1. Introduction and Objectives
Karst in evaporitic rocks presents a large variety of risks associated with any type of infrastructure. The risk component is inevitable. It cannot be totally eliminated, although it can be reduced to an acceptable level if rigorous studies are performed in the initial phases of geotechnical and geological research. Thus, risk can persist not only during the construction phase, but also throughout the useful life of the infrastructure, since detecting in advance all problems of this nature is practically impossible [
1]. Since evaporitic rocks can be karstified in months and/or years, continuous monitoring and surveillance of the hydrogeological behavior of the massif near a tunnel is crucial during its useful life.
Regarding the risk reduction strategy during the previous phases of the investigation, the geological predisposition constitutes the most important factor in determining the karst conditions that could occur on site during construction. Of the set of factors that explains the development of a karst system, the lithological factor is the most important and necessary, since karst can be considered as intrinsically linked to certain lithologies. In evaporitic rocks, the ease of karstification will depend mainly on the solubility of the mineral specimens present. The prevention and prediction of risks related to karst in evaporites is based on a good knowledge of geological formations with minerals susceptible to dissolution, as well as their location. Secondly, it is important to know the hydrogeological conceptual model prior to the infrastructure construction, as well as the factors that are expected to become involved in the evolution of the massif karstification as a consequence of these anthropic changes.
Regarding the importance of hydrogeology, gypsum and evaporite rocks in general have not aroused the interest of hydrogeologists, since they constitute low-quality aquifers for human water supply and use. The hydrogeological interest lies more in the field of applied geology and geotechnics, as they are related to problematic geological formations in the infrastructures construction and its interaction with water [
2].
Among these problems the risk of karstification near tunnels is found. Gypsiferous massifs are not excessively permeable if they are not karstified. However, natural karstification originated in geological times, or was produced in an accelerated way by the alteration of the flow induced by a tunnel construction, for example, which can create a new anisotropic aquifer with a much higher porosity and permeability [
3,
4].
The knowledge of the mechanisms that operate in karstification and other processes of interaction with water in gypsum and other evaporitic rocks has a clear scientific interest and relevant engineering importance. These mechanisms condition the stability of any civil engineering work located in this type of terrain. Given the large extent that gypsum and evaporite soils reach in general in Spain [
5], this becomes one of the problems that most frequently concerns infrastructure construction.
The consequent problems of geological, applied geology, and hydrogeology character are important. Their practical consequences can become even more complex and important if a good geological and hydrogeological knowledge is not available. This also implies knowing the mechanisms that operate between the terrain and the water.
In the case of underground excavations, it is also a problem that is occurring with increasing frequency in the tertiary sedimentary basins of Spain and Europe, as the depth of these new infrastructures reaches more saline units below, where besides gypsum, there may be other more expansive minerals (case of anhydrite) or much more soluble minerals, such as halite, epsomite, glauberite, thenardite, etc. Example cases are tunnels in the French Jura, Wagenburg North in Baden–Württemberg (Germany) [
6,
7], Lilla tunnel (Lérida, Spain) [
8,
9], Fabares (Asturias, Spain), Albertia (Alava, Spain) and Regajal (Madrid, Spain) [
10,
11,
12,
13].
Many infrastructures modify substantially the natural flow of groundwater during their construction, and even during their operation. This is the case in dam foundations on soluble materials, where the speed increase of the seepage can lead to their dissolution. In tunnels dug below the water table, groundwater drainage and karst reactivation of the massif can occur.
The processes and chemical reactions that occur due to interaction of water with the minerals of evaporitic rocks are usually very slow under natural conditions, where hydraulic gradients are usually small, and there is a hydrochemical balance between groundwater and rock. However, the alteration of the underground flow due to the construction of tunnels can accelerate these processes and increase the hydraulic gradient and, consequently, the speed and the water renewal. This can imply loss of tunnel peripheral support mainly by the generation of dissolution voids, which involves important consequences for the stability of the infrastructure and the area around it.
