Empirical Characterization Factors for Life Cycle Assessment of the Impacts of Reservoir Occupation on Macroinvertebrate Richness across the United States
Abstract
:1. Introduction
2. Material and Methods
2.1. Life Cycle Impact Assessment (LCIA) Framework
2.2. Data Collection
2.2.1. Macroinvertebrate Richness
2.2.2. Ecoregions
2.2.3. Native Riverine Taxa Definition
2.2.4. PDFs Calculation
2.3. Data Analysis and Empirical Modelling
2.3.1. Regionalization and ANOVA
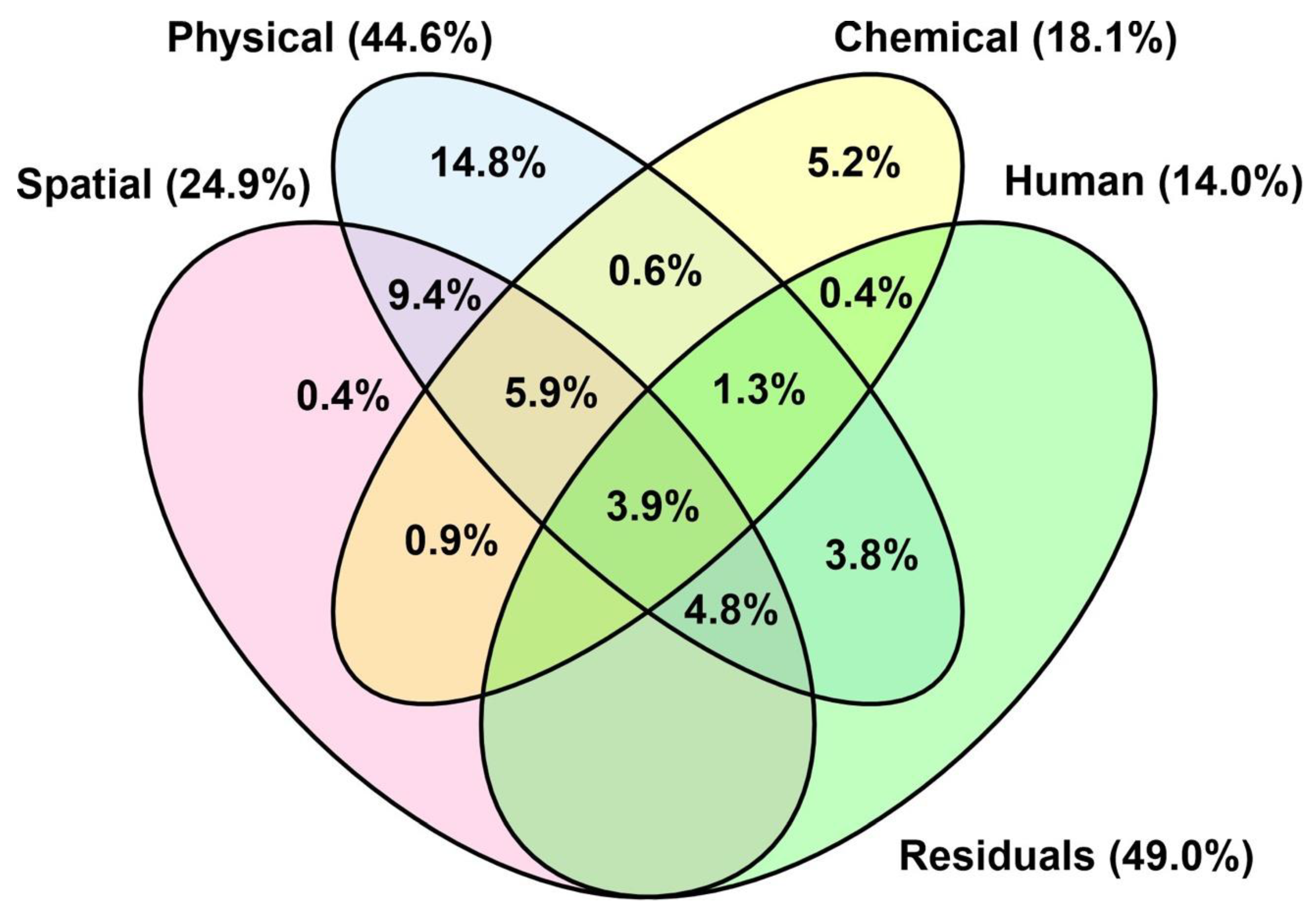
2.3.2. Variation Partitioning to Explain the Variation Observed in Our PDFres
2.3.3. Empirical Modelling
3. Results
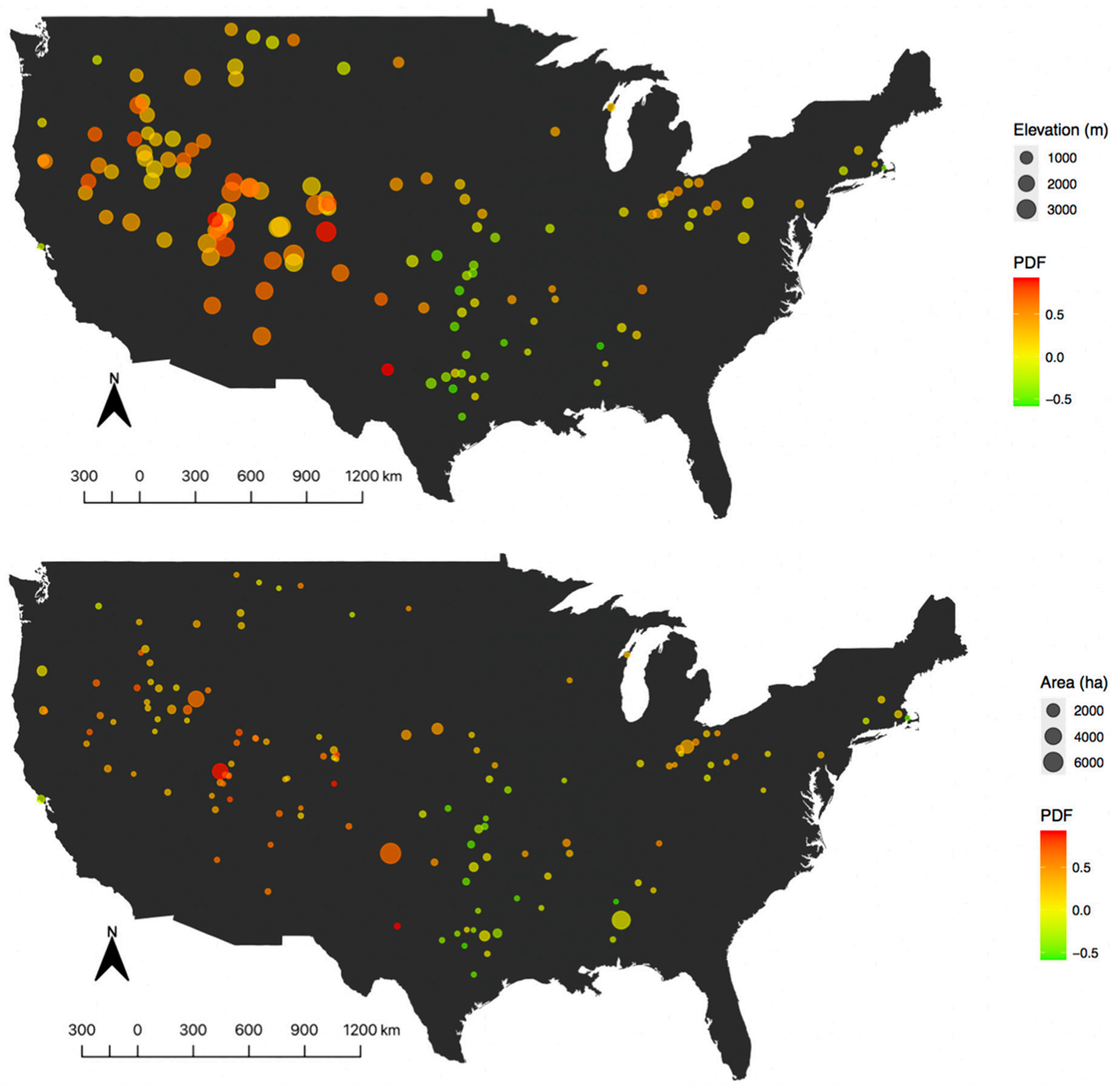
3.1. PDFusa and Variation in PDFeco across Ecoregions
3.2. Variables Explaining the Variation in PDFres
3.3. PDFres Empirical Model
4. Discussion
4.1. United States Taxa Loss and Regionalization
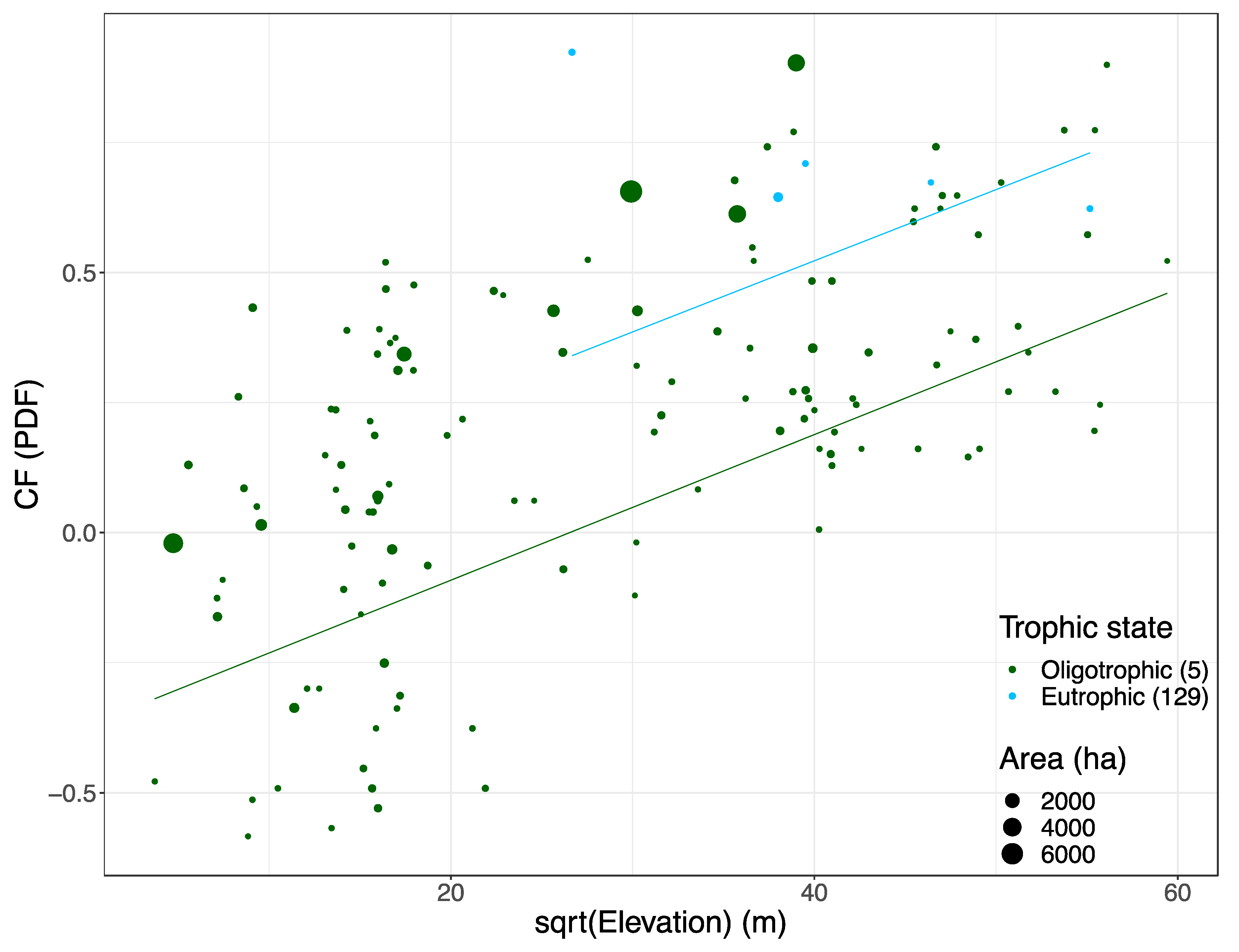
4.2. Elevation, Trophic State and Reservoir Surface Area
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| UID | LAT | LON | ECO | ELE | AREA | TS | N.REF | MEAN.REF.S | SD.REF.S | N.IMP | IMP.S | SD.IMP.S | SD.PDF | LOW.CI | UP.CI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6243 | 38.507965 | −94.673265 | TPL | 295.8 | 110.0 | EUT | 209 | 32.0 | 12.9 | 1 | 42 | 0 | −0.314 | 0.126 | −0.562 | −0.066 |
| 6252 | 40.111767 | −75.861530 | SAP | 186.7 | 63.2 | EUT | 344 | 45.8 | 15.1 | 1 | 35 | 0 | 0.236 | 0.078 | 0.083 | 0.388 |
| 6267 | 31.621546 | −88.353739 | CPL | 50.8 | 32.6 | EUT | 327 | 28.4 | 16.6 | 1 | 32 | 0 | −0.126 | 0.074 | −0.271 | 0.019 |
| 6270 | 35.174388 | −99.077489 | SPL | 499.9 | 141.5 | EUT | 176 | 26.1 | 13.1 | 1 | 14 | 0 | 0.465 | 0.233 | 0.007 | 0.922 |
| 6281 | 35.285013 | −112.154163 | WMT | 2072.1 | 24.8 | EUT | 222 | 39.8 | 12.0 | 1 | 15 | 0 | 0.623 | 0.188 | 0.254 | 0.992 |
| 6319 | 36.831633 | −104.226303 | WMT | 2066.2 | 44.2 | EUT | 222 | 39.8 | 12.0 | 1 | 16 | 0 | 0.598 | 0.181 | 0.243 | 0.952 |
| 6342 | 39.994962 | −105.112227 | SPL | 1620.9 | 18.2 | EUT | 176 | 26.1 | 13.1 | 1 | 26 | 0 | 0.006 | 0.003 | 0.000 | 0.011 |
| 6404 | 39.638354 | −95.456811 | TPL | 321.7 | 26.9 | EUT | 209 | 32.0 | 12.9 | 1 | 22 | 0 | 0.312 | 0.126 | 0.066 | 0.558 |
| 6437 | 39.000154 | −95.779648 | TPL | 350.6 | 101.9 | EUT | 209 | 32.0 | 12.9 | 1 | 34 | 0 | −0.064 | 0.026 | −0.114 | −0.013 |
| 6451 | 40.161787 | −79.052384 | SAP | 551.9 | 18.2 | EUT | 344 | 45.8 | 15.1 | 1 | 43 | 0 | 0.061 | 0.020 | 0.022 | 0.101 |
| 6481 | 39.484355 | −118.723571 | XER | 1202.0 | 164.1 | EUT | 213 | 31.0 | 11.6 | 1 | 19 | 0 | 0.387 | 0.144 | 0.104 | 0.670 |
| 6482 | 41.928985 | −119.179002 | XER | 1678.2 | 100.3 | EUT | 213 | 31.0 | 11.6 | 1 | 16 | 0 | 0.484 | 0.181 | 0.129 | 0.838 |
| 6501 | 35.980828 | −108.931643 | WMT | 2290.2 | 15.4 | EUT | 222 | 39.8 | 12.0 | 1 | 14 | 0 | 0.648 | 0.196 | 0.264 | 1.032 |
| 6525 | 35.992932 | −96.873677 | SPL | 255.6 | 177.7 | EUT | 176 | 26.1 | 13.1 | 1 | 40 | 0 | −0.530 | 0.266 | −1.051 | −0.009 |
| 6550 | 34.953616 | −96.718159 | SPL | 281.1 | 533.1 | EUT | 176 | 26.1 | 13.1 | 1 | 27 | 0 | −0.033 | 0.016 | −0.065 | −0.001 |
| 6556 | 35.412203 | −95.929276 | SPL | 201.3 | 204.3 | EUT | 176 | 26.1 | 13.1 | 1 | 25 | 0 | 0.044 | 0.022 | 0.001 | 0.087 |
| 6570 | 37.655565 | −98.260986 | SPL | 479.3 | 56.8 | EUT | 176 | 26.1 | 13.1 | 1 | 39 | 0 | −0.492 | 0.247 | −0.975 | −0.008 |
| 6575 | 40.723819 | −109.183908 | XER | 2184.0 | 43.3 | EUT | 213 | 31.0 | 11.6 | 1 | 21 | 0 | 0.322 | 0.120 | 0.086 | 0.558 |
| 6586 | 31.787274 | −96.064492 | CPL | 91.4 | 852.5 | EUT | 327 | 28.4 | 16.6 | 1 | 28 | 0 | 0.015 | 0.009 | −0.002 | 0.031 |
| 6599 | 46.543922 | −104.028258 | NPL | 907.4 | 3.8 | EUT | 179 | 29.4 | 10.1 | 1 | 33 | 0 | −0.121 | 0.042 | −0.203 | −0.040 |
| 6606 | 37.390887 | −99.784838 | SPL | 685.8 | 127.3 | EUT | 176 | 26.1 | 13.1 | 1 | 28 | 0 | −0.071 | 0.036 | −0.141 | −0.001 |
| 6617 | 41.757410 | −115.722027 | XER | 2089.2 | 23.6 | EUT | 213 | 31.0 | 11.6 | 1 | 26 | 0 | 0.161 | 0.060 | 0.043 | 0.279 |
| 6618 | 41.198060 | −115.892296 | XER | 1814.2 | 5.0 | EUT | 213 | 31.0 | 11.6 | 1 | 26 | 0 | 0.161 | 0.060 | 0.043 | 0.279 |
| 6622 | 31.889172 | −97.702492 | SPL | 290.0 | 20.7 | EUT | 176 | 26.1 | 13.1 | 1 | 35 | 0 | −0.339 | 0.170 | −0.672 | −0.005 |
| 6668 | 36.823001 | −96.047588 | SPL | 230.6 | 103.5 | EUT | 176 | 26.1 | 13.1 | 1 | 38 | 0 | −0.453 | 0.228 | −0.899 | −0.007 |
| 6695 | 36.705443 | −96.419109 | TPL | 266.8 | 325.4 | EUT | 209 | 32.0 | 12.9 | 1 | 40 | 0 | −0.251 | 0.101 | −0.450 | −0.053 |
| 6719 | 38.398162 | −115.117053 | XER | 1574.3 | 72.3 | EUT | 213 | 31.0 | 11.6 | 1 | 23 | 0 | 0.258 | 0.096 | 0.069 | 0.446 |
| 6731 | 41.701690 | −113.959671 | XER | 1622.6 | 10.3 | EUT | 213 | 31.0 | 11.6 | 1 | 26 | 0 | 0.161 | 0.060 | 0.043 | 0.279 |
| 6735 | 32.944430 | −96.453752 | SPL | 146.0 | 13.8 | EUT | 176 | 26.1 | 13.1 | 1 | 34 | 0 | −0.300 | 0.151 | −0.596 | −0.005 |
