Developing a Thermally Stable Ester-Based Drilling Fluid for Offshore Drilling Operations by Using Aluminum Oxide Nanorods
Abstract
1. Introduction
1.1. Ester-Based Drilling Fluids
1.2. Nano-Enhanced Drilling Fluids
2. Methodology
2.1. Materials
2.2. Apparatus
2.3. Experimental Procedure
2.4. Mathematical Theory
2.5. Coding
3. Results and Discussion
3.1. Visual Inspection for the Invert Emulsion (EBDF) Stability
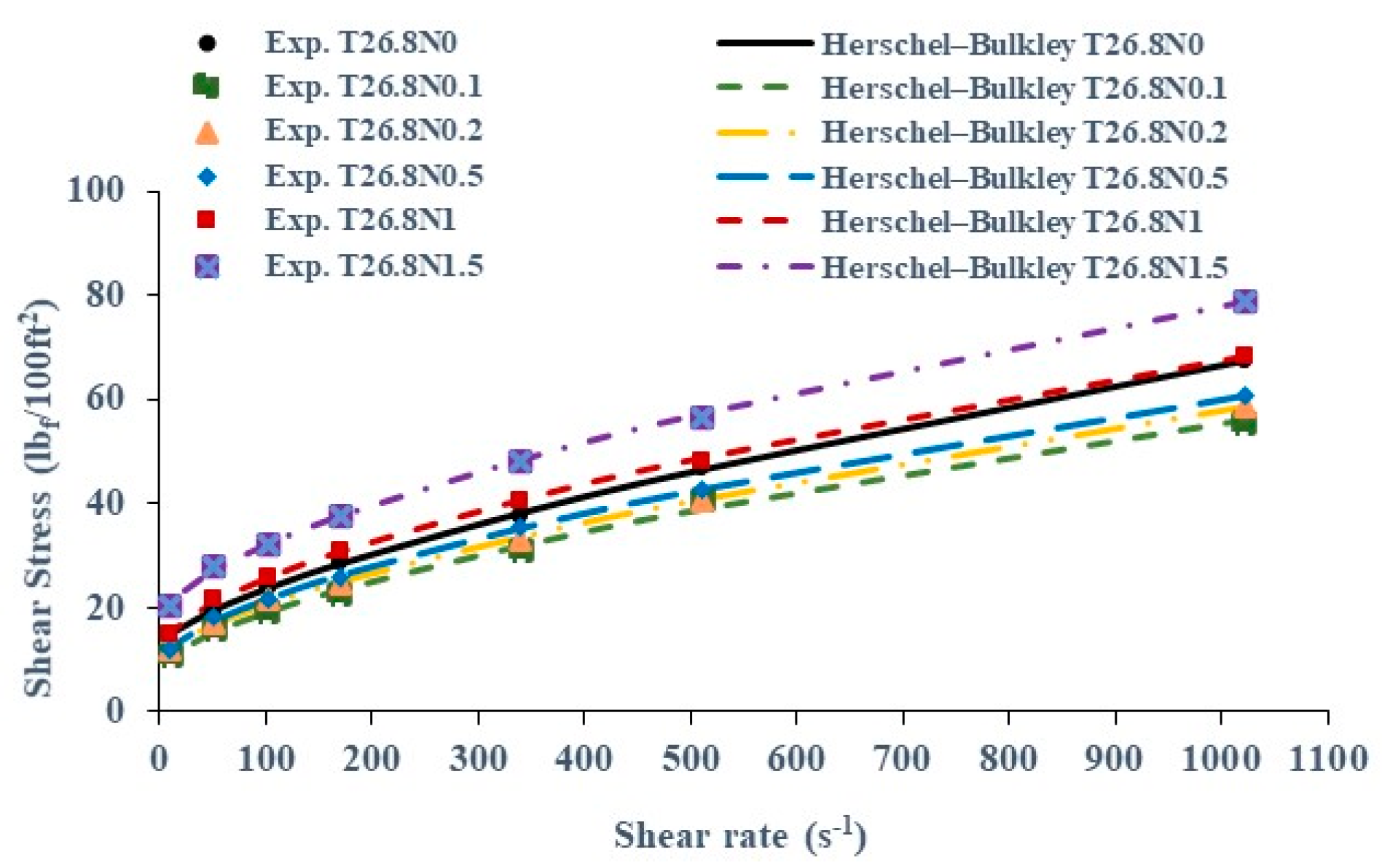
3.2. Rheological Properties in NPNT Conditions
