Effects of Spent Drilling Fluids from Natural Gas Fields on Seed Germination and Root Development of Maize (Zea mays L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Experimental Design
2.2. Physicochemical Analysis
2.3. Germination Assays
2.4. Root Growth
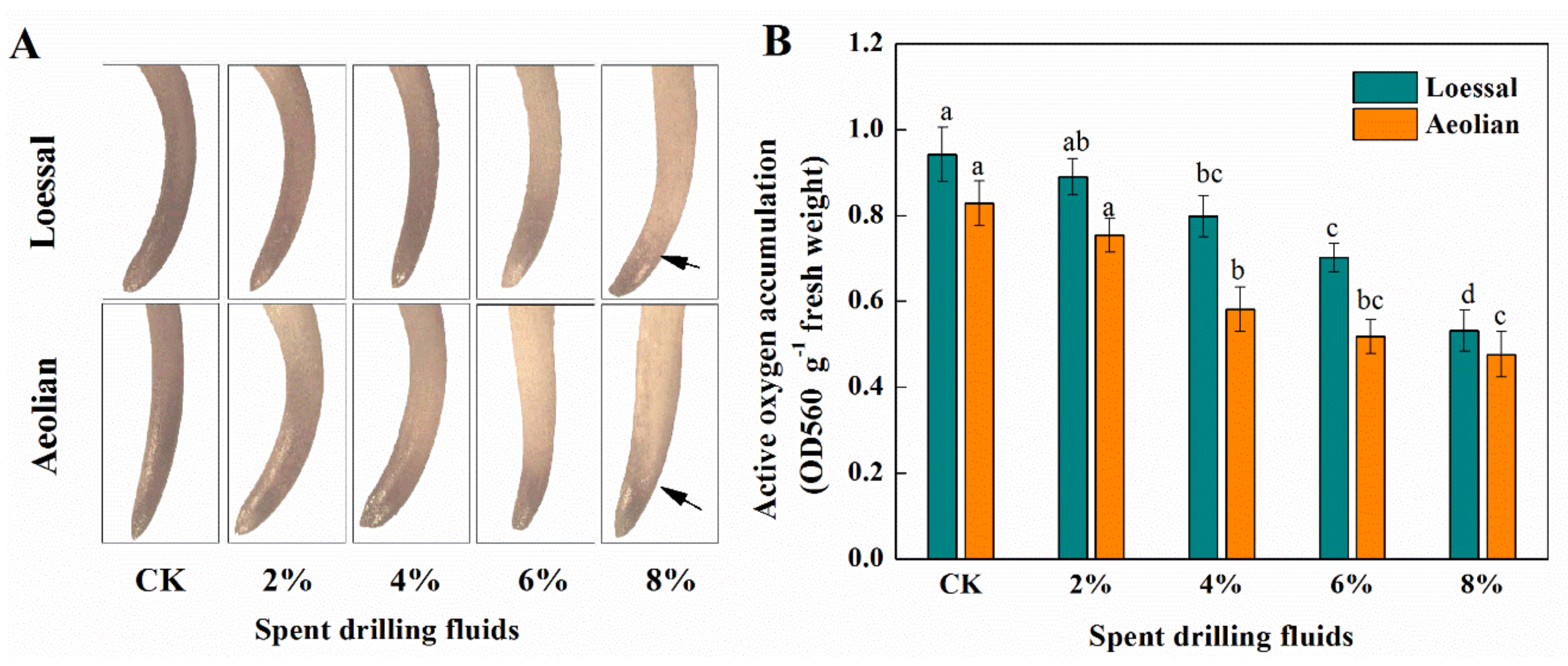
2.5. Distribution and Accumulation of Superoxide Anion in Roots
2.6. Determination of Antioxidants Activity
2.7. Lipid Peroxidation, Membrane Leakage, and Cell Viability Analysis
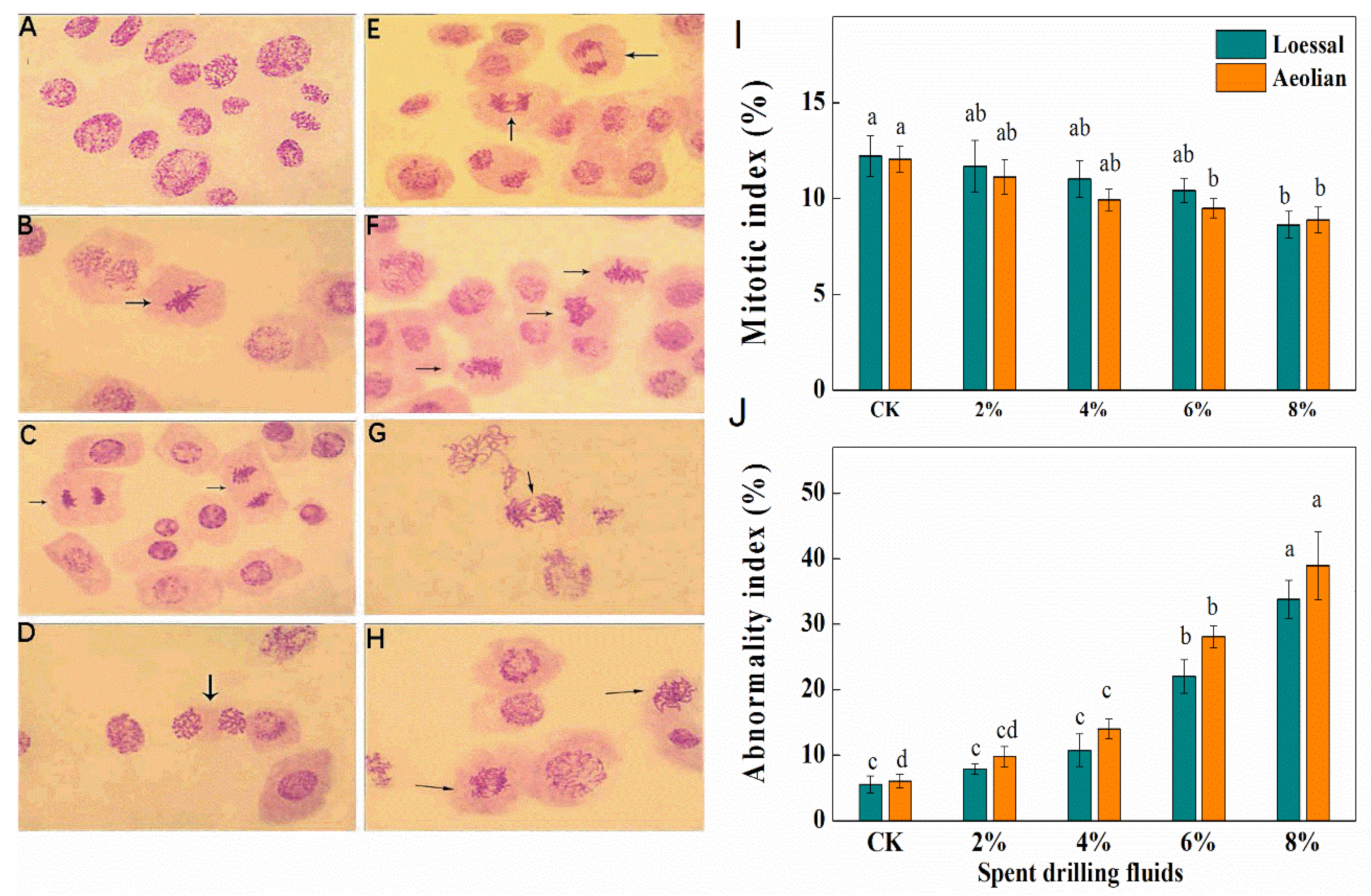
2.8. Cytogenetic Analysis
2.9. Statistical Analysis
3. Results
3.1. Spent Drilling Fluids and Soil Properties Prior to Application
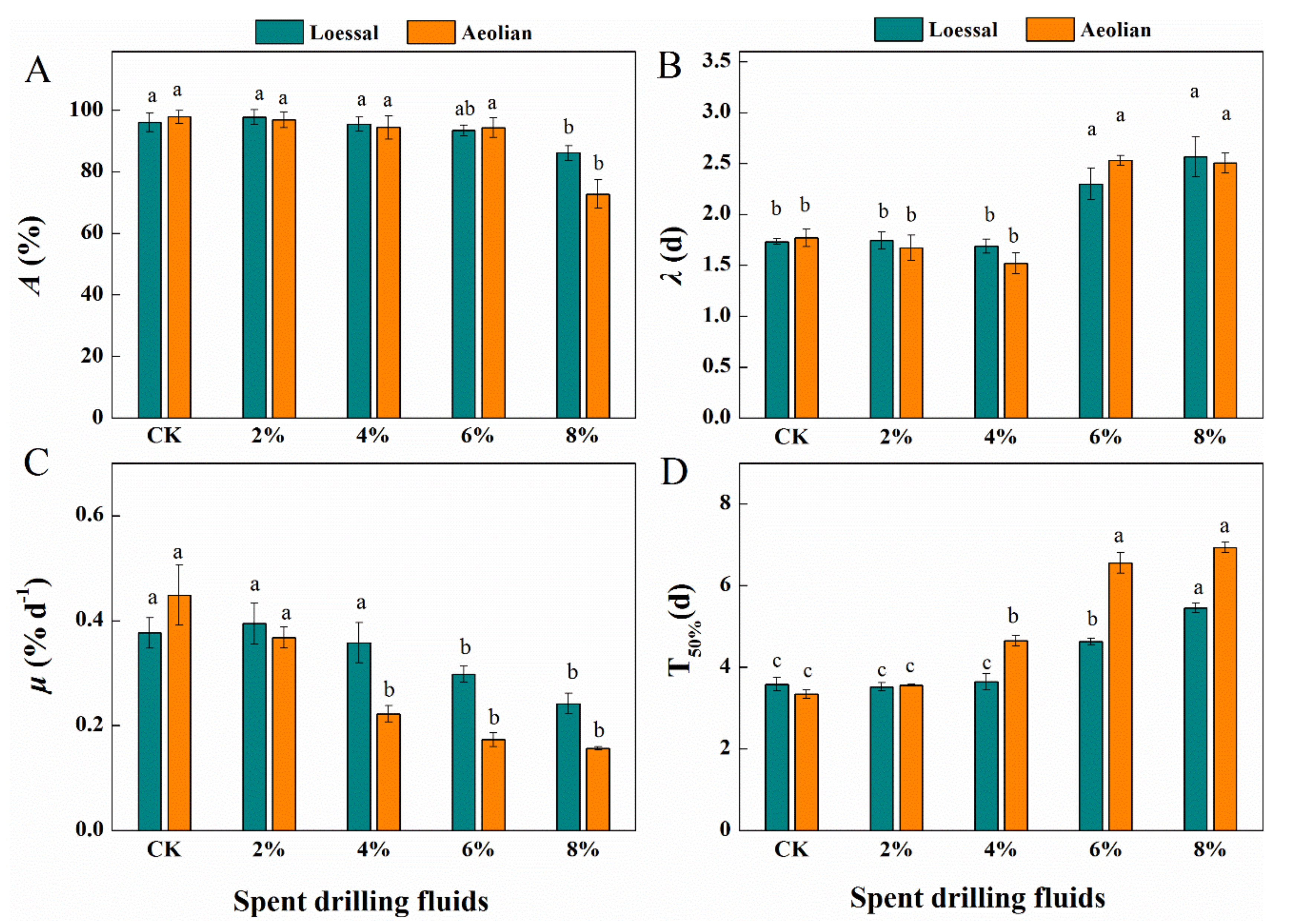
3.2. Responses of Seed Germination to Spent Drilling Fluids
3.3. Responses of Root Development to Spent Drilling Fluids
3.3.1. Root Growth
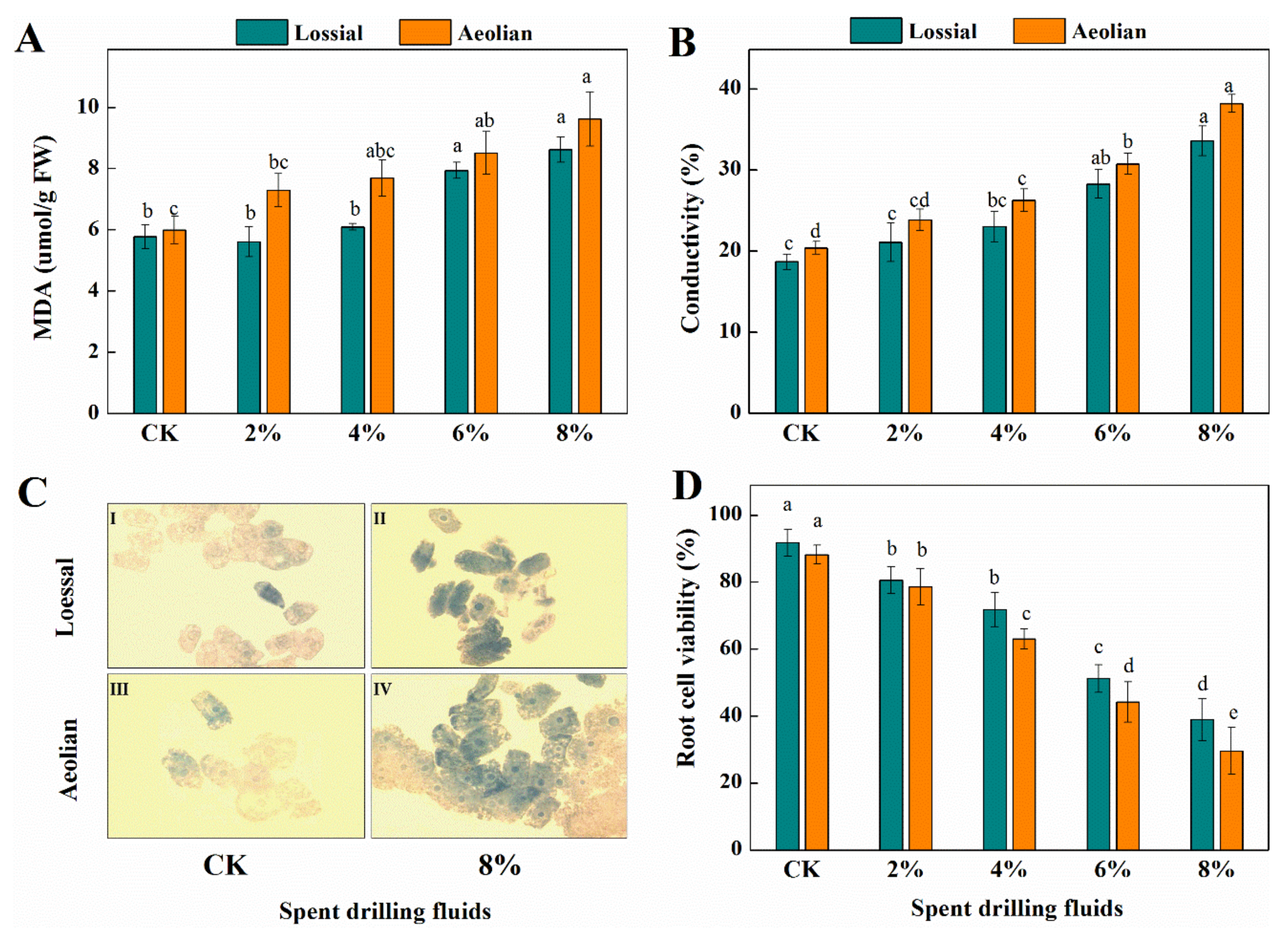
3.3.2. Biochemical Responses
3.3.3. ROS Generation in Maize Roots
3.3.4. Membrane Stability and Root Cell Viability
3.3.5. Cytogenetic Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ismail, A.R.; Alias, A.H.; Sulaiman, W.R.W.; Jaafar, M.Z.; Ismail, I. Drilling fluid waste management in drilling for oil and gas wells. Chem. Eng. Trans. 2017, 56, 1351–1356. [Google Scholar]
- Ball, A.S.; Stewart, R.J.; Schliephake, K. A review of the current options for the treatment and safe disposal of drill cuttings. Waste Manag. Res. 2012, 30, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Petri, I.; Martins, A.L.; Duarte, C.R.; Ataíde, C.H. Development and performance of a continuous industrial microwave dryer for remediation of drill cuttings. J. Pet. Sci. Eng. 2019, 176, 362–368. [Google Scholar] [CrossRef]
- Chang, X.; Sun, J.; Xu, Z.; Lv, K.; Dai, Z.; Zhang, F.; Huang, X.; Liu, J. Synthesis of a novel environment-friendly filtration reducer and its application in water-based drilling fluids. Colloids Surf. A 2019, 568, 284–293. [Google Scholar] [CrossRef]
