Effect of the Human Utilization of Northern Snakehead (Channa argus Cantor, 1842) on the Settlement of Exotic Fish and Cladoceran Community Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Monitoring Strategy
2.3. Stable Isotope Analysis
2.4. Data Analysis
3. Results
3.1. Environmental Variables and Fish Distribution
3.2. Microabitat Utilization and Food Preference of Channa Argus
3.3. Utilization of the Cladoceran Community by Lepomis Macrochirus
4. Discussion
4.1. Effects of Environmental Characteristics on the Settlement of Exotic Fish in Freshwater Wetlands
4.2. Effect of the Fishing Activity by Humans on the Settlement of Exotic Fish
4.3. Effect of the Cladoceran Community on the Settlement of Exotic Fish
4.4. Human Culture and Freshwater Ecosystems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jonsson, T.; Ebenman, B. Effects of predator–prey body size ratios on the stability of food chains. J. Theor. Biol. 1998, 193, 407–417. [Google Scholar] [CrossRef]
- Emmerson, M.C.; Raffaelli, D. Predator–prey body size, interaction strength and the stability of a real food web. J. Anim. Ecol. 2004, 73, 399–409. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Harwood, J.D.; Rypstra, A.L. Foraging activity of a dominant epigeal predator: Molecular evidence for the effect of prey density on consumption. Oikos 2012, 121, 1715–1724. [Google Scholar] [CrossRef]
- Eklöv, P.; Jonsson, P. Pike predators induce morphological changes in young perch and roach. J. Fish Biol. 2007, 70, 155–164. [Google Scholar] [CrossRef]
- Dodson, S.L.; Cáceres, C.E.; Rogers, D.C. Cladocera and other Branchiopoda. In Ecology and Classification of North American Freshwater Invertebrates; Academic Press: Cambridge, MA, USA, 2010; pp. 773–827. [Google Scholar]
- Geffeney, S.; Brodie, E.D.; Ruben, P.C. Mechanisms of adaptation in a predator-prey arms race: TTX-resistant sodium channels. Science 2002, 297, 1336–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fryxell, J.M.; Mosser, A.; Sinclair, A.R.; Packer, C. Group formation stabilizes predator–prey dynamics. Nature 2007, 449, 1041–1043. [Google Scholar] [CrossRef] [PubMed]
- Chivers, D.P.; Zhao, X.; Brown, G.E.; Marchant, T.A.; Ferrari, M.C. Predator-induced changes in morphology of a prey fish: The effects of food level and temporal frequency of predation risk. Evol. Ecol. 2008, 22, 561–574. [Google Scholar] [CrossRef]
- Brandl, Z. Freshwater copepods and rotifers: Predators and their prey. Hydrobiologia 2005, 546, 475–489. [Google Scholar] [CrossRef]
- Lohrer, A.M.; Whitlatch, R.B. Interactions among aliens: Apparent replacement of one exotic species by another. Ecology 2002, 83, 719–732. [Google Scholar] [CrossRef]
- Jenkins, M. Prospects for biodiversity. Science 2003, 302, 1175–1177. [Google Scholar] [CrossRef]
