Plutonium-Doped Monazite and Other Orthophosphates—Thermodynamics and Experimental Data on Long-Term Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Experimental Details
2.2. Atomistic Simulation
3. Results
3.1. Optical Microscopy and Spectroscopy
3.2. Thermodynamic Properties
3.2.1. Estimation of Interaction Energy and Enthalpy of Mixing
3.2.2. Calculation of the Properties of Mixing for Solid Solutions in Systems LaPO4–PuPO4, EuPO4–PuPO4, GdPO4–PuPO4 and TbPO4–PuPO4 by the Supercell Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karkhanavala, M.D.; Shankar, J. An X-ray study of natural monazite: I. Proc. Indian Acad. Sci. 1954, 40, 67–71. [Google Scholar] [CrossRef]
- Meldrum, A.; Boatner, L.A.; Weber, W.J.; Ewing, R.C. Radiation damage in zircon and monazite. Geochim. Cosmochim. Acta 1998, 62, 2509–2520. [Google Scholar] [CrossRef]
- Seydoux-Guillaume, A.M.; Wirth, R.; Nasdala, L.; Gottschalk, M.; Montel, J.M.; Heinrich, W. An XRD, TEM and Raman study of experimentally annealed natural monazite. Phys. Chem. Miner. 2002, 29, 240–253. [Google Scholar] [CrossRef]
- Meldrum, A.; Boatner, L.A.; Ewing, R.C. Displacive irradiation effects in the monazite- and zircon-structure orthophosphates. Phys. Rev. B 1997, 56, 13805–13814. [Google Scholar] [CrossRef]
- Ewing, R.C.; Meldrum, A.; Wang, L.; Wang, S.X. Radiation-Induced Amorphization. Rev. Miner. Geochem. 2000, 39, 319–361. [Google Scholar] [CrossRef]
- Nasdala, L.; Akhmadaliev, S.; Burakov, B.E.; Chanmuang, N.C.; Škoda, R. The absence of metamictisation in natural monazite. Sci. Rep. 2020, 10, 14676. [Google Scholar] [CrossRef]
- Clavier, N.; Podor, R.; Dacheux, N. Crystal chemistry of the monazite structure. J. Eur. Ceram. Soc. 2011, 31, 941–976. [Google Scholar]
- McCarthy, G.L.; White, W.B.; Pfoertsch, D.E. Synthesis of nuclear waste monazites, ideal actinide hosts for geological disposal. Mater. Res. Bull. 1978, 13, 1239–1245. [Google Scholar] [CrossRef]
- Boatner, L.A.; Beall, G.W.; Abraham, M.M.; Finch, C.B.; Huray, P.C.; Rapaz, M. Monazite and other lanthanide orthophosphates as alternative actinide waste forms. In Scientific Basis for Nuclear Waste Management; Northrup, C.J.M., Jr., Ed.; Plenum Press: New York, NY, USA, 1980; Volume 2, pp. 289–296. [Google Scholar]
- Dacheux, N.; Clavier, N.; Podor, R. Monazite as a promising long-term radioactive waste matrix: Benefits of high-structural flexibility and chemical durability. Am. Miner. 2013, 98, 833–847. [Google Scholar] [CrossRef]
