Precision Agriculture Digital Technologies for Sustainable Fungal Disease Management of Ornamental Plants
Abstract
:1. Introduction
1.1. The Ornamental Plant Sector
1.1.1. Open-Field and Protected Crops: The Current Agronomic Practices
1.1.2. The Use of Pesticides for Ornamental Plant Productions
1.2. Fungal Disease Incidence in Ornamental Sector and Digital Tool Implementation for Their Early Detection
2. Materials and Methods of Case Studies
3. Conventional Disease Management in Ornamental Plant Productions
4. Traditional and Novel Approaches for Fungal Disease Detection and Monitoring
4.1. Molecular Biology Methods
4.2. Non-Imaging and Imaging Sensor-Based Methods
4.3. Fungal Risk Models Based on Microclimate Trends
5. Integration of Multidisciplinary Approaches for a Sustainable Management of Ornamentals
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volckaert, E.; Gobin, B. Ornamental plants and floriculture. In Soils, Plant Growth and Crop Production; Verheye, W.H., Ed.; EOLSS Publications: Paris, France, 2010; Volume III. [Google Scholar]
- Cardoso, B.F.; Rasetti, M.; Giampietri, E.; Finco, A.; Shikida, P.F.A. Trade dynamics in the Italian floriculture sector within EU borders: A gravity model analysis. AGRIS On-Line Pap. Econ. Inform. 2017, 9, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Yao, B.; Shanoyan, A.; Peterson, H.H.; Boyer, C.; Baker, L. The use of new-media marketing in the green industry: Analysis of social media use and impact on sales. Agribusiness 2019, 35, 281–297. [Google Scholar] [CrossRef]
- Havardi-Burger, N.; Mempel, H.; Bitsch, V. Sustainability challenges and innovations in the value chain of flowering potted plants for the German market. Sustainability 2020, 12, 1905. [Google Scholar] [CrossRef] [Green Version]
- DGAGRI-G2, 2017. Horticultural products. In Flowers and Ornamental Plants—Production; Statistics 2006–2016; European Commission Working Document. Publications Office of the European Union: Luxembourg, Luxembourg, 2017. [Google Scholar]
- DGAGRI-G2, 2020. Horticultural products. In Flowers and Ornamental Plants—Production; Statistics 2010–2019; European Commission Working Document. Publications Office of the European Union: Luxembourg, Luxembourg, 2020. [Google Scholar]
- Hendricks, J.; Briercliffe, T.; Oosterom, B.; Treer, A.; Kok, G.; Edwards, T.; Kong, H. Productions and Markets, the Future of Ornamentals; AIPH, International Association of Horticultural Producers: Oxfordshire, UK, 2019. [Google Scholar]
- Xia, Y.; Deng, X.; Zhou, P.; Shima, K.; Teixeira Da Silva, J.A. The world floriculture industry: Dynamics of production and markets. In Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues; Teixeira Da Silva, J.A., Ed.; Global Science Books: Isleworth, UK, 2006; pp. 336–347. [Google Scholar]
- Waliczek, T.M.; Byrne, D.H.; Holeman, D.J. Growers’ and consumers’ knowledge, attitudes and opinions regarding roses available for purchase. Acta Hortic. 2015, 1064, 235–239. [Google Scholar] [CrossRef]
- Kocian, A.; Massa, D.; Cannazzaro, S.; Incrocci, L.; Di Lonardo, S.; Milazzo, P.; Chessa, S. Dynamic Bayesian network for crop growth prediction in greenhouses. Comput. Electron. Agr. 2020, 169, 105167. [Google Scholar] [CrossRef]
- Incrocci, L.; Marzialetti, P.; Incrocci, G.; Di Vita, A.; Balendonck, J.; Bibbiani, C.; Spagnol, S.; Pardossi, A. Sensor-based management of container nursery crops irrigated with fresh or saline water. Agric. Water Manag. 2019, 213, 49–61. [Google Scholar] [CrossRef]
- Cardarelli, M.; Rouphael, Y.; Muntean, D.; Colla, G. Growth, quality index, and mineral composition of five ornamental cabbage cultivars grown under different nitrogen fertilization rates. HortScience 2015, 50, 688–693. [Google Scholar] [CrossRef]
- An, S.; Arakawa, O.; Tanaka, N.; Zhang, S.; Kobayashi, M. Effects of blue and red light irradiations on flower colouration in cherry blossom (Prunus × yedoensis ‘Somei-yoshino’). Sci. Hortic. 2020, 263, 109093. [Google Scholar] [CrossRef]
- Lee, R.; Den Uyl, R.; Runhaar, H. Assessment of policy instruments for pesticide use reduction in Europe; learning from a systematic literature review. Crop Prot. 2019, 126, 104929. [Google Scholar] [CrossRef]
- Gullino, M.L.; Gilardi, G.; Bertetti, D.; Garibaldi, A. Emerging soilborne pathogens and trends in their management. Acta Hortic. 2020, 1270, 9–22. [Google Scholar] [CrossRef]
- Pretty, J.; Bharucha, Z.P. Integrated pest management for sustainable intensification of agriculture in Asia and Africa. Insects 2015, 6, 152–182. [Google Scholar] [CrossRef] [PubMed]
- Gullino, M.L.; Tavella, L. Chemical and natural pesticides in IPM: Side-effects and application. In Integrated Pest and Disease Management in Greenhouse Crops; Plant Pathology in the 21st Century; Gullino, M.L., Albajes, R., Nicot, P.C., Eds.; Springer: Cham, The Netherlands, 2020; pp. 441–454. [Google Scholar]
- Alwang, J.; Norton, G.; Larochelle, C. Obstacles to widespread diffusion of IPM in developing countries: Lessons from the field. J. Integr. Pest Manag. 2019, 10, 10. [Google Scholar] [CrossRef]
- Wei, X.; Khachatryan, H.; Rihn, A. Production costs and profitability for selected greenhouse grown annual and perennial crops: Partial enterprise budgeting and sensitivity analysis. HortScience 2020, 55, 637–646. [Google Scholar] [CrossRef] [Green Version]
- Lentola, A.; David, A.; Abdul-Sada, A.; Tapparo, A.; Goulson, D.; Hill, E.M. Ornamental plants on sale to the public are a significant source of pesticide residues with implications for the health of pollinating insects. Env. Pollut. 2017, 228, 297–304. [Google Scholar] [CrossRef]
- Bika, R.; Baysal-Gurel, F.; Jennings, C. Botrytis cinerea management in ornamental production: A continuous battle. Can. J. Plant Pathol. 2020, 1–21. [Google Scholar] [CrossRef]
- Devappa, V.; Archith, T.C. Wilt diseases of ornamental crops and their management. In Wilt Diseases of Crops; Bhattacharyya, A., Chakraborty, B.N., Pandey, R.N., Singh, D., Dubey, S.C., Eds.; Today and Tomorrow Printers and Publisher: New Delhi, India, 2019; pp. 141–164. [Google Scholar]
- Agrios, G.N. Plant Pathology, 5rd ed.; Elsevier: Cambridge, MA, USA, 2005. [Google Scholar]
- Scott, J.C.; Gordon, T.R.; Shaw, D.V.; Koike, S.T. Effect of temperature on severity of Fusarium wilt of lettuce caused by Fusarium oxysporum f. sp. lactucae. Plant Dis. 2010, 94, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Soesanto, L.; Termorshuizen, A.J. Effect of temperature on the formation of microsclerotia of Verticillium dahliae. J. Phytopathol. 2001, 149, 685–691. [Google Scholar] [CrossRef]