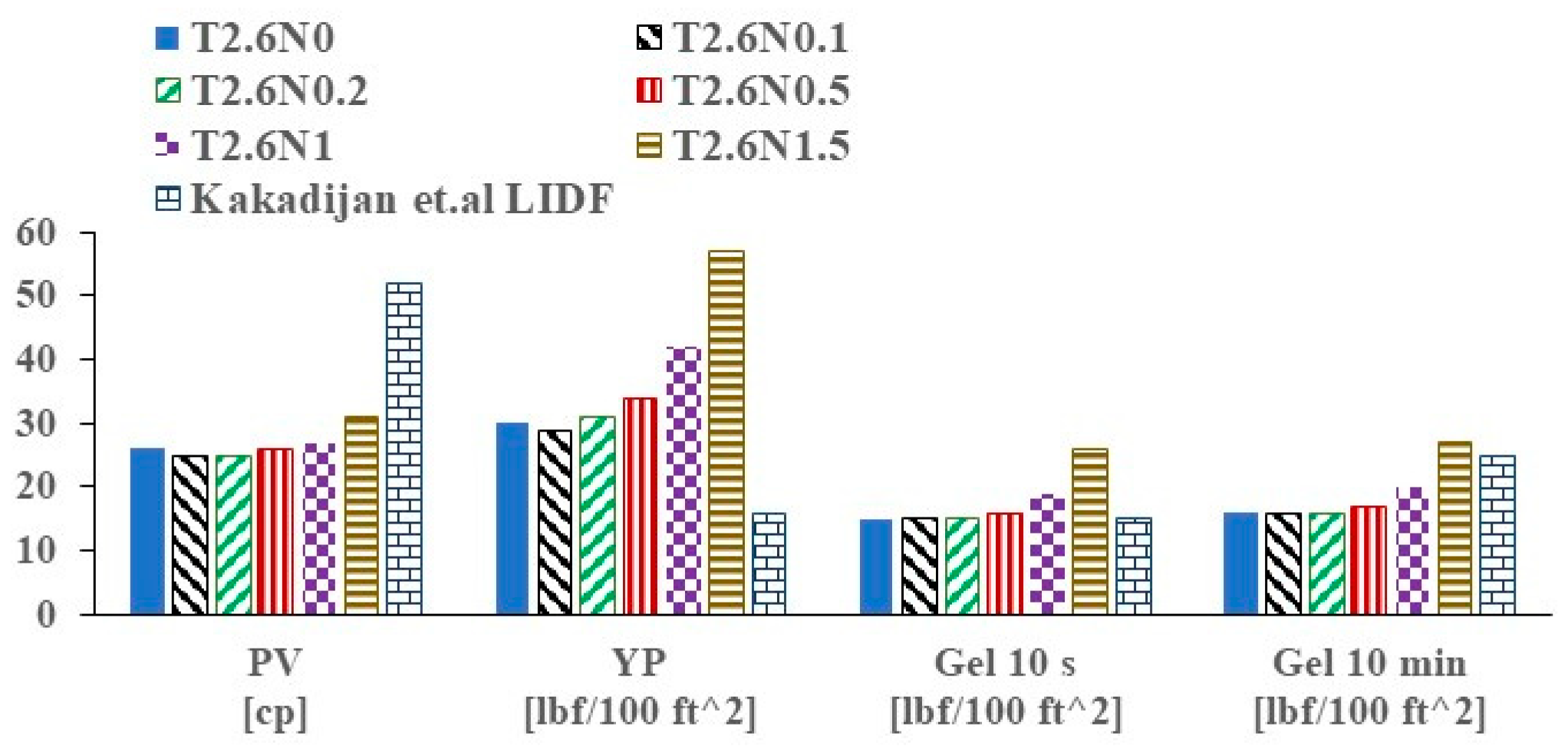
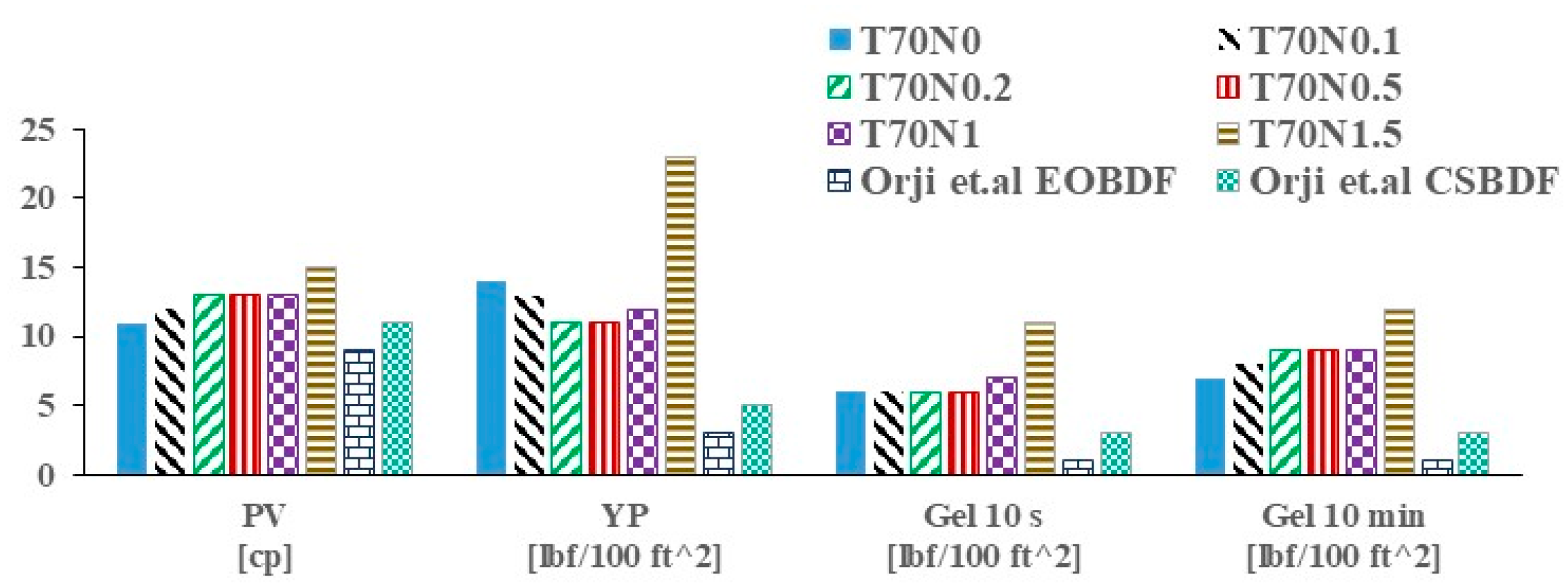
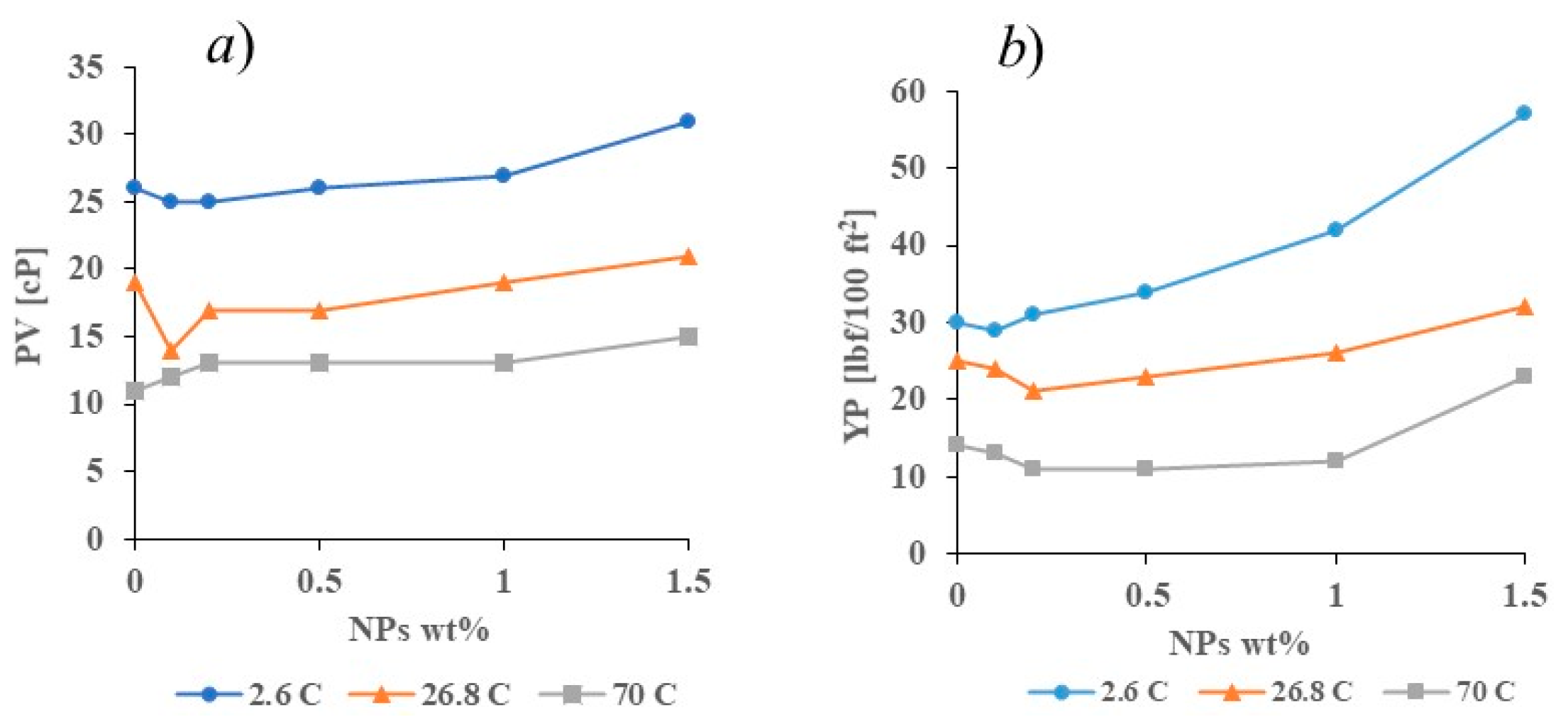
3.3. Rheological Properties in Simulated Deepwater Conditions (2.6 °C) and an Elevated Temperature (70 °C)
