Quantitative Dissection of Salt Tolerance for Sustainable Wheat Production in Sodic Agro-Ecosystems through Farmers’ Participatory Approach: An Indian Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Locations
2.2. Delineation of Soil Sodicity, Nutrient Status and Irrigation Water Quality
2.3. Experimental Procedure and Treatment Details
2.4. Assessment of Physiological Parameters and Yield-Related Traits (FPT–I)
2.5. Farmers’ Preference for Crop Variety Traits
2.6. Statistical Analysis
3. Results
3.1. Morpho-Physiological Adaptation to Sodicity Stress
3.1.1. Plant Water Relations and Membrane Stability
3.1.2. Gas Exchange Parameters
3.1.3. Biochemical and Ionic Balances
3.1.4. Morphometric Observations (FPT–I)
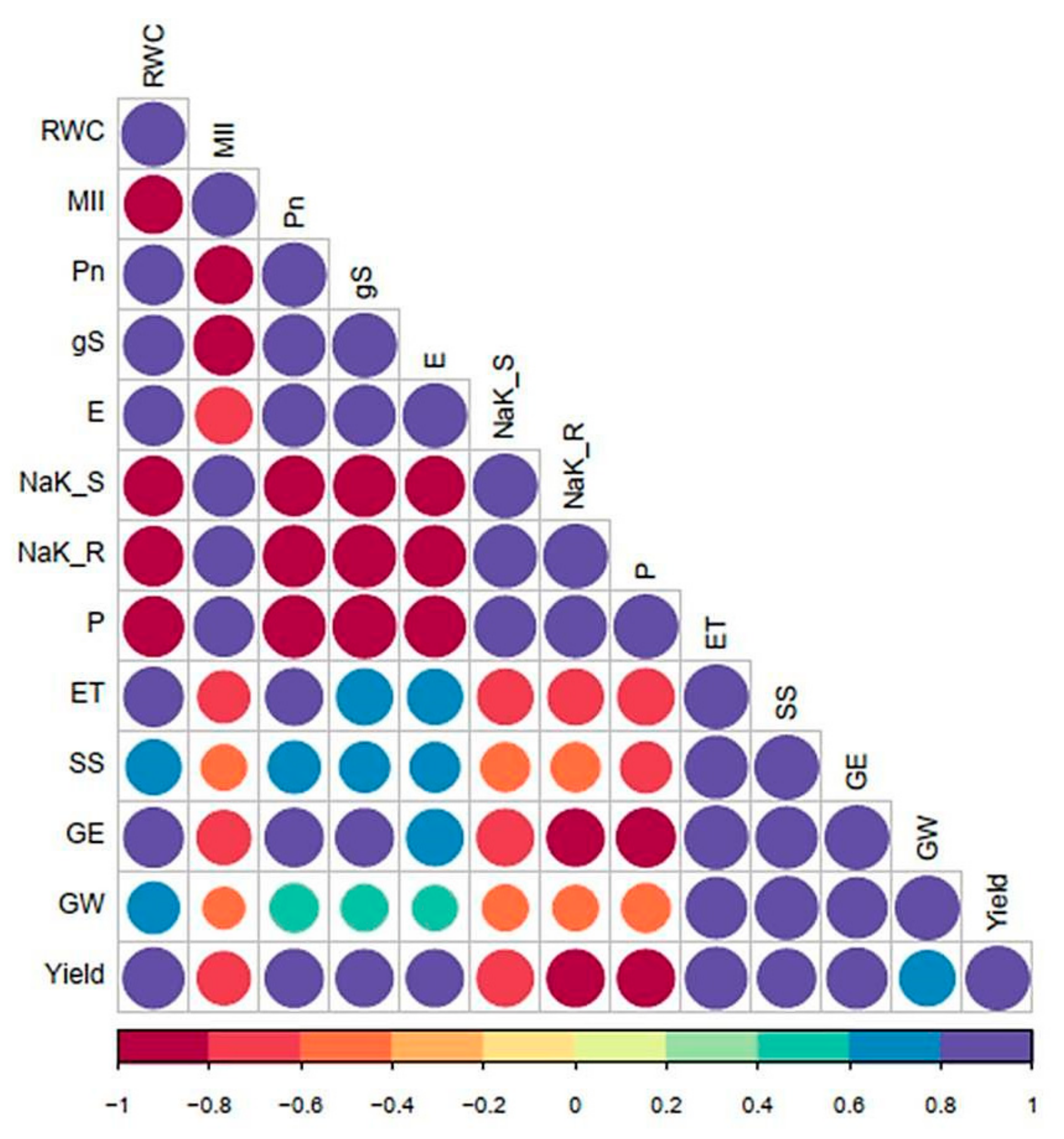
3.2. Correlation Studies
3.3. Farmers’ Preference and Trait Analysis
3.4. Dissection of Salt Tolerance and Yield Assessments (FPT–II)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ait-Mouheb, N.; Bahri, A.; Thayer, B.B.; Benyahia, B.; Bourrie, G.; Cherki, B.; Kitir, N. The reuse of reclaimed water for irrigation around the Mediterranean Rim: A step towards a more virtuous cycle? Reg. Environ. Chang. 2018, 18, 693–705. [Google Scholar] [CrossRef]
- Angelakιs, A.N.; Zaccaria, D.; Krasilnikoff, J.; Salgot, M.; Bazza, M.; Roccaro, P.; Jimenez, B.; Kumar, A.; Yinghua, W.; Baba, A.; et al. Irrigation of World Agricultural Lands: Evolution through the Millennia. Water 2020, 12, 1285. [Google Scholar] [CrossRef]
- Jury, W.A.; Vaux, H.J., Jr. The emerging global water crisis: Managing scarcity and conflict between water users. Adv. Agron. 2007, 95, 1–76. [Google Scholar]
- Qadir, M.; Sharma, B.R.; Bruggeman, A.; Choukr-Allah, R.; Karajeh, F. Non-conventional water resources and opportunities for water augmentation to achieve food security in water scarce countries. Agric. Water Manag. 2007, 87, 2–22. [Google Scholar] [CrossRef]
- Shani, U.; Dudley, L.M. Field studies of crop response to water and salt stress. Soil Sci. Soc. Am. J. 2001, 65, 1522–1528. [Google Scholar] [CrossRef]
- Minhas, P.S.; Qadir, M.; Yadav, R.K. Groundwater irrigation induced soil sodification and response options. Agric. Water Manag. 2019, 215, 74–85. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Sharma, D.K.; Thimmappa, K.; Chinchmalatpure, A.R.; Mandal, A.K.; Yadav, R.K.; Chaudhari, S.K.; Kumar, S.; Sikka, A.K. Assessment of Production and Monetary Losses from Salt Affected Soils in India; Technical Bulletin; ICAR-CSSRI: Karnal, India, 2015; pp. 1–99. [Google Scholar]
- Singh, Y.P.; Mishra, V.K.; Singh, S.; Sharma, D.K.; Singh, D.; Singh, U.S.; Singh, R.K.; Haefele, S.M.; Ismail, A.M. Productivity of sodic soils can be enhanced through the use of salt tolerant rice varieties and proper agronomic practices. Field Crops Res. 2016, 190, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Minhas, P.S.; Bali, A.; Bhardwaj, A.K.; Singh, A.; Yadav, R.K. Structural stability and hydraulic characteristics of soils irrigated for two decades with water having residual alkalinity and its neutralization with gypsum and sulphuric acid. Agric. Water Manag. 2021, 244, 106609. [Google Scholar] [CrossRef]