| 6742 | 47.761716 | −108.432829 | NPL | 912.2 | 4.3 | EUT | 179 | 29.4 | 10.1 | 1 | 30 | 0 | −0.019 | 0.007 | −0.032 | −0.006 |
| 6753 | 39.931042 | −104.973296 | SPL | 1600.5 | 9.2 | EUT | 176 | 26.1 | 13.1 | 1 | 20 | 0 | 0.235 | 0.118 | 0.004 | 0.466 |
| 6762 | 33.516175 | −94.125132 | CPL | 82.4 | 17.1 | EUT | 327 | 28.4 | 16.6 | 1 | 43 | 0 | −0.513 | 0.301 | −1.103 | 0.076 |
| 6774 | 46.826042 | −100.634208 | NPL | 523.4 | 3.9 | EUT | 179 | 29.4 | 10.1 | 1 | 16 | 0 | 0.456 | 0.157 | 0.149 | 0.764 |
| 6795 | 38.997241 | −108.051180 | WMT | 3070.9 | 15.0 | EUT | 222 | 39.8 | 12.0 | 1 | 32 | 0 | 0.195 | 0.059 | 0.080 | 0.311 |
| 6796 | 37.193346 | −95.988976 | SPL | 252.1 | 13.5 | EUT | 176 | 26.1 | 13.1 | 1 | 36 | 0 | −0.377 | 0.189 | −0.748 | −0.006 |
| 6806 | 38.491412 | −79.314781 | SAP | 604.1 | 3.8 | EUT | 344 | 45.8 | 15.1 | 1 | 43 | 0 | 0.061 | 0.020 | 0.022 | 0.101 |
| 6823 | 43.165878 | −115.652476 | XER | 997.2 | 163.9 | EUT | 213 | 31.0 | 11.6 | 1 | 24 | 0 | 0.225 | 0.084 | 0.060 | 0.390 |
| 6868 | 38.235087 | −112.463009 | WMT | 2680.7 | 9.6 | EUT | 222 | 39.8 | 12.0 | 1 | 26 | 0 | 0.346 | 0.105 | 0.141 | 0.551 |
| 6869 | 38.847537 | −111.961390 | XER | 1589.5 | 93.2 | EUT | 213 | 31.0 | 11.6 | 1 | 16 | 0 | 0.484 | 0.181 | 0.129 | 0.838 |
| 6874 | 39.036703 | −107.911131 | WMT | 3105.2 | 5.7 | EUT | 222 | 39.8 | 12.0 | 1 | 30 | 0 | 0.246 | 0.074 | 0.100 | 0.391 |
| 6875 | 40.944919 | −106.011968 | XER | 2410.4 | 17.4 | EUT | 213 | 31.0 | 11.6 | 1 | 26 | 0 | 0.161 | 0.060 | 0.043 | 0.279 |
| 6923 | 40.039991 | −81.013888 | SAP | 322.7 | 43.0 | EUT | 344 | 45.8 | 15.1 | 1 | 24 | 0 | 0.476 | 0.157 | 0.168 | 0.784 |
| 6940 | 44.329096 | −116.184107 | WMT | 1507.0 | 71.6 | EUT | 222 | 39.8 | 12.0 | 1 | 29 | 0 | 0.271 | 0.082 | 0.110 | 0.431 |
| 6944 | 39.169507 | −111.450721 | WMT | 2837.7 | 18.8 | EUT | 222 | 39.8 | 12.0 | 1 | 29 | 0 | 0.271 | 0.082 | 0.110 | 0.431 |
| 6959 | 44.964115 | −116.463019 | WMT | 1453.0 | 211.4 | EUT | 222 | 39.8 | 12.0 | 1 | 32 | 0 | 0.195 | 0.059 | 0.080 | 0.311 |
| 6966 | 39.142411 | −111.452546 | WMT | 2889.5 | 27.9 | EUT | 222 | 39.8 | 12.0 | 1 | 9 | 0 | 0.774 | 0.234 | 0.315 | 1.232 |
| 6970 | 44.796705 | −116.732688 | WMT | 2154.4 | 12.4 | OLI | 222 | 39.8 | 12.0 | 1 | 13 | 0 | 0.673 | 0.204 | 0.274 | 1.072 |
| 6971 | 43.191413 | −116.959804 | XER | 1399.8 | 73.0 | EUT | 213 | 31.0 | 11.6 | 1 | 8 | 0 | 0.742 | 0.277 | 0.199 | 1.285 |
| 6976 | 38.791149 | −105.106361 | WMT | 3147.0 | 10.2 | EUT | 222 | 39.8 | 12.0 | 1 | 4 | 0 | 0.899 | 0.272 | 0.366 | 1.433 |
| 7020 | 38.078326 | −122.743359 | XER | 51.1 | 335.3 | EUT | 213 | 31.0 | 11.6 | 1 | 36 | 0 | −0.162 | 0.061 | −0.281 | −0.043 |
| 7057 | 39.204737 | −111.668912 | WMT | 1789.8 | 24.8 | EUT | 222 | 39.8 | 12.0 | 1 | 30 | 0 | 0.246 | 0.074 | 0.100 | 0.391 |
| 7097 | 38.788187 | −111.774878 | WMT | 2203.2 | 6.7 | EUT | 222 | 39.8 | 12.0 | 1 | 15 | 0 | 0.623 | 0.188 | 0.254 | 0.992 |
| 7100 | 43.965218 | −122.683968 | WMT | 255.3 | 709.5 | EUT | 222 | 39.8 | 12.0 | 1 | 37 | 0 | 0.070 | 0.021 | 0.028 | 0.111 |
| 7105 | 41.110516 | −82.083872 | NAP | 258.0 | 21.0 | EUT | 225 | 46.0 | 13.8 | 1 | 28 | 0 | 0.391 | 0.118 | 0.160 | 0.622 |
| 7108 | 30.963438 | −95.903504 | CPL | 86.9 | 27.3 | EUT | 327 | 28.4 | 16.6 | 1 | 27 | 0 | 0.050 | 0.029 | −0.007 | 0.107 |
| 7109 | 32.072696 | −97.129773 | SPL | 186.9 | 12.6 | EUT | 176 | 26.1 | 13.1 | 1 | 24 | 0 | 0.082 | 0.041 | 0.001 | 0.163 |
| 7136 | 41.633291 | −118.389357 | XER | 1311.6 | 15.4 | EUT | 213 | 31.0 | 11.6 | 1 | 23 | 0 | 0.258 | 0.096 | 0.069 | 0.446 |
| 7205 | 32.240254 | −101.313303 | SPL | 711.1 | 56.8 | OLI | 176 | 26.1 | 13.1 | 1 | 2 | 0 | 0.924 | 0.464 | 0.015 | 1.832 |
| 7207 | 42.157825 | −122.607634 | WMT | 684.2 | 256.5 | EUT | 222 | 39.8 | 12.0 | 1 | 26 | 0 | 0.346 | 0.105 | 0.141 | 0.551 |
| 7226 | 42.130013 | −122.478277 | WMT | 1344.3 | 4.4 | EUT | 222 | 39.8 | 12.0 | 1 | 19 | 0 | 0.522 | 0.158 | 0.213 | 0.832 |
| 7228 | 34.227816 | −86.843449 | SAP | 247.0 | 73.0 | EUT | 344 | 45.8 | 15.1 | 1 | 44 | 0 | 0.039 | 0.013 | 0.014 | 0.065 |
| 7229 | 40.631585 | −120.002870 | XER | 1329.5 | 37.1 | EUT | 213 | 31.0 | 11.6 | 1 | 20 | 0 | 0.354 | 0.132 | 0.095 | 0.614 |
| 7232 | 40.703407 | −83.378745 | TPL | 269.7 | 102.7 | EUT | 209 | 32.0 | 12.9 | 1 | 17 | 0 | 0.468 | 0.189 | 0.099 | 0.838 |
| 7276 | 41.168583 | −119.817451 | XER | 1560.8 | 28.9 | OLI | 213 | 31.0 | 11.6 | 1 | 9 | 0 | 0.710 | 0.265 | 0.190 | 1.229 |
| 7294 | 39.056042 | −82.690673 | SAP | 211.5 | 65.1 | EUT | 344 | 45.8 | 15.1 | 1 | 47 | 0 | −0.026 | 0.009 | −0.043 | −0.009 |
| 7304 | 31.587497 | −98.622503 | SPL | 448.8 | 27.8 | EUT | 176 | 26.1 | 13.1 | 1 | 36 | 0 | −0.377 | 0.189 | −0.748 | −0.006 |
| 7306 | 39.241149 | −117.165818 | XER | 2255.5 | 5.8 | EUT | 213 | 31.0 | 11.6 | 1 | 19 | 0 | 0.387 | 0.144 | 0.104 | 0.670 |
| 7325 | 40.337080 | −105.126694 | SPL | 1562.4 | 189.8 | EUT | 176 | 26.1 | 13.1 | 1 | 19 | 0 | 0.273 | 0.137 | 0.004 | 0.542 |
