Fouling Identification for Nanofiltration Membrane and the Potential Reduction of Pollutants in the Leachate by Using Fe/Al/PAC Coagulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site
2.2. Coagulation Process
2.3. Measurements and Methods
2.3.1. Fundamental Parameters
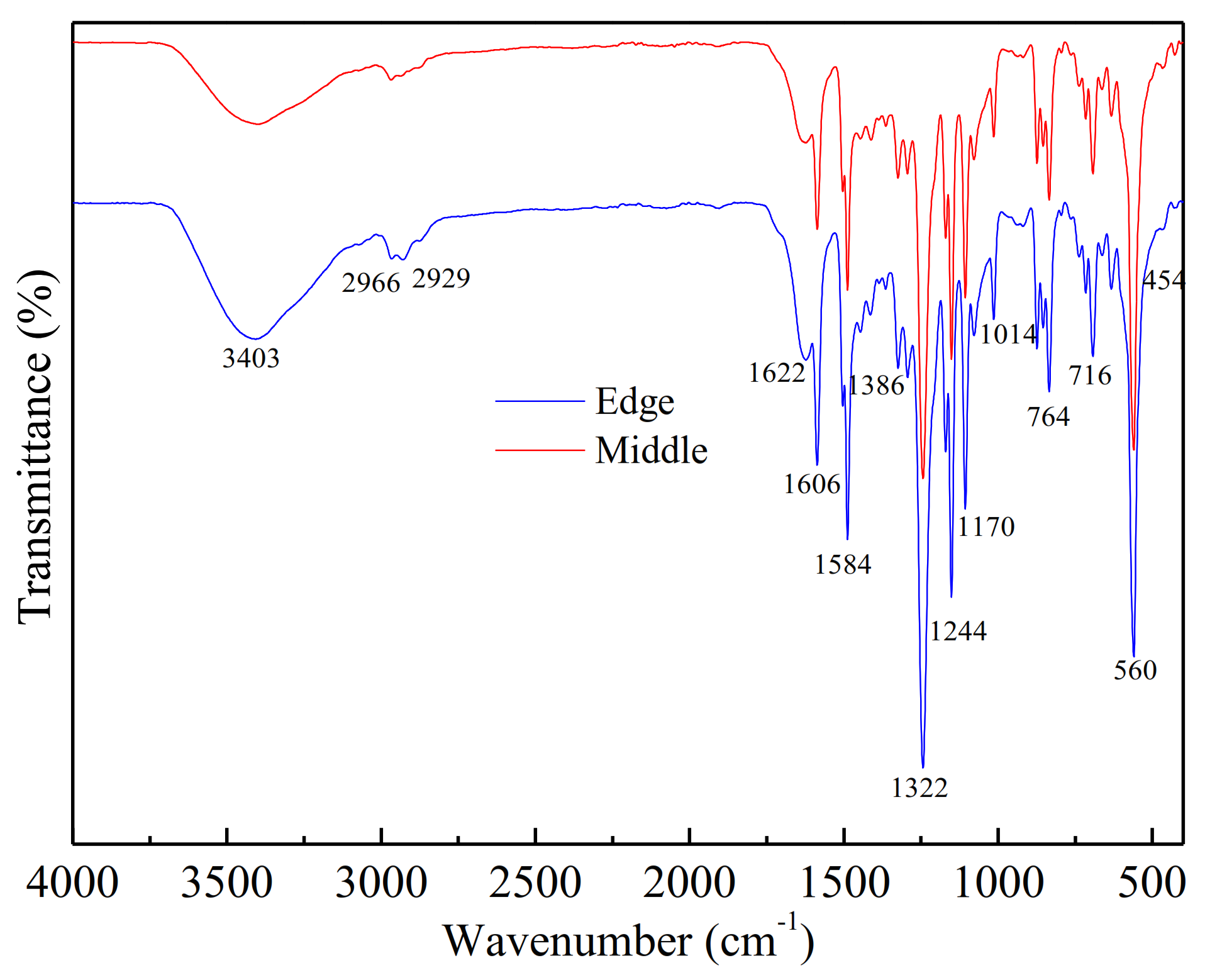
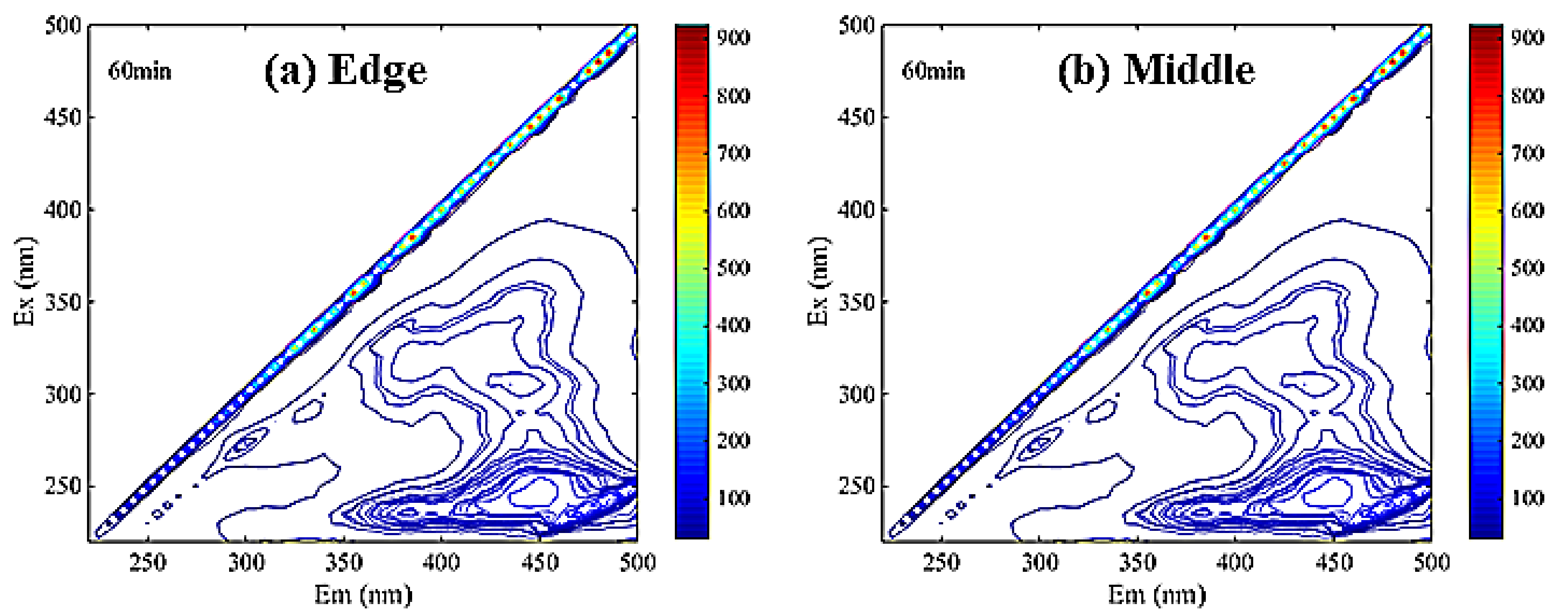
2.3.2. Spectroscopy Analysis of DOM
3. Results and Discussion
3.1. Fouling Components Identification
3.1.1. SEM Appearance
3.1.2. Fouling Components
3.1.3. Membrane Soaking Solution Analysis
Metal Ion Contents
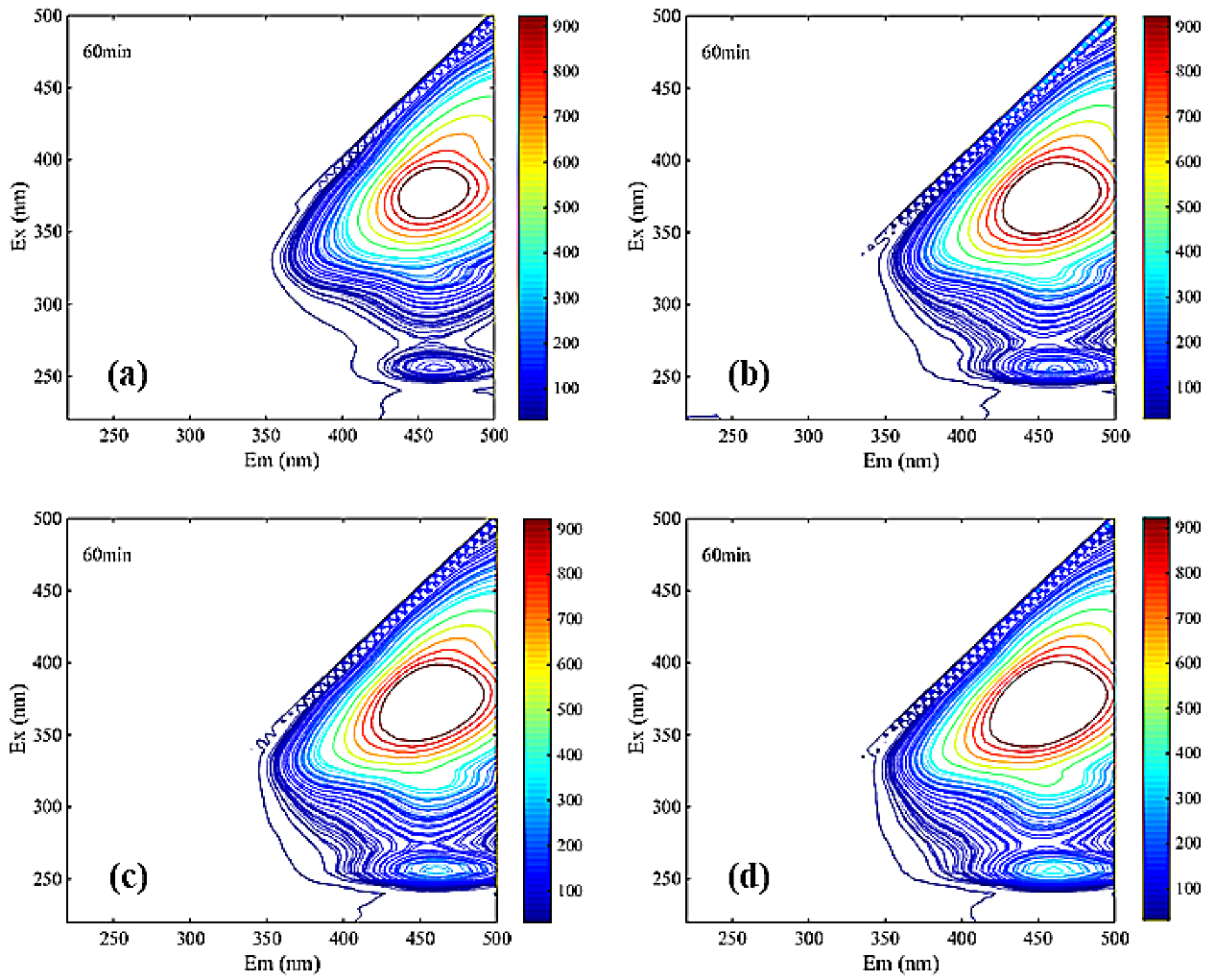
Dissolved Organic Matter Contents
3.2. Coagulators and the Operation Conditions Opitimized
3.2.1. Removal Potential
3.2.2. DOM Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguiar, A.; Andrade, L.; Grossi, L. Acid mine drainage treatment by nanofiltration: A study of membrane fouling, chemical cleaning, and membrane ageing. Sep. Purif. Technol. 2018, 192, 185–195. [Google Scholar] [CrossRef]
- Al-Amoudi, A. Nanofiltration membrane cleaning characterization. Desalination Water Treat. 2016, 57, 323–334. [Google Scholar] [CrossRef]
