 0.12 | 0.14 |
| Centaurea stoebe L. | 2.81 | 1.84 | 2.32 | 1.16 | 0.83 | 0.99 | 0.78 | 0.61 | 0.69 |
| Eryngium planum L. | 3.28 | 2.20 | 2.74 | 1.32 | 0.93 | 1.12 | 0.90 | 0.52 | 0.71 |
| Scabiosa ochroleuca L. | 3.23 | 2.18 | 2.7 | 1.94 | 1.25 | 1.59 | 1.92 | 1.18 | 1.55 |
| Verbascum thapsus L. | 0.75 | 0.57 | 0.66 | 0.52 | 0.42 | 0.47 | 0.51 | 0.38 | 0.44 |
| Galium boreale L. | 1.87 | 1.29 | 1.58 | 1.67 | 1.06 | 1.36 | 1.22 | 0.83 | 1.02 |
| Iris sibirica L. | 3.12 | 3.20 | 3.16 | 2.74 | 2.50 | 2.62 | 1.45 | 1.43 | 1.44 |
| Lychnis flos-cuculi L. | 0.79 | 0.53 | 0.66 | 0.62 | 0.43 | 0.52 | 0.42 | 0.37 | 0.39 |
| Sanguisorba officinalis L. | 2.92 | 1.99 | 2.45 | 1.72 | 1.15 | 1.43 | 1.60 | 1.03 | 1.31 |
| Allium angulosum L. | 2.03 | 1.48 | 1.75 | 1.23 | 0.88 | 1.05 | 0.96 | 0.66 | 0.81 |
| Cnidium dubium (Schkuhr) Thell. | 2.05 | 1.71 | 1.88 | 1.28 | 0.99 | 1.13 | 0.84 | 0.61 | 0.72 |
| Achillea millefolium L. | 1.69 | 1.26 | 1.47 | 0.58 | 0.44 | 0.51 | 0.16 | 0.09 | 0.12 |
| Campanula patula L. | 0.47 | 0.31 | 0.39 | 0.32 | 0.18 | 0.25 | 0.16 | 0.10 | 0.13 |
| Centaurea jacea L. | 2.47 | 2.06 | 2.26 | 1.12 | 0.93 | 1.02 | 0.85 | 0.65 | 0.75 |
| Daucus carota L. | 2.78 | 1.80 | 2.29 | 1.28 | 0.85 | 1.06 | 0.64 | 0.44 | 0.54 |
| Geranium pratense L. | 3.01 | 2.35 | 2.68 | 1.76 | 1.48 | 1.62 | 1.51 | 1.31 | 1.41 |
| Lathyrus pratensis L. | 2.89 | 2.23 | 2.56 | 2.54 | 1.91 | 2.22 | 2.04 | 1.26 | 1.65 |
| Leontodon autumnalis L. | 4.53 | 3.43 | 3.98 | 0.47 | 0.45 | 0.46 | 0.41 | 0.33 | 0.37 |
| Plantago lanceolata L. | 2.33 | 1.75 | 2.04 | 1.04 | 0.75 | 0.89 | 0.56 | 0.44 | 0.50 |
| Rumex acetosa L. | 1.57 | 1.17 | 1.37 | 0.95 | 0.65 | 0.8 | 0.91 | 0.64 | 0.77 |
| Tragopogon orientalis L. | 9.09 | 3.20 | 6.14 | 1.08 | 0.15 | 0.61 | 1.04 | 0.13 | 0.58 |
| Trifolium pratense L. | 1.73 | 1.18 | 1.45 | 1.38 | 0.88 | 1.13 | 1.06 | 0.59 | 0.82 |
| Armeria maritima (MILL.) WILLD | 5.35 | 4.09 | 4.72 | 1.48 | 0.85 | 1.16 | 1.19 | 0.66 | 0.92 |
| Hypericum perforatum L. | 0.99 | 0.76 | 0.87 | 0.41 | 0.35 | 0.38 | 0.41 | 0.31 | 0.36 |
| Linaria vulgaris L. | 1.96 | 1.20 | 1.58 | 1.67 | 1.00 | 1.33 | 0.11 | 0.14 | 0.12 |
| Lysimachia vulgaris L. | 1.38 | 1.10 | 1.24 | 1.03 | 0.70 | 0.86 | 0.82 | 0.52 | 0.67 |
| Potentilla erecta (L.) RAEUSCH | 0.85 | 0.60 | 0.72 | 0.62 | 0.44 | 0.53 | 0.33 | 0.30 | 0.31 |
| Veronica longifolia L. | 0.72 | 0.52 | 0.62 | 0.51 | 0.35 | 0.43 | 0.05 | 0.12 | 0.08 |