| 7368 | 32.515869 | −87.861085 | CPL | 22.3 | 4731.5 | EUT | 327 | 28.4 | 16.6 | 1 | 29 | 0 | −0.021 | 0.012 | −0.044 | 0.003 |
| 7369 | 41.035420 | −96.837727 | TPL | 391.7 | 29.2 | EUT | 209 | 32.0 | 12.9 | 1 | 26 | 0 | 0.187 | 0.075 | 0.039 | 0.334 |
| 7375 | 33.364862 | −88.166880 | CPL | 78.1 | 5.6 | EUT | 327 | 28.4 | 16.6 | 1 | 45 | 0 | −0.584 | 0.342 | −1.254 | 0.086 |
| 7392 | 34.534351 | −92.268826 | CPL | 74.1 | 105.8 | EUT | 327 | 28.4 | 16.6 | 1 | 26 | 0 | 0.085 | 0.050 | −0.013 | 0.182 |
| 7402 | 34.284778 | −97.170972 | SPL | 245.4 | 160.7 | EUT | 176 | 26.1 | 13.1 | 1 | 39 | 0 | −0.492 | 0.247 | −0.975 | −0.008 |
| 7405 | 40.328099 | −96.532001 | TPL | 425.9 | 32.1 | EUT | 209 | 32.0 | 12.9 | 1 | 25 | 0 | 0.218 | 0.088 | 0.046 | 0.390 |
| 7409 | 33.075563 | −92.660596 | CPL | 55.4 | 7.3 | EUT | 327 | 28.4 | 16.6 | 1 | 31 | 0 | −0.091 | 0.053 | −0.196 | 0.013 |
| 7459 | 33.882010 | −85.931618 | SAP | 171.0 | 18.1 | EUT | 344 | 45.8 | 15.1 | 1 | 39 | 0 | 0.148 | 0.049 | 0.052 | 0.244 |
| 7471 | 46.040623 | −110.692175 | NPL | 1556.2 | 96.7 | EUT | 179 | 29.4 | 10.1 | 1 | 23 | 0 | 0.219 | 0.075 | 0.071 | 0.366 |
| 7472 | 46.624624 | −110.738336 | NPL | 1672.9 | 150.7 | EUT | 179 | 29.4 | 10.1 | 1 | 25 | 0 | 0.151 | 0.052 | 0.049 | 0.252 |
| 7533 | 43.078399 | −112.693659 | XER | 1338.8 | 21.3 | EUT | 213 | 31.0 | 11.6 | 1 | 14 | 0 | 0.548 | 0.205 | 0.147 | 0.949 |
| 7572 | 40.372482 | −84.340110 | TPL | 291.8 | 327.1 | EUT | 209 | 32.0 | 12.9 | 1 | 22 | 0 | 0.312 | 0.126 | 0.066 | 0.558 |
| 7579 | 39.608156 | −84.971507 | TPL | 254.8 | 72.8 | EUT | 209 | 32.0 | 12.9 | 1 | 21 | 0 | 0.343 | 0.138 | 0.072 | 0.614 |
| 7643 | 39.706824 | −111.293369 | WMT | 2569.0 | 31.5 | EUT | 222 | 39.8 | 12.0 | 1 | 29 | 0 | 0.271 | 0.082 | 0.110 | 0.431 |
| 7652 | 40.176639 | −84.265220 | TPL | 275.4 | 15.3 | EUT | 209 | 32.0 | 12.9 | 1 | 29 | 0 | 0.093 | 0.037 | 0.020 | 0.166 |
| 7684 | 37.673281 | −107.112778 | WMT | 3530.5 | 2.1 | EUT | 222 | 39.8 | 12.0 | 1 | 19 | 0 | 0.522 | 0.158 | 0.213 | 0.832 |
| 7686 | 37.316232 | −107.112994 | WMT | 2348.4 | 35.2 | EUT | 222 | 39.8 | 12.0 | 1 | 34 | 0 | 0.145 | 0.044 | 0.059 | 0.231 |
| 7698 | 41.152339 | −110.824953 | XER | 2180.1 | 90.9 | EUT | 213 | 31.0 | 11.6 | 1 | 8 | 0 | 0.742 | 0.277 | 0.199 | 1.285 |
| 7713 | 46.118216 | −113.374640 | WMT | 1847.6 | 152.3 | EUT | 222 | 39.8 | 12.0 | 1 | 26 | 0 | 0.346 | 0.105 | 0.141 | 0.551 |
| 7800 | 41.677573 | −73.144698 | NAP | 198.8 | 56.2 | EUT | 225 | 46.0 | 13.8 | 1 | 51 | 0 | −0.109 | 0.033 | −0.174 | −0.045 |
| 7810 | 43.413998 | −119.410472 | XER | 1268.6 | 107.8 | EUT | 213 | 31.0 | 11.6 | 1 | 10 | 0 | 0.677 | 0.253 | 0.181 | 1.173 |
| 7812 | 35.562459 | −93.637568 | SAP | 203.7 | 44.7 | EUT | 344 | 45.8 | 15.1 | 1 | 28 | 0 | 0.389 | 0.128 | 0.137 | 0.640 |
| 8016 | 33.829304 | −109.090421 | WMT | 2403.4 | 48.4 | EUT | 222 | 39.8 | 12.0 | 1 | 17 | 0 | 0.573 | 0.173 | 0.233 | 0.912 |
| 8121 | 36.067203 | −91.142428 | SAP | 82.7 | 222.6 | EUT | 344 | 45.8 | 15.1 | 1 | 26 | 0 | 0.432 | 0.143 | 0.153 | 0.712 |
| 8144 | 35.583189 | −90.962941 | CPL | 69.0 | 95.9 | EUT | 327 | 28.4 | 16.6 | 1 | 21 | 0 | 0.261 | 0.153 | −0.038 | 0.560 |
| 8151 | 41.088461 | −82.729015 | TPL | 249.8 | 80.7 | EUT | 209 | 32.0 | 12.9 | 1 | 26 | 0 | 0.187 | 0.075 | 0.039 | 0.334 |
| 8184 | 40.055586 | −105.747080 | WMT | 3029.0 | 51.0 | EUT | 222 | 39.8 | 12.0 | 1 | 17 | 0 | 0.573 | 0.173 | 0.233 | 0.912 |
| 8207 | 39.653475 | −82.473781 | SAP | 240.2 | 47.7 | EUT | 344 | 45.8 | 15.1 | 1 | 44 | 0 | 0.039 | 0.013 | 0.014 | 0.065 |
| 8250 | 48.380621 | −110.985266 | NPL | 913.5 | 9.5 | EUT | 179 | 29.4 | 10.1 | 1 | 20 | 0 | 0.321 | 0.110 | 0.105 | 0.537 |
| 8256 | 39.775971 | −81.522472 | SAP | 241.9 | 24.2 | EUT | 344 | 45.8 | 15.1 | 1 | 36 | 0 | 0.214 | 0.071 | 0.076 | 0.352 |
| 8278 | 48.026569 | −109.623760 | NPL | 1128.8 | 9.8 | EUT | 179 | 29.4 | 10.1 | 1 | 27 | 0 | 0.083 | 0.028 | 0.027 | 0.138 |
| 8325 | 37.416782 | −108.405651 | WMT | 2213.0 | 65.4 | EUT | 222 | 39.8 | 12.0 | 1 | 14 | 0 | 0.648 | 0.196 | 0.264 | 1.032 |
| 8342 | 32.056309 | −96.731783 | SPL | 162.6 | 5.5 | EUT | 176 | 26.1 | 13.1 | 1 | 34 | 0 | −0.300 | 0.151 | −0.596 | −0.005 |
| 8360 | 40.674602 | −110.970699 | WMT | 3043.5 | 39.4 | OLI | 222 | 39.8 | 12.0 | 1 | 15 | 0 | 0.623 | 0.188 | 0.254 | 0.992 |
| 8395 | 40.889268 | −109.846108 | WMT | 2622.7 | 32.5 | EUT | 222 | 39.8 | 12.0 | 1 | 24 | 0 | 0.397 | 0.120 | 0.161 | 0.632 |
| 8409 | 39.720767 | −86.720223 | TPL | 255.2 | 124.2 | EUT | 209 | 32.0 | 12.9 | 1 | 30 | 0 | 0.062 | 0.025 | 0.013 | 0.110 |
| 8413 | 31.910344 | −95.301856 | CPL | 129.5 | 481.4 | EUT | 327 | 28.4 | 16.6 | 1 | 38 | 0 | −0.337 | 0.198 | −0.725 | 0.050 |
| 8414 | 40.121695 | −104.945911 | SPL | 1509.8 | 24.7 | EUT | 176 | 26.1 | 13.1 | 1 | 6 | 0 | 0.771 | 0.387 | 0.012 | 1.529 |
| 8416 | 38.939348 | −91.282857 | TPL | 226.5 | 4.3 | EUT | 209 | 32.0 | 12.9 | 1 | 37 | 0 | −0.157 | 0.063 | −0.282 | −0.033 |