- Thorpe, D.J.; Harrington, T.C.; Uchida, J.Y. Pathogenicity, internal transcribed spacer-rDNA variation, and human dispersal of Ceratocystis fimbriata on the family Araceae. Phytopathology 2005, 95, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Stahr, M.; Quesada-Ocampo, L.M. Assessing the role of temperature, inoculum density, and wounding on disease progression of the fungal pathogen Ceratocystis fimbriata causing black rot in sweetpotato. Plant Dis. 2020, 104, 930–937. [Google Scholar] [CrossRef]
- Benson, D.M.; Cartwright, D.K. Ornamental diseases incited by Rhizoctonia spp. In Rhizoctonia Species: Taxonomy, Molecular Biology, Ecology, Pathology and Disease Control; Sneh, B., Jabaji-Hare, S., Neate, S., Dijst, G., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 303–314. [Google Scholar]
- Ritchie, F.; Bain, R.A.; Mc Quilken, M.P. Effects of nutrient status, temperature and pH on mycelial growth, sclerotial production and germination of Rhizoctonia solani from potato. J. Plant Pathol. 2009, 91, 589–596. [Google Scholar]
- Hayek, S.; Gianinazzi-Pearson, V.; Gianinazzi, S.; Franken, P. Elucidating mechanisms of mycorrhiza-induced resistance against Thielaviopsis basicola via targeted transcript analysis of Petunia hybrida genes. Physiol. Mol. Plant Pathol. 2014, 88, 67–76. [Google Scholar] [CrossRef]
- Wu, T.L.; Huang, J.W. Carrot black root rot caused by Thielaviopsis basicola—identification of the pathogen and factors affecting its occurrence in Taiwan. Plant Pathol. Bulletin 2015, 24, 53–66. [Google Scholar]
- Clarkson, J.P.; Fawcett, L.; Anthony, S.G.; Young, C. A model for Sclerotinia sclerotiorum infection and disease development in lettuce, based on the effects of temperature, relative humidity and ascospore density. PLoS ONE 2014, 9, e94049. [Google Scholar]
- Grabowski, M.A.; Malvick, D.K. Evaluation of ornamental tropical plants for resistance to white mold caused by Sclerotinia sclerotiorum. HortScience 2017, 52, 1375–1379. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, M.E.; Caetano, P.; Ferraz, J.; Trapero, A. Phytophthora disease of Quercus ilex in south-western Spain. Forest Pathol. 2002, 32, 5–18. [Google Scholar] [CrossRef]
- Moralejo, E.; Pérez-Sierra, A.M.; Álvarez, L.A.; Belbahri, L.; Lefort, F.; Descals, E. Multiple alien Phytophthora taxa discovered on diseased ornamental plants in Spain. Plant Pathol. 2009, 58, 100–110. [Google Scholar] [CrossRef]
- Rodríguez-Rajo, F.J.; Iglesias, I.; Jato, V. Variation assessment of airborne Alternaria and Cladosporium spores at different bioclimatical conditions. Mycol. Res. 2005, 109, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Nagrale, D.T.; Gaikwad, A.P.; Goswami, S.; Sharma, L. Fungicidal management of Alternaria alternata (Fr.) Keissler causing blight of gerbera (Gerbera jamesonii H. Bolus ex J.D. Hook). J. App. Nat. Sci. 2012, 4, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Amsalem, L.; Freeman, S.; Rav-David, D.; Nitzani, Y.; Sztejnberg, A.; Pertot, I.; Elad, Y. Effect of climatic factors on powdery mildew caused by Sphaerotheca macularis f. sp. fragariae on strawberry. Eur. J. Plant Pathol. 2006, 114, 283–292. [Google Scholar] [CrossRef]
- Talgø, V.; Sundheim, L.; Gjærum, H.B.; Luz Herrero, M.; Suthaparan, A.; Toppe, B.; Stensvand, A. Powdery mildews on ornamental trees and shrubs in Norway. Eur. J. Plant Sci. Biotec. 2011, 5, 86–92. [Google Scholar]
- Salgado-Salazar, C.; Shiskoff, N.; Daughtrey, M.; Palmer, C.L.; Crouch, J.A. Downy mildew: A serious disease threat to rose health worldwide. Plant Dis. 2018, 102, 1873–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fillinger, S.; Elad, Y. Botrytis: The Fungus, the Pathogen and Its Management in Agricultural Systems; Springer: New York, NY, USA, 2016. [Google Scholar]
- Cao, X.; Shi, S.; Zhang, Z. First report of Botrytis leaf blight on lily (Lilium longiflorum) caused by Botrytis cinerea in Beijing, China. Plant Dis. 2018, 102, 1033. [Google Scholar] [CrossRef]
- Danti, R.; Della Rocca, G. Epidemiological history of cypress canker disease in source and invasion sites. Forests 2017, 8, 121. [Google Scholar] [CrossRef] [Green Version]
- Guarnaccia, V.; Gilardi, G.; Martino, I.; Garibaldi, A.; Gullino, M.L. Species diversity in Colletotrichum causing anthracnose of aromatic and ornamental Lamiaceae in Italy. Agronomy 2019, 9, 613. [Google Scholar] [CrossRef] [Green Version]
- Estrada, A.B.; Dodd, J.C.; Jeffries, P. Effect of humidity and temperature on conidial germination and appressorium development of two Philippine isolates of the mango anthracnose pathogen Colletotrichum gloeosporioides. Plant Pathol. 2000, 49, 608–618. [Google Scholar] [CrossRef]
- Hollier, C.A.; King, S.B. Effects of temperature and relative humidity on germinability and infectivity of Puccinia polysora uredospores. Plant Dis. 1985, 69, 937–939. [Google Scholar]
- Mueller, D.S.; Jeffers, S.N.; Buck, J.W. Toxicity of fungicides to urediniospores of six rust fungi that occur on ornamental crops. Plant Dis. 2005, 89, 3. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Li, H.M.; Eschen, R.; Morales-Rodriguez, C.; Vannini, A. The sentinel tree nursery as an early warning system for pathway risk assessment: Fungal pathogens associated with Chinese woody plants commonly shipped to Europe. PLoS ONE 2017, 12, e0188800. [Google Scholar] [CrossRef] [Green Version]
- EUROPHYT—Interceptions. European Union Notification System for Plant Health Interceptions—Annual Report 2018; European Commission Document; Publications Office of the European Union: Luxembourg., Luxembourg, 2018. [Google Scholar]
- Baysal-Gurel, F.; Kabir, N. Comparative performance of fungicides and biocontrol products in suppression of Rhizoctonia root rot in viburnum. J. Plant Pathol. Microbiol. 2018, 9, 2. [Google Scholar]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for management of soilborne diseases in crop production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Sierra, A.; Jung, T. Phytophthora in woody ornamental nurseries. In Phytophthora: A Global Perspective; Lamour, K., Ed.; CABI: Wallingford, UK, 2013; pp. 166–177. [Google Scholar]
- Rooney-Latham, S.; Blomquist, C.L.; Kosta, K.L.; Gou, Y.Y.; Woods, P.W. Phytophthora species are common on nursery stock grown for restoration and revegetation purposes in California. Plant Dis. 2019, 103, 448–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, S.; Kadooka, C.; Uchida, J.Y. Fusarium species as pathogen on orchids. Microbiol. Res. 2018, 207, 188–195. [Google Scholar] [CrossRef]
- Mc Govern, R.J.; Elmer, W.H. Disease of tulip in Handbook of Florists’ Crops Diseases; Mc Govern, R.J., Elmer, W.H., Eds.; Springer: Cham, Switzerland, 2018; pp. 1313–1337. [Google Scholar]
- Little, E.L. 2017-Georgia Plant Disease Loss Estimates. UGA Coop. Ext. Annu. Publ. 2019, 102–110. Available online: https://secure.caes.uga.edu/extension/publications/files/pdf/AP%20102-10_1.PDF (accessed on 2 February 2021).