- Parizad, A.; Shahbazi, K.; Ayatizadeh, T.A. Enhancement of polymeric water-based drilling fluid properties using nanoparticles. J. Pet. Sci. Eng. 2018, 170, 813–828. [Google Scholar] [CrossRef]
- Ghazali, N.A.; Alias, N.H.; Mohd, T.A.T.; Adeib, S.I.; Noorsuhana, M.Y. Potential of corn starch as fluid loss control agent in drilling mud. Appl. Mech. Mater. 2015, 754–755, 682–687. [Google Scholar] [CrossRef]
- Jain, R.; Paswan, B.K.; Mahto, T.K.; Mahto, V. Study the effect of synthesized graft copolymer on the inhibitive water based drilling fluid system. Egypt. J. Pet. 2017, 26, 875–883. [Google Scholar] [CrossRef] [Green Version]
- Ukachukwu, C.O.; Ogbobe, O.; Umoren, S.A. Preparation and characterization of biodegradable polymer mud based on millet starch. Chem. Eng. Commun. 2010, 197, 1126–1139. [Google Scholar] [CrossRef]
- Salehnezhad, L.; Heydari, A.; Fattahi, M. Experimental investigation and rheological behaviors of water-based drilling mud contained starch-ZnO nanofluids through response surface methodology. J. Mol. Liq. 2019, 276, 417–430. [Google Scholar] [CrossRef]
- Cole, E.L.; Mark, S. E and P waste: Manage it cost effectively through landfarming. World Oil 2000, 221, 132–134. [Google Scholar]
- Bauder, T.; Barbarick, K.A.; Shanahan, J.F.; Ayers, P.D.; Chapman, P.L. Drilling fluid effects on crop growth and iron and zinc availability. J. Environ. Qual. 1999, 28, 744–749. [Google Scholar] [CrossRef]
- Suthar, S.; Singh, S. Feasibility of vermicomposting in biostabilization of sludge from a distillery industry. Sci. Total. Environ. 2008, 394, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Zvomuya, F.; Larney, F.J.; Willms, W.D.; Beck, R.K.; Olson, A.F. Vegetation response to a one-time spent drilling mud application to semiarid, mixed-grass prairie. Rangel. Ecol. Manag. 2011, 64, 375–383. [Google Scholar] [CrossRef]