| 8427 | 44.698479 | −87.499926 | UMW | 179.8 | 35.5 | EUT | 167 | 39.3 | 12.7 | 1 | 30 | 0 | 0.237 | 0.077 | 0.087 | 0.388 |
| 8435 | 47.876688 | −107.125232 | NPL | 758.1 | 13.8 | EUT | 179 | 29.4 | 10.1 | 1 | 14 | 0 | 0.524 | 0.180 | 0.171 | 0.878 |
| 8437 | 42.646907 | −72.218497 | NAP | 195.1 | 138.4 | EUT | 225 | 46.0 | 13.8 | 1 | 40 | 0 | 0.130 | 0.039 | 0.053 | 0.206 |
| 8443 | 41.812487 | −70.638227 | CPL | 13.7 | 10.8 | EUT | 327 | 28.4 | 16.6 | 1 | 42 | 0 | −0.478 | 0.280 | −1.027 | 0.071 |
| 8480 | 30.006973 | −96.709810 | SPL | 109.8 | 21.4 | EUT | 176 | 26.1 | 13.1 | 1 | 39 | 0 | −0.492 | 0.247 | −0.975 | −0.008 |
| 8487 | 39.661693 | −84.646207 | TPL | 287.3 | 7.1 | EUT | 209 | 32.0 | 12.9 | 1 | 20 | 0 | 0.374 | 0.151 | 0.079 | 0.670 |
| 8494 | 41.989559 | −71.205066 | NAP | 30.9 | 202.4 | EUT | 225 | 46.0 | 13.8 | 1 | 40 | 0 | 0.130 | 0.039 | 0.053 | 0.206 |
| 8495 | 46.941268 | −119.278530 | XER | 263.5 | 53.1 | EUT | 213 | 31.0 | 11.6 | 1 | 34 | 0 | −0.097 | 0.036 | −0.169 | −0.026 |
| 8504 | 38.070619 | −111.375127 | WMT | 3074.1 | 11.2 | EUT | 222 | 39.8 | 12.0 | 1 | 9 | 0 | 0.774 | 0.234 | 0.315 | 1.232 |
| 8614 | 36.044443 | −85.586295 | SAP | 269.0 | 30.2 | EUT | 344 | 45.8 | 15.1 | 1 | 22 | 0 | 0.520 | 0.171 | 0.184 | 0.856 |
| 8635 | 40.495536 | −83.899880 | TPL | 303.7 | 2018.9 | EUT | 209 | 32.0 | 12.9 | 1 | 21 | 0 | 0.343 | 0.138 | 0.072 | 0.614 |
| 8765 | 37.595562 | −112.254669 | WMT | 2389.9 | 70.2 | EUT | 222 | 39.8 | 12.0 | 1 | 25 | 0 | 0.371 | 0.112 | 0.151 | 0.592 |
| 8766 | 40.863781 | −109.811815 | WMT | 2528.1 | 18.1 | EUT | 222 | 39.8 | 12.0 | 1 | 13 | 0 | 0.673 | 0.204 | 0.274 | 1.072 |
| 8777 | 39.360351 | −111.963708 | XER | 1521.4 | 3220.7 | EUT | 213 | 31.0 | 11.6 | 1 | 3 | 0 | 0.903 | 0.337 | 0.242 | 1.565 |
| 8811 | 31.331826 | −97.268438 | SPL | 180.5 | 14.8 | EUT | 176 | 26.1 | 13.1 | 1 | 41 | 0 | −0.568 | 0.285 | −1.127 | −0.009 |
| 1000019 | 43.460190 | −116.141301 | XER | 973.1 | 33.4 | EUT | 213 | 31.0 | 11.6 | 1 | 25 | 0 | 0.193 | 0.072 | 0.052 | 0.334 |
| 1000025 | 43.198407 | −114.599726 | XER | 1678.7 | 44.6 | EUT | 213 | 31.0 | 11.6 | 1 | 27 | 0 | 0.129 | 0.048 | 0.034 | 0.223 |
| 1000029 | 42.677045 | −113.407296 | XER | 1278.7 | 3395.5 | EUT | 213 | 31.0 | 11.6 | 1 | 12 | 0 | 0.613 | 0.229 | 0.164 | 1.061 |
| 1000030 | 42.206372 | −114.878852 | XER | 1593.0 | 393.0 | EUT | 213 | 31.0 | 11.6 | 1 | 20 | 0 | 0.354 | 0.132 | 0.095 | 0.614 |
| 1000068 | 46.206071 | −116.834367 | XER | 1034.1 | 38.1 | EUT | 213 | 31.0 | 11.6 | 1 | 22 | 0 | 0.290 | 0.108 | 0.078 | 0.502 |
| 1000073 | 35.582330 | −101.717139 | SPL | 894.9 | 6559.4 | EUT | 176 | 26.1 | 13.1 | 1 | 9 | 0 | 0.656 | 0.329 | 0.011 | 1.301 |
| 1000084 | 41.037407 | −100.775775 | SPL | 916.0 | 641.0 | EUT | 176 | 26.1 | 13.1 | 1 | 15 | 0 | 0.426 | 0.214 | 0.007 | 0.846 |
| 1000086 | 41.321987 | −98.900675 | SPL | 657.7 | 1117.9 | EUT | 176 | 26.1 | 13.1 | 1 | 15 | 0 | 0.426 | 0.214 | 0.007 | 0.846 |
| 1000122 | 42.264258 | −116.310496 | XER | 1690.0 | 35.5 | EUT | 213 | 31.0 | 11.6 | 1 | 25 | 0 | 0.193 | 0.072 | 0.052 | 0.334 |
| 1000126 | 42.531475 | −116.364513 | XER | 1773.6 | 29.4 | EUT | 213 | 31.0 | 11.6 | 1 | 23 | 0 | 0.258 | 0.096 | 0.069 | 0.446 |
| 1000137 | 42.187306 | −113.924080 | XER | 1444.7 | 407.4 | OLI | 213 | 31.0 | 11.6 | 1 | 11 | 0 | 0.645 | 0.241 | 0.173 | 1.117 |
| 1000223 | 43.537123 | −90.959353 | UMW | 277.4 | 14.8 | EUT | 167 | 39.3 | 12.7 | 1 | 25 | 0 | 0.364 | 0.118 | 0.133 | 0.595 |
| % | [a] | [b] | [c] | [d] | [e] | [f] | [g] | [h] | [i] | [j] | [k] | [l] | [m] | [n] | [o] | Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 51 | 0.2 | 14.8 | 5.2 | 0.0 | 9.4 | 0.6 | 0.9 | −0.2 | 3.8 | 0.4 | 4.8 | 5.9 | 1.3 | −0.1 | 3.9 | 51.0 |
| 45 | 0.2 | 14.8 | 9.4 | 0.6 | 0.9 | −0.2 | 3.8 | 4.8 | 5.9 | 1.3 | −0.1 | 3.9 | 45.4 | |||
| 25 | 0.2 | 9.4 | 0.9 | −0.2 | 4.8 | 5.9 | −0.1 | 3.9 | 24.9 | |||||||
| 24 | 9.4 | 4.8 | 5.9 | 3.9 | 24.1 | |||||||||||
| 11 | 0.9 | 5.9 | −0.1 | 3.9 | 10.7 | |||||||||||
| 8 | −0.2 | 4.8 | −0.1 | 3.9 | 8.4 | |||||||||||
| 45 | 14.8 | 9.4 | 0.6 | 3.8 | 4.8 | 5.9 | 1.3 | 3.9 | 44.6 | |||||||
| 15 | 14.8 | 14.8 | ||||||||||||||
| 18 | 5.2 | 0.6 | 0.9 | 0.4 | 5.9 | 1.3 | −0.1 | 3.9 | 18.1 | |||||||
| 14 | 0.0 | −0.2 | 3.8 | 0.4 | 4.8 | 1.3 | −0.1 | 3.9 | 14.0 |
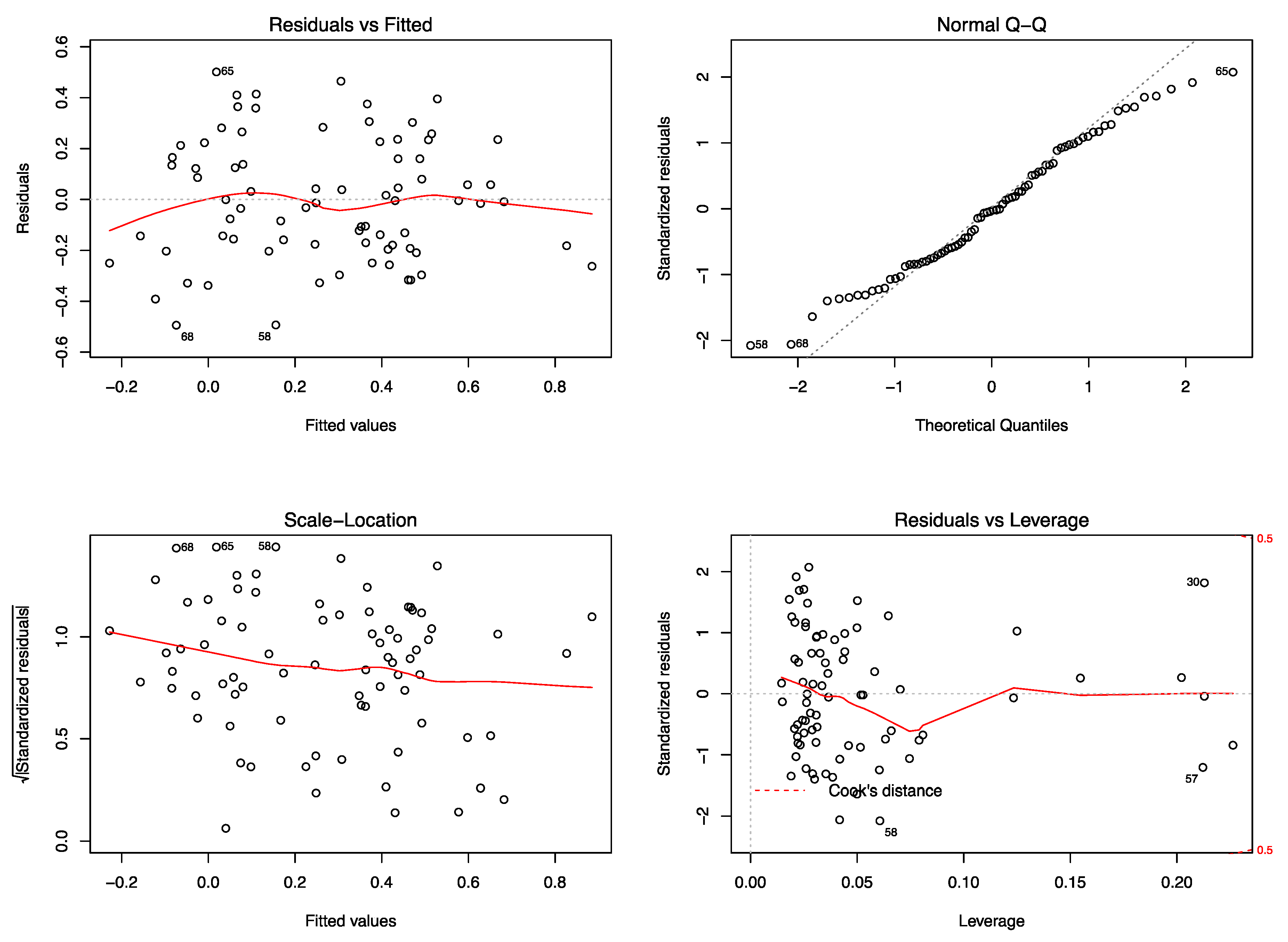
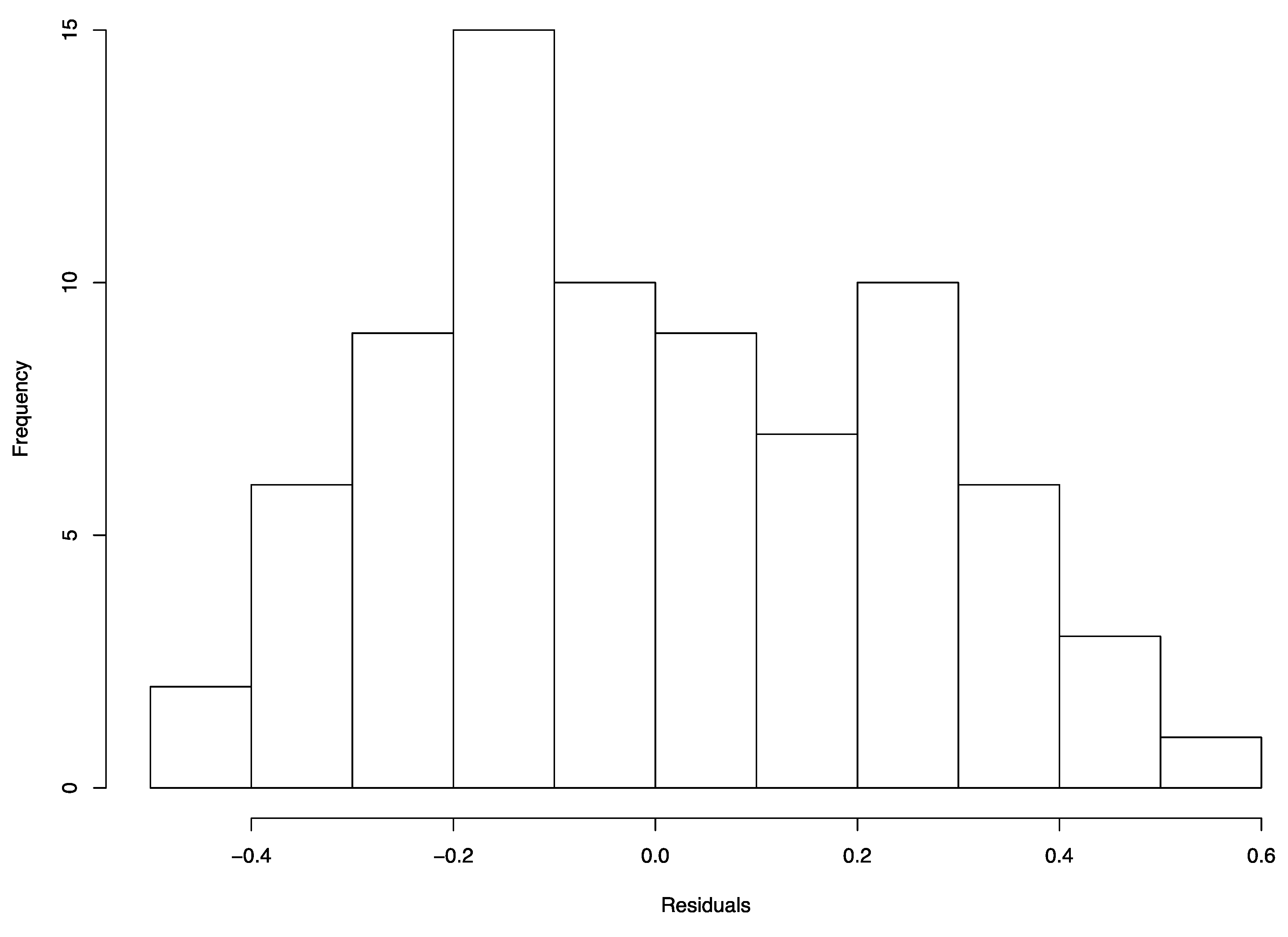
References
- Richter, B.D.; Mathews, R.; Harrison, D.L.; Wigington, R. Ecologically sustainable water management: Managing river flows for ecological integrity. Ecol. Appl. 2003, 13, 206–224. [Google Scholar] [CrossRef]
- Lehner, B.; Liermann, C.R.; Revenga, C.; Vörösmarty, C.J.; Fekete, B.M.; Crouzet, P.; Döll, P.; Endejan, M.; Frenken, K.; Magome, J.; et al. High-resolution mapping of the world’s reservoirs and dams for sustainable river-flow management. Front. Ecol. Environ. 2011, 9, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Shi, H.; Sivakumar, B.; Peart, M.R. Population, water, food, energy and dams. Renew. Sustain. Energy Rev. 2016, 56, 18–28. [Google Scholar] [CrossRef]