- Ilyas, S.; De Grooth, J.; Nijmeijer, K. Multifunctional polyelectrolyte multilayers as nanofiltration membranes and as sacrificial layers for easy membrane cleaning. J. Colloid Interface Sci. 2015, 446, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.L.; Gao, X.; Xu, L. Investigation of synchronous arsenic and salinity rejection via nanofiltration system and membrane cleaning. Desalination Water Treat. 2016, 57, 6554–6565. [Google Scholar] [CrossRef]
- Liu, Z.P.; Wu, W.H.; Shi, P. Characterization of dissolved organic matter in landfill leachate during the combined treatment process of air stripping, Fenton, SBR and coagulation. Waste Manag. 2015, 41, 111–118. [Google Scholar] [CrossRef]
- Renou, S.; Givaudan, J.G.; Poulain, S. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef]
- da Costa, F.M.; Alves Daflon, S.D.; Bila, D.M. Evaluation of the biodegradability and toxicity of landfill leachates after pretreatment using advanced oxidative processes. Waste Manag. 2018, 76, 606–613. [Google Scholar] [CrossRef]
- Andrade, L.H.; Aguiar, A.O.; Pires, W.L. Nanofiltration and Reverse Osmosis Applied to Gold Mining Effluent Treatment and Reuse. Braz. J. Chem. Eng. 2017, 34, 93–107. [Google Scholar] [CrossRef] [Green Version]
- Labiadh, L.; Fernandes, A.; Ciriaco, L. Electrochemical treatment of concentrate from reverse osmosis of sanitary landfill leachate. J. Environ. Manag. 2016, 181, 515–521. [Google Scholar] [CrossRef]