- Yao, L.; Naeth, M.A. Soil and plant response to used potassium silicate drilling fluid application. Ecotoxicol. Environ. Saf. 2015, 120, 326–333. [Google Scholar] [CrossRef]
- Yao, L.; Naeth, M.A. Soil and plant response to unused potassium silicate drilling fluid application. Ecol. Eng. 2014, 73, 461–468. [Google Scholar] [CrossRef]
- Debez, A.; Hamed, K.B.; Grignon, C.; Abdelly, C. Salinity effects on germination, growth, and seed production of the halophyte Cakile maritima. Plant Soil 2004, 262, 179–189. [Google Scholar] [CrossRef]
- Li, W.; Khan, M.A.; Yamaguchi, S.; Kamiya, Y. Effects of heavy metals on seed germination and early seedling growth of Arabidopsis thaliana. Plant Growth Regul. 2005, 46, 45–50. [Google Scholar] [CrossRef]
- Smith, M.J.; Flowers, T.H.; Duncan, H.J.; Alder, J. Effects of polycyclic aromatic hydrocarbons on germination and subsequent growth of grasses and legumes in freshly contaminated soil and soil with aged PAHs residues. Environ. Pollut. 2005, 141, 519–525. [Google Scholar] [CrossRef]
- Strazisar, T.; Koch, M.S.; Madden, C.J.; Filina, J.; Lara, P.U.; Mattair, A. Salinity effects on Ruppia maritima L. seed germination and seedling survival at the Everglades-Florida Bay ecotone. J. Exp. Mar. Biol. Ecol. 2013, 445, 129–139. [Google Scholar] [CrossRef]
- Hubbard, M.; Germida, J.; Vujanovic, V. Fungal endophytes improve wheat seed germination under heat and drought stress. Botany 2012, 90, 137–149. [Google Scholar] [CrossRef]
- Gong, X.; Cai, L.; Li, S.; Chang, S.X.; Sun, X.; An, Z. Bamboo biochar amendment improves the growth and reproduction of Eisenia fetida and the quality of green waste vermicompost. Ecotoxicol. Environ. Saf. 2018, 156, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Bellino, A.; Lofrano, G.; Carotenuto, M.; Libralato, G.; Baldantoni, D. Antibiotic effects on seed germination and root development of tomato (Solanum lycopersicum L.). Ecotoxicol. Environ. Saf. 2018, 148, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xing, M.; Yang, J.; Huang, Z. Compositional and functional features of humic acid-like fractions from vermicomposting of sewage sludge and cow dung. J. Hazard. Mater. 