- Sheoran, P.; Basak, N.; Kumar, A.; Yadav, R.K.; Singh, R.; Sharma, R.; Kumar, S.; Singh, R.K.; Sharma, P.C. Ameliorants and salt tolerant varieties improve rice-wheat production in soils undergoing sodification with alkali water irrigation in Indo–Gangetic Plains of India. Agric. Water Manag. 2021, 243, 106492. [Google Scholar] [CrossRef]
- Choudhary, O.P.; Ghuman, B.S.; Thuy, N.; Buresh, R.J. Effects of long-term use of sodic water irrigation, amendments and crop residues on soil properties and crop yields in rice–wheat cropping system in a calcareous soil. Field Crops Res. 2011, 121, 363–372. [Google Scholar] [CrossRef]
- Murtaza, G.; Ghafoor, A.; Qadir, M. Irrigation and soil management strategies for using saline-sodic water in a cotton–wheat rotation. Agric. Water Manag. 2006, 81, 98–114. [Google Scholar] [CrossRef]
- Bagarello, V.; Iovino, M.; Palazzolo, E.; Panno, M.; Reynolds, W.D. Field and laboratory approaches for determining sodicity effects on saturated soil hydraulic conductivity. Geoderma 2006, 130, 1–13. [Google Scholar] [CrossRef]
- Shaw, R.J.; Coughlin, K.J.; Bell, L.C. Root zone sodicity. In Distribution, Management and Environmental Consequences; Oxford University Press: New York, NY, USA, 1998; pp. 95–106. [Google Scholar]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.K.; Singh, A.; Sharma, P.C.; Dagar, J.C.; Chaudhari, S.K. Sustainable management of sodic soils for crop production: Opportunities and challenges. J. Soil Salin. Water Qual. 2016, 8, 109–130. [Google Scholar]
- Machado, R.; Serralheiro, R. Soil Salinity: Effect on vegetable crop growth management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Qadir, M.; Oster, J.D. Crop and irrigation management strategies for saline-sodic soils and waters aimed at environmentally sustainable agriculture. Sci. Total Environ. 2004, 323, 1–19. [Google Scholar] [CrossRef]
- Sheoran, P.; Kumar, A.; Kumar, A.; Raju, R.; Sharma, R.; Parjapat, K.; Barman, A.; Singh, R.K.; Kumar, S.; Sharma, P.C.; et al. Impact of pressmud application in reclamation of high RSC irrigation water induced soil sodification and sustaining rice (Oryza sativa)–wheat (Triticum aestivum) production in Indo-Gangetic Plains. Indian J. Agric. Sci. 2020, 90, 206–211. [Google Scholar]
- Hammer, G.L.; Broad, I.J. Genotype and environment effects on dynamics of harvest index during grain filling sorghum. Agron. J. 2003, 95, 199–206. [Google Scholar] [CrossRef]
- Ismail, A.M.; Horie, M. Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Ann. Rev. Plant Biol. 2017, 68, 405–434. [Google Scholar] [CrossRef]