- Rosenberg, D.M.; McCully, P.; Pringle, C.M. Global-scale environmental effects of hydrological alterations: Introduction. Bioscience 2000, 50, 746–751. [Google Scholar] [CrossRef] [Green Version]
- Vörösmarty, C.J. Chapter 7: Fresh Water. In Millennium Ecosystem Assessment, Volume 1: Conditions and Trends Working Group Report; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Balderas, S.C.; Bussing, W.; et al. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. Bioscience 2008, 58, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Renöfält, B.M.; Jansson, R.; Nilsson, C. Effects of hydropower generation and opportunities for environmental flow management in Swedish riverine ecosystems. Freshw. Biol. 2010, 55, 49–67. [Google Scholar] [CrossRef]
- Gracey, E.O.; Verones, F. Impacts from hydropower production on biodiversity in an LCA framework—review and recommendations. Int. J. Life Cycle Assess. 2016, 21, 412–428. [Google Scholar] [CrossRef]
- Agostinho, A.; Pelicice, F.; Gomes, L. Dams and the fish fauna of the Neotropical region: Impacts and management related to diversity and fisheries. Braz. J. Biol. 2008, 68, 1119–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryder, R.A. The morphoedaphic index—use, abuse, and fundamental concepts. Trans. Am. Fish. Soc. 1982, 111, 154–164. [Google Scholar] [CrossRef]
- Youngs, W.D.; Heimbuch, D.G. Another consideration of the morphoedaphic index. Trans. Am. Fish. Soc. 1982, 111, 151–153. [Google Scholar] [CrossRef]
- Jackson, D.A.; Harvey, H.H.; Somers, K.M. Ratios in aquatic sciences: Statistical shortcomings with mean depth and the morphoedaphic index. Can. J. Fish. Aquat. Sci. 1990, 47, 1788–1795. [Google Scholar] [CrossRef]
- Rempel, R.S.; Colby, P.J. A statistically valid model of the morphoedaphic index. Can. J. Fish. Aquat. Sci. 1991, 48, 1937–1943. [Google Scholar] [CrossRef]
- Poff, N.L.; Allan, J.D.; Bain, M.B.; Karr, J.R.; Prestegaard, K.L.; Richter, B.D.; Sparks, R.E.; Stromberg, J.C. The natural flow regime. Bioscience 1997, 47, 769–784. [Google Scholar] [CrossRef]
- Bunn, S.E.; Arthington, A.H. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environ. Manag. 2002, 30, 492–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Kraft, K.J. Effect of Increased Winter Drawdown on Benthic Macroinvertebrates in Namakan Reservoir, Voyeurs National Park; Michigan Techonological University: Houghton, MI, USA, 1988; p. 86. [Google Scholar]
- Englund, G.; Malmqvist, B. Effects of flow regulation, habitat area and isolation on the macroinvertebrate fauna of rapids in North Swedish rivers. Regul. Rivers Res. Manag. 1996, 12, 433–445. [Google Scholar] [CrossRef]
- Malmqvist, B.; Englund, G. Effects of hydropower-induced flow perturbations on mayfly (Ephemeroptera) richness and abundance in north Swedish river rapids. Hydrobiology 1996, 341, 145–158. [Google Scholar] [CrossRef]
- Valdovinos, C.; Moya, C.; Olmos, V.; Parra, O.; Karrasch, B.; Buettner, O. The importance of water-level fluctuation for the conservation of shallow water benthic macroinvertebrates: An example in the Andean zone of Chile. Biodivers. Conserv. 2007, 16, 3095–3109. [Google Scholar] [CrossRef]
- Aroviita, J.; Hämäläinen, H. The impact of water-level regulation on littoral macroinvertebrate assemblages in boreal lakes. Hydrobiol. 2008, 613, 45–56. [Google Scholar] [CrossRef]
- White, M.S.; Xenopoulos, M.A.; Metcalfe, R.A.; Somers, K.M. Water level thresholds of benthic macroinvertebrate richness, structure, and function of boreal lake stony littoral habitats. Can. J. Fish. Aquat. Sci. 2011, 68, 1695–1704. [Google Scholar] [CrossRef]
- Behrend, R.D.L.; Takeda, A.; Gomes, L.; Fernandes, S.E.P. Using oligochaeta assemblages as an indicator of environmental changes. Braz. J. Biol. 2012, 72, 873–884. [Google Scholar] [CrossRef] [Green Version]
- Jackson, H.M.; Gibbins, C.N.; Soulsby, C. Role of discharge and temperature variation in determining invertebrate community structure in a regulated river. River Res. Appl. 2007, 23, 651–669. [Google Scholar] [CrossRef]
- Kullasoot, S.; Intrarasattayapong, P.; Phalaraksh, C. Use of benthic macroinvertebrates as bioindicators of anthropogenic impacts on water quality of Mae Klong River, Western Thailand. Chiang Mai J. Sci. 2017, 44, 1356–1366. [Google Scholar]
- Takao, A.; Kawaguchi, Y.; Minagawa, T.; Kayaba, Y.; Morimoto, Y. The relationships between benthic macroinvertebrates and biotic and abiotic environmental characteristics downstream of the Yahagi Dam, Central Japan, and the State Change Caused by inflow from a Tributary. River Res. Appl. 2008, 24, 580–597. [Google Scholar] [CrossRef]
- Głowacki, Ł.; Grzybkowska, M.; Dukowska, M.; Penczak, T. Effects of damming a large lowland river on chironomids and fish assessed with the (multiplicative partitioning of) true/Hill biodiversity measure. River Res. Appl. 2010, 27, 612–629. [Google Scholar] [CrossRef]