2011, 185, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Garg, V.K. Industrial wastes and sludges management by vermicomposting. Rev. Environ. Sci. Bio/Technol. 2011, 10, 243–276. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Wang, Q.; Huang, H.; Li, R.; Shen, F.; Lahori, A.H.; Wang, P.; Guo, D.; Guo, Z.; Jiang, S.; et al. Effect of biochar amendment on greenhouse gas emission and bio-availability of heavy metals during sewage sludge co-composting. J. Clean. Prod. 2016, 135, 829–835. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Daud, M.K.; Quiling, H.; Lei, M.; Ali, B.; Zhu, S.J. Ultrastructural, metabolic and proteomic changes in leaves of upland cotton in response to cadmium stress. Chemosphere 2015, 120, 309–320. [Google Scholar] [CrossRef]
- Siddiqui, H.; Ahmed, K.B.M.; Hayat, S. Comparative effect of 28-homobrassinolide and 24-epibrassinolide on the performance of different components influencing the photosynthetic machinery in Brassica juncea L. Plant Physiol. Biochem. 2008, 129, 198–212. [Google Scholar] [CrossRef]
- Verma, S.; Dubey, R.S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Shakir, S.K.; Irfan, S.; Akhtar, B.; Rehman, S.U.; Daud, M.K.; Taimur, N.; Azizullah, A. Pesticide-induced oxidative stress and antioxidant responses in tomato (Solanum lycopersicum L.) seedlings. Ecotoxicology 2018, 27, 919–935. [Google Scholar] [CrossRef]
- Diaz-Tielas, C.; Grana, E.; Sotelo, T.; Reigosa, M.J.; Sanchez-Moreiras, A.M. The natural compound trans-chalcone induces programmed cell death in Arabidopsis thaliana roots. Plant Cell Environ. 