- Karioris, F.G.; Gowda, K.; Cartz, L. Heavy ion bombardment on monoclinic ThSiO4, ThO2, and monazite. Radiat. Eff. Lett. 1981, 58, 1–3. [Google Scholar] [CrossRef]
- Burakov, B.E.; Yagovkina, M.A.; Garbuzov, V.M.; Kitsay, A.A.; Zirlin, V.A. Self-irradiation of monazite ceramics: Contrasting behavior of PuPO4 and (La,Pu)PO4 doped with Pu-238. In Mater. Res. Symp. Proc. Scientific Basis for Nuclear Waste Management XXVIII; Hanchar, J.M., Stroes-Gascoyne, S., Browning, L., Eds.; Materials Research Society: Warrendale, PA, USA, 2004; Volume 824, p. CC4.1. [Google Scholar] [CrossRef]
- Nasdala, L.; Grötzschel, R.; Probst, S.; Bleisteiner, B. Irradiation damage in monazite (CePO4): An example to establish the limits of Raman confocality and depth resolution. Can. Mineral. 2010, 48, 351–359. [Google Scholar] [CrossRef]
- Poitrasson, F.; Chenery, S.; Shepherd, T.J. Electron microprobe and LA-ICP-MS study of monazite hydrothermal alteration: Implications for U-Th-Pb geochronology and nuclear ceramics. Geochim. Cosmochim. Acta 2000, 64, 3283–3297. [Google Scholar] [CrossRef]
- Williams, M.L.; Jercinovic, M.J.; Harlov, D.E.; Budzyń, B.; Hetherington, C.J. Resetting monazite ages during fluid-related alteration. Chem. Geol. 2011, 283, 218–225. [Google Scholar]
- Shiryaev, A.A.; Nickolsky, M.S.; Averin, A.A.; Grigoriev, M.S.; Zubavichus, Y.V.; Vlasova, I.E.; Petrov, V.G.; Burakov, B.E. Structural peculiarities of aged 238Pu-doped monazite. MRS Adv. 2016, 1, 4275. [Google Scholar] [CrossRef]
- Shiryaev, A.A.; Burakov, B.E.; Nickolsky, M.S.; Yapaskurt, V.O.; Pavlushin, A.D.; Grigoriev, M.S.; Vlasova, I.E. Surface features on aged 238Pu-doped Eu-monazite. Radiochim. Acta 2020, 108, 353–360. [Google Scholar] [CrossRef]
- Shiryaev, A.A.; Burakov, B.E.; Yapaskurt, V.O.; Egorov, A.V.; Vlasova, I.E. Microstructure of Aged 238Pu-doped La-monazite Ceramic and Peculiarities of its X-ray Emission Spectra. MRS Adv. 2020, 5, 1–7. [Google Scholar] [CrossRef]
- Du Fou de Kerdaniel, E.; Clavier, N.; Dacheux, N.; Terra, O.; Podor, R. Actinide solubility-controlling phases during the dissolution of phosphate ceramics. J. Nucl. Mater. 2007, 362, 451–458. [Google Scholar] [CrossRef]
- Shelyug, A.; Mesbah, A.; Szenknect, S.; Clavier, N.; Dacheux, N.; Navrotsky, A. Thermodynamics and stability of rhabdophanes, hydrated rare earth phosphates REPO4 n H2O. Front. Chem. 2018, 6, 604. [Google Scholar] [CrossRef]