- Rhoades, J.D. Intercepting, isolating and reusing drainage water for irrigation to conserve water and protect water quality. Agric. Water Manag. 1989, 16, 37–52. [Google Scholar] [CrossRef]
- FAO. Characterization of Small Farmers in Asia and the Pacific. Asia and Pacific Commission on Agricultural Statistics: Twenty Third Sessions. Siem Reap, Cambodia. 2010. Available online: http://www.fao.org/fileadmin/templates/ess/documents/meetings_and_workshops/APCAS23/documents_OCT10/APCAS-10-28_-Small_farmers.pdf (accessed on 15 March 2020).
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils. USDA Handbook No. 60; Oxford & IBH Publishing Co.: New Delhi, India, 1954. [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis, Part-2, 2nd ed.; Soil Science Society of America: Madison, WI, USA, 1982. [Google Scholar]
- Subbiah, B.V.; Asija, G.L. A rapid procedure for the determination of available nitrogen in soils. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Olsen, S.R.; Col, C.W.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA: Washington, DC, USA, 1954. [Google Scholar]
- Hanway, J.J.; Heidel, H. Soil analysis methods as used in Iowa State College, Soil Testing Laboratory. Iowa Agric. 1952, 54, 1–31. [Google Scholar]
- Mandal, A.K. Mapping and characterization of salt-affected and waterlogged soils in the Gangetic plain of central Haryana (India) for reclamation and management. Cogent Geosci. 2016, 2, 1213689. [Google Scholar] [CrossRef]
- Minhas, P.S.; Gupta, R.K. Quality of Irrigation Water: Assessment and Management; Indian Council of Agricultural Research: New Delhi, India, 1992; p. 123. [Google Scholar]
- Weatherley, P.E. Studies in the water relations of the cotton plant. I. The field measurement of water deficits in leaves. New Phytol. 1950, 49, 81–87. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Effects of salinity on sodium content and photosynthetic responses of rice seedlings differing in salt tolerance. J. Plant Physiol. 2000, 157, 54–58. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the AOAC, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Qadir, M.; Tubeileh, A.; Akhtar, J.; Larbi, A.; Minhas, P.S.; Khan, M.A. Productivity enhancement of salt-affected environments through crop diversification. Land Degrad. Dev. 2008, 19, 429–453. [Google Scholar] [CrossRef]
- De Souza, E.R.; dos Santos Freire, M.B.G.; da Cunha, K.P.V.; do Nascimento, C.W.A.; Ruiz, H.A.; Lins, C.M.T. Biomass, anatomical changes and osmotic potential in Atriplex nummularia Lindl. cultivated in sodic saline soil under water stress. Environ. Expt. Bot. 2012, 82, 20–27. [Google Scholar] [CrossRef]
- Saqib, M.; Zörb, C.; Schubert, S. Silicon-mediated improvement in the salt resistance of wheat (Triticum aestivum) results from increased sodium exclusion and resistance to oxidative stress. Funct. Plant Biol. 2008, 35, 633–639. [Google Scholar] [CrossRef]
- Lutts, S.; Majerus, V.; Kinet, J.M. NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiol. Plant. 1999, 105, 450–458. [Google Scholar] [CrossRef]