2012, 35, 1500–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leme, D.M.; Marin-Morales, M.A. Allium cepa test in environmental monitoring: A review on its application. Mutat. Res. 2009, 682, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.P.; Kim, S.H.; Bang, J.W.; Lee, H.S.; Kwak, S.S.; Kwon, S.Y. Enhanced tolerance to oxidative stress in transgenic tobacco plants expressing three antioxidant enzymes in chloroplasts. Plant Cell Rep. 2007, 26, 591–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2007, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Zavariyan, A.M.; Rad, M.; Asghari, M. Effect of seed priming by potassium nitrate on germination and biochemical indices in Silybumm arianum L. under salinity stress. Int. J. Life Sci. 2015, 9, 23–29. [Google Scholar]
- Dunand, C.; Crevecoeur, M.; Penel, C. Distribution of superoxide and hydrogenperoxide in Arabidopsis root and their influence on root development: Possible interaction with peroxidases. New Phytol. 2007, 174, 332–341. [Google Scholar] [CrossRef]
- Liszkay, A.; vander Zalm, E.; Schopfer, P. Production of reactive oxygen intermediates (O2.-, H2O2, and .OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol. 2004, 136, 3114–3123. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Dat, J.F.; Vandenabeele, S.; Vranova, E.; Montagu, M.V.; Inze, D.; Breusegem, F.V. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 2009, 57, 779–795. [Google Scholar] [CrossRef]
- Schafer, F.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Vivancos, P.D.; Driscoll, S.P.; Bulman, C.A.; Ying, L.; Emami, K.; Treumann, A.; Mauve, C.; Noctor, G.; Foyer, C.H. Perturbations of amino acid metabolism associated with glyphosate-dependent inhibition of shikimic acid metabolism affect cellular redox homeostasis and alter the abundance of proteins involved in photosynthesis and photorespiration. Plant Physiol. 2011, 157, 256–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.B.; Kim, Y.H.; Lee, H.S.; Kim, K.Y.; Deng, X.P.; Kwak, S.S. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol. Biochem. 2009, 47, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Türkan, İ.; Bor, M.; Özdemir, F.; Koca, H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci. 2005, 168, 223–231. [Google Scholar]