- Fudala-Ksiazek, S.; Pierpaoli, M.; Luczkiewicz, A. Efficiency of landfill leachate treatment in a MBR/UF system combined with NF, with a special focus on phthalates and bisphenol A removal. Waste Manag. 2018, 78, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Sir, M.; Podhola, M.; Patocka, T. The effect of humic acids on the reverse osmosis treatment of hazardous landfill leachate. J. Hazard. Mater. 2012, 207, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Ang, W.L.; Mohammad, A.W.; Benamor, A.; Hilal, N. Hybrid coagulation-NF membrane processes for brackish water treatment: Effect of pH and salt/calcium concentration. Desalination 2016, 390, 25–32. [Google Scholar] [CrossRef]
- Smol, M.; Włodarczyk-Makuła, M. Effectiveness in the Removal of Organic Compounds from Municipal Landfill Leachate in Integrated Membrane Systems: Coagulation—NF/RO. Polycycl. Aromat. Compd. 2016, 37, 456–474. [Google Scholar] [CrossRef]
- Alfaia, R.G.S.M.; Nascimento, M.M.P.; Bila, D.M. Coagulation/flocculation as a pretreatment of landfill leachate for minimizing fouling in membrane processes. Desalination Water Treat. 2019, 159, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Mariam, T.; Nghiem, L.D. Landfill leachate treatment using hybrid coagulation-nanofiltration processes. Desalination 2010, 250, 677–681. [Google Scholar] [CrossRef]