- Floss, E.C.S.; Secretti, E.; Kotzian, C.B.; Spies, M.R.; Pires, M.M. Spatial and temporal distribution of non-biting midge larvae assemblages in streams in a mountainous region in Southern Brazil. J. Insect Sci. 2013, 13, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Smokorowski, K.E.; Metcalfe, R.A.; Finucan, S.D.; Jones, N.; Marty, J.; Power, M.; Pyrce, R.S.; Steele, R. Ecosystem level assessment of environmentally based flow restrictions for maintaining ecosystem integrity: A comparison of a modified peaking versus unaltered river. Ecohydrology 2010, 4, 791–806. [Google Scholar] [CrossRef]
- Marchetti, M.P.; Esteban, E.; Smith, A.N.; Pickard, D.; Richards, A.B.; Slusark, J. Measuring the ecological impact of long-term flow disturbance on the macroinvertebrate community in a large Mediterranean climate river. J. Freshw. Ecol. 2011, 26, 1–22. [Google Scholar] [CrossRef]
- ISO. ISO 14040: Environmental Management—Life Cycle Assessment; BSI: London, UK, 2006. [Google Scholar]
- Hellweg, S.; Milà I Canals, L. Emerging approaches, challenges and opportunities in life cycle assessment. Science 2014, 344, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.; De Baan, L.; De Schryver, A.M.; Van Zelm, R.; Hellweg, S.; Koellner, T.; Sonnemann, G.; Huijbregts, M.A.J. Toward meaningful end points of biodiversity in life cycle assessment. Environ. Sci. Technol. 2011, 45, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Verones, F.; Bare, J.; Bulle, C.; Frischknecht, R.; Hauschild, M.; Hellweg, S.; Henderson, A.; Jolliet, O.; Laurent, A.; Liao, X.; et al. LCIA framework and cross-cutting issues guidance within the UNEP-SETAC Life Cycle Initiative. J. Clean. Prod. 2017, 161, 957–967. [Google Scholar] [CrossRef] [Green Version]
- Turgeon, K.; Trottier, G.; Turpin, C.; Bulle, C.; Margni, M. Empirical characterization factors to be used in LCA and assessing the effects of hydropower on fish richness. Ecol. Indic. 2021, 121, 107047. [Google Scholar] [CrossRef]
- Dorber, M.; Mattson, K.R.; Sandlund, O.T.; May, R.; Verones, F. Quantifying net water consumption of Norwegian hydropower reservoirs and related aquatic biodiversity impacts in Life Cycle Assessment. Environ. Impact Assess. Rev. 2019, 76, 36–46. [Google Scholar] [CrossRef]
- Xenopoulos, M.A.; Lodge, D.M. Going with the flow: Using species–discharge relationships to forecast losses in fish biodiversity. Ecology 2006, 87, 1907–1914. [Google Scholar] [CrossRef]
- Pickett, S.T.A. Space-for-time substitution as an alternative to long-term studies. In Long-Term Studies in Ecology; Likens, G.E., Ed.; Springer: New York, NY, USA, 1989; pp. 110–135. ISBN 978-1-4615-7360-9. [Google Scholar]
- Rosenbaum, R.K.; Bachmann, T.M.; Gold, L.S.; Huijbregts, M.A.J.; Jolliet, O.; Juraske, R.; Koehler, A.; Larsen, H.F.; MacLeod, M.; Margni, M.; et al. USEtox—the UNEP-SETAC toxicity model: Recommended characterisation factors for human toxicity and freshwater ecotoxicity in life cycle impact assessment. Int. J. Life Cycle Assess. 2008, 13, 532–546. [Google Scholar] [CrossRef] [Green Version]
- De Baan, L.; Mutel, C.L.; Curran, M.; Hellweg, S.; Koellner, T. Land use in life cycle assessment: Global characterization factors based on regional and global potential species extinction. Environ. Sci. Technol. 2013, 47, 9281–9290. [Google Scholar] [CrossRef]
- Jolliet, O.; Margni, M.; Charles, R.; Humbert, S.; Payet, J.; Rebitzer, G.; Rosenbaum, R. IMPACT 2002+: A new life cycle impact assessment methodology. Int. J. Life Cycle Assess. 2003, 8, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Goedkoop, M.; Heijungs, R.; Huijbregts, M.; De Schryver, A.; Struijs, J.; van Zelm, R. ReCiPe 2008—Life Cycle Impact Assessment Which Comprises Harmonized Category Indicators at the Midpoint and Endpoint Level. 2009. Available online: https://www.researchgate.net/profile/Mark_Goedkoop/publication/230770853_Recipe_2008/links/09e4150dc068ff22e9000000.pdf (accessed on 2 March 2021).
- Chaudhary, A.; Verones, F.; De Baan, L.; Hellweg, S. Quantifying Land Use Impacts on Biodiversity: Combining Species–Area Models and Vulnerability Indicators. Environ. Sci. Technol. 2015, 49, 9987–9995. [Google Scholar] [CrossRef]
- Lindeijer, E.; Müller-Wenk, R.; Steen, B. Impact assessment of resources and land use. In Life Cycle Impact Assessment: Striving towards Best Practice; SETAC: Pensacola, FL, USA, 2002; pp. 11–64. [Google Scholar]
- Milà i Canals, L.M.I.; Bauer, C.; Depestele, J.; Dubreuil, A.; Freiermuth Knuchel, R.; Gaillard, G.; Michelsen, O.; Müller-Wenk, R.; Rydgren, B. Key elements in a framework for land use impact assessment within LCA (11 pp). Int. J. Life Cycle Assess. 2007, 12, 5–15. [Google Scholar] [CrossRef] [Green Version]
- USEPA. National Lakes Assessment. Available online: https://www.epa.gov/national-aquatic-resource-surveys/nla (accessed on 16 January 2019).