- Covich, A.P. Winning the biodiversity arms race among freshwater gastropods: Competition and coexistence through shell variability and predator avoidance. Hydrobiologia 2010, 653, 191–215. [Google Scholar] [CrossRef]
- Dudgeon, D.; Smith, R.E. Exotic species, fisheries and conservation of freshwater biodiversity in tropical Asia: The case of the Sepik River, Papua New Guinea. Aquat. Conserv. 2006, 16, 203–215. [Google Scholar] [CrossRef]
- Roemer, G.W.; Donlan, C.J.; Courchamp, F. Golden eagles, feral pigs, and insular carnivores: How exotic species turn native predators into prey. Proc. Natl. Acad. Sci. USA 2002, 99, 791–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.S.; Shin, H.W. Studies on phyto-and-zooplankton composition and its relation to fish productivity in a west coast fish pond ecosystem. J. Environ. Biol. 2007, 28, 415. [Google Scholar]
- Carey, M.; Wahl, D.H. Interactions of multiple predators with different foraging modes in an aquatic food web. Oecologia 2010, 2, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, D.B.; Davis, B.M.; Warner, D.M.; Chriscinske, M.A.; Roseman, E.F. Planktivory in the changing Lake Huron zooplankton community: Bythotrephes consumption exceeds that of Mysis and fish. Freshw. Biol. 2011, 56, 1281–1296. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jeong, K.S.; Joo, G.J. Spatio-temporal distribution of Diaphanosoma brachyurum (Cladocera: Sididae) in freshwater reservoir ecosystems: Importance of maximum water depth and macrophyte beds for avoidance of fish predation. J. Limnol. 2015, 74, 403–413. [Google Scholar] [CrossRef] [Green Version]
- Jeppesen, E.; Lauridsen, T.L.; Kairesalo, T.; Perrow, M.R. Impact of submerged macrophytes on fish-zooplankton interactions in lakes. In The Structuring Role of Submerged Macrophytes in Lakes; Jeppesen, E., Søndergaard, M., Søndergaard, M., Christoffersen, K., Eds.; Springer: New York, NY, USA, 1998; pp. 91–114. [Google Scholar]
- Sagrario, G.; LosÁneles, M.D.; Balseiro, E.; Ituarte, R.; Spivak, E. Macrophytes as refuge or risky area for zooplankton: A balance set by littoral predacious macroinvertebrates. Freshw. Biol. 2009, 54, 1042–1053. [Google Scholar] [CrossRef]
- Leprieur, F.; Hickey, M.A.; Arbuckle, C.J.; Closs, G.P.; Brosse, S.; Townsend, C.R. Hydrological disturbance benefits a native fish at the expense of an exotic fish. J. Appl. Ecol. 2006, 43, 930–939. [Google Scholar] [CrossRef]
- Leuven, R.S.E.W.; Hendriks, A.J.; Huijbregts, M.A.J.; Lenders, H.J.R.; Matthews, J.; Velde, G.V.D. Differences in sensitivity of native and exotic fish species to changes in river temperature. Curr. Zool. 2011, 57, 852–862. [Google Scholar] [CrossRef] [Green Version]