Some of the processes that can occur in the evaporitic massifs that contain the abovementioned minerals are related to mineral expansiveness and to rock porosity changes. The main processes are dissolution, transformation from glauberite to gypsum, physical erosion, expansiveness of anhydrite, and salt crystallization [
10,
11]. Among all, it is worth mentioning dissolution, which greatly affects halite, thenardite, and mirabilite, and moderately affects gypsum and anhydrite. These modifying processes in evaporitic rock massifs are accelerated near dams, tunnels, and other structures and could compromise their stability due to the alteration of the initial hydrogeological conditions.
During tunnel construction, water can significantly accelerate karstification phenomena. Expansion phenomena are also frequent due to the change in anhydrite volume, creeping, corrosion, etc. They are phenomena that, in some way, are interrelated with each other, but are not the subject of this work. This paper deals exclusively with the first generation of karstification. All these phenomena influence, when planning technical solutions in the excavation system to limit the active zone [
10] as much as possible, the waterproofing of the tunnel and the support-lining.
The chemistry, flow, and groundwater temperature play fundamental roles in the processes of dissolution, precipitation, and swelling [
14].
In addition to mineralogy, the hydrogeological context knowledge and the conceptual model of hydrogeological functioning is essential, as has been stated already. The water mobility is key to estimating the stability of the rock through which it circulates or is stored. In this sense, to identify supersaturated watertight zones without dissolution, it may be convenient to use isotopes to determine the water age, such as tritium. It is convenient, however, to carry out water stability diagrams with respect to calcium and sodium sulphates that allow one to know if such water can cause dissolution in different mineral species existing in the massif.
To determine and predict the progress of karstification, it is important to verify if all the ions are active, since their activity is conditioned by the electrical charges of other ions. For this reason, it is necessary to obtain the activity coefficients and subsequently determine the chemical equilibrium of the reactions of the different materials existing and involved in the dissolution process (saturation index), which will allow the dissolution reaction direction for each material to be deduced. It is about knowing the residual dissolution capacity and the tendency to saturation. Additionally, in a particular way, the solid mass loss and, consequently, the karstification increase due to the drainage to the tunnel or to the pumping wells, as occurred in the case here studied.
The karst processes and the dissolution of gypsum and evaporitic rocks were discussed by [
15,
16,
17,
18,
19,
20,
21], but the saturation index is a novel methodological technique in the tunneling and civil engineering field and has not been used before, although it has been applied from a speleogenetic point of view in geomorphology [
17]. The need for a saturation index calculation with respect to several mineral species is justified. It helps to determine the water capacity to continue dissolving materials and therefore allows the increase in karst progress to be predicted.
Thus, the main objective of this work is to contribute to a greater knowledge in the gypsum and evaporitic rocks karstification field in those massifs crossed by tunnels by applying the saturation index of the mineral specimens present in them. To reach this main final objective, we address, in an orderly manner, the following specific objectives: (i) the definition of the geological and hydrogeochemical conceptual model of the site, characterizing the local petrology and mineralogy; (ii) the chemical characterization of the groundwater and its evolution over time; (iii) the study of the groundwater chemical stratification and the potential relationship between the stratification and the geology and mineralogy of the area; (iv) the determination of equilibrium states of the saturation reactions of the groundwater regarding the different evaporitic minerals; and (v) the identification of the modifying processes of mineral transformation as a consequence of the flow alteration caused by the drainage operations.
2. Description of the Case Study
The case study in this work is the south portal of the El Regajal Tunnel, on the high-speed railway line from Madrid to Valencia (
Figure 1). The area is located between the provinces of Madrid and Toledo, in its rise from the Tagus river plain to the Ontígola plateau. The drainage network in this plain is quasi-endorheic, and at its head there are (and were) damp areas prone to flooding. On the eastern edge of these highlands are the so-called Ontígola springs. Their flow drains to the north through the Arroyo de la Vega to the Aranjuez and Tagus River. The tunnel is part of the Aranjuez–Ocaña section of the Levante high speed railway access. Work on the tunnel lasted from 2008 to 2010. It is a double track tunnel with an approximate inner diameter of 8.50 m. The useful section interior is 85.7 m
2, but the excavation section reaches 180 m
2. The maximum cap is 65 m with an average cap of 40 m. The average slope is 25 thousandths. The total length, including false tunnels, is 2445 m.