- Rock, S. Survey of powdery mildew and gray mold disease management in Virginia and North Carolina cut flower farms. In Graduate Research Project, Online Master of Agricultural and Life Sciences; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2019. [Google Scholar]
- Soulioti, N.; Tsopelas, P.; Woodward, S. Platypus cylindrus, a vector of Ceratocystis platani in Platanus orientalis stands in Greece. Forest Pathol. 2015, 45, 367–372. [Google Scholar] [CrossRef]
- LeBlanc, N.; Salgado-Salazar, C.; Crouch, J.A. Boxwood blight: An ongoing threat to ornamental and native boxwood. Appl. Microbiol. Biotechnol. 2018, 102, 4371–4380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daughtrey, M.L. Boxwood blight: Threat to ornamentals. Ann. Rev. Phytopathol. 2019, 57, 189–209. [Google Scholar] [CrossRef]
- Borah, M.; Rajkhowa, M.; Ali, S. Occurrence of diseases in floricultural crops in and around Jorhat, Assam. Int. J. Econ. Plants 2019, 6, 54–63. [Google Scholar] [CrossRef]
- Kruidhof, H.M.; Elmer, W.H. Cultural methods for greenhouse pest and disease management. In Integrated Pest and Disease Management in Greenhouse Crops; Plant Pathology in the 21st Century; Gullino, M.L., Albajes, R., Nicot, P.C., Eds.; Springer: Cham, The Netherlands, 2020; pp. 285–330. [Google Scholar]
- Grigatti, M.; Giorgioni, M.E.; Ciavatta, C. Compost-based growing media: Influence on growth and nutrient use of bedding plants. Bioresour. Technol. 2007, 98, 3526–3534. [Google Scholar] [CrossRef]
- Bonanomi, G.; Antignani, V.; Pane, C.; Scala, F. Suppression of soilborne fungal diseases with organic amendments. J. Plant Pathol. 2007, 89, 311–324. [Google Scholar]
- Gebremedhin, H. Effects of Aluminum Sulphate, Ethanol, Sucrose and their combination on the longevity and physiological properties of rose (Rosa hybrida L.) cut flowers. J. Hortic. Res. 2020, 28, 29–38. [Google Scholar] [CrossRef]
- Percival, G.C.; Haynes, I. The influence of calcium sprays to reduce fungicide inputs against apple scab (Venturia inaequalis (Cooke) G. Wint.). Arboriculture Urban For. 2009, 35, 263–270. [Google Scholar]
- Deliopoulos, T.; Kettlewell, P.S.; Hare, M.C. Fungal disease suppression by inorganic salts: A review. Crop Prot. 2010, 29, 1059–1075. [Google Scholar] [CrossRef]
- Bardin, M.; Pugliese, M. Biocontrol agents against diseases. In Integrated Pest and Disease Management in Greenhouse Crops; Plant Pathology in the 21st Century; Gullino, M.L., Albajes, R., Nicot, P.C., Eds.; Springer: Cham, The Netherlands, 2020; pp. 385–407. [Google Scholar]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galán, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar] [CrossRef]
- Sreenivasaprasad, S.; Manibhushanrao, K. Antagonistic potential of Gliocladium virens and Trichoderma longibrachiatum to phytopathogenic fungi. Mycopathologia 1990, 109, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Segarra, G.; Casanova, E.; Avilés, M.; Trillas, I. Trichoderma asperellum strain T34 controls Fusarium wilt disease in tomato plants in soilless culture through competition for iron. Microb. Ecol. 2010, 59, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Yedidia, I.; Chet, I. Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathol. 2005, 95, 76–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic amendments, beneficial microbes, and soil microbiota: Toward a unified framework for disease suppression. Ann. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Baysal-Gurel, F.; Oliver, J.B.; Addesso, K.M. Evaluation of fungicides and biofungicide to control Phytophthora root rot (Phytophthora cinnamomi Rands) and ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) on flowering dogwoods exposed to simulated flood events. Crop Prot. 2019, 124, 104834. [Google Scholar] [CrossRef]
- Stewart, A.; Hill, R. Applications of Trichoderma in plant growth promotion. In Biotechnology and biology of Trichoderma; Gupta, V., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 415–428. [Google Scholar]
- Necha, L.L.B.; Bautista-Baños, S. Prospects for the use of chitosan and other alternatives in ornamental conservation. In Chitosan in the Preservation of Agricultural Commodities; Bautista-Baños, S., Romanazzi, G., Jiménez-Aparicio, A., Eds.; Elsevier: Cambridge, MA, USA, 2016; pp. 221–249. [Google Scholar]
- Van Huylenbroeck, J.; Leus, L.; Luypaert, G.; Van Laere, K. Durable disease resistance in woody ornamentals: The breeders’ challenge. Acta Hortic. 2018, 1191, 1–8. [Google Scholar] [CrossRef]
- Deng, Z. Breeding for disease resistance in florists’ crops. In Handbook of Florists’ Crops Diseases; Mc Govern, R.J., Elmer, W.H., Eds.; Springer: Cham, Switzerland, 2018; pp. 87–118. [Google Scholar]