- Lu, F.; Shen, Y.; Sun, X.; Dong, Z.; Ewing, R.C.; Lian, J. Size dependence of radiation-induced amorphization and recrystallization of synthetic nanostructured CePO4 monazite. Acta Mater. 2013, 61, 2984–2992. [Google Scholar] [CrossRef]
- Woodward, J.; Kennel, S.J.; Stuckey, A.; Osborne, D.; Wall, J.; Rondinone, A.J.; Standaert, R.F.; Mirzadeh, S. LaPO4 Nanoparticles Doped with Actinium-225 that Partially Sequester Daughter Radionuclides. Bioconjugate Chem. 2011, 22, 766–776. [Google Scholar] [CrossRef]
- Shiryaev, A.A.; Hinks, J.A.; Marks, N.A.; Greaves, G.; Valencia, F.J.; Donnelly, S.E.; González, R.I.; Kiwi, M.; Trigub, A.L.; Bringa, E.M.; et al. Ion implantation in nanodiamonds: Size effect and energy dependence. Sci. Rep. 2018, 8, 5099. [Google Scholar] [CrossRef] [PubMed]
- Burakov, B.E.; Ojovan, M.I.; Lee, W.E. Crystalline Materials for Actinide Immobilisation, Materials for Engineering; Imperial College Press: London, UK, 2010; Volume 1, p. 197. [Google Scholar]
- Eremin, N.N.; Marchenko, E.I.; Petrov, V.G.; Mitrofanov, A.A.; Ulanova, A.S. Solid solutions of monazites and xenotimes of lanthanides and plutonium: Atomistic model of crystal structures, point defects and mixing properties. Comput. Mater. Sci. 2019, 157, 43–50. [Google Scholar] [CrossRef]
- Boatner, L.A. Synthesis, Structure, and Properties of Monazite, Pretulite, and Xenotime. Rev. Miner. Geochem. 2002, 48, 87–121. [Google Scholar] [CrossRef]
- Jardin, R.; Pavel, C.C.; Raison, P.E.; Bouëxière, D.; Santa-Cruz, H.; Konings, R.J.; Popa, K. The high-temperature behaviour of PuPO4 monazite and some other related compounds. J. Nucl. Mat. 2008, 378, 167–171. [Google Scholar] [CrossRef]
- Ni, Y.; Hughes, J.M.; Mariano, A.M. Crystal chemistry of the monazite and xenotime structures. Amer. Miner. 2005, 80, 21–26. [Google Scholar] [CrossRef]
- Gale, J.D.; Rohl, A.L. The general utility lattice program (GULP). Mol. Simul. 2003, 29, 291–341. [Google Scholar] [CrossRef]
- Hardwick, J.L.; Brand, J.C.D. Anharmonic potential constants and the large amplitude bending vibration in nitrogen dioxide. Can. J. Phys. 1976, 54, 80–91. [Google Scholar] [CrossRef]
- Reed, D.; Van Konynenburg, R. Effect of Ionizing Radiation on Moist Air Systems. MRS Proc. 1987, 112, 393–404. [Google Scholar] [CrossRef] [Green Version]
- Mesbah, A.; Clavier, N.; Elkaim, E.; Gausse, C.; Kacem, I.B.; Szenknect, S.; Dacheux, N. Monoclinic Form of the Rhabdophane Compounds: REEPO4·0.667H2O. Cryst. Growth Des. 2014, 14, 5090–5098. [Google Scholar] [CrossRef]