The tunnel was built with robust sections, preventing decompression of the massif, and avoiding all possible water contributions, both from the very excavation (which was carried out with the least amount of water possible), and from all types of water flow into the tunnel.
For this, the excavation was performed mainly in phases by a roadheader in saline soils and hydraulic hammers in clay soils. The lining was designed as a full section, very close to the excavation front, to achieve a rapid closure of the section [
22], avoiding movements and decompressions that would promote incoming water. In the project, a series of standard sections were proposed, which in the construction phase were simplified to four very similar designs (
Figure 2), varying only in the inverted vault thickness and its reinforcement. These sections are (1) the excavation in clay and clayey marls (I–M
1), (2) the section of glauberite and halite without water (I–R
N), (3) the anhydrite and expansive materials section (I–M
EXP), and (4) the sections with risk of dissolution (I–M
PIL) [
22].
The tunnel was executed as a false tunnel for the last 334 m of the southern mouth. The southern portal was carried out by a trench excavation 527 m long with a maximum depth of 15 m and an intermediate berm, a width that varies from 80 m on the surface to 40 m at the bottom of the excavation, and slopes of a 1:1 ratio.
In this area, this underground work crosses evaporitic Miocene materials. The final stretch of the tunnel ends in a false tunnel, as mentioned, and a large trench (
Figure 3 and
Figure 4), both in an ascending slope. This track intersects a subhorizontal geological contact between a lower layer of saline materials (in which halite and glauberite predominate, sometimes in a massive form and others interbedded with clay) and an upper horizon rich in gypsum and clay (
Figure 4). During the construction of the southern portal, it was observed that this stratigraphic discontinuity acts as a water exudation zone, and waterproofing and other measures were taken, such as waterproofing of concrete modules joints, injections, etc. [
22,
23].
In order to avoid problems during construction and exploitation of the tunnel, a drainage well was drilled to the northeast of the trench, near the tunnel portal, to drain the massif. A small wall of cut-off piles was built parallel to the tunnel axis to stop or reduce water infiltration into the tunnel from the ground. The groundwater, pumped since 2011 from the well, was first poured into a decanter before joining the drainage waters from the trench located in the last stretch. Analysis of the drained groundwater indicated an electrical conductivity of about 56,000 μS/cm due to the dissolved salts.
The drainage well was drilled during the construction of the excavation of the false tunnel to work in dry conditions, and it is still (when this work was being delivered) in use to avoid infiltration to the tunnel. Nevertheless, the groundwater extraction, heavily loaded with salts, caused material loss in the tunnel environment due to dissolution in the evaporitic massif (
Figure 4D).
To study the system as a whole, a hydrogeological investigation of the tunnel and the trench environment was carried out. The main objective was to determine the hydrogeological development after the construction of the tunnel and inauguration of the high speed Madrid–Valencia line. In a retrospective way, the hydrogeological process was inferred before and during the construction of the tunnel [
24,
25]. This hydrogeological study addressed two fundamental questions: the analysis of the drained groundwater origin, and geotechnical risk mitigation. Subsequently, the saturation indices research presented here was developed.
This work also aims to reflect on the need to carry out prior hydrogeological studies during the project phase of any tunnel with a regional character without being restricted to the narrow strip defined around the tunnel axis [
26].
3. Methodology
To achieve the objectives proposed above and given the hydrogeochemical nature of the research, the following methodology was followed:
Compilation of background and historical data available (construction project and data that were collected at the time of construction of the tunnel).
Mineralogy identification of the evaporite massif in order to define potentially karstifiable mineralogical species.