- Kishi-Kaboshi, M.; Aida, R.; Sasaki, K. Genome engineering in ornamental plants: Current status and future prospects. Plant Physiol. Biochem. 2018, 131, 47–52. [Google Scholar] [CrossRef]
- Luchi, N.; Ioos, R.; Santini, A. Fast and reliable molecular methods to detect fungal pathogens in woody plants. Appl. Microbiol. Biotechnol. 2020, 104, 2453–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanasamy, P. Microbial Plant Pathogens-Detection and Disease Diagnosis: Fungal Pathogens; Springer: New York, NY, USA, 2011; Volume 1. [Google Scholar]
- West, J.S.; Atkins, S.D.; Emberlin, J.; Fitt, B.D. PCR to predict risk of airborne disease. Trends Microbiol. 2008, 16, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Torres-Calzada, C.; Tapia-Tussell, R.; Quijano-Ramayo, A.; Martin-Mex, R.; Rojas-Herrera, R.; Higuera-Ciapara, I.; Perez-Brito, D. A species-specific polymerase chain reaction assay for rapid and sensitive detection of Colletotrichum capsici. Mol. Biotechnol. 2011, 49, 48–55. [Google Scholar] [CrossRef]
- Pasquali, M.; Acquadro, A.; Balmas, V.; Migheli, Q.; Gullino, M.L.; Garibaldi, A. Development of PCR primers for a new Fusarium oxysporum pathogenic on Paris daisy (Argyranthemum frutescens L.). Eur. J. Plant Pathol. 2004, 110, 7–11. [Google Scholar] [CrossRef]
- Mmbaga, M.T.; Kim, M.S.; Mackasmiel, L.; Klopfenstein, N.B. Differentiation of Corynespora cassiicola and Cercospora sp. in leaf-spot diseases of Hydrangea macrophylla using a PCR-mediated method. Can. J. Plant Sci. 2015, 95, 711–717. [Google Scholar] [CrossRef] [Green Version]
- Inderbitzin, P.; Davis, R.M.; Bostock, R.M.; Subbarao, K.V. Identification and differentiation of Verticillium species and V. longisporum lineages by simplex and multiplex PCR assays. PLoS ONE 2013, 8, e65990. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elmagid, A.; Garrido, P.A.; Hunger, R.; Lyles, J.L.; Mansfield, M.A.; Gugino, B.K.; Smith, D.L.; Melouk, H.A.; Garzon, C.D. Discriminatory simplex and multiplex PCR for four species of the genus Sclerotinia. J. Microbiol. Methods 2013, 92, 293–300. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Li Destri Nicosia, M.G.; Faedda, R.; Cacciola, S.O.; Schena, L. Use of quantitative PCR detection methods to study biocontrol agents and phytopathogenic fungi and oomycetes in environmental samples. J. Phytopathol. 2014, 162, 1–13. [Google Scholar] [CrossRef]
- Yadav, M.K.; Singh, B.P. Real-time polymerase chain reaction (PCR) based identification and detection of fungi belongs to genus Fusarium. In Molecular Markers in Mycology; Singh, B.P., Gupta, V.K., Eds.; Springer: Cham, The Netherlands, 2017; pp. 65–85. [Google Scholar]
- Minerdi, D.; Moretti, M.; Li, Y.; Gaggero, L.; Garibaldi, A.; Gullino, M.L. Conventional PCR and real time quantitative PCR detection of Phytophthora cryptogea on Gerbera jamesonii. Eur. J. Plant Pathol. 2008, 122, 227–237. [Google Scholar] [CrossRef]
- Gautam, A.K.; Kumar, S. Techniques for the detection, identification, and diagnosis of agricultural pathogens and diseases. In Natural Remedies for Pest, Disease and Weed Control; Egbuna, C., Sawicka, B., Eds.; Elsevier: Cambridge, MA, USA, 2020; pp. 135–142. [Google Scholar]
- Rani, A.; Donovan, N.; Mantri, N. The future of plant pathogen diagnostics in a nursery production system. Biosens. Bioelectron. 2019, 145, 111631. [Google Scholar] [CrossRef]
- Le, D.T.; Vu, N.T. Progress of loop-mediated isothermal amplification technique in molecular diagnosis of plant diseases. Appl. Biol. Chem. 2017, 60, 169–180. [Google Scholar] [CrossRef]
- Koo, C.; Malapi-Wight, M.; Kim, H.S.; Cifci, O.S.; Vaughn-Diaz, V.L.; Ma, B.; Kim, S.; Abdel-Raziq, H.; Ong, K.; Jo, Y.; et al. Development of a real-time microchip PCR system for portable plant disease diagnosis. PLoS ONE 2013, 8, e82704. [Google Scholar] [CrossRef] [PubMed]
- Aglietti, C.; Luchi, N.; Pepori, A.L.; Bartolini, P.; Pecori, F.; Raio, A.; Capretti, P.; Santini, A. Real-time loop-mediated isothermal amplification: An early-warning tool for quarantine plant pathogen detection. AMB Express 2019, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Olmos, A.; Boonham, N.; Candresse, T.; Gentit, P.; Giovani, B.; Kutnjak, D.; Liefting, L.; Maree, H.J.; Minafra, A.; Moreira, A.; et al. High-throughput sequencing technologies for plant pest diagnosis: Challenges and opportunities. EPPO Bulletin 2018, 48, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Mahlein, A.K.; Kuska, M.T.; Thomas, S.; Bohnenkamp, D.; Alisaac, E.; Behmann, J.; Wahabzada, M.; Kersting, K. Plant disease detection by hyperspectral imaging: From the lab to the field. Adv. Animal Biosc. 2017, 8, 238–243. [Google Scholar] [CrossRef]