- Maas, E.V.; Hoffman, G.J.; Chaba, G.D.; Poss, J.A.; Shannon, M.C. Salt sensitivity of corn at various growth stages. Irrig. Sci. 1983, 4, 45–57. [Google Scholar] [CrossRef]
- Koyama, M.L.; Levesley, A.; Koebner, R.M.; Flowers, T.J.; Yeo, A.R. Quantitative trait loci for component physiological traits determining salt tolerance in rice. Plant Physiol. 2001, 125, 406–422. [Google Scholar] [CrossRef]
- Hussain, S.; Zhang, J.H.; Zhong, C.; Zhu, L.F.; Cao, X.C.; Yu, S.M.; James, A.B.; Hu, J.J.; Jin, Q.Y. Effects of salt stress on rice growth, development characteristics, and the regulating ways: A review. J. Integr. Agric. 2017, 16, 2357–2374. [Google Scholar] [CrossRef]
- Qadir, M.; Oster, J.D. Vegetative bioremediation of calcareous sodic soils: History, mechanisms, and evaluation. Irrig. Sci. 2002, 21, 91–101. [Google Scholar]
- Maas, E.V. Plant growth response to salt stress. In Towards the Rational Use of High Salinity Tolerant Plants; Springer: Dordrecht, The Netherlands, 1993; pp. 279–291. [Google Scholar]
- Van Straten, G.; de Vos, A.C.; Rozema, J.; Bruning, B.; van Bodegom, P.M. An improved methodology to evaluate crop salt tolerance from field trials. Agric. Water Manag. 2019, 213, 375–387. [Google Scholar] [CrossRef]
- Vysotskaya, L.; Hedley, P.E.; Sharipova, G.; Veselov, D.; Kudoyarova, G.; Morris, J.; Jones, H.G. Effect of salinity on water relations of wild barley plants differing in salt tolerance. Acta Plants 2010, plq006. [Google Scholar] [CrossRef]
- Saqib, M.; Akhtar, J.; Abbas, G.; Nasim, M. Salinity and drought interaction in wheat (Triticum aestivum L.) is affected by the genotype and plant growth stage. Acta Physiol. Plant. 2013, 35, 2761–2768. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Sudhir, P.; Murthy, S.D.S. Effects of salt stress on basic processes of photosynthesis. Photosynthetica 2004, 42, 481–486. [Google Scholar] [CrossRef]
- Zhang, M.; Smith, J.A.C.; Harberd, N.P.; Jiang, C. The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol. Biol. 2016, 91, 651–659. [Google Scholar] [CrossRef]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Plasma membrane permeability as an indicator of salt tolerance in plants. Biol. Plant. 2013, 57, 1–10. [Google Scholar] [CrossRef]
- Dadshani, S.; Sharma, R.C.; Baum, M.; Ogbonnaya, F.C.; Le’on, J.; Ballvora, A. Multidimensional evaluation of response to salt stress in wheat. PLoS ONE 2019, 14, e0222659. [Google Scholar] [CrossRef]