Analysis of the hydrogeological situation before and especially shortly after the execution of the tunnel works. The features of the existing aquifers in the area and the natural hydrological behavior of the streams were defined in order to identify the piezometric changes and the influence of the water flow in the massif.
Modeling of the behavior of the waters of the massif in relation to its dissolution capacity. This model was made based on the calculation of solute content activities in those waters. The objective was to fix the saturation index of each salt at the time of its sample collection. This index established a specific state in the dissolution process of each salt and, therefore, allowed the karstification degree at that moment to be estimated and, where appropriate and with more data, the evolution over time to be determined.
This methodology entailed, therefore, some field work and cabinet and laboratory work as well.
3.1. Field Work and Laboratory Tests
Firstly, a site geological study (geological cartography) and in depth geological cross section analysis were performed, which involved a detailed stratigraphy of the different geological formations. For that, intense field work was carried out, taking advantage of the outcrops of trench clearings, etc., and the information provided from ten railway tunnel project boreholes and twenty other boreholes fitted with piezometers (open-pipe type) specifically carried out later for this specific current work (
Figure 3). The borehole depths ranged between 20 and 45 m. Since it was not possible to visually determine all the mineralogical species in the sounding recognition obtained from these boreholes, careful sampling was carried out, identifying and making comparisons with the mineralogical analysis collected in the preliminary geotechnical tunnel project studies.
The field work included an inventory of forty-five existing water-points in the wider environment of the study area, which included springs, wells, other boreholes, and streams. Complementing this information and to determine in detail the hydrogeology of the site, a piezometric network of 20 boreholes/piezometers was designed. This network of piezometers helped, among other things, to measure water levels and draw isopieze maps, thus determining the induced underground flow system. Among the water points, in addition to the piezometers, the following should be mentioned: the abovementioned pumping well, the drainage pump of the ditch, and the Ontígola springs, which are located at 2725 m in the center of the abovementioned village at the public park of Los Manantiales (Los Manantiales), (
Figure 1 and
Figure 5). A specific explanation and its results were studied in depth in the
Section 4.2. Hydrogeological Synthesis of this work.
The evolution of hydrochemistry was monitored by taking samples at different depths in the most representative piezometers in the network (PZ1, PZ2, PZ3, PZ5, PZ6, PZ7, PZ8, PZ10, and PZ11). In some piezometers, only one sample was performed. These samples were distributed in each piezometer covering the entire studied area in order to collect the ion concentrations in the water. These samples provided results of hydrochemical processes in the field.
Likewise, and given that the mineralogical quality of the ground varies depending on the stratification, at different depths in some piezometers (number 6—PZ6, for example) water samples were also carefully taken. Some of the samples were taken during the excavation of the borehole or piezometer.
Water samples were also taken from the water-points around the tunnel, in the drainage well, and at the pump exits. Samples were analyzed, characterizing their composition, activity, saturation, and dissolution capacity in the laboratory (
Table 1).
The field works were performed in 2011–12, including sampling. In winter 2012, mineralogical studies were carried out, and, finally, modelling was fulfilled in 2015.
3.2. Modeling
Based on the sample analyses, the evaluation of the chemical activity of the salts was estimated in order to determine the different saturation indices and, therefore, water capacity to continue dissolving saline minerals. In this technique, a calculation model of the ionic activities, the saturation degree, and the dissolution capacity of materials was obtained. For that, a specific and simple model was developed by using an Excel spreadsheet, which allowed us to achieve all calculations. By using this model, the theoretical development of which is described below, mineral transformation of modifying processes in the field were identified. It was then possible to explain them by the flow alteration caused by the tunnel drainage operations.
3.3. Saturation Index Calculation
In saline waters, not all ions are active, but their activity is conditioned by the electrical charges of other ions. The activity coefficients are calculated applying the Debye–Hückel formula [
27], and the activity is determined in mol/L. Subsequently, the chemical equilibrium of the reactions with the different salts are determined, and then the saturation index is obtained in order to determine the direction of each reaction. The process followed to determine the saturation index (SI) is shown below.
