Strength Performance and Microstructure of Calcium Sulfoaluminate Cement-Stabilized Soft Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials of the Experiments
2.2. Specimen Preparation
2.3. Methods of Tests
3. Results and Discussion
3.1. UCS of CSA-Stabilized Soil
3.2. Hydration Products of CSA-Stabilized Soil
3.3. Distinction of Microstructures Between OPC- and CSA-Stabilized Soils
4. Conclusions
- (1)
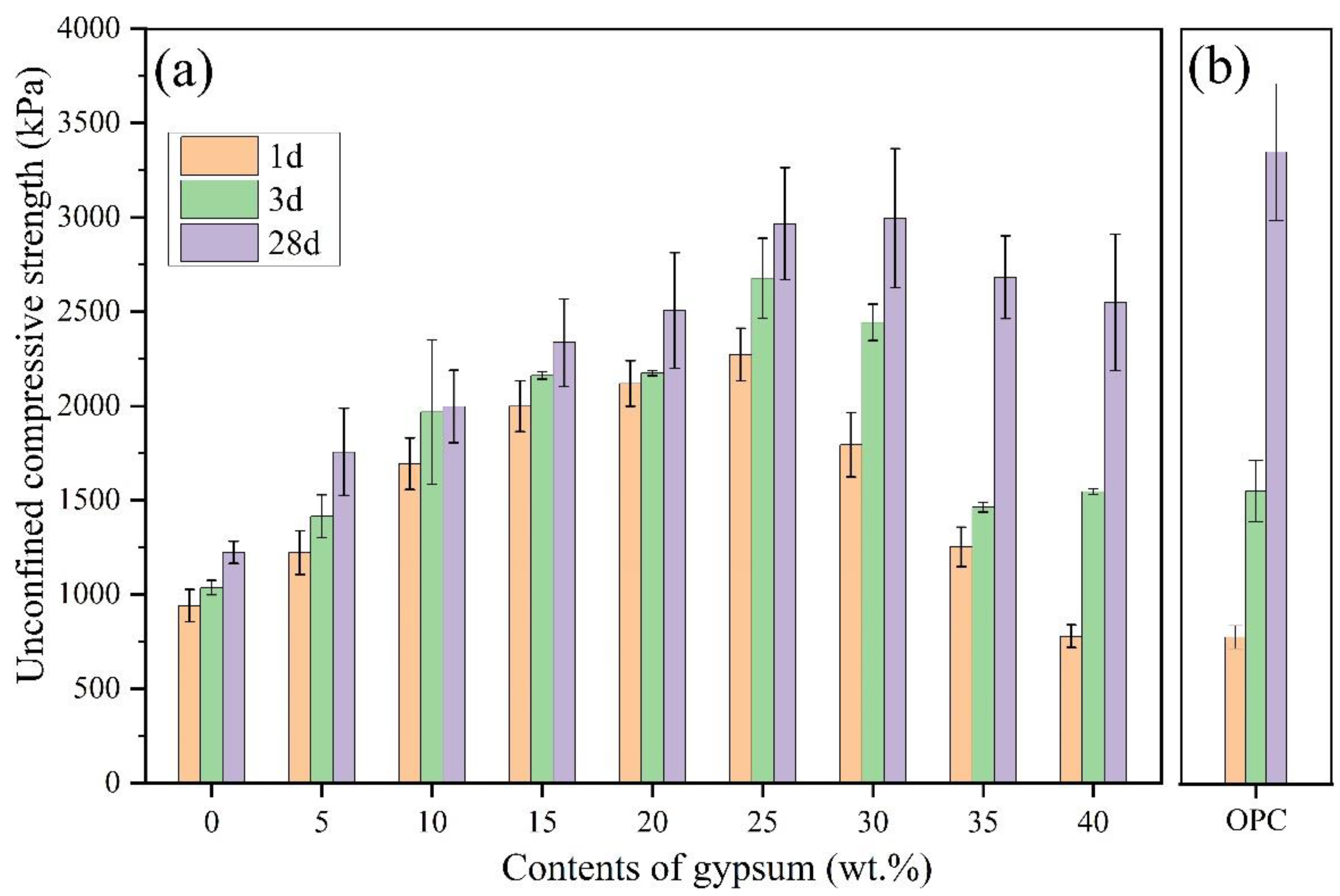