- Zengeya, T.A.; Booth, A.J.; Bastos, A.D.; Chimimba, C.T. Trophic interrelationships between the exotic Nile tilapia, Oreochromis niloticus and indigenous tilapiine cichlids in a subtropical African river system (Limpopo River, South Africa). Environ. Biol. Fish. 2011, 92, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Castaldelli, G.; Pluchinotta, A.; Milardi, M.; Lanzoni, M.; Giari, L.; Rossi, R.; Fano, E.A. Introduction of exotic fish species and decline of native species in the lower Po basin, north-eastern Italy. Aquat. Conserv. 2013, 23, 405–417. [Google Scholar] [CrossRef]
- Park, T.J.; Lee, S.H.; Lee, M.S.; Lee, J.K.; Lee, S.H.; Zoh, K.D. Occurrence of microplastics in the Han River and riverine fish in South Korea. Sci. Total Environ. 2020, 708, 134535. [Google Scholar] [CrossRef]
- Maezono, Y.; Kobayashi, R.; Kusahara, M.; Miyashita, T. Direct and indirect effects of exotic bass and bluegill on exotic and native organisms in farm ponds. Ecol. Appl. 2005, 15, 638–650. [Google Scholar] [CrossRef]
- Maezono, Y.; Miyashita, T. Community-level impacts induced by introduced largemouth bass and bluegill in farm ponds in Japan. Biol. Conserv. 2003, 109, 111–121. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, S.K. Effects of aquatic macrophytes on spatial distribution and feeding habits of exotic fish species Lepomis macrochirus and Micropterus salmoides in shallow reservoirs in South Korea. Sustainability 2020, 12, 1447. [Google Scholar] [CrossRef] [Green Version]
- Jolley, J.C.; Willis, D.W.; DeBates, T.J.; Graham, D.D. The effects of mechanically reducing northern pike density on the sport fish community of West Long Lake, Nebraska, USA. Fish. Manag. Ecol. 2008, 15, 251–258. [Google Scholar] [CrossRef]
- Nicholson, M.E.; Rennie, M.D.; Mills, K.H. Apparent extirpation of prey fish communities following the introduction of Northern Pike (Esox lucius). Can. Field Nat. 2015, 129, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Jang, M.H.; Kim, J.G.; Park, S.B.; Jeong, K.S.; Cho, G.I.; Joo, G.J. The current status of the distribution of introduced fish in large river systems of South Korea. Int. Rev. Hydrobiol. 2002, 87, 319–328. [Google Scholar] [CrossRef]
- Yorisue, T.; Ellrich, J.A.; Momota, K. Mechanisms underlying predator-driven biotic resistance against introduced barnacles on the Pacific coast of Hokkaido, Japan. Biol. Invasions 2019, 21, 2345–2356. [Google Scholar] [CrossRef]
- Anton, A.; Geraldi, N.R.; Ricciardi, A.; Dick, J.T. Global determinants of prey naiveté to exotic predators. Proc. R. Soc. B 2020, 287, 20192978. [Google Scholar] [CrossRef]
- Son, M.W.; Jeon, Y.G. Physical geographical characteristics of natural wetlands on the downstream reach of Nakdong River. J. KARG 2003, 9, 66–76. [Google Scholar]
- Choi, J.Y.; Jeong, K.S.; Kim, S.K.; La, G.H.; Chang, K.H.; Joo, G.J. Role of macrophytes as microhabitats for zooplankton community in lentic freshwater ecosystems of South Korea. Ecol. Inform. 2014, 24, 177–185. [Google Scholar] [CrossRef]