- Racar, M.; Dolar, D.; Spehar, A. Optimization of coagulation with ferric chloride as a pretreatment for fouling reduction during nanofiltration of rendering plant secondary effluent. Chemosphere 2017, 181, 485–491. [Google Scholar] [CrossRef]
- De Haan, H.; De Boer, T. Applicability of light absorbance and fluorescence as measures of concentration and molecular size of dissolved organic carbon in humic Lake Tjeukemeer. Water Res. 1987, 21, 731–734. [Google Scholar] [CrossRef]
- Peuravuori, J.; Pihlaja, K. Molecular size distribution and spectroscopic properties of aquatic humic substances. Anal. Chim. Acta 1997, 337, 133–149. [Google Scholar] [CrossRef]
- Choudhry, G.G.; Hutzinger, O. Photochemical formation and degradation of polychlorinated dibenzofurans and dibenzo-p-dioxins. Residue reviews. 1982, 84, 113–161. [Google Scholar]
- Dilling, J.; Kaiser, K. Estimation of the hydrophobic fraction of dissolved organic matter in water samples using UV photometry. Water Res. 2002, 36, 5037–5044. [Google Scholar] [CrossRef]
- Amir, S.; Jouraiphy, A.; Meddich, A. Structural study of humic acids during composting of activated sludge-green waste: Elemental analysis, FTIR and 13C NMR. J. Hazard Mater. 2010, 177, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, L.F.; Ong, S.L. Fouling behavior and foulant characteristics of reverse osmosis membranes for treated secondary effluent reclamation. J. Membr. Sci. 2010, 349, 65–74. [Google Scholar] [CrossRef]