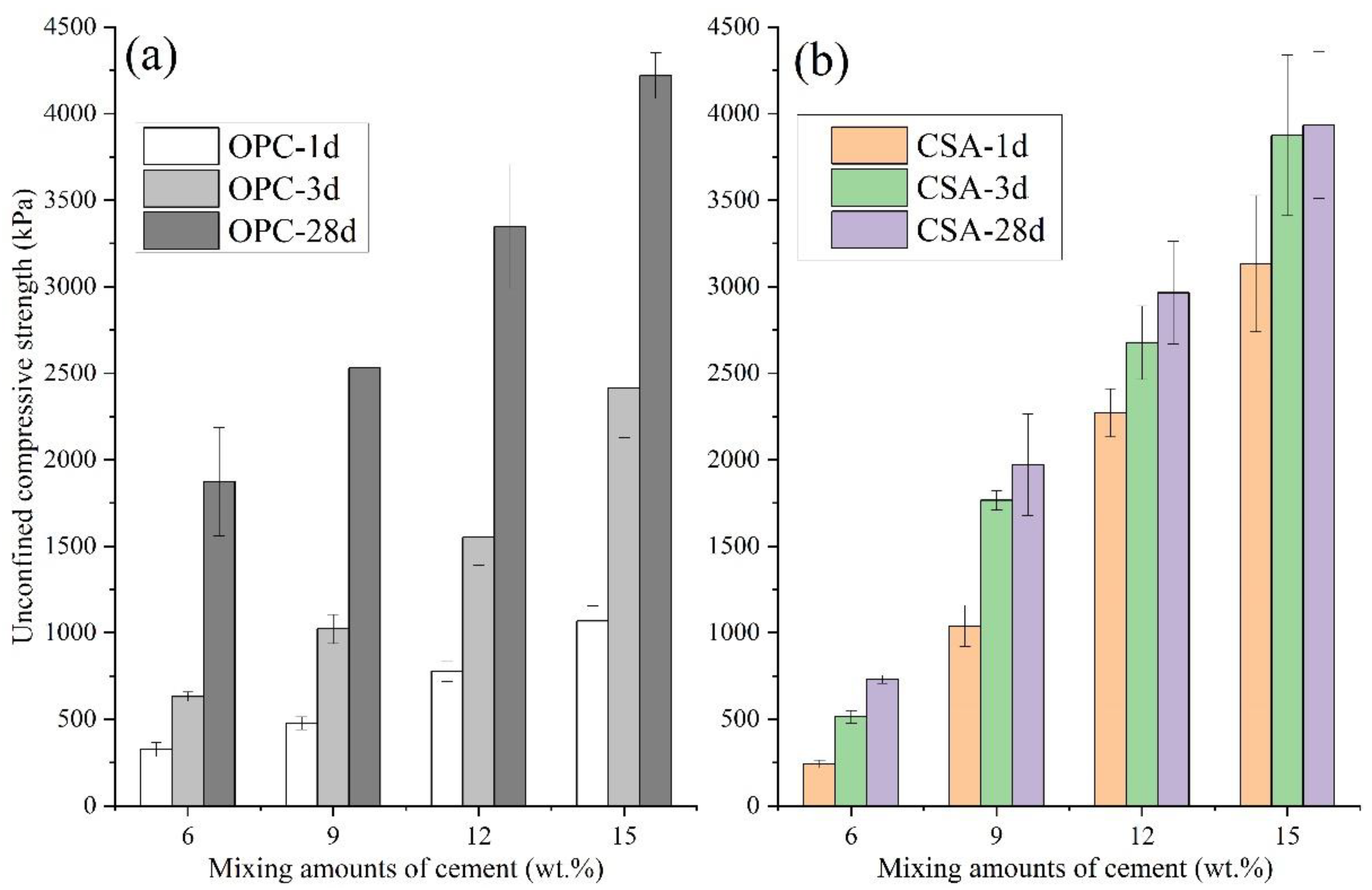
- UCS of CSA-stabilized soils at 1, 3, and 28 d firstly increased and then decreased with contents of C$·H2 increasing from 0 to 40 wt.%. The optimum C$·H2 content for CSA-stabilized soils was 25 wt.%, which means the stabilized soils had the highest UCS. When the mixing amounts of OPC and CSA were the same, CSA-stabilized soils had significantly higher early strength (1 and 3 d) than OPC and similar strength at 28 d.