- Mahlein, A.K. Plant disease detection by imaging sensors-parallels and specific demands for precision agriculture and plant phenotyping. Plant Dis. 2016, 100, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, R.L.; et al. Advanced methods of plant disease detection. A review. Agron. Sustain. Dev. 2015, 35, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.; Kuska, M.T.; Bohnenkamp, D.; Brugger, A.; Alisaac, E.; Wahabzada, M.; Behmann, J.; Mahlein, A.K. Benefits of hyperspectral imaging for plant disease detection and plant protection: A technical perspective. J. Plant Dis. Prot. 2018, 125, 5–20. [Google Scholar] [CrossRef]
- Kuska, M.T.; Mahlein, A.K. Aiming at decision making in plant disease protection and phenotyping by the use of optical sensors. Eur. J. Plant Pathol. 2018, 152, 987–992. [Google Scholar] [CrossRef]
- Bock, C.H.; Barbedo, J.G.; Del Ponte, E.M.; Bohnenkamp, D.; Mahlein, A.K. From visual estimates to fully automated sensor-based measurements of plant disease severity: Status and challenges for improving accuracy. Phytopathol. Res. 2020, 2, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Marín Ortiz, J.C.; Gutierrez Toro, N.; Botero Fernández, V.; Hoyos Carvajal, L.M. Linking physiological parameters with visible/near-infrared leaf reflectance in the incubation period of vascular wilt disease. Saudi J. Biol. Sci. 2020, 27, 88–99. [Google Scholar] [CrossRef]
- Marín Ortiz, J.C.; Hoyos Carvajal, L.M.; Botero Fernández, V. Detection of significant wavelengths for identifying and classifying Fusarium oxysporum during the incubation period and water stress in Solanum lycopersicum plants using reflectance spectroscopy. J. Plant Prot. Res. 2019, 59, 244–254. [Google Scholar]
- Poona, N.K.; Ismail, R. Using Boruta-selected spectroscopic wavebands for the asymptomatic detection of Fusarium circinatum stress. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 3764–3772. [Google Scholar] [CrossRef]
- Newby, Z.; Murphy, R.J.; Guest, D.I.; Ramp, D.; Liew, E.Y. Detecting symptoms of Phytophthora cinnamomi infection in Australian native vegetation using reflectance spectrometry: Complex effects of water stress and species susceptibility. Australas. Plant Pathol. 2019, 48, 409–424. [Google Scholar] [CrossRef]
- Poona, N.K.; Ismail, R. Developing optimized spectral indices using machine learning to model Fusarium circinatum stress in Pinus radiata seedlings. J. Appl. Remote Sens. 2019, 13, 034515. [Google Scholar] [CrossRef]
- Heim, R.H.J.; Wright, I.J.; Allen, A.P.; Geedicke, I.; Oldeland, J. Developing a spectral disease index for myrtle rust (Austropuccinia psidii). Plant Pathol. 2019, 68, 738–745. [Google Scholar] [CrossRef]
- Barry, K.M.; Corkrey, R.; Pham Thi, H.; Ridge, S.; Mohammed, C.L. Spectral characterization of necrosis from reflectance of Eucalyptus globulus leaves with Mycosphaerella leaf disease or subjected to artificial lesions. Int. J. Remote Sens. 2011, 32, 9243–9259. [Google Scholar] [CrossRef]
- Arens, N.; Backhaus, A.; Döll, S.; Fischer, S.; Seiffert, U.; Mock, H.P. Non-invasive presymptomatic detection of Cercospora beticola infection and identification of early metabolic responses in sugar beet. Front. Plant Sci. 2016, 7, 1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitić, G.; Tagarakis, A.; Cselyuszka, N.; Panić, M.; Birgermajer, S.; Sakulski, D.; Matović, J. A new low-cost portable multispectral optical device for precise plant status assessment. Comput. Electron. Agr. 2019, 162, 300–308. [Google Scholar] [CrossRef]
- Polder, G.; Pekkeriet, E.J.; Snikkers, M. A spectral imaging system for detection of botrytis in greenhouses. In Proceedings of the EFITA-WCCA-CIGR Conference “Sustainable Agriculture through ICT Innovation”, Turin, Italy, 24–27 June 2013. [Google Scholar]
- Pethybridge, S.J.; Hay, F.; Esker, P.; Groom, T.; Wilson, C.; Nutter, F.W., Jr. Visual and radiometric assessments for yield losses caused by ray blight in pyrethrum. Crop Sci. 2008, 48, 343–352. [Google Scholar] [CrossRef]
- Polder, G.; Van De Westeringh, N.; Kool, J.; Khan, H.A.; Kootstra, G.; Nieuwenhuizen, A. Automatic detection of tulip breaking virus (TBV) using a deep convolutional neural network. IFAC-PapersOnLine 2019, 52, 12–17. [Google Scholar] [CrossRef]
- Buitrago, M.F.; Groen, T.A.; Hecker, C.A.; Skidmore, A.K. Changes in thermal infrared spectra of plants caused by temperature and water stress. ISPRS J. Photogramm. Remote Sens. 2016, 111, 22–31. [Google Scholar] [CrossRef]
- Jafari, M.; Minaei, S.; Safaie, N. Detection of pre-symptomatic rose powdery-mildew and gray-mold diseases based on thermal vision. Infrared Phys. Technol. 2017, 85, 170–183. [Google Scholar] [CrossRef]