- Chun, Y.; Zaviska, F.; Kim, S.J.; Mulcahy, D.; Yang, E.; Kim, I.S.; Zou, L. Fouling characteristics and their implications on cleaning of a FO-RO pilot process for treating brackish surface water. Desalination 2016, 394, 91–100. [Google Scholar] [CrossRef]
- Antony, A.; Low, J.H.; Gray, S. Scale formation and control in high pressure membrane water treatment systems: A review. J. Membr. Sci. 2011, 383, 1–16. [Google Scholar] [CrossRef]
- Xu, P.; Bellona, C.; Drewes, J.E. Fouling of nanofiltration and reverse osmosis membranes during municipal wastewater reclamation: Membrane autopsy results from pilot-scale investigations. J. Membr. Sci. 2010, 353, 111–121. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Chemical and physical aspects of organic fouling of forward osmosis membranes. J. Membr. Sci. 2008, 320, 292–302. [Google Scholar] [CrossRef]
- Li, Q.; Elimelech, M. Organic fouling and chemical cleaning of nanofiltration membranes: Measurements and mechanisms. Environ. Sci. Technol. 2004, 38, 4683–4693. [Google Scholar] [CrossRef]
- Kerry, J.; Howe, K.P.I.; Clark, M.M. Use of ATR/FTIR spectrometry to study fouling of microfiltration membranes by natural waters. Desalination 2002, 147, 251–255. [Google Scholar]