- (2)
- For CSA-stabilized soil with 0 wt.% C$·H2, AFm was detected as a major hydration product. As for CSA-stabilized soil with certain amounts of C$·H2, the intensity of AFt was significantly higher than that in the sample hydrating without C$·H2; meanwhile, a tiny peak of AFm could be also detected in the sample with 15 wt.% C$·H2 at 28 d. Additionally, the intensity of AFt increased with the contents of gypsum increasing from 0 to 25 wt.%. When contents of C$·H2 increased from 25 to 40 wt.%, the intensity of AFt tended to decrease slightly, and residual C$·H2 could be detected in the sample with 40 wt.% C$·H2 at 28 d.
- (3)
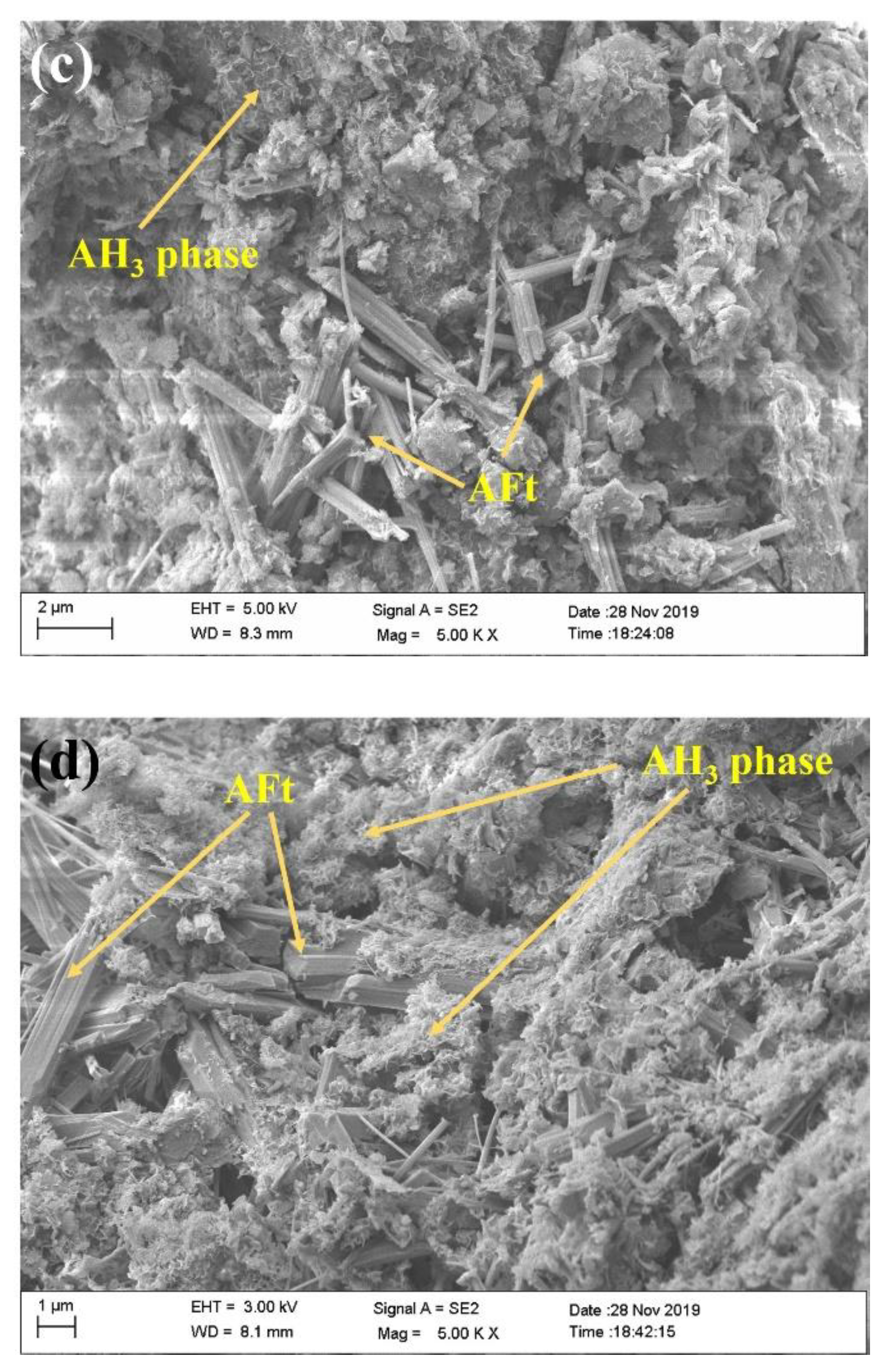
- In the microstructure of OPC-stabilized soils, hexagonal plate-shaped CH constituted skeleton structures, and clusters of C-S-H gel adhered to particles of soils. In the microstructure of CSA-stabilized soils, AFt constituted skeleton structures, and the crystalline sizes of ettringite increased with contents of C$·H2 increasing, meanwhile, clusters of AH3 phase could be observed to adhere to particles of soils and strengthen the interaction.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ekinci, A. Effect of preparation methods on strength and microstructural properties of cemented marine clay. Constr. Build. Mater. 2019, 227, 116690. [Google Scholar] [CrossRef]
- Kang, G.; Tsuchida, T.; Kim, Y. Strength and stiffness of cement-treated marine dredged clay at various curing stages. Constr. Build. Mater. 2017, 132, 71–84. [Google Scholar] [CrossRef]
- Kun, M.; Chunyi, C.; Haijiang, L. An Ontology Framework for Pile Integrity Evaluation Based on Analytical Methodology. IEEE Access 2020, 99, 72158–72168. [Google Scholar]
- Yi, Y.; Li, C.; Liu, S.; Al-Tabbaa, A. Resistance of MgO–GGBS and CS–GGBS stabilised marine soft clays to sodium sulfate attack. Géotechnique 2014, 64, 673–679. [Google Scholar] [CrossRef]
- Ho, T.O.; Chen, W.B.; Yin, J.H.; Wu, P.C.; Tsang, D.C. Stress-Strain behaviour of Cement-Stabilized Hong Kong marine deposits. Constr. Build. Mater. 2021, 274, 122103. [Google Scholar] [CrossRef]
- Mickovski, S.B.; Stokes, A.; Van Beek, R.; Ghestem, M.; Fourcaud, T. Simulation of direct shear tests on rooted and non-rooted soil using finite element analysis. Ecol. Eng. 2011, 37, 1523–1532. [Google Scholar] [CrossRef]