| LSD0.05 (S) | 0.404 | 0.134 | 0.133 | ||||||
| Mean (Y) | 2.41 | 1.67 | 1.17 | 0.83 | 0.82 | 0.56 | |||
| LSD0.05 (Y) | 0.057 | 0.019 | 0.019 | ||||||
| Species (S) | Year of Harvest (Y) | CV (%) | ||
|---|---|---|---|---|
| 2016 | 2017 | Mean | ||
| Artemisia campestris L. | 0.049 | 0.067 | 0.058 | 19.5 |
| Centaurea stoebe L. | 1.407 | 1.155 | 1.282 | 17.4 |
| Eryngium planum L. | 1.302 | 1.133 | 1.218 | 12.9 |
| Scabiosa ochroleuca L. | 1.963 | 1.625 | 1.794 | 16.8 |
| Verbascum thapsus L. | 0.103 | 0.117 | 0.110 | 9.2 |
| Galium boreale L. | 1.258 | 1.097 | 1.177 | 22.8 |
| Iris sibirica L. | 3.797 | 11.527 | 7.662 | 55.8 |
| Lychnis flos-cuculi L. | 0.105 | 0.130 | 0.118 | 21.5 |
| Sanguisorba officinalis L. | 1.347 | 1.790 | 1.568 | 17.7 |
| Allium angulosum L. | 1.337 | 1.283 | 1.310 | 8.2 |
| Cnidium dubium (Schkuhr) Thell. | 0.387 | 0.550 | 0.468 | 22.4 |
| Achillea millefolium L. | 0.095 | 0.103 | 0.099 | 9.3 |
| Campanula patula L. | 0.018 | 0.030 | 0.024 | 27.6 |
| Centaurea jacea L. | 1.343 | 1.489 | 1.416 | 11.7 |
| Daucus carota L. | 0.665 | 0.755 | 0.710 | 9.4 |
| Geranium pratense L. | 5.850 | 9.300 | 7.575 | 25.7 |
| Lathyrus pratensis L. | 12.293 | 11.154 | 11.724 | 7.2 |
| Leontodon autumnalis L. | 0.663 | 0.537 | 0.600 | 15.0 |
| Plantago lanceolata L. | 1.257 | 1.244 | 1.250 | 13.3 |
| Rumex acetosa L. | 0.403 | 0.378 | 0.390 | 11.1 |
| Tragopogon orientalis L. | 3.387 | 7.411 | 5.399 | 41.0 |
| Trifolium pratense L. | 1.183 | 1.255 | 1.219 | 14.0 |
| Armeria maritima (MILL.) WILLD | 1.080 | 1.177 | 1.128 | 10.1 |
| Hypericum perforatum L. | 0.072 | 0.133 | 0.102 | 34.1 |
| Linaria vulgaris L. | 0.137 | 0.120 | 0.128 | 12.5 |
| Lysimachia vulgaris L. | 0.271 | 0.320 | 0.295 | 24.2 |
| Potentilla erecta (L.) RAEUSCH | 0.080 | 0.052 | 0.066 | 31.0 |
| Veronica longifolia L. | 0.038 | 0.042 | 0.040 | 17.8 |
| LSD0.05 (S) | 2.11 | |||
| Mean (Y) | 1.496 | 1.999 | ||
| LSD0.05 (Y) | 0.29 | |||
| Species (S) | Year of Harvest (Y) | Mean | ||
|---|---|---|---|---|
| 2015 | 2016 | 2017 | ||
| Artemisia campestris L. | 65.3 | 64.0 | 70.7 | 66.7 |
| Centaurea stoebe L. | 10.7 | 44.0 | 36.0 | 30.2 |
| Eryngium planum L. | 48.0 | 41.3 | 71.3 | 53.6 |
| Scabiosa ochroleuca L. | 34.7 | 58.7 | 68.7 | 54.0 |
| Verbascum thapsus L. | 83.3 | 92.0 | 88.7 | 88.0 |
| Galium boreale L. | 34.0 | 60.0 | 38.7 | 44.2 |
| Iris sibirica L. | 87.3 | 38.7 | 72.7 | 66.2 |
| Lychnis flos-cuculi L. | 72.7 | 70.7 | 68.0 | 70.5 |