- USEPA. National Lake Assessment 2012: Technical Report; U.S. Environmental Protection Agency: Washington, DC, USA, 2017.
- USEPA. National Rivers and Streams Assessment. Available online: https://www.epa.gov/national-aquatic-resource-surveys/nrsa (accessed on 16 January 2019).
- Banet, A.I.; Trexler, J.C. Space-for-time substitution works in everglades ecological forecasting models. PLoS ONE 2013, 8, e81025. [Google Scholar] [CrossRef]
- USEPA. 2012 National Lakes Assessment. Field Operations Manual; U.S. Environmental Protection Agency: Washington, DC, USA, 2011; p. 234.
- USEPA. National Rivers and Streams Assessment: Field Operations Manual; U.S. Environmental Protection Agency: Washington, DC, USA, 2007.
- Omernik, J.M. Ecoregions of the conterminous United States. Ann. Assoc. Am. Geogr. 1987, 77, 118–125. [Google Scholar] [CrossRef]
- Herlihy, A.T.; Paulsen, S.G.; Van Sickle, J.; Stoddard, J.L.; Hawkins, C.P.; Yuan, L.L. Striving for consistency in a national assessment: The challenges of applying a reference-condition approach at a continental scale. J. N. Am. Benthol. Soc. 2008, 27, 860–877. [Google Scholar] [CrossRef]
- USEPA. Ecoregions Used in the National Aquatic Resource Surveys. Available online: https://www.epa.gov/national-aquatic-resource-surveys/ecoregions-used-national-aquatic-resource-surveys (accessed on 16 January 2019).
- Patouillard, L.; Bulle, C.; Margni, M. Ready-to-use and advanced methodologies to prioritise the regionalisation effort in LCA. Matériaux Tech. 2016, 104, 105. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y. Two sides of the same coin: Consequential life cycle assessment based on the attributional framework. J. Clean. Prod. 2016, 127, 274–281. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Okumura, Y. Rpsychi: Statistics for Psychiatric Research; 2012. [Google Scholar]
- Legendre, P. Studying beta diversity: Ecological variation partitioning by multiple regression and canonical analysis. J. Plant Ecol. 2007, 1, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; et al. Vegan: Community Ecology Package; 2019. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2004; ISBN 978-0-387-95364-9. [Google Scholar]
- Schwarz, G. Estimating the dimension of a model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- García-Vega, D.; Newbold, T. Assessing the effects of land use on biodiversity in the world’s drylands and Mediterranean environments. Biodivers. Conserv. 2019, 29, 393–408. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.; Noss, R. Endemism hotspots are linked to stable climatic refugia. Ann. Bot. 2017, 119, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Mykrä, H.; Heino, J. Decreased habitat specialization in macroinvertebrate assemblages in anthropogenically disturbed streams. Ecol. Complex. 2017, 31, 181–188. [Google Scholar] [CrossRef]
- Viterbi, R.; Cerrato, C.; Bassano, B.; Bionda, R.; Von Hardenberg, A.; Provenzale, A.; Bogliani, G. Patterns of biodiversity in the northwestern Italian Alps: A multi-taxa approach. Community Ecol. 2013, 14, 18–30. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystems; Academic Press: San Diego, CA, USA, 2001; ISBN 978-0-12-744760-5. [Google Scholar]
- Dodson, S.I.; Arnott, S.E.; Cottingham, K.L. The relationship in lake communities between primary productivity and species richness. Ecology 2000, 81, 2662–2679. [Google Scholar] [CrossRef]
- Heino, J.; Tolonen, K.T. Ecological drivers of multiple facets of beta diversity in a lentic macroinvertebrate metacommunity. Limnol. Oceanogr. 2017, 62, 2431–2444. [Google Scholar] [CrossRef]
- Jackson, D.A.; Peres-Neto, P.R.; Olden, J.D. What controls who is where in freshwater fish communities—the roles of biotic, abiotic, and spatial factors. Can. J. Fish. Aquat. Sci. 2001, 58, 157–170. [Google Scholar] [CrossRef]
- Tonn, W.M.; Magnuson, J.J. Patterns in the species composition and richness of fish assemblages in Northern Wisconsin Lakes. Ecology 1982, 63, 1149–1166. [Google Scholar] [CrossRef]
- Heino, J. Lentic macroinvertebrate assemblage structure along gradients in spatial heterogeneity, habitat size and water chemistry. Hydrobiologia 2000, 418, 229–242. [Google Scholar] [CrossRef]
- Connor, E.F.; McCoy, E.D. The statistics and biology of the species-area relationship. Am. Nat. 1979, 113, 791–833. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 2001; ISBN 978-0-691-08836-5. [Google Scholar]
- Verones, F.; Hanafiah, M.M.; Pfister, S.; Huijbregts, M.A.J.; Pelletier, G.J.; Koehler, A. Characterization factors for thermal pollution in freshwater aquatic environments. Environ. Sci. Technol. 2010, 44, 9364–9369. [Google Scholar] [CrossRef]
- Williams, C.B. Patterns in the Balance of Nature and Related Problems in Quantitative Ecology; Academic Press: Cambridge, MA, USA, 1964. [Google Scholar]
- Horwitz, R.J. Temporal variability patterns and the distributional patterns of stream fishes. Ecol. Monogr. 1978, 48, 307–321. [Google Scholar] [CrossRef]
- Moyle, P.B.; Li, H.W. Community ecology and predator-prey relations in warmwater streams. In Predator-Prey Systems in Fisheries Management; Clepper, H., Ed.; Sport Fishing Institute: Washington, DC, USA, 1979; pp. 171–180. [Google Scholar]
- Eadie, J.M.; Hurly, T.A.; Montgomerie, R.D.; Teather, K.L. Lakes and rivers as islands: Species-area relationships in the fish faunas of Ontario. Environ. Biol. Fishes 1986, 15, 81–89. [Google Scholar] [CrossRef]
- Gorman, O.T.; Karr, J.R. Habitat structure and stream fish communities. Ecology 1978, 59, 507–515. [Google Scholar] [CrossRef]
- Matthews, W.J. Small fish community structure in Ozark streams: Structured assembly patterns or random abundance of species? Am. Midl. Nat. 1982, 107, 42. [Google Scholar] [CrossRef]
- Walker, K.F.; Thoms, M.C.; Sheldon, F. Effects of weirs on the littoral environment of the River Murray, South Australia. In River Conservation and Management; Boon, P.J., Calow, P., Petts, G.E., Eds.; John Wiley & Sons: Chichester, UK, 1992; pp. 271–292. [Google Scholar]