- Lang, L.; Liu, N.; Chen, B. Strength development of solidified dredged sludge containing humic acid with cement, lime and nano-SiO2. Constr. Build. Mater. 2020, 230, 116971. [Google Scholar] [CrossRef]
- Jamshidi, R.J.; Lake, C.B. Hydraulic and strength properties of unexposed and freeze–thaw exposed cement-stabilized soils. Can. Geotech. J. 2015, 52, 283–294. [Google Scholar] [CrossRef]
- Yi, Y.; Gu, L.; Liu, S.; Puppala, A.J. Carbide slag–activated ground granulated blastfurnace slag for soft clay stabilization. Can. Geotech. J. 2015, 52, 656–663. [Google Scholar] [CrossRef]
- Robayo, R.A.; Mulford, A.; Munera, J.; de Gutiérrez, R.M. Alternative cements based on alkali-activated red clay brick waste. Constr. Build. Mater. 2016, 128, 163–169. [Google Scholar] [CrossRef]
- Cui, C.; Meng, K.; Xu, C.; Liang, Z.; Li, H.; Pei, H. Analytical solution for longitudinal vibration of a floating pile in saturated porous media based on a fictitious saturated soil pile model. Comput. Geotech. 2021, 131, 103942. [Google Scholar] [CrossRef]
- Yi, Y.; Li, C.; Liu, S. Alkali-Activated Ground-Granulated Blast Furnace Slag for Stabilization of Marine Soft Clay. J. Mater. Civil Eng. 2015, 27, 04014146. [Google Scholar] [CrossRef]
- Subramanian, S.; Moon, S.W.; Moon, J.; Ku, T. CSA-treated sand for geotechnical application: Microstructure analysis and rapid strength development. J. Mater. Civil Eng. 2018, 30, 04018313. [Google Scholar] [CrossRef]
- Kim, G.M.; Jang, J.G.; Khalid, H.R.; Lee, H.K. Water purification characteristics of pervious concrete fabricated with CSA cement and bottom ash aggregates. Constr. Build. Mater. 2017, 136, 1–8. [Google Scholar] [CrossRef]
- Ghadir, P.; Ranjbar, N. Clayey soil stabilization using geopolymer and Portland cement. Constr. Build. Mater. 2018, 188, 361–371. [Google Scholar] [CrossRef]
- Uchima, J.S.; Restrepo-Baena, O.J.; Tobón, J.I. Mineralogical evolution of portland cement blended with metakaolin obtained in simultaneous calcination of kaolinitic clay and rice husk. Constr. Build. Mater. 2016, 118, 286–293. [Google Scholar] [CrossRef]
- Behnood, A. Soil and clay stabilization with calcium- and non-calcium-based additives: A state-of-the-art review of challenges, approaches and techniques. Transp. Geotech. 2018, 17, 14–32. [Google Scholar] [CrossRef]