- Sharma, P.; Dubey, R.S. Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Rep. 2007, 26, 2027–2038. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox. Sign. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.L.; Lan, S.S.; Gong, M. Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. J. Plant Physiol. 2009, 166, 1694–1699. [Google Scholar] [CrossRef]
- Kuchy, A.H.; Wani, A.A.; Kamili, A.N. Cytogenetic effects of three commercially formulated pesticides on somatic and germ cells of Allium cepa. Environ. Sci. Pollut. Res. 2016, 23, 6895–6906. [Google Scholar] [CrossRef]
- Hossain, Z.; Mandal, A.K.A.; Shukla, R.; Datta, S.K. NaCl stress—Its chromotoxic effects and antioxidant behavior in roots of Chrysanthemum morifolium Ramat. Plant Sci. 2003, 166, 215–220. [Google Scholar] [CrossRef]
- West, G.; Inze, D.; Beemster, G.T. Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol. 2004, 135, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.V.A.; Wettern, A.C.; Caligari, P.D.S. Cell and nuclear degradation in root meristems following exposure of potatoes (Solanum tuberosum L.) to salinity. Potato Res. 2001, 44, 389–390. [Google Scholar] [CrossRef]
- Kim, J.C.; Bendixen, L.E. Effects of Haloxyfop and Cga-82725 On Cell Cycle and Cell Division of Oat (Avena sativa) Root Tips. Weed Sci. 1978, 35, 769–774. [Google Scholar] [CrossRef]
- Sudhakar, R.; Gowda, K.N.N.; Venu, G. Mitotic Abnormalities Induced by Silk Dyeing Industry Effluents in the Cells of Allium cepa. Cytologia 2001, 66, 235–239. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, L.K.S.; Gupta, S.K. Combined cytogenetic and ultrastructural effects of substituted urea herbicides and synthetic pyrethroid insecticide on the root meristem cells of Allium cepa. Pestic. Biochem. Phys. 2005, 82, 27–35. [Google Scholar] [CrossRef]