- El-Hendawy, S.; Hassan, W.M.; Al-Suhaibani, N.A.; Refay, Y.; Abdella, K.A. Comparative performance of multivariable agro-physiological parameters for detecting salt tolerance of wheat cultivars under simulated saline field growing conditions. Front. Plant Sci. 2017, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Chunthaburee, S.; Dongsansuk, A.; Sanitchon, J.; Pattanagul, W.; Theerakulpisut, P. Physiological and biochemical parameters for evaluation and clustering of rice cultivars differing in salt tolerance at seedling stage. Saudi J. Biol. Sci. 2016, 23, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.; Kumar, A.; Saha, M.; Lata, C.; Kumar, A. Stress induced changes in osmoprotectants, ionic relations, antioxidants activities and protein profilling characterize Sporobolus marginatus Hochst. Ex A. Rich. Salt tolerance mechanism. Indian J. Expt. Biol. 2019, 57, 672–679. [Google Scholar]
- Munns, R.; James, R.A.; Lauchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Expt. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Fatehi, F.; Coventry, S.; Rengasamy, P.; McDonald, G.K. Additive effects of Na and Cl–ions on barley growth under salinity stress. J. Exp. Bot. 2011, 62, 2189–2203. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Norouzi, F.; Poschenrieder, C. Growth, physiological, biochemical and ionic responses of pistachio seedlings to mild and high salinity. Trees 2014, 28, 1065–1078. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R.M. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.M.; Murtaza, G.; Saqib, Z.A.; Rashid, A. Growth and physiological responses of two rice varieties to applied lead in normal and salt-affected soils. Int. J. Agric. Biol. 2015, 17, 901–910. [Google Scholar] [CrossRef]
- Monneveux, P.; Rekika, D.; Acevedo, E.; Merah, O. Effect of drought on leaf gas exchange, carbon isotope discrimination, transpiration efficiency and productivity in field grown durum wheat genotypes. Plant Sci. 2006, 170, 867–872. [Google Scholar] [CrossRef]
- Liu, T.; Bruins, R.J.; Heberling, M.T. Factors influencing farmers’ adoption of best management practices: A review and synthesis. Sustainability 2018, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.; Wu, W. Soil and crop management strategies to ensure higher crop productivity within sustainable environments. Sustainability 2019, 11, 1485. [Google Scholar] [CrossRef]
- Nikam, V.R.; Singh, R.K.; Chinchmalatpure, A. Salt tolerant varieties: A biological intervention to manage saline and sodic environment and sustain livelihoods. J. Soil Salin. Water Qual. 2016, 8, 37–44. [Google Scholar]




| Varieties | Sodicity Stress * | Mean | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| SS1 | SS2 | SS3 | SS4 | SS5 | SS6 | |||
| Relative water content (RWC, %) | ||||||||
| KRL 210 | 81.3 | 80.1 | 78.6 | 76.3 | 73.6 | 71.4 | 76.8 A | S: <0.0001 V: 0.0145 S × V: 0.0327 |
| HD 2967 | 80.9 | 79.9 | 77.1 | 74.8 | 70.6 | 68.6 | 75.4 B | |
| Mean | 81.1 A | 80.0 A | 77.9 B | 75.6 B | 72.1 D | 70.0 E | ||
| Membrane injury index (MII, %) | ||||||||
| KRL 210 | 28.1 | 28.4 | 28.8 | 29.4 | 31.9 | 34.1 | 30.1 B | S: <0.0001 V: <0.0001 S × V: 0.0003 |
| HD 2967 | 29.5 | 29.6 | 30.1 | 32.7 | 36.9 | 38.3 | 32.9 A | |
| Mean | 28.8 D | 29.0 D | 29.5 D | 31.1 C | 34.4 B | 36.2 A | ||