- Muvuna, J.; Boutaleb, T.; Mickovski, S.B.; Baker, K.; Mohammad, G.S.; Cools, M.; Selmi, W. Information integration in a smart city system—A case study on air pollution removal by green infrastructure through a vehicle smart routing system. Sustainability 2020, 12, 5099. [Google Scholar] [CrossRef]
- Yoon, H.N.; Seo, J.; Kim, S.; Lee, H.K.; Park, S. Hydration of calcium sulfoaluminate cement blended with blast-furnace slag. Constr. Build. Mater. 2021, 268, 121214. [Google Scholar] [CrossRef]
- Horpibulsuk, S.; Rachan, R.; Chinkulkijniwat, A.; Raksachon, Y.; Suddeepong, A. Analysis of strength development in cement-stabilized silty clay from microstructural considerations. Constr. Build. Mater. 2010, 24, 2011–2021. [Google Scholar] [CrossRef]
- Lorenzo, G.A.; Bergado, D.T. Fundamental parameters of cement-admixed clay—New approach. J. Geotech. Geoenvironmental Eng. 2004, 130, 1042–1050. [Google Scholar] [CrossRef]
- Wu, J.; Deng, Y.; Zheng, X.; Cui, Y.; Zhao, Z.; Chen, Y.; Zha, F. Hydraulic conductivity and strength of foamed cement-stabilized marine clay. Constr. Build. Mater. 2019, 222, 688–698. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, A.; Deng, Y.; Cui, Y.; Yu, Z.; Yu, C. Strength performance of cement/slag-based stabilized soft clays. Constr. Build. Mater. 2019, 211, 909–918. [Google Scholar] [CrossRef]
- Shi, C.; Jiménez, A.F.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Zhou, Q.; Milestone, N.B.; Hayes, M. An alternative to Portland Cement for waste encapsulation—The calcium sulfoaluminate cement system. J. Hazard. Mater. 2006, 136, 120–129. [Google Scholar] [CrossRef]
- Muvuna, J.; Boutaleb, T.; Baker, K.J.; Mickovski, S.B. A methodology to model integrated smart city system from the information perspective. Smart Cities 2019, 2, 496–511. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cement Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Seo, J.; Kim, S.; Park, S.; Yoon, H.N.; Lee, H.K. Carbonation of calcium sulfoaluminate cement blended with blast furnace slag. Cement Concr. Compos. 2021, 118, 103918. [Google Scholar] [CrossRef]