- Saglam, A.; Chaerle, L.; Van Der Straeten, D.; Valcke, R. Promising monitoring techniques for plant science: Thermal and chlorophyll fluorescence imaging. Photosynth. Product. Environ. Stress 2019, 241–266. [Google Scholar] [CrossRef]
- Jafari, M.; Minaei, S.; Safaie, N.; Torkamani-Azar, F.; Sadeghi, M. Classification using radial-basis neural networks based on thermographic assessment of Botrytis cinerea infected cut rose flowers treated with methyl jasmonate. J. Crop Prot. 2016, 5, 591–602. [Google Scholar] [CrossRef]
- Minaei, S.; Jafari, M.; Safaie, N. Design and development of a rose plant disease-detection and site-specific spraying system based on a combination of infrared and visible images. J. Agr. Sci. Tech. 2018, 20, 23–36. [Google Scholar]
- Gorbe, E.; Calatayud, A. Applications of chlorophyll fluorescence imaging technique in horticultural research: A review. Sci. Hortic. 2012, 138, 24–35. [Google Scholar] [CrossRef]
- Casa, R.; Pignatti, S.; Pascucci, S.; Castaldi, F.V.M. Il telerilevamento in agricoltura di precisione. In Agricoltura di Precisione; Casa, R., Ed.; Edagricole-New Business Media: Milano, Italy, 2016. [Google Scholar]
- Kopacki, M.; Wagner, A.; Michałek, W. Pathogenicity of Fusarium oxysporum, Fusarium avenaceum and Sclerotinia sclerotiorum and their effect on photosynthetic activity of chrysanthemum plants. Acta Sci. Pol. Hortoru. 2016, 15, 59–70. [Google Scholar]
- Alaei Shah Vali Anar, H.; Baeyen, S.; Lemeire, E.; Lootens, P.; Höfte, M.; Maes, M.; Heungens, K. Detection of Puccinia horiana, the causal agent of Chrysanthemum white rust, with PCR and chlorophyll fluorescence image analysis. In Proceedings of the Programme & Abstracts 57th International Symposium on crop Protection, Gent, Belgium, 10 May 2005; p. 153. [Google Scholar]
- Polder, G.; Van Der Heijden, G.W.; Van Doorn, J.; Baltissen, T.A. Automatic detection of tulip breaking virus (TBV) in tulip fields using machine vision. Biosyst. Eng. 2014, 117, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Velázquez-López, N.; Sasaki, Y.; Nakano, K.; Mejía-Muñoz, J.M.; Romanchik Kriuchkova, E. Detección de cenicilla en rosa usando procesamiento de imágenes por computadora. Rev. Chapingo Ser. Hortic. 2011, 17, 151–160. [Google Scholar] [CrossRef]
- Rossi, V.; Giosuè, S.; Caffi, T. Modelling plant diseases for decision making in crop protection. In Precision Crop Protection-the Challenge and Use of Heterogeneity; Oerke, E.C., Gerhards, R., Menz, G., Sikora, R.A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 241–258. [Google Scholar]
- Magarey, R.D.; Sutton, T.B.; Thayer, C.L. A simple generic infection model for foliar fungal plant pathogens. Phytopathology 2005, 95, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Shah, D.A.; Paul, P.A.; De Wolf, E.D.; Madden, L.V. Predicting plant disease epidemics from functionally represented weather series. Philos. Trans. R. Soc. B 2019, 374, 20180273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donatelli, M.; Magarey, R.D.; Bregaglio, S.; Willocquet, L.; Whish, J.P.; Savary, S. Modelling the impacts of pests and diseases on agricultural systems. Agric. Syst. 2017, 155, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Magarey, R.D.; Sutton, T.B. How to create and deploy infection models for plant pathogens. In General Concepts in Integrated Pest and Disease Management; Ciancio, A., Mukerji, K.G., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 3–25. [Google Scholar]
- Launay, M.; Caubel, J.; Bourgeois, G.; Huard, F.; De Cortazar-Atauri, I.G.; Bancal, M.O.; Brisson, N. Climatic indicators for crop infection risk: Application to climate change impacts on five major foliar fungal diseases in Northern France. Agric. Ecosyst. Env. 2014, 197, 147–158. [Google Scholar] [CrossRef]
- Bergot, M.; Cloppet, E.; Pérarnaud, V.; Déqué, M.; Marcais, B.; Desprez-Loustau, M.L. Simulation of potential range expansion of oak disease caused by Phytophthora cinnamomi under climate change. Global Change Biol. 2004, 10, 1539–1552. [Google Scholar] [CrossRef] [Green Version]
- Caubel, J.; Launay, M.; Lannou, C.; Brisson, N. Generic response functions to simulate climate-based processes in models for the development of airborne fungal crop pathogens. Ecol. Model. 2012, 242, 92–104. [Google Scholar] [CrossRef]
- Manici, L.M.; Bregaglio, S.; Fumagalli, D.; Donatelli, M. Modelling soil borne fungal pathogens of arable crops under climate change. Int. J. Biomet. 2014, 58, 2071–2083. [Google Scholar] [CrossRef]