The activity of a species or material according to its concentration is defined as
where
(Xi): activity of the species or material under study (mol/L),
mi: concentration (mol/L), and
γi: ionic activity coefficient (values are between 0 and 1)
where the activity coefficient depends on
the radius and the ion charge,
the dissolution temperature, and
the water salinity (the higher the salinity, the lower the active proportion of the element).
The activity coefficient is obtained using the Debye–Hückel formula:
where
A and
B are constants that depend on the temperature. They are tabulated, although they can also be obtained in the following way, expressing
T in °C:
The influence of temperature on the activity coefficient is irrelevant for the usual range of temperatures, so that the variation of the saturation index against temperature was not significant in our case of study. This point was checked and validated in piezometer number 6 (
Figure 3) for the range of 10 to 19 °C.
Parameter I using the Debye–Hückel formula expresses the ionic force, which also indicates the water salinity:
where
The saturation index is related to the chemical equilibrium of the reaction, and it is expressed using the following reaction:
When the reaction is in equilibrium, by the law of mass action, the following is obtained:
where
K is a constant (theoretical) that characterizes a reaction at a given temperature (25 °C, if no other is indicated); for example, for gypsum,
K = 10
−4.58 (
Table 3).
K is also called the dissociation constant (equation in equilibrium).
To evaluate the imbalance (no equilibrium) of a dissolution sample and, therefore, the direction of a reaction in such an unsaturated dissolution sample, the ion activity product (IAP) is used. Note that in Equation (7),
K expresses equilibrium in the sample:
Saturation index (
SI) is a function of the
IAP/
K ratio, where
K is the dissociation constant.
Regarding the SI, the following cases in relation to a specific material dissolution can occur:
SI > 0, reaction to the left: supersaturated water, therefore precipitation,
SI = 0, equilibrium reaction, and
SI < 0, reaction to the right: subsaturated water, therefore dissolution.
6. Discussion
From the results obtained and considering the evolution observed in groundwater (piezometers PZ-1 to PZ-17) and in the surface waters analyzed, some aspects can be highlighted. The water flowing through the evaporitic deposits to the drainage well has two origins: (i) rain infiltration water (vertical flow), and (ii) lateral incoming flow, mostly from the aquifer of the Ontígola springs, located outside the piezometric contour map (
Figure 7).
In its circulation, the infiltrated water quickly reaches equilibrium or becomes slightly saturated in sulphated calcium salts in the upper part of the aquifer. For the rest of the evaporitic minerals, the waters remain unsaturated, and rapid dissolution takes place at depth. Even so, water is only saturated in glauberite, anhydrite, and thenardite at levels below 16–20 m, and it remains unsaturated in epsomite and halite. This process is showed in
Figure 12, where the prevalence of linear trends shows this phenomenon.
The incoming water to the system affected by the pumping should originally have the average chemical characteristics of the Ontígola springs. The characteristics of the outgoing water are different than those of the Ontígola spring due to the pumping operations. The chemistry variation from the original water (Ontígola springs) to the outgoing water (average of the two main catchment points, the drainage well, and drainage of the trench) is shown in
Table 10. The resulting increase in salt concentration is 58.8 g/L.
Table 11 shows the variation of the saturation index of the different salts before and after the water infiltration process of the Ontígola springs and its subsequent pumping and draining through the massif.
Figure 13 shows the clear trend of the subsaturated waters towards saturation in several salts. However, water can only be considered saturated in gypsum and anhydrite with saturation indexes close to zero, being at equilibrium reactions. The presence of ions of different saline compounds in dissolution leads to a general subsaturation state for the rest of the salts at the drainage points, although less obvious than in the original water.
6.1. Porosity Increase in the Evaporitic Massif Due to the Drainage from the Well and the Trench
The variation of saline concentration between the incoming water to the system and the water collected from the catchment points allows the total amount of dissolved evaporitic minerals and the volume that it occupied in the terrain to be estimated. The input flow data between the two-catchment points averages 4 L/s (drainage well: 2.5 L/s, trench: 1.5 L/s). The average dissolution is 58.800 g/L with 90.188 g/L in the trench and 33.787 g/L in the drainage well.