- Le Saoût, G.; Lothenbach, B.; Hori, A.; Higuchi, T.; Winnefeld, F. Hydration of Portland cement with additions of calcium sulfoaluminates. Cement Concr. Res. 2013, 43, 81–94. [Google Scholar] [CrossRef]
- Juenger, M.C.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cement Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Meng, K.; Cui, C.; Liang, Z.; Li, H.; Pei, H. A new approach for longitudinal vibration of a large-diameter floating pipe pile in visco-elastic soil considering the three-dimensional wave effects. Comput. Geotech. 2020, 128, 103840. [Google Scholar] [CrossRef]
- Quillin, K. Performance of belite–sulfoaluminate cements. Cement Concr. Res. 2001, 31, 1341–1349. [Google Scholar] [CrossRef]
- Guotang, Z.; Wei, S.; Guotao, Y.; Li, P.; Degou, C.; Jinyang, J.; Hao, H. Mechanism of cement on the performance of cement stabilized aggregate for high speed railway roadbed. Constr. Build. Mater. 2017, 144, 347–356. [Google Scholar] [CrossRef]
- Michel, M.; Georgin, J.F.; Ambroise, J.; Péra, J. The influence of gypsum ratio on the mechanical performance of slag cement accelerated by calcium sulfoaluminate cement. Constr. Build. Mater. 2011, 25, 1298–1304. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate–belite compositions. Cement Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Gastaldi, D.; Paul, G.; Marchese, L.; Irico, S.; Boccaleri, E.; Mutke, S.; Buzzi, L.; Canonico, F. Hydration products in sulfoaluminate cements: Evaluation of amorphous phases by XRD/solid-state NMR. Cement Concr. Res. 2016, 90, 162–173. [Google Scholar] [CrossRef]
- Vinoth, G.; Moon, S.W.; Moon, J.; Ku, T. Early strength development in cement-treated sand using low-carbon rapid-hardening cements. Soils Found. 2018, 58, 1200–1211. [Google Scholar] [CrossRef]
- Li, J.; Chang, J. Effect of crystal/amorphous ratio on mechanical properties in a C4A3 $-C2S hydration system with or without gypsum addition. Constr. Build. Mater. 2019, 208, 36–45. [Google Scholar] [CrossRef]
- Lan, W.; Glasser, F.P. Hydration of calcium sulphoaluminate cements. Advances in Cement Research 1996, 8, 127–134. [Google Scholar] [CrossRef]
- Tang, S.W.; Zhu, H.G.; Li, Z.J.; Chen, E.; Shao, H.Y. Hydration stage identification and phase transformation of calcium sulfoaluminate cement at early age. Constr. Build. Mater. 2015, 75, 11–18. [Google Scholar] [CrossRef]