- Bulle, C.; Margni, M.; Patouillard, L.; Boulay, A.-M.; Bourgault, G.; De Bruille, V.; Cao, V.; Hauschild, M.; Henderson, A.; Humbert, S.; et al. IMPACT World+: A globally regionalized life cycle impact assessment method. Int. J. Life Cycle Assess. 2019, 24, 1653–1674. [Google Scholar] [CrossRef] [Green Version]
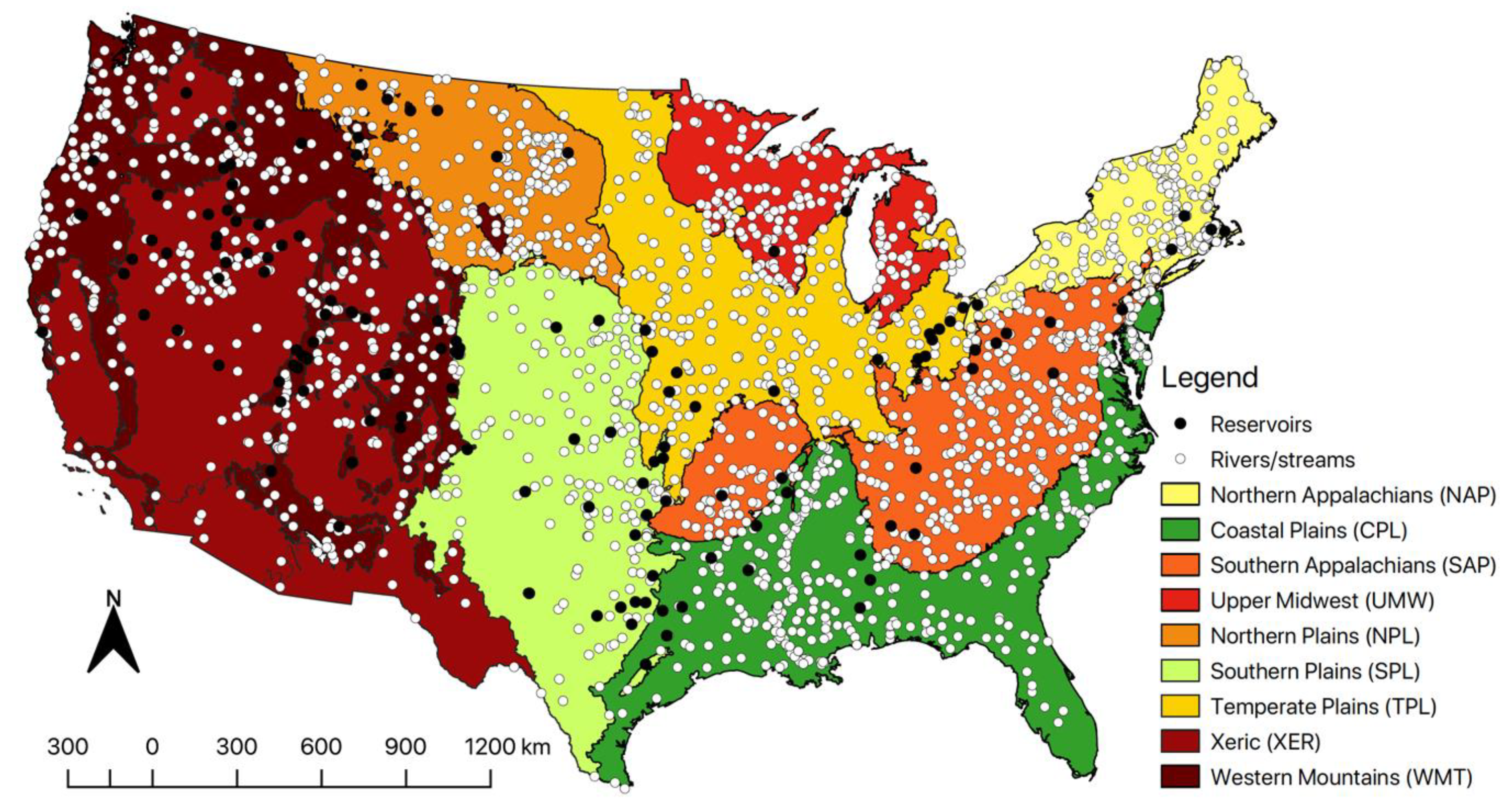
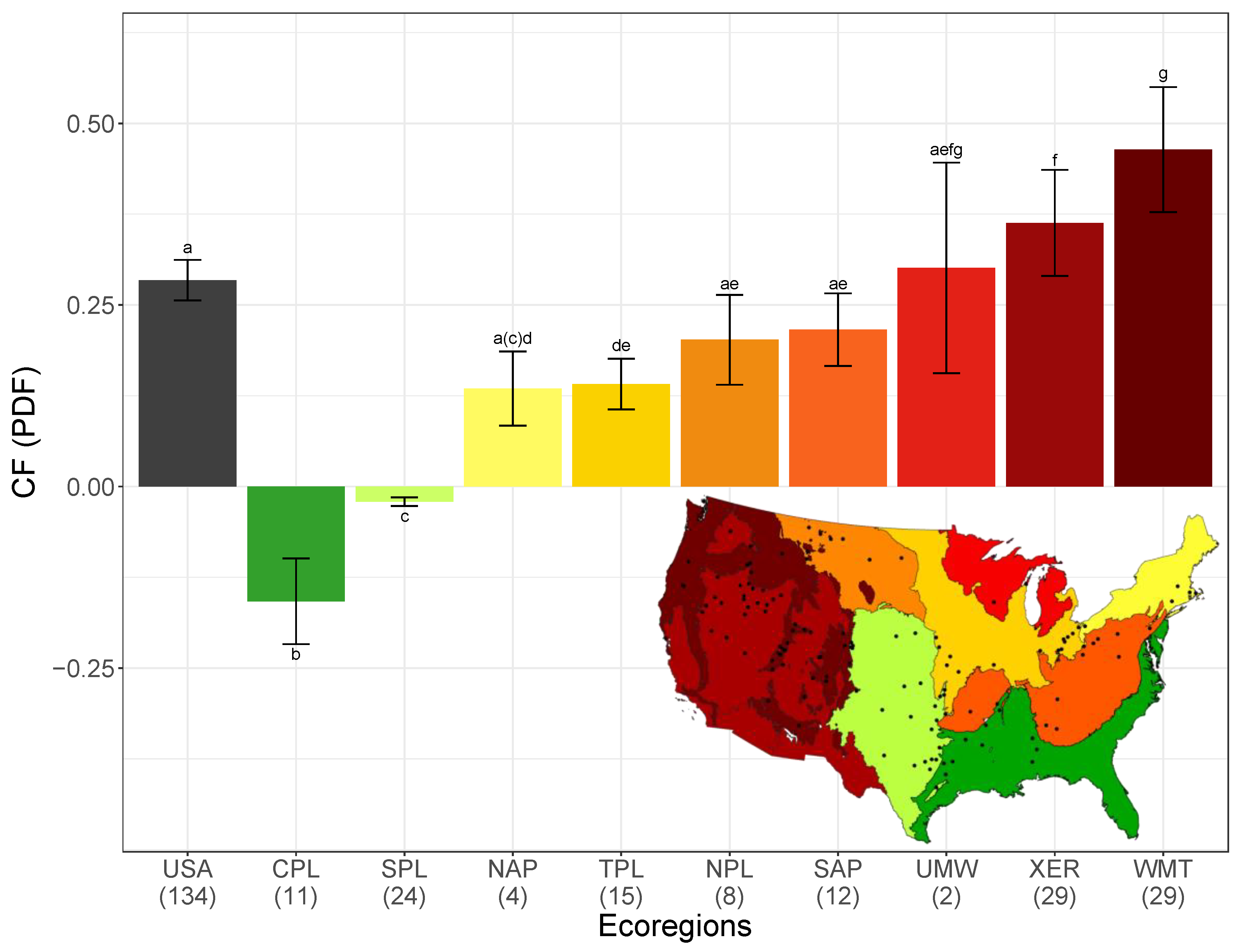
| Matrix | Variable | Definition | Units | Type |
|---|---|---|---|---|
| Spatial | Latitude | Latitude of reservoir | Decimal degrees | N |
| Longitude | Longitude of reservoir | Decimal degrees | N | |
| Ecoregion | National Aquatic Resource Surveys (NARS) 9-level reporting regions, based on aggregated Omernik [52] level III ecoregions | - | F | |
| Temperature | Annual mean air temperature, specific to ecoregion | °C | N | |
| Precipitation | Annual mean precipitations, specific to ecoregion | mm | N | |
| Forested | Percentage of land cover in ecoregion that is forested | % | N | |
| Cultivated pasture | Percentage of land cover in ecoregion that is cultivated pastures | % | N | |
| Wetlands | Percentage of land cover in ecoregion that is wetlands | % | N | |
| Grassland and shrubs | Percentage of land cover in ecoregion that is grasslands and shrubs | % | N | |
| Developed | Percentage of land cover in ecoregion that is developed | % | N | |
| Water or barren | Percentage of land cover in ecoregion that is water or barren | % | N | |
| Physical | Area | Surface area of reservoir | ha | N |
| Elevation | Elevation reservoir coordinates | m | N | |
| Macrophytes | Index of total cover of aquatic macrophytes of reservoir | - | N | |
| Shallow water | Shallow water habitat condition indicator | - | N | |
| Riparian vegetation | Riparian vegetation condition indicator | - | N | |
| Chemical | Trophic state | Trophic state of reservoir (oligotrophic and eutrophic) | - | F |
| Secchi | Secchi depth | m | N | |
| DOC | Dissolved Organic Carbon level | mg/L | N | |
| PTL | Total Phosphorus Level | µg/L | N | |
| Color | Water color | PCU | N | |
| Conductivity | Water conductivity level | µs/cm | N | |
| NTL | Total Nitrogen Level | mg/L | N | |
| pH | pH level | pH scale | N | |
| Methylmercury | Top sediment methylmercury level | ng/L | N | |
| Chl-α | Chlorophyll-α measurement result of reservoir | µg/L | N | |
| Human | Buildings | Human influence by buildings around reservoir shoreline | - | N |
| Commercial | Human influence by commercial activities around reservoir shoreline | - | N | |
| Crops | Human influence by crops around reservoir shoreline | - | N | |
| Docks | Human influence by docks around reservoir shoreline | - | N | |
| Landfill | Human Influence by landfill around reservoir shoreline | - | N | |
| Lawn | Human influence by lawn around reservoir shoreline | - | N | |
| Park | Human influence by parks around reservoir shoreline | - | N | |
| Pasture | Human influence by pastures around reservoir shoreline | - | N | |
| Powerlines | Human influence by powerlines around reservoir shoreline | - | N | |
| Roads | Human influence by roads around reservoir shoreline | - | N | |
| Walls | Human influence by walls around reservoir shoreline | - | N | |
| Other | Human influence by other around reservoir shoreline | - | N |
| Models | Non-Significant Variables | ΔAIC | BIC |
|---|---|---|---|
| (A) PDF~bint + bELE*sqrt(ELE) + bAREA*log10(AREA) + bT.S.*T.S. + bPH*PH + bLAWN*log10(LAWN) + bROAD*log10(ROAD) | PH §, LAWN and ROAD | 2 | 27 |
| (B) PDF~bint + bELE*sqrt(ELE) + bAREA*log10(AREA) + bT.S.*T.S. + bPH*PH + bLAWN*log10(LAWN) | PH § and LAWN | 1 | 24 |
| (C) PDF~bint + bELE*sqrt(ELE) + bAREA*log10(AREA) + bT.S.*T.S. + bPH*PH | PH § | 0 | 20 |
| (D) PDF~bint + bELE*sqrt(ELE) + bAREA*log10(AREA) + bT.S.*T.S. | None | 2 | 20 |
| (E) PDF~bint + bELE*sqrt(ELE) + bAREA*log10(AREA) | None | 8 | 23 |
| (F) PDF~bint + bELE*sqrt(ELE) | None | 19 | 32 |
| (G) PDF~bint | - | 52 | 63 |
| Ecoregion or Country | Mean Nat. Riv. Richness | ±SD | n.riv | Mean Imp. Res. Richness | ±SD | PDF · m2·yr/m2·yr | ±SD | ±95% CI | n.res |
|---|---|---|---|---|---|---|---|---|---|
| USA | 35.9 | 15.5 | 2062 | 25.7 | 10.4 | 0.284 | 0.168 | 0.028 | 134 |
| CPL | 28.4 | 16.6 | 327 | 32.9 | 7.9 | −0.158 | −0.100 | −0.059 | 11 |
| SPL | 26.1 | 13.1 | 176 | 26.7 | 11.8 | −0.021 | −0.014 | −0.006 | 24 |
| NAP | 46.0 | 13.8 | 225 | 39.8 | 9.4 | 0.135 | 0.052 | 0.051 | 4 |
| TPL | 32.0 | 12.9 | 209 | 27.5 | 7.7 | 0.141 | 0.069 | 0.035 | 15 |
| NPL | 29.4 | 10.1 | 179 | 23.5 | 6.6 | 0.202 | 0.090 | 0.062 | 8 |
| SAP | 45.8 | 15.1 | 344 | 35.9 | 8.8 | 0.216 | 0.089 | 0.050 | 12 |
| UMW | 39.3 | 12.7 | 167 | 27.5 | 3.5 | 0.301 | 0.105 | 0.145 | 2 |
| XER | 31.0 | 11.6 | 213 | 19.7 | 7.9 | 0.363 | 0.199 | 0.073 | 29 |
| WMT | 39.8 | 12.0 | 222 | 21.3 | 8.6 | 0.464 | 0.235 | 0.086 | 29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trottier, G.; Turgeon, K.; Verones, F.; Boisclair, D.; Bulle, C.; Margni, M. Empirical Characterization Factors for Life Cycle Assessment of the Impacts of Reservoir Occupation on Macroinvertebrate Richness across the United States. Sustainability 2021, 13, 2701. https://doi.org/10.3390/su13052701
Trottier G, Turgeon K, Verones F, Boisclair D, Bulle C, Margni M. Empirical Characterization Factors for Life Cycle Assessment of the Impacts of Reservoir Occupation on Macroinvertebrate Richness across the United States. Sustainability. 2021; 13(5):2701. https://doi.org/10.3390/su13052701
Chicago/Turabian StyleTrottier, Gabrielle, Katrine Turgeon, Francesca Verones, Daniel Boisclair, Cécile Bulle, and Manuele Margni. 2021. "Empirical Characterization Factors for Life Cycle Assessment of the Impacts of Reservoir Occupation on Macroinvertebrate Richness across the United States" Sustainability 13, no. 5: 2701. https://doi.org/10.3390/su13052701