- Sari, M.A.; Chellam, S. Reverse osmosis fouling during pilot-scale municipal water reuse: Evidence for aluminum coagulant carryover. J. Membr. Sci. 2016, 520, 231–239. [Google Scholar] [CrossRef]
- He, X.S.; Xi, B.D.; Li, X. Fluorescence excitation-emission matrix spectra coupled with parallel factor and regional integration analysis to characterize organic matter humification. Chemosphere 2013, 93, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- Zbytniewski, R.; Buszewski, B. Characterization of natural organic matter (NOM) derived from sewage sludge compost. Part 1: Chemical and spectroscopic properties. Bioresour. Technol. 2005, 96, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, R.; Le Petit, J.; Terrom, G.; Périssol, C. Comparison between UV spectroscopy and nirs to assess humification process during sewage sludge and green wastes co-composting. Bioresour. Technol. 2011, 102, 4495–4500. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Khan, M.T.; Croue, J.P. Contribution of effluent organic matter (EfOM) to ultrafiltration (UF) membrane fouling: Isolation, characterization, and fouling effect of EfOM fractions. Water Res. 2014, 65, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Uyguner, C.S.; Bekbolet, M. Evaluation of humic acid photocatalytic degradation by UV–vis and fluorescence spectroscopy. Catal. Today 2005, 101, 267–274. [Google Scholar] [CrossRef]