- Eremin, N.N.; Deyanov, R.Z.; Urusov, V.S. Choice of the supercell with the optimum atomic configuration in simulation of disordered solid solutions. Glass Phys. Chem. 2008, 34, 9–18. [Google Scholar] [CrossRef]
- Arinicheva, Y.; Popa, K.; Scheinost, A.C.; Rossberg, A.; Dieste-Blanco, O.; Raison, P.; Cambriani, A.; Somers, J.; Bosbach, D.; Neumeier, S. Structural investigations of (La,Pu)PO4 monazite solid solutions: XRD and XAFS study. J. Nucl. Mat. 2017, 493, 404–411. [Google Scholar] [CrossRef]
- Kowalski, P.M.; Beridze, G.; Vinograd, V.; Bosbach, D. Heat capacities of lanthanide and actinide monazite-type ceramics. J. Nucl. Mater. 2015, 464, 147–154. [Google Scholar] [CrossRef]
- Ji, Y.; Kowalski, P.M.; Kegler, P.; Huittinen, N.; Marks, N.A.; Vinograd, V.L.; Arinicheva, Y.; Neumeier, S.; Bosbach, D. Rare-Earth Orthophosphates from Atomistic Simulations. Front. Chem. 2019, 7, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, A.; Panda, R.; Jha, M.K.; Kumar, J.R.; Lee, J.Y. Process development to recover rare earth metals from monazite mineral: A review. Miner. Eng. 2015, 79, 102–115. [Google Scholar] [CrossRef]
- Arinicheva, Y.; Gausse, C.; Neumeier, S.; Brandt, F.; Rozov, K.; Szenknect, S.; Dacheux, N.; Bosbach, D.; Deissmann, G. Influence of temperature on the dissolution kinetics of synthetic LaPO4-monazite in acidic media between 50 and 130 °C. J. Nucl. Mater. 2018, 509, 488–495. [Google Scholar] [CrossRef]
- Neumeier, S.; Arinicheva, Y.; Clavier, N.; Podor, R.; Bukaemskiy, A.; Modolo, G.; Dacheux, N.; Bosbach, D. The effect of the synthesis route of monazite precursors on the microstructure of sintered pellets. Progr. Nucl. Energy 2016, 92, 298–305. [Google Scholar] [CrossRef]
- Bryukhanova, K.I.; Nikiforova, G.E.; Tyurin, A.V.; Kondrat’eva, O.N.; Gavrichev, K.S. Effect of the Particle Habit on the Heat Capacity and Thermodynamic Functions of EuPO4 in the Range 7–1600 K. Russ. J. Inorg. Chem. 2020, 65, 681–687. [Google Scholar] [CrossRef]




| Parameter | LaPO4 | CePO4 | PuPO4 | PrPO4 | NdPO4 | SmPO4 | SmPO4 (Exp., [32]) | EuPO4 | GdPO4 |
|---|---|---|---|---|---|---|---|---|---|
| Volume, Å3 | 594.05 | 580.70 | 573.78 | 571.41 | 562.57 | 545.70 | 540.97 (1) | 539.80 | 534.56 |
| a, Å | 12.6454 | 12.5428 | 12.4998 | 12.4710 | 12.4169 | 12.2862 | 12.1443 (1) | 12.2275 | 12.1754 |
| b, Å | 7.3008 | 7.2416 | 7.2167 | 7.2001 | 7.1689 | 7.0935 | 7.0178 (1) | 7.0596 | 7.0295 |
| c, Å | 6.4346 | 6.3933 | 6.3607 | 6.3637 | 6.3199 | 6.2614 | 6.3476 (1) | 6.2534 | 6.2459 |