Considering an average density of the evaporitic minerals of 2.47 t/m3, the yearly volume of salts dissolved is about 2.772 m3. This figure is obtained from the monthly dissolution estimated in the trench area of 142 m3/month (350.7 t/month) and from the drainage well (88.6 m3/month, 218.94 t/month).
6.2. Porosity Increase in the Evaporitic Massif Due to the Transformation of Glauberite to Gypsum
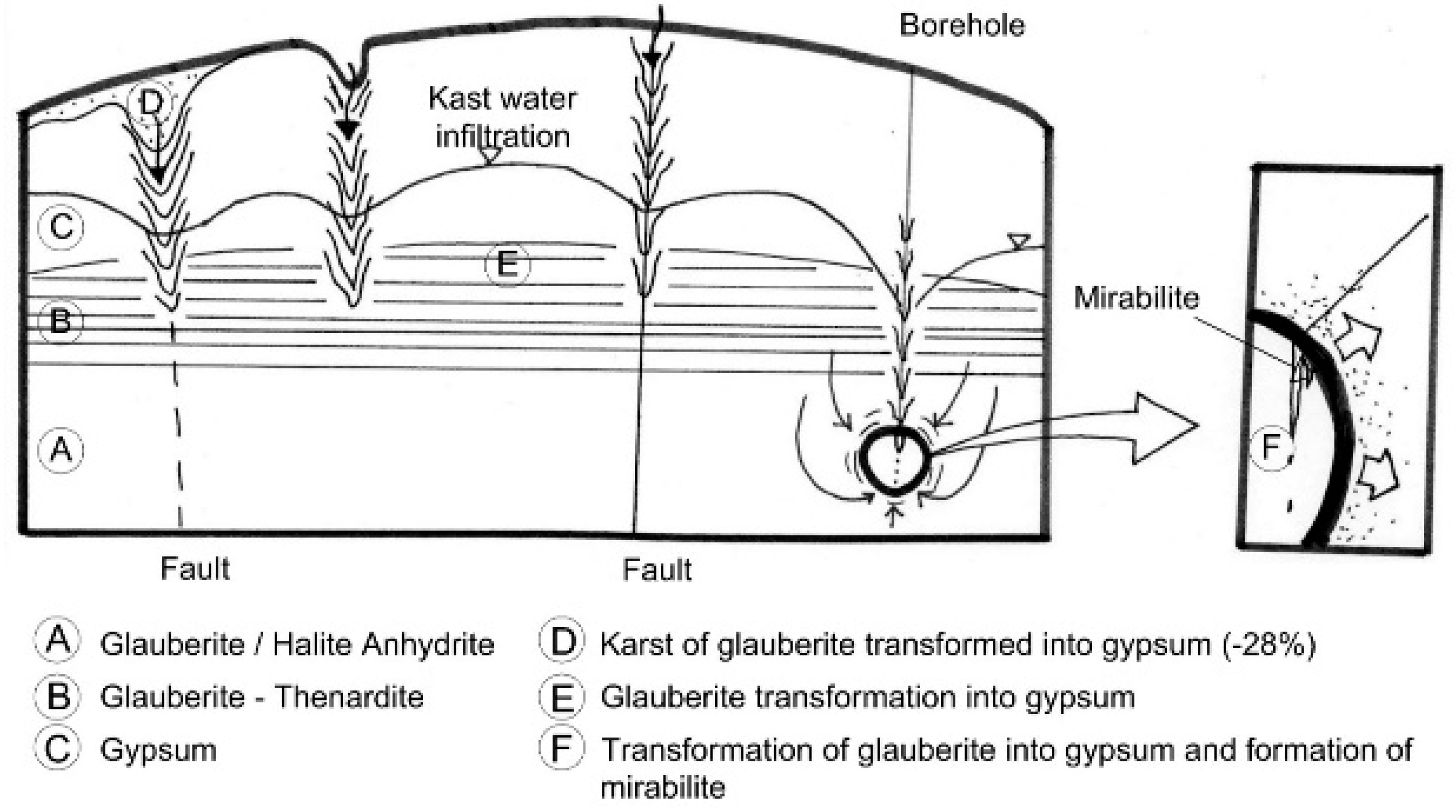
The water presence or simply the moisture can produce the replacement of glauberite into gypsum (solid) and mirabilite (dissolved) by incongruent dissolution. The transformation of glauberite into gypsum is a prototype of an incongruous dissolution reaction, the result of which is the neoformation of amorphous gypsum and the enrichment of groundwater in sulfate anion and sodium cation. This transformation entails a 27% decrease in volume and favors the generation of moderate voids in the ground, generally from pre-existing joints and cracks [
33]. The diameter of the created voids can range from a few millimeters to several decimeters. These processes can be increased by the massif permeability achievement, which will tend to promote the renewed groundwater circulation and, therefore, karstification. This transformation process has slow kinetics in natural conditions, but it can be fast (days or months) in the presence of renewed “fresh” waters with high capacity to dissolve and transform the glauberite. The following possible patterns have been inferred from the behavior of each mineral observed in this study.
Figure 14 represents a vertical section through the complex CaSO
4Na SO
4–NaCl–H
2O units. The water turns glauberite into gypsum by these ways: (1) through the upper gypsum aquifer with the formation of an horizon of secondary gypsum after glauberite, (2) through karstic formations caused by the volume reduction in the transformation process of the glauberite into gypsum, (3) by karst caused by the gypsum dissolution, (4) through faults, (5) by the “water call” process caused by the excavation of the tunnel, and (6) through non-closed drill holes, which reduce the phreatic level.
Both the well and the trench lowered the phreatic level below the contact level between gypsum and glauberite at 590 m a.s.l. in a considerable area. Therefore, during the pumping process, the transformation of glauberite into gypsum was produced, which implied a reduction of 28% of the initial volume. The effects of this process are difficult to quantify, but certainly it contributed hastily to the formation of a new aquifer, in the form of a semi-circular sinkhole around the well, where the porosity and permeability were significantly increased.