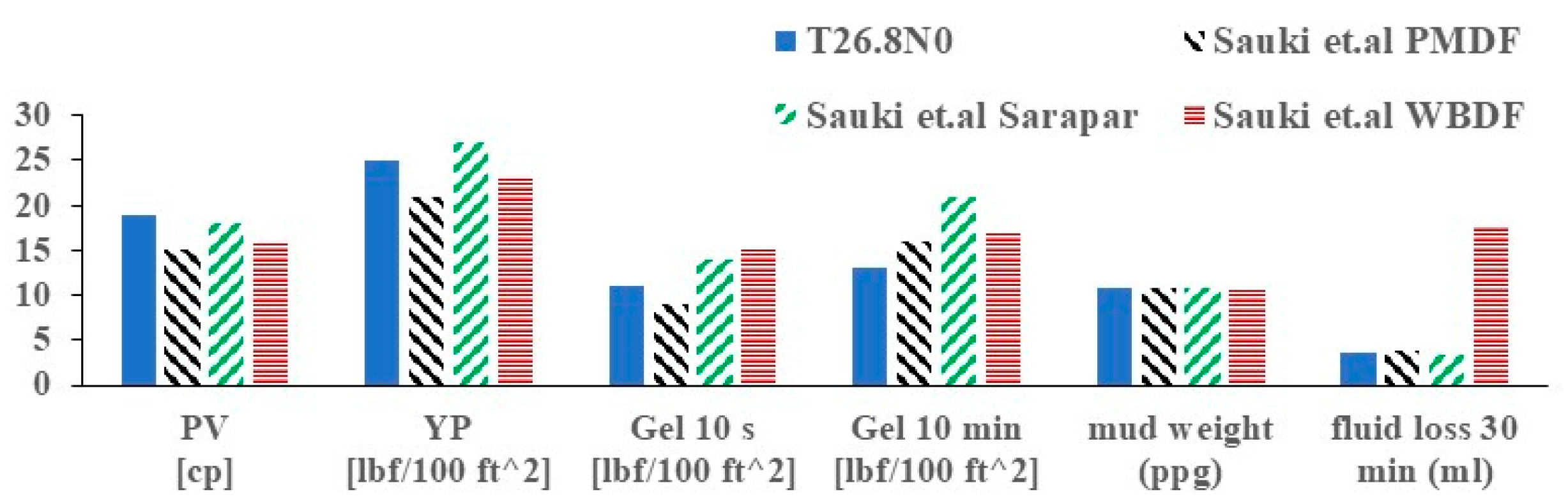
3.4. Filtration Loss at NPNT
4. Conclusions and Recommendations
- Comparison of the performance of aluminum oxide nanoparticles with other types of nanoparticles that are compatible with esters, such as MWCNT nanoparticles.