- Wetzel, R.G.; Likens, G.E. Composition and biomass of phytoplankton. In Limnological Analyses; Springer: New York, NY, USA, 2000; pp. 147–174. [Google Scholar]
- Kim, I.S.; Park, J.Y. Freshwater Fish of Korea; Kyo Hak Publishing: Seoul, Korean, 2002. [Google Scholar]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V. Fishes of the World; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Haney, J.F.; Hall, D.J. Sugar-coated Daphnia: A preservation technique for Cladocera 1. Limnol. Oceanogr. 1973, 18, 331–333. [Google Scholar] [CrossRef]
- Mizuno, T.; Takahashi, E. An Illustration Guide to Freshwater Zooplankton in Japan; Tokai University: Tokyo, Japan, 1999. [Google Scholar]
- Sakuma, M.; Hanazato, T.; Nakazato, R.; Haga, H. Methods for quantitative sampling of epiphytic microinvertebrates in lake vegetation. Limnology 2002, 3, 115–119. [Google Scholar] [CrossRef]
- Gyllström, M.; Hansson, L.A.; Jeppesen, E.; García-Criado, F.; Gross, E.; Irvine, K.; Kairesalo, T.; Kornijow, R.; Miracle, M.R.; Nykänen, M.; et al. The role of climate in shaping zooplankton communities of shallow lakes. Limnol. Oceanogr. 2005, 50, 2008–2021. [Google Scholar] [CrossRef] [Green Version]
- Post, D.M.; Layman, C.A.; Arrington, D.A.; Takimoto, G.; Quattrochi, J.; Montaña, C.G. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 2007, 152, 179–189. [Google Scholar] [CrossRef]
- Im, R.Y.; Kim, J.Y.; Joo, G.J.; Do, Y. Process of wetland loss in the lower Nakdong River, South Korea. Appl. Ecol. Environ. Res. 2017, 15, 69–78. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, S.K. Responses of rotifer community to microhabitat changes caused by summer-concentrated rainfall in a shallow reservoir, South Korea. Diversity 2020, 12, 113. [Google Scholar] [CrossRef] [Green Version]
- Jeppesen, E.; Søndergaard, M.; Søndergaard, M.; Christoffersen, K. The Structuring role of Submerged Macrophytes in Lakes; Springer Science & Business Media: Berlin, Germany, 2012; Volume 131. [Google Scholar]
- Manatunge, J.; Asaeda, T.; Priyadarshana, T. The influence of structural complexity on fish–zooplankton interactions: A study using artificial submerged macrophytes. Environ. Biol. Fish. 2000, 58, 425–438. [Google Scholar] [CrossRef]
- Warfe, D.M.; Barmuta, L.A.; Wotherspoon, S. Quantifying habitat structure: Surface convolution and living space for species in complex environments. Oikos 2008, 117, 1764–1773. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jeong, K.S.; La, G.H.; Joo, G.J. Effect of removal of free-floating macrophytes on zooplankton habitat in shallow wetland. Knowl. Manag. Aquat. Ecosyst. 2014, 414, 11. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, G.M.; Hines, A.H.; Posey, M.H. Shallow water as a refuge habitat for fish and crustaceans in non-vegetated estuaries: An example from Chesapeake Bay. Mar. Ecol. Prog. Ser. 1993, 99, 1–16. [Google Scholar] [CrossRef]