| β,° | 90.00 | 90.00 | 90.00 | 90.00 | 90.00 | 90.00 | 90.02 (1) | 90.00 | 90.00 |
| Structural energy, eV | −34.314 | −34.582 | −34.687 | −34.802 | −35.220 | −35.506 | - | −35.568 | −35.648 |
| K, GPa | 94.58 | 93.01 | 39.26 | 39.86 | 46.17 | 52.69 | - | 50.98 | 50.01 |
| G, GPa | 41.94 | 43.29 | 45.38 | 44.05 | 47.50 | 50.82 | - | 49.83 | 49.18 |
| Q1(TRPO4-PuPO4), kJ/mol | 0.964 | 0.097 | - | 0.039 | 0.264 | 1.767 | - | 2.687 | 3.624 |
| Q2(PuPO4-TRPO4), kJ/mol | 0.987 | 0.101 | - | 0.039 | 0.263 | 1.745 | 2.742 | 3.856 | |
| ΔH0,5 (TRPO4-PuPO4), kJ/mol | 0.2439 | 0.0248 | - | 0.0097 | 0.0658 | 0.4390 | - | 0.6786 | 0.9350 |
| Structure | Parameter | LaPO4 | CePO4 | PrPO4 | NdPO4 | SmPO4 | EuPO4 | GdPO4 | TbPO4 | DyPO4 | YPO4 | HoPO4 | ErPO4 | TmPO4 | YbPO4 | LuPO4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monazite | Q1 | 1.06 | 0.1 | 0.04 | 0.46 | 2.21 | 3.28 | 4.38 | 10.42 | 17.55 | 19.55 | 24.01 | 31.11 | 37.76 | 44.17 | 51.71 |
| Q2 | 1.06 | 0.12 | 0.05 | 0.46 | 2.15 | 3.13 | 4.16 | 10.08 | 16.00 | 17.71 | 21.36 | 27.18 | 32.58 | 37.71 | 43.66 | |
| η | 1.00 | 0.91 | 0.89 | 1.00 | 1.03 | 1.05 | 1.05 | 1.03 | 1.10 | 1.10 | 1.12 | 1.16 | 1.16 | 1.17 | 1.18 | |
| Xenotime | Q1 | 1.47 | 0.11 | 0.06 | 0.31 | 2.51 | 4.41 | 6.55 | 24.74 | 32.15 | 34.48 | 39.72 | 47.90 | 55.35 | 63.04 | 72.13 |
| Q2 | 1.44 | 0.11 | 0.06 | 0.31 | 2.46 | 4.19 | 6.04 | 18.79 | 23.98 | 25.76 | 29.19 | 34.78 | 39.81 | 44.90 | 50.82 | |
| η | 1.02 | 1.00 | 1.04 | 1.00 | 1.02 | 1.05 | 1.08 | 1.32 | 1.34 | 1.34 | 1.36 | 1.38 | 1.39 | 1.40 | 1.42 | |
| Rhabdophane | Q1 | 0.964 | 0.097 | 0.039 | 0.264 | 1.767 | 2.687 | 3.624 | 11.753 | 16.651 | 17.897 | 21.502 | 26.933 | 32.149 | 37.361 | 43.553 |
| Q2 | 0.987 | 0.101 | 0.039 | 0.263 | 1.745 | 2.742 | 3.856 | 12.655 | 16.288 | 17.609 | 20.299 | 24.833 | 29.092 | 33.343 | 38.344 | |
| η | 0.98 | 0.96 | 1.00 | 1.00 | 1.01 | 0.98 | 0.94 | 0.93 | 1.02 | 1.02 | 1.06 | 1.08 | 1.11 | 1.12 | 1.14 |
| Monazite Solid Solution | ΔH0,5, kJ/mol | Xenotime Solid Solution | ΔH0,5, kJ/mol | Rhabdophane Solid Solution | ΔH0,5, kJ/mol |
|---|---|---|---|---|---|
| LaPO4–PuPO4 | 0.26 | LaPO4 *–PuPO4 * | 0.36 | LaPO4–PuPO4 * | 0.24 |
| CePO4–PuPO4 | 0.03 | CePO4 *–PuPO4 * | 0.03 | CePO4–PuPO4 * | 0.02 |
| PuPO4–PrPO4 | 0.01 | PuPO4 *–PrPO4 * | 0.01 | PuPO4 *–PrPO4 | 0.01 |
| PuPO4–NdPO4 | 0.12 | PuPO4 *–NdPO4 * | 0.08 | PuPO4 *–NdPO4 | 0.07 |