- Conducting experiments using different esters (preferably, branched-chain ones) to compare and determine the preeminent ester as a base fluid, depending on the drilling operations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| API | American Petroleum Institute |
| AV | Apparent viscosity, centipoise (cP) |
| EBDF | Ester-based drilling fluid |
| ECD | Equivalent circulating density |
| Gel 10 min | Gel strength after 10 min, lbf/100ft2 |
| Gel 10 s | Gel strength after 10 s, lbf/100ft2 |
| HPHT | High pressure and high temperature |
| NPNT | Normal pressure and normal temperature; this means that a measurement is performed at ambient temperature and under 100 psi differential pressure; also refers to the API Filter Press standard condition |
| LT | Low temperature conditions, representing deepwater conditions |
| NPs | Nanoparticles |
| OBDF | Oil-based drilling fluid |
| PAC | Polyanionic cellulose |
| ppb | Pounds per barrel |
| ppg | Pounds per gallon |
| PV | Plastic viscosity, centipoise (cP) |
| ROP | Rate of penetration |
| RPM | Revolutions per minute |
| WBDF | Water-based drilling fluid |
| wt% | Concentration by weight |
| XG | Xanthan gum |
| YPBP | Yield Point (Bingham Plastic model), lbf/100ft2 |
| γ | Shear rate, s−1 |
| Shear stress, lbf/100ft2 |
References
- Razali, S.; Yunus, R.; Rashid, S.A.; Lim, H.; Jan, B.M. Review of biodegradable synthetic-based drilling fluid: Progression, performance and future prospect. Renew. Sustain. Energy Rev. 2018, 90, 171–186. [Google Scholar] [CrossRef]
- Knothe, G.H. Chapter 2: Biodiesel and its properties. In Industrial Oil Crops, 1st ed.; McKeon, T.A., Hayes, D.G., Hildebrand, D.F., Weselake, R.J., Eds.; AOCS Press: Urbana, IL, USA, 2016; pp. 15–42. [Google Scholar]
- Lyons, W.C.; Plisga, G.J.; Lorenz, M.D. Standard Handbook of Petroleum and Natural Gas Engineering; Chapter 4—Drilling and Well Completions; Gulf Professional Publishing: Woburn, MA, USA, 2015; pp. 4-1–4-584. [Google Scholar]
- Burrows, K.; Evans, J.; Hall, J.; Kirsner, J. New low viscosity ester is suitable for drilling fluids in deepwater applications. In Proceedings of the SPE/EPA/DOE Exploration and Production Environmental Conference, San Antonio, TX, USA, 26–28 February 2001. [Google Scholar]
- Eckhout, D.; Dolan, S.; Gogan, R.; Ledgister, H.; Mowat, C.; Tipton, P.; Dye, W. Development process and field applications of a new ester-based mud system for ERD wells on Australia’s northwest shelf. In Proceedings of the ADC/SPE Asia Pacific Drilling Technology, Kuala Lumpur, Malaysia, 11–13 September 2000. [Google Scholar]
- Rudnick, L. Chapter 3: Esters-Synthetics, Mineral Oils, and Bio-Based Lubricants: Chemistry and Technology; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2013; pp. 47–63. [Google Scholar]
- Ismail, A.; Kamis, A.; Foo, K. Performance of the mineral blended ester oil-based drilling fluid systems. In Proceedings of the Canadian International Petroleum Conference, Calgary, AB, Canada, 12–14 June 2001. [Google Scholar]
- Mueller, H.; Herold, C.P.; Von Tapavicza, S.; Grimes, D.J.; Braun, J.M.; Smith, S.P. Use of Selected Ester Oils in Drilling Fluids and Muds. U.S. Patent 5232910A, 1999. [Google Scholar]
- Imevbore, V.O.; Nwankwo, J.N.; Ifeadi, C.N.; Ladan, M.D. Laboratory assessment of biodegradation of non soluble drilling mud base fluids under Nigerian environmental conditions. In Proceedings of the SPE International Conference on Health, Safety and Environment in Oil and Gas Exploration and Production, Stavanger, Norway, 26–28 June 2000. [Google Scholar]
- Neff, J.; Mckelvie, S.; Ayers, R.C., Jr. Environmental Impacts of Synthetic Based Drilling Fluids; U.S. Department of the Interior Minerals Management Service Gulf of Mexico OCS Region: Washington, DC, USA, 2000; pp. 30–52.