- Pascual, M.A.; Cussac, V.; Dyer, B.; Soto, D.; Vigliano, P.; Ortubay, S.; Macchi, P. Freshwater fishes of Patagonia in the 21st century after a hundred years of human settlement, species introductions, and environmental change. Aquat. Ecosyst. Health Manag. 2007, 10, 212–227. [Google Scholar] [CrossRef]
- Chapman, D.S.; Gunn, I.D.; Pringle, H.E.; Siriwardena, G.M.; Taylor, P.; Thackeray, S.J.; Willby, N.J.; Carvalho, L. Invasion of freshwater ecosystems is promoted by network connectivity to hotspots of human activity. Glob. Ecol. Biogeogr. 2020, 29, 645–655. [Google Scholar] [CrossRef] [Green Version]
- Jeppesen, E.; Meerhoff, M.; Holmgren, K.; González-Bergonzoni, I.; Teixeira-de Mello, F.; Declerck, S.A.; De Meester, L.; Søndergaard, M.; Lauridsen, T.L.; Bjerring, R.; et al. Impacts of climate warming on lake fish community structure and potential effects on ecosystem function. Hydrobiologia 2010, 646, 73–90. [Google Scholar] [CrossRef]
- Rowe, D.K. Exotic fish introductions and the decline of water clarity in small North Island, New Zealand lakes: A multi-species problem. Hydrobiologia 2007, 583, 345–358. [Google Scholar] [CrossRef]
- Gerry, S.P.; Vogelzang, M.; Ascher, J.M.; Ellerby, D.J. Variation in the diet and feeding morphology of polyphenic Lepomis macrochirus. J. Fish Biol. 2013, 82, 338–346. [Google Scholar] [CrossRef]
- Yokogawa, K. Food habits of bluegill Lepomis macrochirus populations in reservoirs in Kagawa, Japan. Zool. Ecol. 2018, 28, 185–204. [Google Scholar] [CrossRef]
- Lemke, A.M.; Stoeckel, J.A.; Pegg, M.A. Utilization of the exotic cladoceran Daphnia lumholtzi by juvenile fishes in an Illinois River floodplain lake. J. Fish Biol. 2003, 62, 938–954. [Google Scholar] [CrossRef]
- Diehl, S.; Kornijów, R. Influence of submerged macrophytes on trophic interactions among fish and macroinvertebrates. In The Structuring Role of Submerged Macrophytes in Lakes; Springer: New York, NY, USA, 1998; pp. 24–46. [Google Scholar]
- Theel, H.J.; Dibble, E.D. An experimental simulation of an exotic aquatic macrophyte invasion and its influence on foraging behavior of bluegill. J. Freshw. Ecol. 2008, 23, 79–89. [Google Scholar] [CrossRef]
- Bastos Filho, C.J.; de Lima Neto, F.B.; Lins, A.J.; Nascimento, A.I.; Lima, M.P. A novel search algorithm based on fish school behavior. In Proceedings of the 2008 IEEE International Conference on Systems, Man and Cybernetics, Singapore, 12–15 October 2008; pp. 2646–2651. [Google Scholar]