The new drainage level of the trench increased the underground flow in the saline layers down to 20 to 25 m deep, a zone where water previously barely circulated. This is the reason why waters collected at the drainage points are so saline. The large volume of additional terrain involved is large enough to maintain the current salinity levels for years.
Water comes out with a higher saline concentration in the trench than in the drainage well because the matrix flow is more important in the trench (the catchment is linear and not punctual, as in the well). Additionally, the saturated zone of the source basin of the trench is entirely located in the glauberite layer.
6.3. Distribution and Development of Porosity and Karstification in Space and Time
The most likely consequence of drainage by pumping is the development of a conduit network near the well caused by direct dissolution and erosion. It remains unknown how this porosity increase is distributed, although it is plausible that the voids are concentrated around the pumping. In the initial stages, a conduit network would be established in the preferred direction oriented (i) to the maximum hydraulic slope, and (ii) along the horizontal stratification planes, where the flow resistance is lower, taking advantage of the levels of halite and sodium sulphate with higher solubility.
Figure 7,
Figure 8,
Figure 9,
Figure 10 and
Figure 11 support these observations.
In the first phase, the stimulated water circulation caused the decrease of the phreatic level, the evacuation of the underground water stored in the upper part of the evaporitic aquifer, and in the overlying terrace, radially propagating the development of the porosity.
The analyzed karst system has a single outgoing point and multiple incoming points along the aquifer’s feeding area (
Figure 15). Hydrochemistry also plays an important role, because from the subsaturated water from the rain or irrigation recharge, with the capacity to dissolve the saline minerals, it becomes saturated in sulphates when interacting with gypsum. However, water remains subsaturated and with the capacity to dissolve halite, thenardite, mirabilite, and epsomite, up to the pumping point, inclusive. It was verified that the main factor in karstification is the presence of hyper-soluble materials, in which the water is predominantly subsaturated and the dissolution process continues due to the change of saturation point, in some cases extraordinarily (
Figure 11 and
Figure 12).