- Steber, J.; Herold, C.-P.; Limia, J. Comparative evaluation ofanaerobic biodegradability of hydrocarbons and fatty derivatives currently used as drilling fluids. Chemosphere 1995, 31, 3105–3118. [Google Scholar] [CrossRef]
- Cordes, E.E.; Jones, D.O.B.; Schlacher, T.A.; Amon, D.J.; Bernardino, A.F.; Brooke, S.; Carney, R.; DeLeo, D.M.; Dunlop, K.M.; Escobar-Briones, E.G.; et al. Environmental Impacts of the Deep-Water Oil and Gas Industry: A Review to Guide Management Strategies. Front. Environ. Sci. 2016, 4. [Google Scholar] [CrossRef]
- Tornero, V.; Hanke, G. Chemical contaminants entering the marine environment from sea-based sources: A review with a focus on European seas. Mar. Pollut. Bull. 2016, 112, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Ellice, M.; Helmy, S.; Shumate, T. Base Oil for Well-Bore Fluids. WO1996022342, 25 July 1996. [Google Scholar]
- Sauki, A.; Shah, M.S.Z.; Bakar, W.Z.W. Application of Ester based drilling fluid for shale gas drilling. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2015; Volume 83, p. 012012. [Google Scholar]
- Said, M.M.; El-Sayed, A.A.H. The use of palm oil fatty acid methyl ester as a base fluid for a flat rheology high-performance drilling fluid. J. Petroleum Sci. Eng. 2018, 166, 969–983. [Google Scholar] [CrossRef]
- Orji, I.; Ibezim-Ezeani, M.U.; Akaranta, O. Evaluation of C10 Esters as Synthetic Base Fluids for Drilling Mud Formulation. IOSR J. Appl. Chem. 2016, 9, 31–38. [Google Scholar] [CrossRef]
- Ghasemi, N.; Mirzaee, M.; Aghayari, R.; Maddah, H. Investigating Created Properties of Nanoparticles Based Drilling Mud. Heat Mass Transf. 2017, 54, 1381–1393. [Google Scholar] [CrossRef]
- Boyou, N.V.; Ismail, I.; Sulaiman, W.R.W.; Haddad, A.S.; Husein, N.; Hui, H.T.; Nadaraja, K. Experimental investigation of hole cleaning in directional drilling by using nano-enhanced water-based drilling fluids. J. Pet. Sci. Eng. 2019, 176, 220–231. [Google Scholar] [CrossRef]
- Krishnan, S.; Abyat, Z.; Chok, C. Characterization of boron-based nanomaterial enhanced additive in water-based drilling fluids: A study on lubricity, drag, ROP and fluid loss improvement. In Proceedings of the SPE/IADC Middle East Drilling Technology Conference and Exhibition, Abu Dhabi, United Arab Emirates, 26 January 2016. [Google Scholar]
- Cheraghian, G. Application of Nano-Particles of Clay to Improve Drilling Fluid. Int. J. Nanosci. Nanotechnol. 2017, 13, 177–186. [Google Scholar]
- Rafati, R.; Smith, S.R.; Haddad, A.S.; Novara, R.; Hamidi, H. Effect of nanoparticles on the modifications of drilling fluids properties: A review of recent advances. J. Pet. Sci. Eng. 2018, 161, 61–76. [Google Scholar] [CrossRef]
- Smith, S.R.; Rafati, R.; Haddad, A.S.; Cooper, A.; Hamidi, H. Application of aluminium oxide nanoparticles to enhance rheological and filtration properties of water based muds at HPHT conditions. Colloids Surfaces Physicochem. Eng. Asp. 2018, 537, 361–371. [Google Scholar] [CrossRef]
- Yu-Hua, L.; Wei, Q.; Jian-Chao, F. Temperature Dependence of Thermal Conductivity of Nanofluids. Chin. Phys. Lett. 2008, 25, 3319–3322. [Google Scholar] [CrossRef]
- Wu, J.; Ma, G.-H. Recent Studies of Pickering Emulsions: Particles Make the Difference. Small 2016, 12, 4633–4648. [Google Scholar] [CrossRef]
- Destribats, M.; Gineste, S.; Laurichesse, E.; Tanner, H.; Leal-Calderon, F.; Héroguez, V.; Schmitt, V. Pickering Emulsions: What Are the Main Parameters Determining the Emulsion Type and Interfacial Properties? Langmuir 2014, 30, 9313–9326. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P.; Kirkland, M. Interfacial structure of solid-stabilised emulsions studied by scanning electron microscopy. Phys. Chem. Chem. Phys. 2002, 4, 3727–3733. [Google Scholar] [CrossRef]