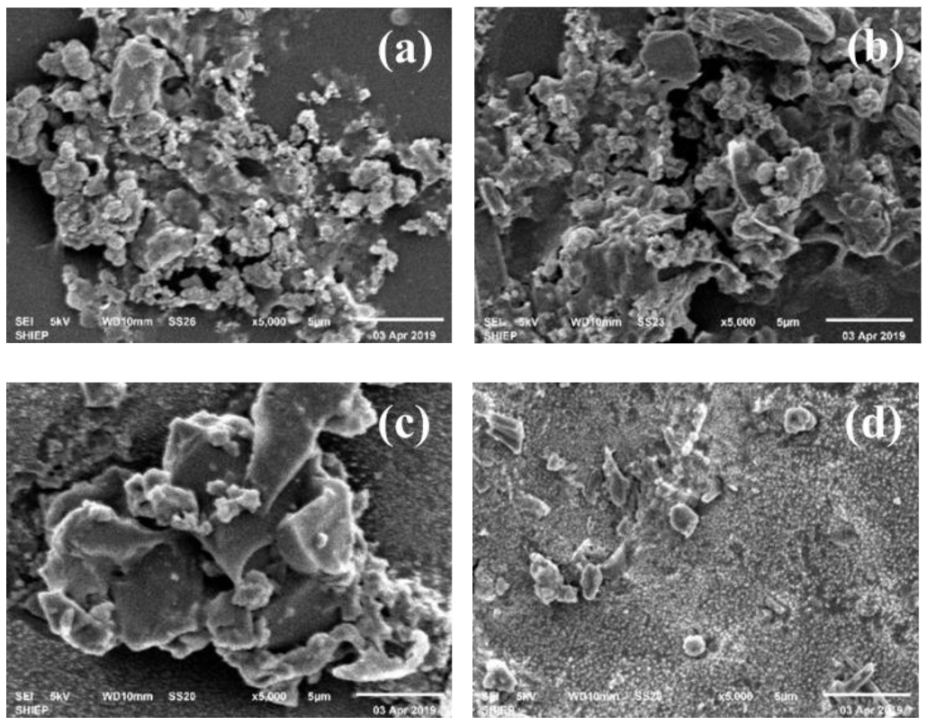
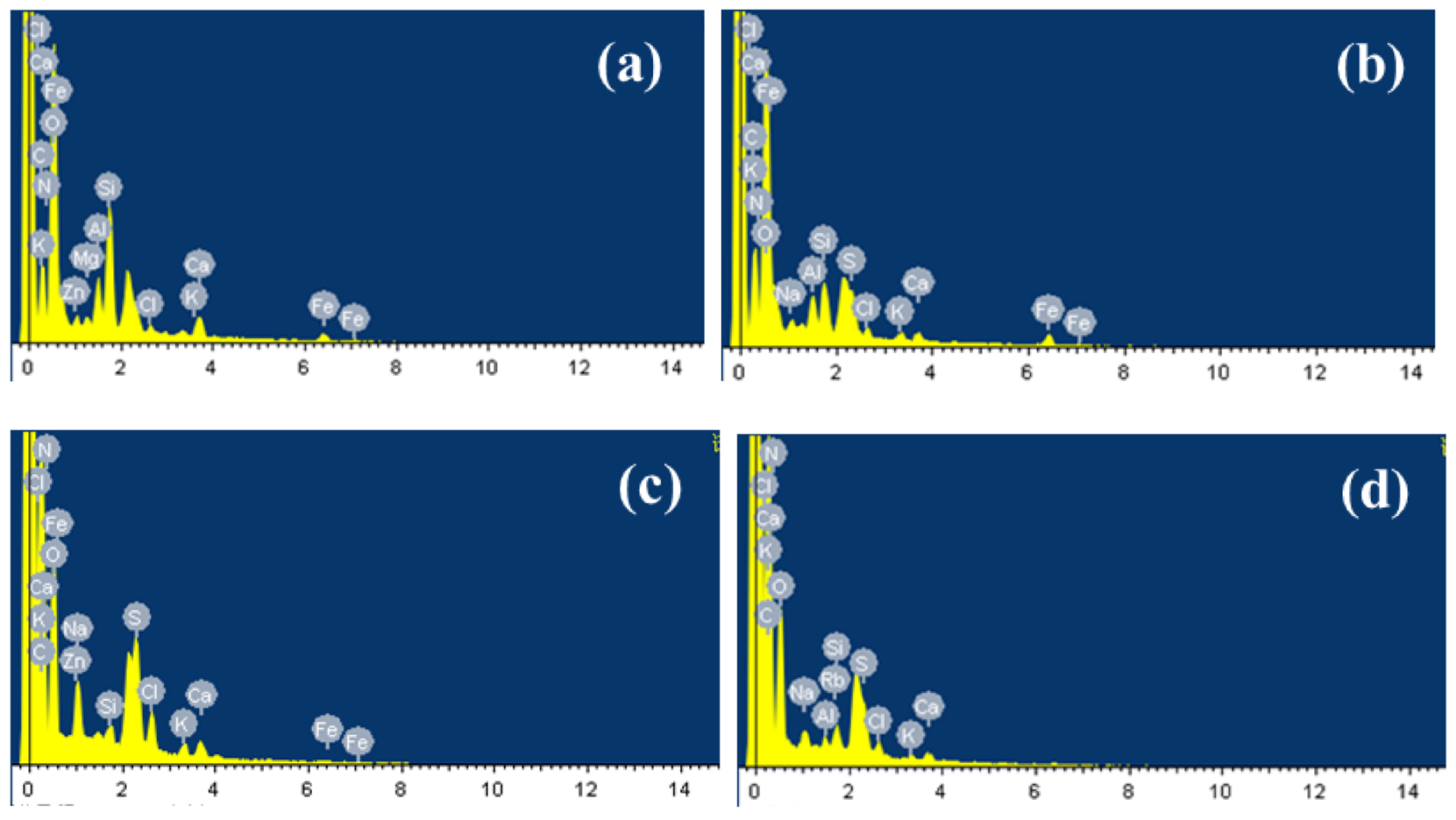
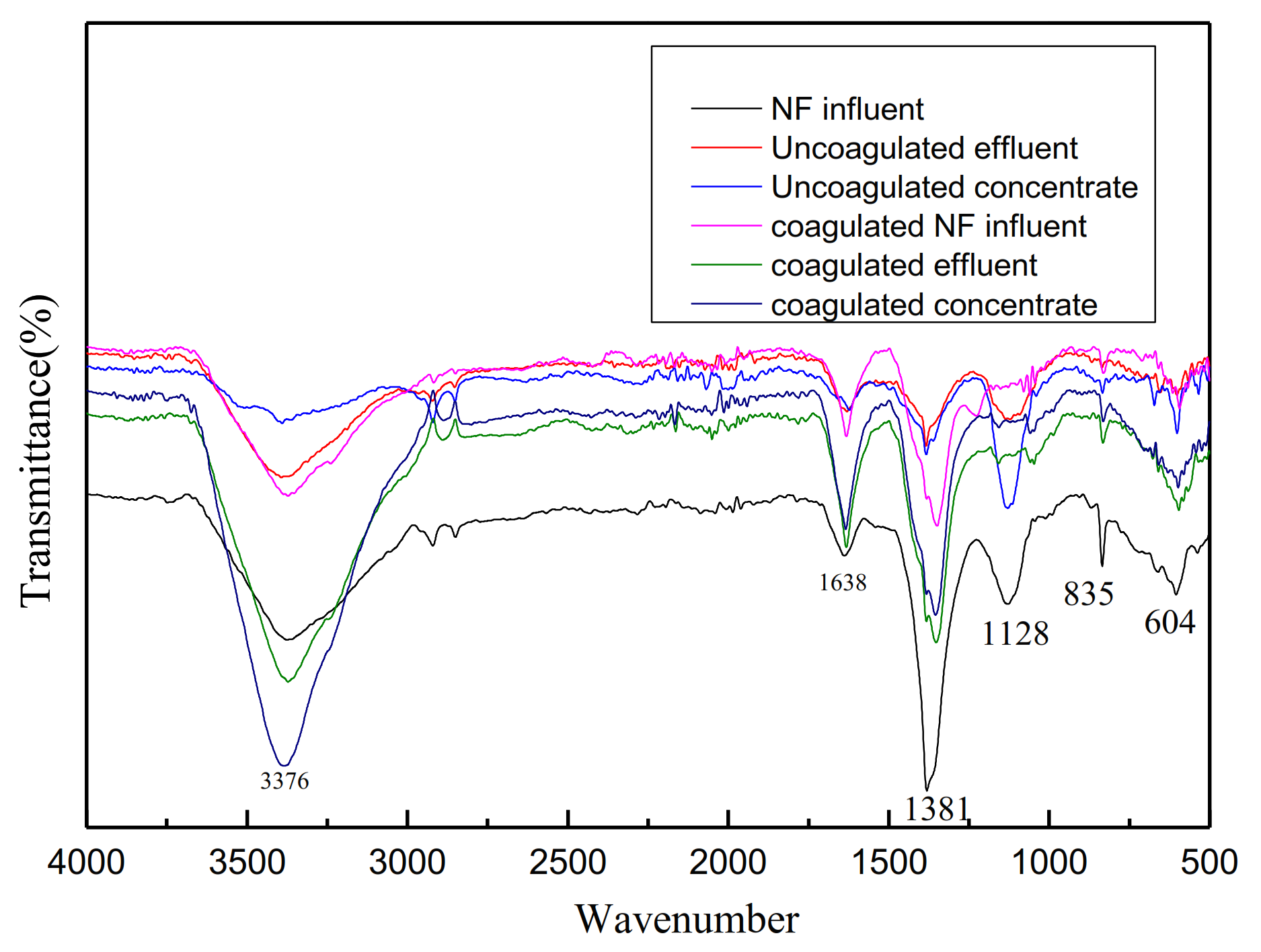

| Fe3+ (mg/L) | Al3+ (mg/L) | Zn2+ (mg/L) | Mg2+ (mg/L) | Ca2+ (mg/L) | |
|---|---|---|---|---|---|
| Edge | 3.1 | 4.9 | 1.7 | 12.6 | 157.0 |
| Middle | 6.1 | 8.2 | 2.9 | 11.6 | 213.2 |
| E254 | E2/E3 | E3/E4 | E4/E6 | |
|---|---|---|---|---|
| Edge | 0.10 | 6.53 | 6.33 | 1.50 |
| Middle | 0.12 | 5.71 | 5.30 | 2.50 |
| Experimental Index | Uncoagulated Raw Water | After Coagulation |
|---|---|---|
| COD of influent, mg/L | 272 ± 11 | 122 ± 5 |
| Pressure, MPa | 0.4 ± 0.1 | 0.4 ± 0.1 |
| flow velocity, L/h | 81 ± 3 | 110 ± 5 |
| Influent load, L | 1 ± 0.005 | 1 ± 0.005 |
| Effluent load, L | 0.9 ± 0.005 | 0.935 ± 0.005 |
| COD of effluent, mg/L | 215 ± 9 | 106 ± 5 |
| COD of concentrate, mg/L | 835 ± 36 | 762 ± 33 |
| Pharmacy | Unit Price (¥/ton) | Optimal pH | Optimal Dosage (mg/L) | Effluent COD (mg/L) | Processing Costv (¥/ton) | Processing Cost (¥/g*COD) |
|---|---|---|---|---|---|---|
| PFS | 2.8 | 5.0 | 100 | 131 | 0.28 | 1.98 |
| PAC | 2 | 5.0 | 100 | 106 | 0.20 | 1.2 |
| PAFC | 9 | 8.0 | 100 | 171 | 0.30 | 3 |
| AlCl3 | 66 | 6.0 | 30 | 106 | 1.98 | 12 |
| FeCl3 | 28 | 4.5 | 150 | 147 | 4.2 | 33.6 |