- You, M.P.; Rensing, K.; Renton, M.; Barbetti, M.J. Modeling effects of temperature, soil, moisture, nutrition and variety as determinants of severity of Pythium damping-off and root disease in subterranean clover. Front. Microbiol. 2017, 8, 2223. [Google Scholar] [CrossRef]
- Philion, V.; Joubert, V.; Trapman, M.; Hjelkrem, A.G.R.; Stensvand, A. Distribution of the infection time of ascospores of Venturia inaequalis. Plant Dis. 2020, 104, 465–473. [Google Scholar] [CrossRef]
- Singh, M.C.; Yousuf, A.; Singh, J.P. Greenhouse microclimate modeling under cropped conditions: A review. Res. Env. Life Sci. 2016, 9, 1552–1557. [Google Scholar]
- Ali, R.B.; Bouadila, S.; Mami, A. Experimental validation of the dynamic thermal behavior of two types of agricultural greenhouses in the Mediterranean context. Renew. Energy 2020, 147, 118–129. [Google Scholar]
- Mashonjowa, E.; Ronsse, F.; Mubvuma, M.; Milford, J.R.; Pieters, J.G. Estimation of leaf wetness duration for greenhouse roses using a dynamic greenhouse climate model in Zimbabwe. Comput. Electron. Agr. 2013, 95, 70–81. [Google Scholar] [CrossRef]
- Boulard, T.; Roy, J.C.; Fatnassi, H.; Kichah, A.; Lee, I.B. Computer fluid dynamics prediction of climate and fungal spore transfer in a rose greenhouse. Comput. Electron. Agr. 2010, 74, 280–292. [Google Scholar] [CrossRef]
- Granke, L.L.; Crawford, L.E.; Hausbeck, M.K. Factors affecting airborne concentrations of Podosphaera xanthii conidia and severity of gerbera powdery mildew. HortScience 2012, 47, 1068–1072. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.M. Effects of temperature on the length of the incubation period of rose powdery mildew (Sphaerotheca pannosa var. rosae). Eur. J. Plant Pathol. 1999, 105, 13–21. [Google Scholar] [CrossRef]
- Körner, O.; Holst, N.; De Visser, P. A model-based decision support tool for grey mould prediction. Acta Hortic. 2014, 1037, 569–574. [Google Scholar] [CrossRef]
- Xu, X.; Robinson, J. The effects of temperature on the incubation and latent periods of powdery mildew (Erysiphe polygoni) on clematis. J. Phytopathol. 2001, 149, 565–568. [Google Scholar] [CrossRef]
- Copes, W.E. Weather-based forecasting of Rhizoctonia web blight development on container-grown azalea. Plant Dis. 2015, 99, 100–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Harris, D.C.; Berrie, A.M. Modeling infection of strawberry flowers by Botrytis cinerea using field data. Phytopathology 2000, 90, 1367–1374. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.L.; Kerns, J.P.; Walker, N.R.; Payne, A.F.; Horvath, B.; Inguagiato, J.C.; Kaminski, J.E.; Tomaso-Peterson, M.; Koch, P.L. Development and validation of a weather-based warning system to advise fungicide applications to control dollar spot on turfgrass. PLoS ONE 2018, 13, e0194216. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.S.; Beresford, R.M.; Walter, M. Development of a disease risk prediction model for downy mildew (Peronospora sparsa) in boysenberry. Phytopathology 2014, 104, 50–56. [Google Scholar] [CrossRef]
- El Jarroudi, M.; Karjoun, H.; Kouadio, L.; El Jarroudi, M. Mathematical modelling of non-local spore dispersion of wind-borne pathogens causing fungal diseases. Appl. Math. Comput. 2020, 376, 125107. [Google Scholar] [CrossRef]
- Populer, C. Changes in host susceptibility with time. In Plant Disease: An Advanced Treatise: How disease develops in populations; Horsfall, J.G., Cowling, E.B., Eds.; Elsevier: Cambridge, MA, USA, 1978; Volume II, pp. 239–262. [Google Scholar]
- O’Neill, T.M.; Pye, D.; Locke, T. The effect of fungicides, irrigation and plant density on the development of Peronospora sparsa, the cause of downy mildew in rose and blackberry. Ann. App. Biol. 2002, 140, 207–214. [Google Scholar] [CrossRef]
- Harwood, T.D.; Xu, X.; Pautasso, M.; Jeger, M.J.; Shaw, M.W. Epidemiological risk assessment using linked network and grid based modelling: Phytophthora ramorum and Phytophthora kernoviae in the UK. Ecol. Model. 2009, 220, 3353–3361. [Google Scholar] [CrossRef]
- Jeger, M.J.; Xu, X.M. Modelling the dynamics of a plant pathogen and a biological control agent in relation to flowering pattern and populations present on leaves. Ecol. Model. 2015, 313, 13–28. [Google Scholar] [CrossRef]
- Elad, Y. Cultural and integrated control of Botrytis spp. In Botrytis—the Fungus, the Pathogen and its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer: Cham, The Netherlands, 2016; pp. 149–164. [Google Scholar]
- Sharma, S.; Dohroo, N.P.; Veerubommu, S.; Phurailatpam, S.; Thakur, N.; Yadav, A.N. Integrated disease management of storage rot of ginger (Zingiber officinale) caused by Fusarium sp. in Himachal Pradesh, India. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3580–3592. [Google Scholar] [CrossRef]