- Pickering, S.U. CXCV.—The chemistry of bordeaux mixture. J. Chem. Soc. Trans. 1907, 91, 1988–2001. [Google Scholar] [CrossRef][Green Version]
- Khan, A.Y.; Talegaonkar, S.; Iqbal, Z.; Ahmed, F.J.; Khar, R.K. Multiple Emulsions: An Overview. Curr. Drug Deliv. 2006, 3, 429–443. [Google Scholar] [CrossRef]
- Katende, A.; Boyou, N.V.; Ismail, I.; Chung, D.Z.; Sagala, F.; Hussein, N.; Ismail, M.S. Improving the performance of oil based mud and water based mud in a high temperature hole using nanosilica nanoparticles. Colloids Surfaces Physicochem. Eng. Asp. 2019, 577, 645–673. [Google Scholar] [CrossRef]
- Luo, D.; Wang, F.; Chen, J.; Zhang, F.; Yu, L.; Wang, D.; Willson, R.C.; Yang, Z.; Ren, Z. Poly(sodium 4-styrenesulfonate) Stabilized Janus Nanosheets in Brine with Retained Amphiphilicity. Langmuir 2018, 34, 3694–3700. [Google Scholar] [CrossRef]
- Li, M.C.; Wu, Q.; Song, K.; French, A.D.; Mei, C.; Lei, T. pH-responsive water-based drilling fluids containing bentonite and chitin nanocrystals. ACS Sustain. Chem. Eng. 2018, 6, 3783–3795. [Google Scholar] [CrossRef]
- Wang, N.; Hsu, C.; Zhu, L.; Tseng, S.; Hsu, J.-P. Influence of metal oxide nanoparticles concentration on their zeta potential. J. Colloid Interface Sci. 2013, 407, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.; Kumar, P.; Choudhary, P.; Sharma, S. Effect of high temperature ageing on TiO2 nanoparticles enhanced drilling fluids: A rheological and filtration study. Upstream Oil Gas Technol. 2020, 5, 100019. [Google Scholar] [CrossRef]
- Cheraghian, G.; Hemmati, M.; Bazgir, S. Application of TiO2 and fumed silica nanoparticles and improve the performance of drilling fluids. AIP Conf. Proc. 2014, 1590, 266–270. [Google Scholar] [CrossRef]
- Heister, E.; Lamprecht, C.; Neves, V.; Tîlmaciu, C.; Datas, L.; Flahaut, E.; Soula, B.; Hinterdorfer, P.; Coley, H.M.; Silva, S.R.P.; et al. Higher Dispersion Efficacy of Functionalized Carbon Nanotubes in Chemical and Biological Environments. ACS Nano 2010, 4, 2615–2626. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.R.; Rashid, N.M.; Jaafar, M.Z.; Sulaiman, W.R.W.; Buang, N.A. Effect of Nanomaterial on the Rheology of Drilling Fluids. J. Appl. Sci. 2014, 14, 1192–1197. [Google Scholar] [CrossRef]
- Vryzas, Z.; Kelessidis, V.C. Nano-Based Drilling Fluids: A Review. Energies 2017, 10, 540. [Google Scholar] [CrossRef]
- MI SWACO. Drilling Fluids Engineering Manual; MI SWACO: Houston, TX, USA, 2001. [Google Scholar]
- Mitchell, R.F.; Miska, S.Z. Chapter 3: Drilling Fluids. Fundamentals of drilling engineering. Soc. Petroleum Eng. 2017, 12, 144. [Google Scholar]
- Caenn, R.; Darley, H.C.H.; Gray, G.R. Chapter 5—The Rheology of Drilling Fluids. In Composition and Properties of Drilling and Completion Fluids, 6th ed.; Gulf Professional Publishing: Houston, TX, USA, 2011; pp. 179–269. [Google Scholar]
- Ghosn, R.; Mihelic, F.; Hochepied, J.-F.; Dalmazzone, D. Silica Nanoparticles for the Stabilization of W/O Emulsions at HTHP Conditions for Unconventional Reserves Drilling Operations. Oil Gas Sci. Technol. Rev. IFP Energ. Nouv. 2017, 72, 21. [Google Scholar] [CrossRef]
- Kakadjian, E.; Shi, A.; Porter, J.; Yadav, P.; Clapper, D.; Pessanha, W. Low impact drilling fluid for deepwater drilling frontier. In Proceedings of the Offshore Technology Conference, Rio de Janeiro, Brasil, 30 October 2019. [Google Scholar]
- Ismail, A.; Aftab, A.; Ibupoto, Z.; Zolkifile, N. The novel approach for the enhancement of rheological properties of water-based drilling fluids by using multi-walled carbon nanotube, nanosilica and glass beads. J. Pet. Sci. Eng. 2016, 139, 264–275. [Google Scholar] [CrossRef]