| FeSO4 | 24 | 6.0 | 150 | 155 | 6.0 | 51.3 |
| UV254 | E2/E3 | E3/E4 | E4/E6 | |
|---|---|---|---|---|
| NF influent | 4.12 | 3.05 | 3.38 | 1.86 |
| Uncoagulated effluent | 2.88 | 4.40 | 5.96 | 20.13 |
| Uncoagulated concentrate | 2.93 | 4.20 | 5.91 | 20.75 |
| Coagulated NF influent | 0.338 | 0.65 | 6.75 | 10.45 |
| Coagulated effluent | 0.205 | 0.59 | 7.87 | 1.61 |
| Coagulated concentrate | 0.225 | 0.58 | 7.07 | 5.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.-w.; Wang, H.; Wang, L.-c.; Lou, Z.-y.; Bai, L.; Zong, H.-f.; Zhou, Z. Fouling Identification for Nanofiltration Membrane and the Potential Reduction of Pollutants in the Leachate by Using Fe/Al/PAC Coagulation. Sustainability 2021, 13, 1114. https://doi.org/10.3390/su13031114
He C-w, Wang H, Wang L-c, Lou Z-y, Bai L, Zong H-f, Zhou Z. Fouling Identification for Nanofiltration Membrane and the Potential Reduction of Pollutants in the Leachate by Using Fe/Al/PAC Coagulation. Sustainability. 2021; 13(3):1114. https://doi.org/10.3390/su13031114
Chicago/Turabian StyleHe, Chang-wei, Hui Wang, Luo-chun Wang, Zi-yang Lou, Li Bai, Hai-feng Zong, and Zhen Zhou. 2021. "Fouling Identification for Nanofiltration Membrane and the Potential Reduction of Pollutants in the Leachate by Using Fe/Al/PAC Coagulation" Sustainability 13, no. 3: 1114. https://doi.org/10.3390/su13031114