- D’Errico, G.; Marra, R.; Crescenzi, A.; Davino, S.W.; Fanigliulo, A.; Woo, S.L.; Lorito, M. Integrated management strategies of Meloidogyne incognita and Pseudopyrenochaeta lycopersici on tomato using a Bacillus firmus-based product and two synthetic nematicides in two consecutive crop cycles in greenhouse. Crop Prot. 2019, 122, 159–164. [Google Scholar] [CrossRef]
- Vandana, U.K.; Singha, P.B.; Chakraborthy, S.; Mazumder, P.B. Integrated fungal foliar diseases of arid legumes: Challenges and strategies of their management in rain-fed areas. In Management of Fungal Pathogens in Pulses; Singh, B.P., Singh, G., Kumar, K., Nayak, S.C., Srinivasa, N., Eds.; Springer: Cham, The Netherlands, 2020; pp. 35–55. [Google Scholar]
- Simko, I.; Jimenez-Berni, J.A.; Sirault, X.R. Phenomic approaches and tools for phytopathologists. Phytopathol. 2017, 107, 6–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bevacqua, D.; Génard, M.; Lescourret, F.; Martinetti, D.; Vercambre, G.; Valsesia, P.; Mirás-Avalos, J.M. Coupling epidemiological and tree growth models to control fungal diseases spread in fruit orchards. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]

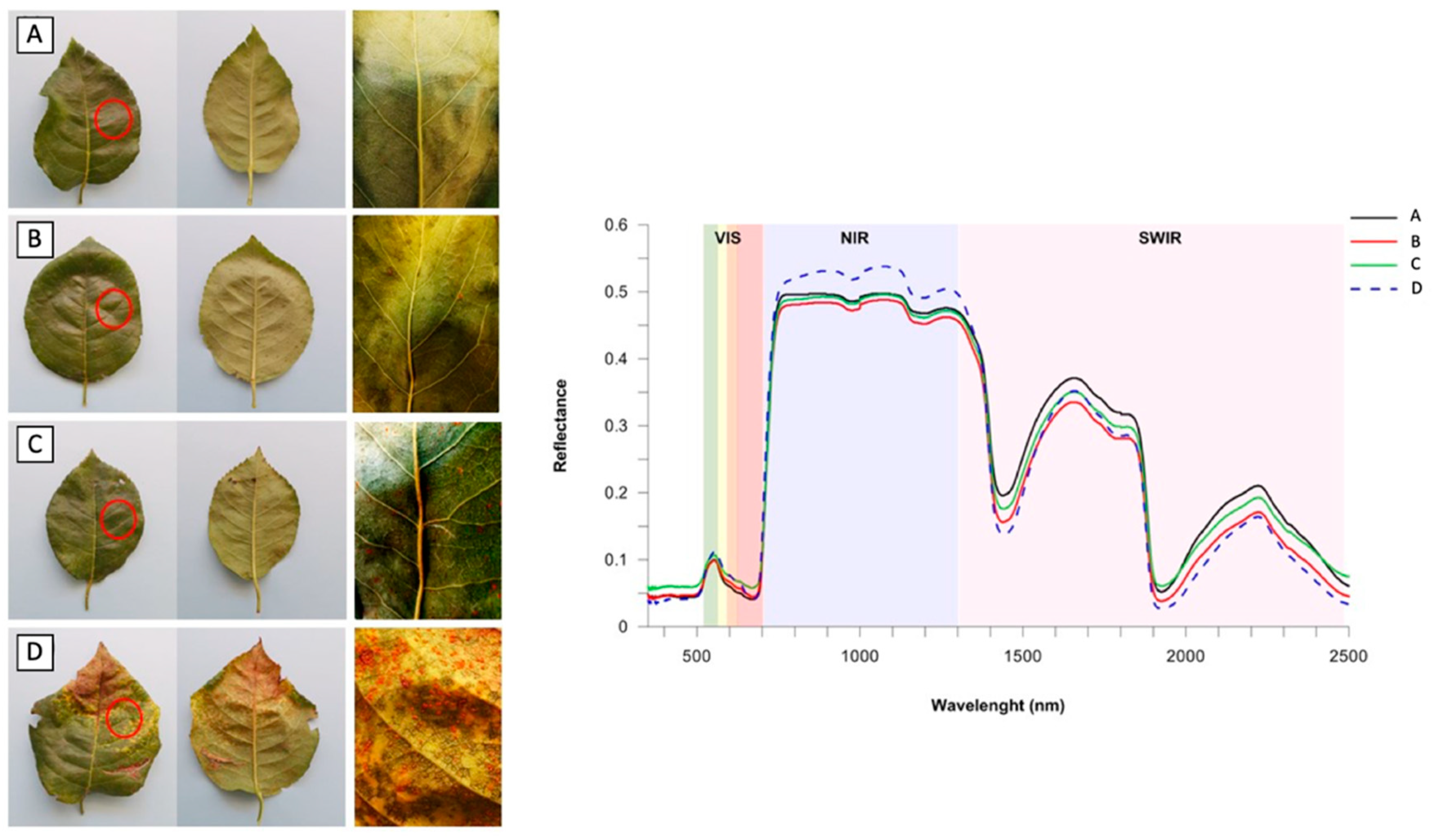

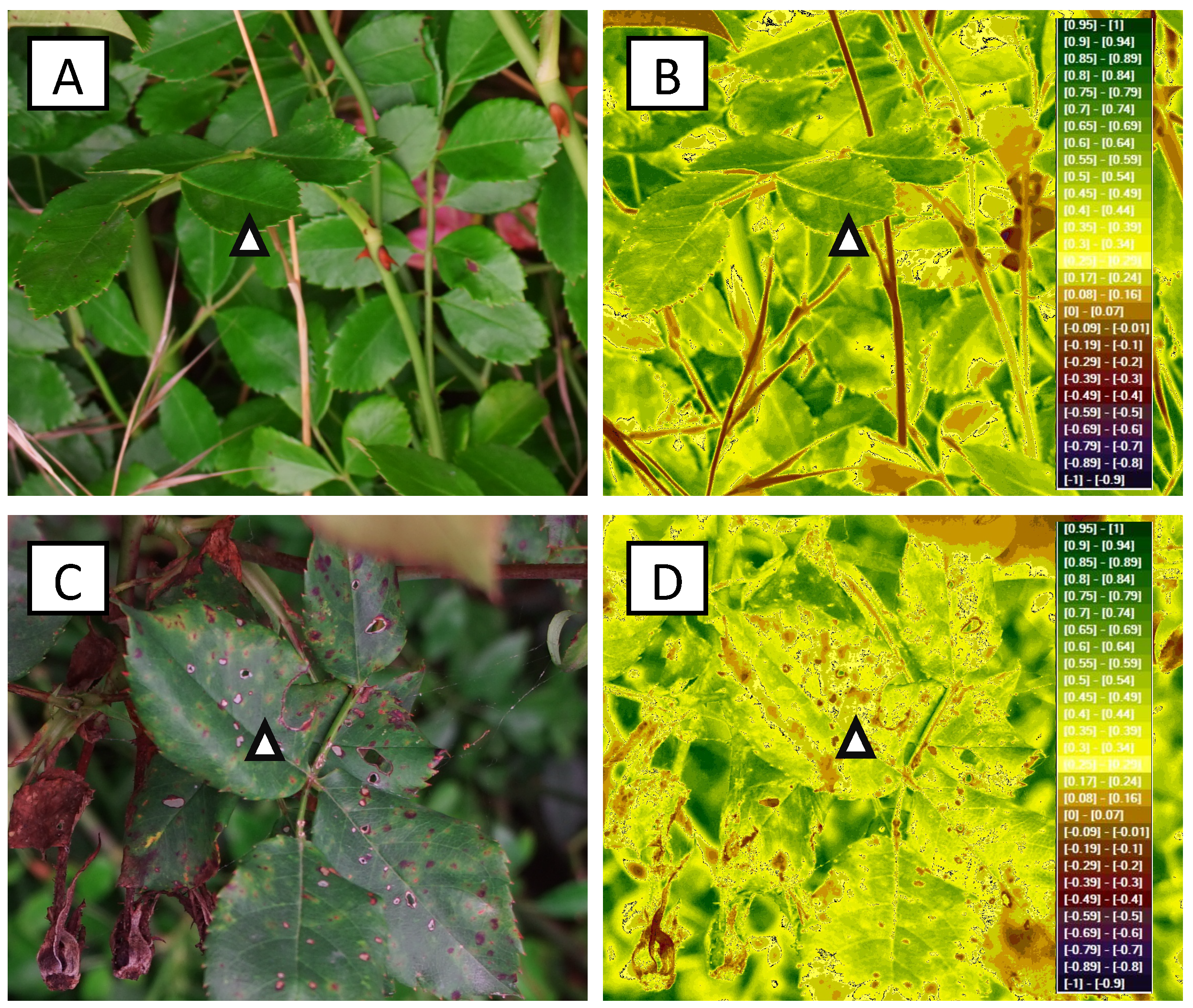



| Fungus | Host | VIsual Signs and Symptoms | Optimal Climatic Parameters | References |
|---|---|---|---|---|
| Soilborne Fungal and Oomycete Diseases | ||||
| Fusarium spp. | All ornamentals | Vascular wilt | T: 25 °C | [24] |
| Verticillum spp. | All ornamentals | Vascular wilt | T: 15–25 °C High water activity | [25] |
| Ceratocystis fimbriata | Forest ornamentals | Wilt, canker stain, and tissue rot | T: 23 °C Relative humidity 85–97% | [26,27] |
| Rhizoctonia solani | Woody ornamentals | Quick decline resulting in total losses | T: 20–25 °C High water activity | [28,29] |
| Thielaviopsis basicola | Several ornamentals | Black root rot | Relative humidity > 85% | [30,31] |
| Sclerotinia spp. | Herbaceous ornamentals | Stem and crown rot/wilt | T: 15–27 °C Relative humidity > 85% | [32,33] |
| Phytophthora spp. | Woody ornamentals | Root, collar and crown rot till plant decline | Wide range of optimal temperatures Presence of free water | [34,35] |
| Airborne Fungal and Oomycete Diseases | ||||
| Leaf blight (e.g., Alternata alternata) | Gerbera and other ornamentals | Leaf blights, pathogenic spots on leaves, twigs, flowers | T: 23–29 °C Relative humidity ≈ 80% | [36,37] |
| Powdery mildew (e.g., Sphaerotheca spp.) | All ornamentals | White powder on aerial organs, buds fail to open, tips desiccation | Moderate temperatures Relative humidity 75–98% Not too high light intensity | [38,39] |
| Downy mildew (e.g., Peronospora sparsa) | Rose and other ornamentals | Purplish-red, brown or black leaf spots, square or angular | T: 15–25 °C Relative humidity > 85% Presence of free water | [40] |
| Grey mould (e.g., Botrytis cinerea) | All ornamentals | Dark spots developing in soft rotting, grey spore carpet on tissues, blights in dry condition | T: >21 °C Relative humidity > 99% | [41,42] |
| Canker (e.g., Seiridium cardinale) | All ornamentals | Dark brown discoloration and necrotic lesions | Wide range of optimal temperatures High relative humidity | [43] |
| Anthracnose (e.g., Colletotrichum spp.) | Several ornamentals | Irregular, desiccated brown leaf spots | T: 25 °C Relative humidity ≈ 100% | [44,45] |
| Rust (e.g., Puccinia spp.) | Several ornamentals | Orange/yellow to brown/black pustules | T: 12–20 °C | [46,47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traversari, S.; Cacini, S.; Galieni, A.; Nesi, B.; Nicastro, N.; Pane, C. Precision Agriculture Digital Technologies for Sustainable Fungal Disease Management of Ornamental Plants. Sustainability 2021, 13, 3707. https://doi.org/10.3390/su13073707
Traversari S, Cacini S, Galieni A, Nesi B, Nicastro N, Pane C. Precision Agriculture Digital Technologies for Sustainable Fungal Disease Management of Ornamental Plants. Sustainability. 2021; 13(7):3707. https://doi.org/10.3390/su13073707
Chicago/Turabian StyleTraversari, Silvia, Sonia Cacini, Angelica Galieni, Beatrice Nesi, Nicola Nicastro, and Catello Pane. 2021. "Precision Agriculture Digital Technologies for Sustainable Fungal Disease Management of Ornamental Plants" Sustainability 13, no. 7: 3707. https://doi.org/10.3390/su13073707
APA StyleTraversari, S., Cacini, S., Galieni, A., Nesi, B., Nicastro, N., & Pane, C. (2021). Precision Agriculture Digital Technologies for Sustainable Fungal Disease Management of Ornamental Plants. Sustainability, 13(7), 3707. https://doi.org/10.3390/su13073707