| Sanguisorba officinalis L. | 49.3 | 32.0 | 12.0 | 31.1 |
| Allium angulosum L. | 74.0 | 54.7 | 81.3 | 70.0 |
| Cnidium dubium (Schkuhr) Thell. | 2.7 | 18.7 | 44.0 | 21.8 |
| Achillea millefolium L. | 59.3 | 82.7 | 77.3 | 73.1 |
| Campanula patula L. | 31.7 | 64.0 | 18.0 | 37.9 |
| Centaurea jacea L. | 46.7 | 66.7 | 62.7 | 58.7 |
| Daucus carota L. | 74.0 | 74.7 | 88.7 | 79.1 |
| Geranium pratense L. | 17.3 | 22.7 | 19.0 | 19.7 |
| Lathyrus pratensis L. | 2.0 | 66.7 | 4.7 | 24.5 |
| Leontodon autumnalis L. | 64.7 | 82.7 | 79.3 | 75.5 |
| Plantago lanceolata L. | 69.3 | 69.3 | 61.3 | 66.6 |
| Rumex acetosa L. | 60.7 | 76.0 | 62.7 | 66.5 |
| Tragopogon orientalis L. | 14.7 | 40.0 | 96.0 | 50.2 |
| Trifolium pratense L. | 13.3 | 4.0 | 12.0 | 9.8 |
| Armeria maritima (MILL.) WILLD | 2.0 | 17.3 | 24.0 | 14.4 |
| Hypericum perforatum L. | 50.7 | 36.0 | 82.7 | 56.5 |
| Linaria vulgaris L. | 9.3 | 17.3 | 34.7 | 20.4 |
| Lysimachia vulgaris L. | 70.7 | 69.3 | 79.3 | 73.1 |
| Potentilla erecta (L.) RAEUSCH | 5.3 | 68.0 | 8.0 | 27.1 |
| Veronica longifolia L. | 73.3 | 85.3 | 82.0 | 80.2 |
| LSD0.05 (S) | 28.88 | |||
| Mean (Y) | 43.8 | 54.5 | 55.2 | |
| LSD0.05 (Y) | 5.93 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janicka, M.; Pawluśkiewicz, B.; Małuszyńska, E.; Gnatowski, T. Diversity of the Seed Material of Selected Plant Species of Naturally Valuable Grassland Habitats in Terms of the Prognosis of Introduction Success. Sustainability 2021, 13, 13979. https://doi.org/10.3390/su132413979
Janicka M, Pawluśkiewicz B, Małuszyńska E, Gnatowski T. Diversity of the Seed Material of Selected Plant Species of Naturally Valuable Grassland Habitats in Terms of the Prognosis of Introduction Success. Sustainability. 2021; 13(24):13979. https://doi.org/10.3390/su132413979
Chicago/Turabian StyleJanicka, Maria, Bogumiła Pawluśkiewicz, Elżbieta Małuszyńska, and Tomasz Gnatowski. 2021. "Diversity of the Seed Material of Selected Plant Species of Naturally Valuable Grassland Habitats in Terms of the Prognosis of Introduction Success" Sustainability 13, no. 24: 13979. https://doi.org/10.3390/su132413979
APA StyleJanicka, M., Pawluśkiewicz, B., Małuszyńska, E., & Gnatowski, T. (2021). Diversity of the Seed Material of Selected Plant Species of Naturally Valuable Grassland Habitats in Terms of the Prognosis of Introduction Success. Sustainability, 13(24), 13979. https://doi.org/10.3390/su132413979