- Ryer, C.H.; Olla, B.L. Influences of food distribution on fish foraging behaviour. Anim. Behav. 1995, 49, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Cooper, W.E., Jr. The foraging mode controversy: Both continuous variation and clustering of foraging movements occur. J. Zool. 2005, 267, 179–190. [Google Scholar] [CrossRef]
- Leoni, B. Zooplankton predators and preys: Body size and stable isotope to investigate the pelagic food web in a deep lake (Lake Iseo, Northern Italy). J. Limnol. 2017, 76, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.Y.; Jeong, K.S.; La, G.H.; Kim, S.K.; Joo, G.J. Sustainment of epiphytic microinvertebrate assemblage in relation with different aquatic plant microhabitats in freshwater wetlands (South Korea). J. Limnol. 2014, 73, 1. [Google Scholar] [CrossRef] [Green Version]
- Haney, J.F. An in situ method for the measurement of zooplankton grazing rates. Limnol. Oceanogr. 1971, 16, 970–977. [Google Scholar] [CrossRef]
- Cauchie, H.M.; Joaquim-Justo, C.; Hoffmann, L.; Thomé, J.P.; Thys, I. A note on the use of fluorescently labelled algae for the determination of gut passage time in Bosmina and Daphnia. Verh. Int. Ver. Theor. Angew. Limnol. 2000, 27, 2987–2991. [Google Scholar] [CrossRef]
- Kuczyńska-Kippen, N.M.; Nagengast, B. The influence of the spatial structure of hydromacrophytes and differentiating habitat on the structure of rotifer and cladoceran communities. Hydrobiologia 2006, 559, 203–212. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, S.K. A study of the distribution of Daphnia obtusa and Simocephalus vetulus in response to varying environmental conditions using field and microcosm approaches. Water 2020, 12, 815. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.Y.; Kim, S.K.; Kim, J.C.; Yun, J.H. Effect of Microhabitat Structure on the Distribution of an Endangered Fish, Coreoperca kawamebari (Temminck & Schlegel, 1843) in the Geum River, South Korea. Water 2020, 12, 1690. [Google Scholar]
- Campbell, L.; Verburg, P.; Dixon, D.G.; Hecky, R.E. Mercury biomagnification in the food web of Lake Tanganyika (Tanzania, East Africa). Sci. Total Environ. 2008, 402, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Vyas, V.; Damde, D.; Parashar, V. Fish biodiversity of Betwa River in Madhya Pradesh, India with special reference to a sacred ghat. Int. J. Biodivers. Conserv. 2012, 4, 71–77. [Google Scholar]
- Kim, J.H.; Park, S.; Kim, S.H.; Kang, K.; Waldman, B.; Lee, M.H.; Yu, M.; Yang, H.; Chung, H.Y.; Lee, E.J. Structural implications of traditional agricultural landscapes on the functional diversity of birds near the Korean Demilitarized Zone. Ecol. Evol. 2020, 10, 12973–12982. [Google Scholar] [CrossRef]
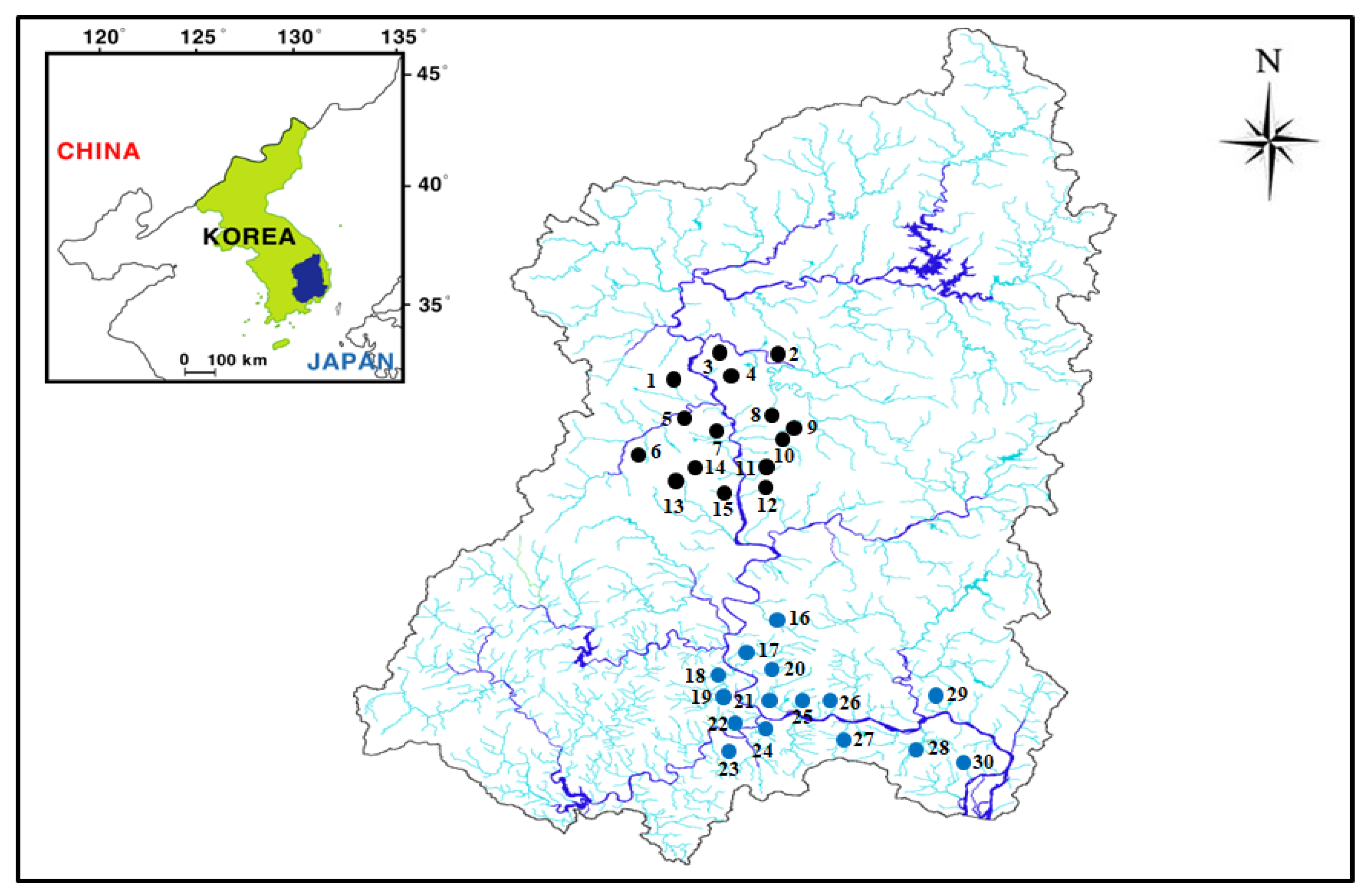





| No. | Main Water Source | Altitude (m) | Area (m2) | Fluctuation (m) | Mean Depth (m) | Maximum Depth (m) | Mean Residence Time (Year) |
|---|---|---|---|---|---|---|---|