7. Conclusions
The study and monitoring of the evaporitic massif drainage of El Regajal (Madrid–Toledo), in the works of the trench and the false tunnel of the Madrid–Valencia high-speed railway line, has allowed the hydrochemical evolution of the water in evaporitic materials to be determined.
The piezometric contours map, obtained from the piezometric control network, shows a macrocone of depression around the pumping and at the catchment of the trench, with an influence radius of at least 1000 m. The topographic height of these two drainage points determines the new hydrogeological base level, which is lower than Ontígola springs, located 2.5 km eastwards, and with whose aquifer they are hydraulically and laterally connected.
The continuity of the potentiometric surface and the existence of the macrocone of depression show that the evaporitic massif behaves hydrogeologically as a single aquifer, without separate compartments or hanging levels in vertical. This does not imply that, on a small scale, the groundwater circulates preferably through the horizontal stratification planes, karstic conduits and fractures [
33].
The minerals composing this evaporitic massif are arranged in horizontal layers with gypsum dominating in the upper and thenardite, glauberite, halite, mirabilite, and polyhalite in the lower. The characteristics and chemical facies of the groundwater reflect the stratigraphy and mineralogy of the area, with an increase of salinity at depth, and groundwater stratification. The sulphated sodic water facies are dominant in the catchment areas.
The hydrochemical study of the water samples in the different piezometers at different depths, including the calculation of their activities, has allowed the saturation index for different saline materials to be identified, and the water residual dissolution capacity was largely surprising despite electrical hyperconductivity, as is shown in
Figure 7,
Figure 8,
Figure 9,
Figure 10 and
Figure 11.
Except for gypsum and anhydrite, which appear saturated in most of the piezometers, especially at the points located at low depth, all the present minerals of evaporitic origin in this massif are susceptible to be dissolved according to the groundwater chemistry, at least to 16 m depth. Below this depth, the water remains subsaturated in relation to thenardite, mirabilite, epsomite, glauberite, and halite.
The alteration of the underground flow and the consequent water renewal of the aquifer by the infiltration of rainwater and irrigation are the cause of the hydrogeochemical imbalance and of the modification of the massif characteristics. These changes, in general, cause an important loss of material, changing the strength of the terrain and the increase of voids index. These processes have been quantified, highlighting the dissolution of halite, thenardite, and mirabilite to an extreme degree, and to the gypsum and anhydrite to a moderate degree. They also include different expansive and recrystallization processes that decrease the massif porosity. Likewise, the transformation of glauberite into gypsum by incongruent dissolution, the physical erosion by water circulation, the anhydrite swelling by hydration, and the crystallization of new salts were identified. It was estimated that the evaporitic material lost in the area from the ground by dissolution is about 2.700 m3/year, extrapolating the results of study as constant over time.
All these processes are originating a new aquifer by karstification and a new evaporitic massif by mineralogical transformation. It is estimated that the porosity near the tunnel increased by 1% during the construction period.
The processes occurring in an evaporitic massif, such as the one under study, are identifiable and in any way quantifiable, in relation to the contents of the different evaporitic minerals (gypsum, halite, thenardite, mirabilite, glauberite, epsomite, and anhydrite). These processes are the increase of voids by erosion, dissolution, and by mineralogical transformations and, on the other hand, recrystallization processes, some of them expansive, which would decrease the rock porosity, neutralizing the increase of the porosity, but with consequences in the geomechanical properties [
34].
Quantifying the magnitude of these processes separately is complex, but generally, the processes increasing the porosity will be dominant over time, since as the circulation of the renewed water increases, the processes of dissolution and mineral transformation are being accelerated.
A measurement campaign of the ions existing in the water at different depths would offer more clarity about the evolution of these phenomena over time, which would facilitate the implementation of preventive and corrective measures in Civil Engineering.