- Subramanian, S.; Khan, Q.; Ku, T. Strength development and prediction of calcium sulfoaluminate treated sand with optimized gypsum for replacing OPC in ground improvement. Constr. Build. Mater. 2019, 202, 308–318. [Google Scholar] [CrossRef]
- Rakhimova, N.R. Recent advances in blended alkali-activated cements: A review. Eur. J. Environ. Civil Eng. 2020, 5, 1–23. [Google Scholar] [CrossRef]
- Ngole, V.M.; Totolo, O.; Ekosse, G.E. Physico-chemical and mineralogical characterisation of subsurface sediments around Gaborone Landfill, Botswana. J. Appl. Sci. Environ. Manag. 2004, 8, 49–53. [Google Scholar] [CrossRef]
- Morin, V.; Termkhajornkit, P.; Huet, B.; Pham, G. Impact of quantity of anhydrite, water to binder ratio, fineness on kinetics and phase assemblage of belite-ye’elimite-ferrite cement. Cem. Concr. Res. 2017, 99, 8–17. [Google Scholar] [CrossRef]
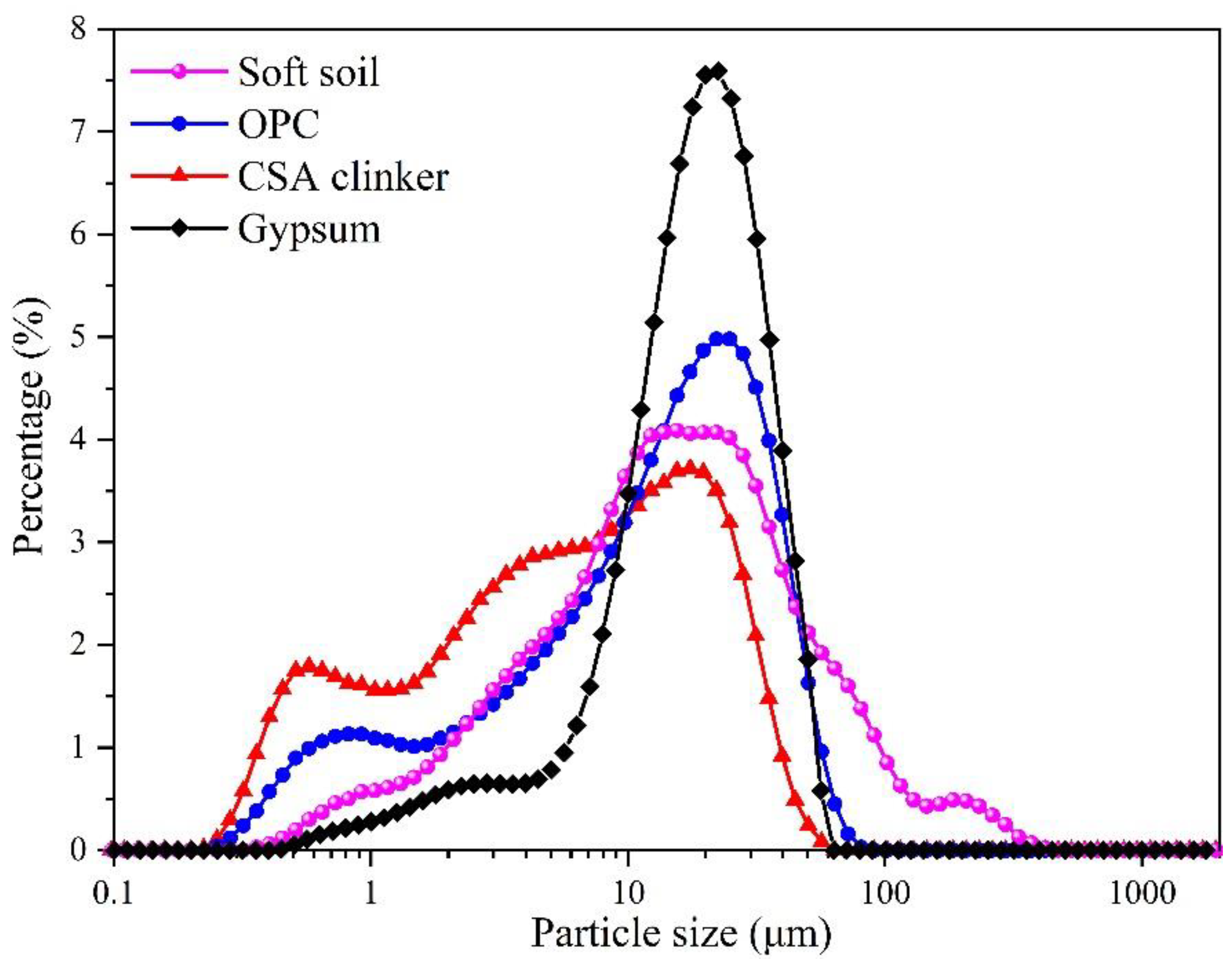



| Raw Materials | CaO | Fe2O3 | MgO | Al2O3 | SiO2 | SO3 | Na2O | K2O | TiO2 | Others |
|---|---|---|---|---|---|---|---|---|---|---|
| Soft soil | 5.55 | 8.65 | 6.41 | 22.48 | 43.88 | 0.83 | 3.42 | 5.77 | 2.19 | 0.82 |
| OPC | 73.74 | 3.42 | 3.50 | 5.82 | 9.15 | 1.86 | 0.29 | 0.90 | 0.88 | 0.44 |
| CSA clinker | 53.95 | 2.23 | 2.60 | 29.04 | 3.28 | 4.85 | 0.13 | 0.75 | 2.93 | 0.24 |
| Gypsum | 41.18 | - | - | - | - | 58.82 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhao, J.; Wang, Y.; Yi, N.; Cui, C. Strength Performance and Microstructure of Calcium Sulfoaluminate Cement-Stabilized Soft Soil. Sustainability 2021, 13, 2295. https://doi.org/10.3390/su13042295
Liu H, Zhao J, Wang Y, Yi N, Cui C. Strength Performance and Microstructure of Calcium Sulfoaluminate Cement-Stabilized Soft Soil. Sustainability. 2021; 13(4):2295. https://doi.org/10.3390/su13042295
Chicago/Turabian StyleLiu, Hailong, Jiuye Zhao, Yu Wang, Nangai Yi, and Chunyi Cui. 2021. "Strength Performance and Microstructure of Calcium Sulfoaluminate Cement-Stabilized Soft Soil" Sustainability 13, no. 4: 2295. https://doi.org/10.3390/su13042295
APA StyleLiu, H., Zhao, J., Wang, Y., Yi, N., & Cui, C. (2021). Strength Performance and Microstructure of Calcium Sulfoaluminate Cement-Stabilized Soft Soil. Sustainability, 13(4), 2295. https://doi.org/10.3390/su13042295