- Maluzynska, J.; Juchimiuk, J. Plant genotoxicity: A molecular cytogenetic approach in plant bioassays. Arh. Hig. Rada Toksiko 2005, 56, 177–184. [Google Scholar]
- Osterberg, R.; Persson, D.; Bjursell, G. The Condensation of DNA by Chromium (III) Ions. J. Biomol. Struct. Dyn. 1984, 2, 285–290. [Google Scholar] [CrossRef]
- Gaulden, M.E. Hypothesis: Some mutagens directly alter specific chromosomal proteins (DNA topoisomerase II and peripheral proteins) to produce chromosome stickiness, which causes chromosome aberrations. Mutagenesis 1987, 2, 357–365. [Google Scholar] [CrossRef]
- Goujon, E.; Richard, C.; Goupil, P.; Ledoigt, G. Cytotoxicity on Allium cepa of the two main sulcotrione photoproducts, xanthene-1, 9-dione-3, 4-dihydro-6-methylsulphonyl and 2-chloro-4-mesylbenzoic acid. Pestic. Biochem. Phys. 2015, 124, 37–42. [Google Scholar] [CrossRef]
- Kalefetoglu, M.T. Investigation of cytotoxicity and genotoxicity of abamectin pesticide in Allium cepa L. Environ. Sci. Pollut. Res. 2020, 28, 2391–2399. [Google Scholar] [CrossRef]
- Mesi, A.; Kopliku, D. Cytotoxic and genotoxic potency screening of two pesticides on Allium cepa L. Procedia Technol. 2013, 8, 19–26. [Google Scholar]

| Properties | Drilling Fluids | Soil | |
|---|---|---|---|
| Aeolian | Loessal | ||
| pH (unitless) | 9.93 | 9.21 | 8.86 |
| EC (dS m−1) | 7.66 | 0.16 | 0.24 |
| SAR (unitless) | 1110.76 | 3.21 | 4.36 |
| Soluble Na (mg kg−1) | 29920.00 | 16.13 | 19.76 |
| Soluble Mg (mg kg−1) | 1435.75 | 11.47 | 15.23 |
| TN (g kg−1) | 1.96 | 0.58 | 0.67 |
| AK (mg kg−1) | 1145.27 | 76.51 | 93.37 |
| AP (mg kg−1) | 1.59 | 5.38 | 5.64 |
| TOC (g kg−1) | 115.17 | 0.22 | 4.73 |
| Cl− (mg kg−1) | 950.55 | 5.74 | 20.22 |
| SO42− (mg kg−1) | 1551.65 | 3.64 | 8.08 |