| 1 | Stream | 11.6 | 26,400 | 2.8 | 3.1 | 3.8 | 0.22 |
| 2 | Drainageway | 6.6 | 7800 | <1 | 2.8 | 3.1 | 0.32 |
| 3 | Stream | 16.4 | 13,700 | 2.5 | 2.7 | 2.9 | 0.31 |
| 4 | Stream | 18.2 | 20,400 | <1 | 4.1 | 4.6 | 0.16 |
| 5 | Rainfall/ground | 27.4 | 17,600 | <1 | 2.4 | 2.7 | 0.36 |
| 6 | Rainfall/ground | 23.2 | 6700 | <1 | 0.8 | 1.1 | 0.21 |
| 7 | Drainageway | 14.5 | 31,600 | <1 | 1.6 | 2.0 | 0.12 |
| 8 | Stream | 12.8 | 13,600 | 1.7 | 3.4 | 3.6 | 0.21 |
| 9 | Stream | 16.5 | 15,900 | 1.1 | 2.8 | 3.2 | 0.15 |
| 10 | Rainfall/ground | 9.2 | 22,600 | <1 | 1.2 | 1.8 | 0.43 |
| 11 | Drainageway | 11.8 | 25,600 | <1 | 0.7 | 1.6 | 0.41 |
| 12 | Stream | 20.7 | 4000 | 3.4 | 2.3 | 3.1 | 0.31 |
| 13 | Stream | 17.6 | 27,600 | 2.8 | 1.1 | 1.6 | 0.22 |
| 14 | Rainfall/ground | 26.7 | 250,000 | <1 | 0.8 | 1.4 | 0.42 |
| 15 | Stream | 24.3 | 137,957 | 1.7 | 1.8 | 2.2 | 0.18 |
| 16 | Stream | 30.5 | 109,000 | 2.5 | 1.6 | 2.1 | 0.11 |
| 17 | Drainageway | 23.4 | 51,000 | 1.2 | 1.3 | 1.6 | 0.44 |
| 18 | Stream | 18.2 | 24,680 | 1.8 | 1.7 | 2.0 | 0.26 |
| 19 | Rainfall/ground | 26.1 | 84,140 | <1 | 1.2 | 1.8 | 0.41 |
| 20 | Stream | 20.7 | 51,700 | <1 | 2.3 | 3.0 | 0.48 |
| 21 | Stream | 24.7 | 67,100 | 2.4 | 2.4 | 2.8 | 0.20 |
| 22 | Stream | 16.2 | 75,000 | <1 | 0.8 | 1.4 | 0.41 |
| 23 | Rainfall/ground | 21.8 | 69,400 | 2.8 | 0.7 | 1.2 | 0.28 |
| 24 | Drainageway | 14.7 | 102,000 | 1.4 | 1.3 | 1.6 | 0.34 |
| 25 | Stream | 20.8 | 84,180 | 2.5 | 1.2 | 1.7 | 0.27 |
| 26 | Rainfall/ground | 12.8 | 98,483 | <1 | 1.8 | 2.4 | 0.31 |
| 27 | Stream | 17.2 | 68,200 | <1 | 2.0 | 2.8 | 0.27 |
| 28 | Drainageway | 20.7 | 49,000 | 1.8 | 1.7 | 2.4 | 0.34 |
| 29 | Stream | 24.1 | 64,570 | 1.2 | 1.4 | 2.3 | 0.29 |
| 30 | Stream | 24.7 | 117,957 | 1.4 | 2.3 | 2.7 | 0.37 |
| Area | Season | WT (°C) | DO (%) | pH | Cond. (µS/cm) | Turbidity (NTU) | TN (mg/L) | TP (µg/L) | MB (g) |
|---|---|---|---|---|---|---|---|---|---|
| Upper | Spring | 20.3 ± 1.2 | 68.3 ± 21.5 | 8.1 ± 0.2 | 335 ± 22.5 | 12.6 ± 3.5 | 1.3 ± 0.6 | 11.4 ± 4.5 | 14.3 ± 8.5 |
| Autumn | 19.8 ± 0.7 | 31.3 ± 13.4 | 7.6 ± 0.6 | 274 ± 30.8 | 24.4 ± 8.4 | 1.8 ± 0.7 | 16.3 ± 3.8 | 32.2 ± 13.5 | |
| Mid-lower | Spring | 19.5 ± 1.1 | 53.8 ± 17.4 | 7.8 ± 0.3 | 389 ± 28.4 | 16.3 ± 5.1 | 1.1 ± 0.5 | 14.3 ± 2.4 | 18.2 ± 6.1 |
| Autumn | 19.8 ± 1.3 | 44.7 ± 10.8 | 7.3 ± 0.4 | 315 ± 21.8 | 28.3 ± 9.1 | 1.6 ± 0.7 | 18.8 ± 4.1 | 46.2 ± 15.4 |
| Region | Season | Fish | df | F | p |
|---|---|---|---|---|---|
| Upper part | Spring | Channa argus | 2 | 3.217 | <0.05 |
| Lepomis macrochirus | 2 | 1.841 | <0.05 | ||
| Micropterus salmoides | 2 | 1.354 | 0.216 | ||
| Autumn | Channa argus | 2 | 3.015 | <0.05 | |
| Lepomis macrochirus | 2 | 1.038 | 0.441 | ||
| Micropterus salmoides | 2 | 1.127 | 0.342 | ||