| Photosynthetic rate (Pn, μmol CO2 m−2 s−1) | ||||||||
| KRL 210 | 18.1 | 17.9 | 17.3 | 16.2 | 15.7 | 13.7 | 16.5 A | S: <0.0001 V: 0.0009 S × V: 0.0001 |
| HD 2967 | 18.6 | 18.4 | 17.1 | 15.6 | 13.8 | 12.1 | 15.7 B | |
| Mean | 18.4 A | 18.2 A | 17.2 B | 15.9 C | 14.8 C | 12.9 D | ||
| Stomatal conductance (gS, mmol H2O m−2 s−1) | ||||||||
| KRL 210 | 1.31 | 1.26 | 1.25 | 1.14 | 1.02 | 0.83 | 1.13 A | S: <0.0001 V: <0.0001 S × V: 0.0001 |
| HD 2967 | 1.34 | 1.31 | 1.19 | 1.01 | 0.86 | 0.65 | 1.06 B | |
| Mean | 1.32 A | 1.28 A | 1.22 B | 1.08 C | 0.94 D | 0.74 E | ||
| Transpiration rate (E, mmol H2O m−2 s−1) | ||||||||
| KRL 210 | 2.62 | 2.58 | 2.42 | 2.23 | 2.02 | 1.82 | 2.28 A | S: <0.0001 V: 0.0008 S × V: 0.0054 |
| HD 2967 | 2.66 | 2.61 | 2.37 | 2.11 | 1.80 | 1.61 | 2.19 B | |
| Mean | 2.64 A | 2.59 A | 2.40 B | 2.17 C | 1.91 D | 1.72 E | ||
| Proline content (P, mg g−1) | ||||||||
| KRL 210 | 0.128 | 0.156 | 0.216 | 0.282 | 0.417 | 0.670 | 0.311 B | S: <0.0001 V: <0.0001 S × V: <0.0001 |
| HD 2967 | 0.097 | 0.143 | 0.204 | 0.312 | 0.572 | 0.856 | 0.381 A | |
| Mean | 0.113 F | 0.150 E | 0.210 D | 0.297 C | 0.494 B | 0.763 A | ||
| Shoot Na+/K+ ratio (NaK_S) | ||||||||
| KRL 210 | 0.147 | 0.158 | 0.172 | 0.194 | 0.216 | 0.269 | 0.193 B | S: <0.0001 V: <0.0001 S × V: <0.0001 |
| HD 2967 | 0.176 | 0.189 | 0.206 | 0.247 | 0.309 | 0.378 | 0.251 A | |
| Mean | 0.161 E | 0.173 DE | 0.189 D | 0.221 | 0.263 B | 0.324 A | ||
| Root Na+/K+ ratio (NaK_R) | ||||||||
| KRL 210 | 0.306 | 0.341 | 0.373 | 0.431 | 0.502 | 0.589 | 0.425 B | S: <0.0001 V: <0.0001 S × V: <0.0001 |
| HD 2967 | 0.359 | 0.402 | 0.446 | 0.527 | 0.632 | 0.766 | 0.523 A | |
| Mean | 0.332 F | 0.371 E | 0.410 D | 0.479 C | 0.567 B | 0.677 A | ||
| Varieties | Sodicity Stress * | Mean | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| SS1 | SS2 | SS3 | SS4 | SS5 | SS6 | |||
| Effective tillers (No. mrl−1) | ||||||||
| KRL 210 | 72.8 | 70.6 | 68.7 | 64.5 | 62.4 | 58.1 | 66.2 A | S: <0.0001 V: 0.0098 S × V: 0.8715 |
| HD 2967 | 71.9 | 69.6 | 66.4 | 62.1 | 57.9 | 54.7 | 63.7 B | |
| Mean | 72.3 A | 70.0 A B | 67.5 B | 63.3 C | 60.1 C | 56.4 D | ||
| Spikelets spike−1 | ||||||||
| KRL 210 | 19.4 | 19.5 | 18.9 | 18.4 | 17.5 | 17.1 | 18.5 | S: <0.0001 V: 0.4517 S × V: 0.6612 |
| HD 2967 | 19.8 | 20.1 | 18.4 | 17.9 | 16.8 | 16.6 | 18.3 | |
| Mean | 19.6 A | 19.8 A | 18.6 B | 18.2 B C | 17.2 C D | 16.9 D | ||
| Grains earhead−1 | ||||||||
| KRL 210 | 65.1 | 64.8 | 62.5 | 59.8 | 53.8 | 47.4 | 58.9 A | S: <0.0001 V: 0.0061 S × V: 0.9188 |
| HD 2967 | 63.8 | 63.6 | 58.4 | 56.2 | 50.7 | 44.6 | 56.2 B | |
| Mean | 64.5 A | 64.2 A | 60.5 B | 58.0 C | 52.3 D | 46.0 E | ||
| 1000-grain weight (g) | ||||||||
| KRL 210 | 42.4 | 42.9 | 41.6 | 40.7 | 39.8 | 38.1 | 40.9 | S: <0.0001 V: 0.0670 S × V: 0.6996 |
| HD 2967 | 42.6 | 42.8 | 41.1 | 40.3 | 39.3 | 37.3 | 40.6 | |
| Mean | 42.5 A | 42.9 A | 41.3 B | 40.5 C | 39.5 D | 37.7 E | ||