| NO3− (mg kg−1) | 12.44 | 3.09 | 4.72 |
| Elements | Zn | Cu | Cr | Cd | Ni | Pb | Hg | |
|---|---|---|---|---|---|---|---|---|
| Content (mg kg−1) | 506.7 | 23.5 | 99.83 | 0.11 | 15.8 | 34.4 | - | |
| CHN Regulation * | I | 1200 | 500 | 500 | 3 | 100 | 300 | 3 |
| II | 3000 | 1000 | 1300 | 4 | 200 | 1000 | 15 | |
| Soil | SDF | H2O2 | Proline | SOD | APX | POD |
|---|---|---|---|---|---|---|
| Loessal | CK | 3.37 c | 9.48 c | 88.07 c | 154.62 b | 122.29 c |
| 2% | 3.32 c | 10.78 c | 105.61 b,c | 165.01 b | 124.79 b,c | |
| 4% | 3.58 c | 13.89 c | 122.28 b,c | 184.68 b | 141.67 b,c | |
| 6% | 5.23 b | 19.83 b | 130.89 b | 226.32 a | 168.75 a,b | |
| 8% | 7.27 a | 38.13 a | 169.58 a | 222.47 a | 207.72 a | |
| Aeolian | CK | 4.25 d | 10.54 c | 107.47 c | 152.41 b | 126.67 c |
| 2% | 4.64 c,d | 11.36 c | 119.94 b,c | 161.42 a,b | 149.17 b,c | |
| 4% | 6.05 c | 18.54 b,c | 136.19 b,c | 173.57 a,b | 164.16 b,c | |
| 6% | 7.95 b | 27.95 b | 155.56 a,b | 230.57 a | 182.08 b | |
| 8% | 9.81 a | 49.48 a | 197.41 a | 244.44 a | 275.83 a | |
| p > F | SDF | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Soil | <0.0001 | 0.0001 | 0.0023 | 0.9246 | 0.0004 | |
| SDF × Soil | 0.0207 | 0.0223 | 0.9160 | 0.9731 | 0.0247 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Hao, M. Effects of Spent Drilling Fluids from Natural Gas Fields on Seed Germination and Root Development of Maize (Zea mays L.). Sustainability 2021, 13, 1510. https://doi.org/10.3390/su13031510
Wang Z, Hao M. Effects of Spent Drilling Fluids from Natural Gas Fields on Seed Germination and Root Development of Maize (Zea mays L.). Sustainability. 2021; 13(3):1510. https://doi.org/10.3390/su13031510
Chicago/Turabian StyleWang, Zhe, and Mingde Hao. 2021. "Effects of Spent Drilling Fluids from Natural Gas Fields on Seed Germination and Root Development of Maize (Zea mays L.)" Sustainability 13, no. 3: 1510. https://doi.org/10.3390/su13031510