| Mid-lower part | Spring | Channa argus | 2 | 0.815 | 0.568 |
| Lepomis macrochirus | 2 | 2.816 | <0.05 | ||
| Micropterus salmoides | 2 | 2.054 | <0.05 | ||
| Autumn | Channa argus | 2 | 0.982 | 0.510 | |
| Lepomis macrochirus | 2 | 2.268 | <0.05 | ||
| Micropterus salmoides | 2 | 1.815 | <0.05 |
| Diet Composition (Cladocerans) | 19 | 26 | 30 | |||
|---|---|---|---|---|---|---|
| Spring | Autumn | Spring | Autumn | Spring | Autumn | |
| Acroperus harpae (Baird, 1834) * | - | 0.7 ± 0.2 | - | - | - | - |
| Alona guttata (Sars, 1862) * | 2.7 ± 0.4 | - | 1.2 ± 0.5 | - | ||
| A. rectangula (Sars, 1862) * | - | - | 0.8 ± 0.1 | 0.3 ± 0.0 | - | - |
| Bosmina longirostris (Müller, 1785) | - | - | 10 ± 3.4 | 5.4 ± 2.1 | 13 ± 4.8 | 15 ± 7.8 |
| Camptocercus rectirostris (Schoedler, 1862) * | 1.6 ± 0.2 | 1.2 ± 0.4 | - | - | - | - |
| Chydorus sphaericus (Müller, 1785) * | - | 2.8 ± 1.1 | - | - | 1.3 ± 2.7 | - |
| Daphnia obtusa (Kurz, 1874) | 21 ± 11.4 | 8.1 ± 2.4 | 13 ± 6.7 | 9.1 ± 4.1 | 24 ± 13.5 | 20 ± 7.1 |
| Diaphanosoma brachyurum (Lievin, 1848) | - | - | 34 ± 21.8 | 41 ± 28.1 | 13 ± 5.1 | 16 ± 12.7 |
| Graptoleveris testudinaria * | - | 2.1 ± 0.8 | - | - | 0.4 ± 0.1 | - |
| Ilyocryptus spinifer (Herrick, 1882) * | - | - | - | 0.2 ± 0.0 | - | - |
| Moina macrocopa (Straus, 1820) | 15 ± 9.4 | 23 ± 18.4 | 20 ± 11.7 | 28 ± 17.4 | 17 ± 11.7 | 22 ± 14.7 |
| Pleuroxus aduncus (Jurine, 1820) * | - | 1.5 ± 0.7 | 0.5 ± 0.1 | - | 2.1 ± 0.8 | - |
| P. laevis (Sars, 1861) * | - | - | 0.7 ± 0.3 | - | - | - |
| Simocephalus expinosus (Koch, 1841) | 10 ± 6.4 | 7 ± 3.7 | - | - | 10 ± 7.4 | 13 ± 8.4 |
| S. vetulus (Müller, 1776) | 24 ± 13.4 | 16 ± 10.4 | 28 ± 20.7 | 30 ± 17.7 | 25 ± 18.2 | 29 ± 23.7 |
| Scapholeberis kingi (Sars, 1903) | 2 ± 0.4 | 6 ± 2.7 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-Y.; Kim, S.-K. Effect of the Human Utilization of Northern Snakehead (Channa argus Cantor, 1842) on the Settlement of Exotic Fish and Cladoceran Community Structure. Sustainability 2021, 13, 2486. https://doi.org/10.3390/su13052486
Choi J-Y, Kim S-K. Effect of the Human Utilization of Northern Snakehead (Channa argus Cantor, 1842) on the Settlement of Exotic Fish and Cladoceran Community Structure. Sustainability. 2021; 13(5):2486. https://doi.org/10.3390/su13052486
Chicago/Turabian StyleChoi, Jong-Yun, and Seong-Ki Kim. 2021. "Effect of the Human Utilization of Northern Snakehead (Channa argus Cantor, 1842) on the Settlement of Exotic Fish and Cladoceran Community Structure" Sustainability 13, no. 5: 2486. https://doi.org/10.3390/su13052486
APA StyleChoi, J.-Y., & Kim, S.-K. (2021). Effect of the Human Utilization of Northern Snakehead (Channa argus Cantor, 1842) on the Settlement of Exotic Fish and Cladoceran Community Structure. Sustainability, 13(5), 2486. https://doi.org/10.3390/su13052486