| Grain yield (t ha−1) | ||||||||
| KRL 210 | 4.61 | 4.44 | 4.22 | 3.98 | 3.78 | 3.53 | 4.09 B | S: <0.0001 V: <0.0001 S × V: <0.0001 |
| HD 2967 | 4.66 | 4.29 | 4.05 | 3.72 | 3.48 | 3.25 | 3.91 A | |
| Mean | 4.63 F | 4.37 E | 4.14 D | 3.85 C | 3.63 B | 3.39 A | ||
| Variables | Preference Score * (Mean ± SE) | |
|---|---|---|
| KRL 210 | HD 2967 | |
| Biomass production | 4.28 ± 0.45 | 4.40 ± 0.50 |
| Tillering capacity | 3.58 ± 0.50 | 3.35 ± 0.48 |
| Sodicity tolerance | 4.15 ± 0.39 | 3.50 ± 0.51 |
| Lodging score | 4.78 ± 0.42 | 4.08 ± 0.35 |
| Disease resistance | 4.13 ± 0.61 | 4.18 ± 0.71 |
| Grain boldness | 3.78 ± 0.59 | 3.98 ± 0.35 |
| Grain quality | 4.35 ± 0.48 | 4.13 ± 0.33 |
| Maturity duration | 4.73 ± 0.45 | 4.45 ± 0.50 |
| Profitability | 3.75 ± 0.48 | 3.63 ± 0.49 |
| Grain yield (t ha−1) | 4.09 ± 0.46 | 3.91 ± 0.55 |
| Preference score | 0.20 | 0.17 |
| Variety | Equation | R2 Value | Per Unit Reduction in Yield at 95% CI | Validation Limits |
|---|---|---|---|---|
| Soil pH | ||||
| KRL 210 | Y = −0.939X + 12.467 | 0.93 | 0.77–1.10 t ha−1 | 7.8–9.3 |
| HD 2967 | Y = −1.446X + 16.768 | 0.98 | 1.30–1.59 t ha−1 | |
| Irrigation water residual alkalinity (RSCiw) | ||||
| KRL 210 | Y = −0.3095X + 5.737 | 0.98 | 0.29–0.33 t ha−1 | 1.7–7.5 |
| HD 2967 | Y = −0.4103X + 6.008 | 0.97 | 0.37–0.48 t ha−1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheoran, P.; Kumar, A.; Sharma, R.; Prajapat, K.; Kumar, A.; Barman, A.; Raju, R.; Kumar, S.; Dar, Y.J.; Singh, R.K.; et al. Quantitative Dissection of Salt Tolerance for Sustainable Wheat Production in Sodic Agro-Ecosystems through Farmers’ Participatory Approach: An Indian Experience. Sustainability 2021, 13, 3378. https://doi.org/10.3390/su13063378
Sheoran P, Kumar A, Sharma R, Prajapat K, Kumar A, Barman A, Raju R, Kumar S, Dar YJ, Singh RK, et al. Quantitative Dissection of Salt Tolerance for Sustainable Wheat Production in Sodic Agro-Ecosystems through Farmers’ Participatory Approach: An Indian Experience. Sustainability. 2021; 13(6):3378. https://doi.org/10.3390/su13063378
Chicago/Turabian StyleSheoran, Parvender, Arvind Kumar, Raman Sharma, Kailash Prajapat, Ashwani Kumar, Arijit Barman, R. Raju, Satyendra Kumar, Yousuf Jaffer Dar, Ranjay K. Singh, and et al. 2021. "Quantitative Dissection of Salt Tolerance for Sustainable Wheat Production in Sodic Agro-Ecosystems through Farmers’ Participatory Approach: An Indian Experience" Sustainability 13, no. 6: 3378. https://doi.org/10.3390/su13063378
APA StyleSheoran, P., Kumar, A., Sharma, R., Prajapat, K., Kumar, A., Barman, A., Raju, R., Kumar, S., Dar, Y. J., Singh, R. K., Sanwal, S. K., Yadav, R. K., Chahal, V. P., & Sharma, P. C. (2021). Quantitative Dissection of Salt Tolerance for Sustainable Wheat Production in Sodic Agro-Ecosystems through Farmers’ Participatory Approach: An Indian Experience. Sustainability, 13(6), 3378. https://doi.org/10.3390/su13063378