| PuPO4–SmPO4 | 0.54 | PuPO4 *–SmPO4 * | 0.62 | PuPO4 *–SmPO4 | 0.44 |
| PuPO4–EuPO4 | 0.80 | PuPO4 *–EuPO4 * | 1.08 | PuPO4 *–EuPO4 | 0.68 |
| PuPO4–GdPO4 | 1.07 | PuPO4 *–GdPO4 * | 1.57 | PuPO4 *–GdPO4 | 0.93 |
| PuPO4–TbPO4 * | 2.56 | PuPO4 *–TbPO4 | 5.44 | PuPO4 *–TbPO4 * | 3.05 |
| PuPO4–DyPO4 * | 4.19 | PuPO4 *–DyPO4 | 7.02 | PuPO4 *–DyPO4 * | 4.12 |
| PuPO4–YPO4 * | 4.66 | PuPO4 *–YPO4 | 7.53 | PuPO4 *–YPO4 * | 4.44 |
| PuPO4–HoPO4 * | 5.67 | PuPO4 *–HoPO4 | 8.61 | PuPO4 *–HoPO4 * | 5.23 |
| PuPO4–ErPO4 * | 7.29 | PuPO4 *–ErPO4 | 10.34 | PuPO4 *–ErPO4 * | 6.47 |
| PuPO4–TmPO4 * | 8.79 | PuPO4 *–TmPO4 | 11.90 | PuPO4 *–TmPO4 * | 7.66 |
| PuPO4–YbPO4 * | 10.23 | PuPO4 *–YbPO4 | 13.49 | PuPO4 *–YbPO4 * | 8.84 |
| PuPO4–LuPO4 * | 11.92 | PuPO4 *–LuPO4 | 15.37 | PuPO4 *–LuPO4 * | 10.24 |
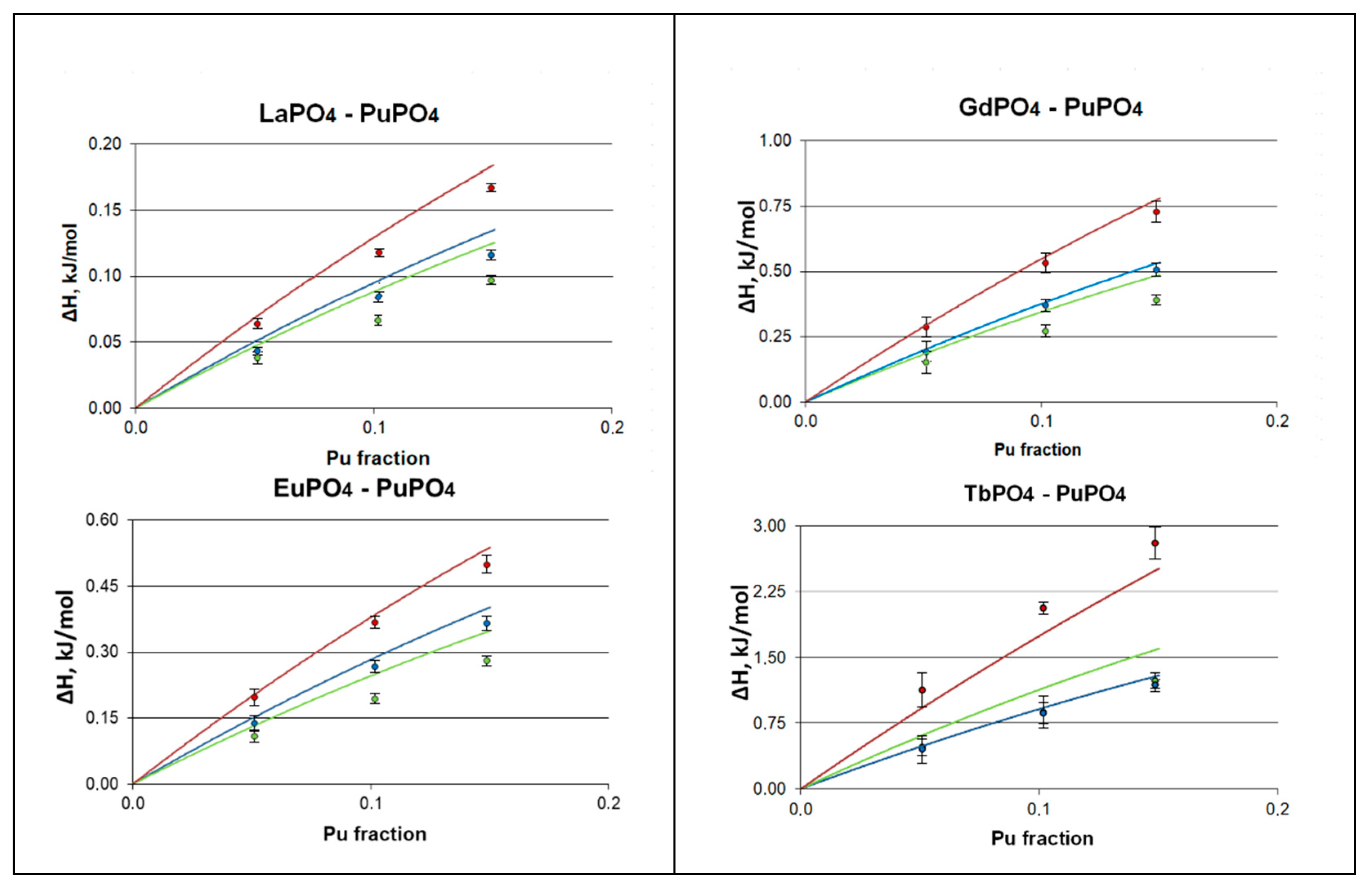
| ΔH0,1 kJ/mol | ΔG0,1 (200 K) kJ/mol | ΔG0,1 (400 K) kJ/mol | ΔG0,1 (600 K) kJ/mol | |
|---|---|---|---|---|
| LaPO4–PuPO4 system | ||||
| Monazite | 0.085 | −0.4588 | −1.0027 | −1.5473 |
| Xenotime | 0.119 | −0.4271 | −0.9743 | −1.5397 |
| Rhabdophane | 0.067 | −0.4722 | −1.0113 | −1.5505 |
| EuPO4–PuPO4 system | ||||
| Monazite | 0.268 | −0.2579 | −0.7845 | −1.3117 |
| Xenotime | 0.367 | −0.1820 | −0.7335 | −1.2850 |
| Rhabdophane | 0.194 | −0.3457 | −0.8862 | −1.4269 |
| GdPO4–PuPO4 system | ||||
| Monazite | 0.376 | −0.1529 | −0.6819 | −1.2111 |
| Xenotime | 0.533 | −0.0235 | −0.5833 | −1.5726 |
| Rhabdophane | 0.269 | −0.2764 | −0.8224 | −1.3684 |
| TbPO4–PuPO4 system | ||||
| Monazite | 0.867 | 0.3088 | −0.2351 | −0.7787 |
| Xenotime | 2.068 | 1.4401 | 0.8101 | −1.7786 |
| Rhabdophane | 0.897 | 0.8257 | 0.2557 | −0.3034 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailova, P.; Burakov, B.; Eremin, N.; Averin, A.; Shiryaev, A. Plutonium-Doped Monazite and Other Orthophosphates—Thermodynamics and Experimental Data on Long-Term Behavior. Sustainability 2021, 13, 1203. https://doi.org/10.3390/su13031203
Mikhailova P, Burakov B, Eremin N, Averin A, Shiryaev A. Plutonium-Doped Monazite and Other Orthophosphates—Thermodynamics and Experimental Data on Long-Term Behavior. Sustainability. 2021; 13(3):1203. https://doi.org/10.3390/su13031203
Chicago/Turabian StyleMikhailova, Polina, Boris Burakov, Nikolai Eremin, Alexei Averin, and Andrey Shiryaev. 2021. "Plutonium-Doped Monazite and Other Orthophosphates—Thermodynamics and Experimental Data on Long-Term Behavior" Sustainability 13, no. 3: 1203. https://doi.org/10.3390/su13031203