- Saboori, R.; Sabbaghi, S.; Kalantariasl, A.; Mowla, D. Improvement in filtration properties of water-based drilling fluid by nanocarboxymethyl cellulose/polystyrene core–shell nanocomposite. J. Pet. Explor. Prod. Technol. 2018, 8, 445–454. [Google Scholar] [CrossRef]







| Material | Color | Molecular Weight | Form | Description | Size (Transmission Electron Microscopes) | Surface Area (Bet) | Melting Point |
|---|---|---|---|---|---|---|---|
| Aluminum Oxide | White | 101.96 | Rod | Gamma phase | <3.28 × 10−9 ft (50 nm) | >210,417 ft2/lbm () | 2040 °C |
| Material | Mixing Order | Concentration | Purpose | Source |
|---|---|---|---|---|
| Ethyl octanoate ester | 1 | 223.2 mL | Base fluid | Sigma Aldrich |
| VG HT | 1 ppb | High temperature Viscosifier | MI SWACO | |
| VG Supreme | 1 ppb | Gelling agent | MI SWACO | |
| Lime | 2 | 10 ppb | Alkalinity agent | |
| Primary emulsifier | 3 | 8 ppb | Emulsifier | MI SWACO |
| Secondary emulsifier | 2 ppb | Wetting agent | MI SWACO | |
| Water | 4 | 55.8 mL | Emulsified fluid Brine Thermal stabilizer Filtration control | |
| Calcium chloride | 17.5 ppb | |||
| Aluminum oxide NPs (<50 nm) | 0.1, 0.2, 0.5, 1, 1.5 wt.% | Sigma Aldrich | ||
| Ecotrol | 5 | 1.5 ppb | Filtration control | MI SWACO |
| Barite | 6 | 180.4 ppb | Weighting agent | |
| Sodium carbonate | 7 | 4 ppb | pH control |
| Wt.% Aluminum Oxide NPs | 2.6 °C | 26.8 °C | 70 °C |
|---|---|---|---|
| 0.0 | T2.6N0 | T26.8N0 | T70N0 |
| 0.1 | T2.6N0.1 | T26.8N0.1 | T70N0.1 |
| 0.2 | T2.6N0.2 | T26.8N0.2 | T70N0.2 |
| 0.5 | T2.6N0.5 | T26.8N0.5 | T70N0.5 |
| 1.0 | T2.6N1 | T26.8N1 | T70N1 |
| 1.5 | T2.6N1.5 | T26.8N1.5 | T70N1.5 |
| EBDF Code | K | n | |
|---|---|---|---|
| T26.8N0 | 12.59 | 0.44 | 0.70 |
| T26.8N0.1 | 8.29 | 0.56 | 0.64 |
| T26.8N0.2 | 9.90 | 0.51 | 0.66 |
| T26.8N0.5 | 9.27 | 0.68 | 0.63 |
| T26.8N1 | 11.97 | 0.80 | 0.61 |
| T26.8N1.5 | 17.54 | 0.78 | 0.63 |
| EBDF Code | K | n | EBDF Code | K | n | ||
|---|---|---|---|---|---|---|---|
| T2.6N0 | 16.3 | 0.74 | 0.66 | T70N0 | 4.9 | 0.34 | 0.66 |
| T2.6N0.1 | 16.3 | 0.73 | 0.65 | T70N0.1 | 7.0 | 0.20 | 0.73 |
| T2.6N0.2 | 17.2 | 0.58 | 0.69 | T70N0.2 | 6.8 | 0.17 | 0.75 |
| T2.6N0.5 | 17.3 | 0.69 | 0.68 | T70N0.5 | 5.2 | 0.19 | 0.75 |
| T2.6N1 | 20.7 | 1.38 | 0.59 | T70N1 | 6.8 | 0.23 | 0.72 |
| T2.6N1.5 | 28.6 | 1.86 | 0.57 | T70N1.5 | 12.1 | 0.92 | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.; Sharifi Haddad, A.; Rafati, R.; Bashir, A.; AlSabagh, A.M.; Aboulrous, A.A. Developing a Thermally Stable Ester-Based Drilling Fluid for Offshore Drilling Operations by Using Aluminum Oxide Nanorods. Sustainability 2021, 13, 3399. https://doi.org/10.3390/su13063399
Ahmed A, Sharifi Haddad A, Rafati R, Bashir A, AlSabagh AM, Aboulrous AA. Developing a Thermally Stable Ester-Based Drilling Fluid for Offshore Drilling Operations by Using Aluminum Oxide Nanorods. Sustainability. 2021; 13(6):3399. https://doi.org/10.3390/su13063399
Chicago/Turabian StyleAhmed, Alaa, Amin Sharifi Haddad, Roozbeh Rafati, Ahmed Bashir, Ahmed M. AlSabagh, and Amany A. Aboulrous. 2021. "Developing a Thermally Stable Ester-Based Drilling Fluid for Offshore Drilling Operations by Using Aluminum Oxide Nanorods" Sustainability 13, no. 6: 3399. https://doi.org/10.3390/su13063399
APA StyleAhmed, A., Sharifi Haddad, A., Rafati, R., Bashir, A., AlSabagh, A. M., & Aboulrous, A. A. (2021). Developing a Thermally Stable Ester-Based Drilling Fluid for Offshore Drilling Operations by Using Aluminum Oxide Nanorods. Sustainability, 13(6), 3399. https://doi.org/10.3390/su13063399






