Characterization of Alkanolamine Blends for Carbon Dioxide Absorption. Corrosion and Regeneration Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Properties Measurements
2.3. Absorption Studies in Stirred Cell
2.4. Absorption Studies in Bubble Column Reactor
2.5. Corrosion Experiments
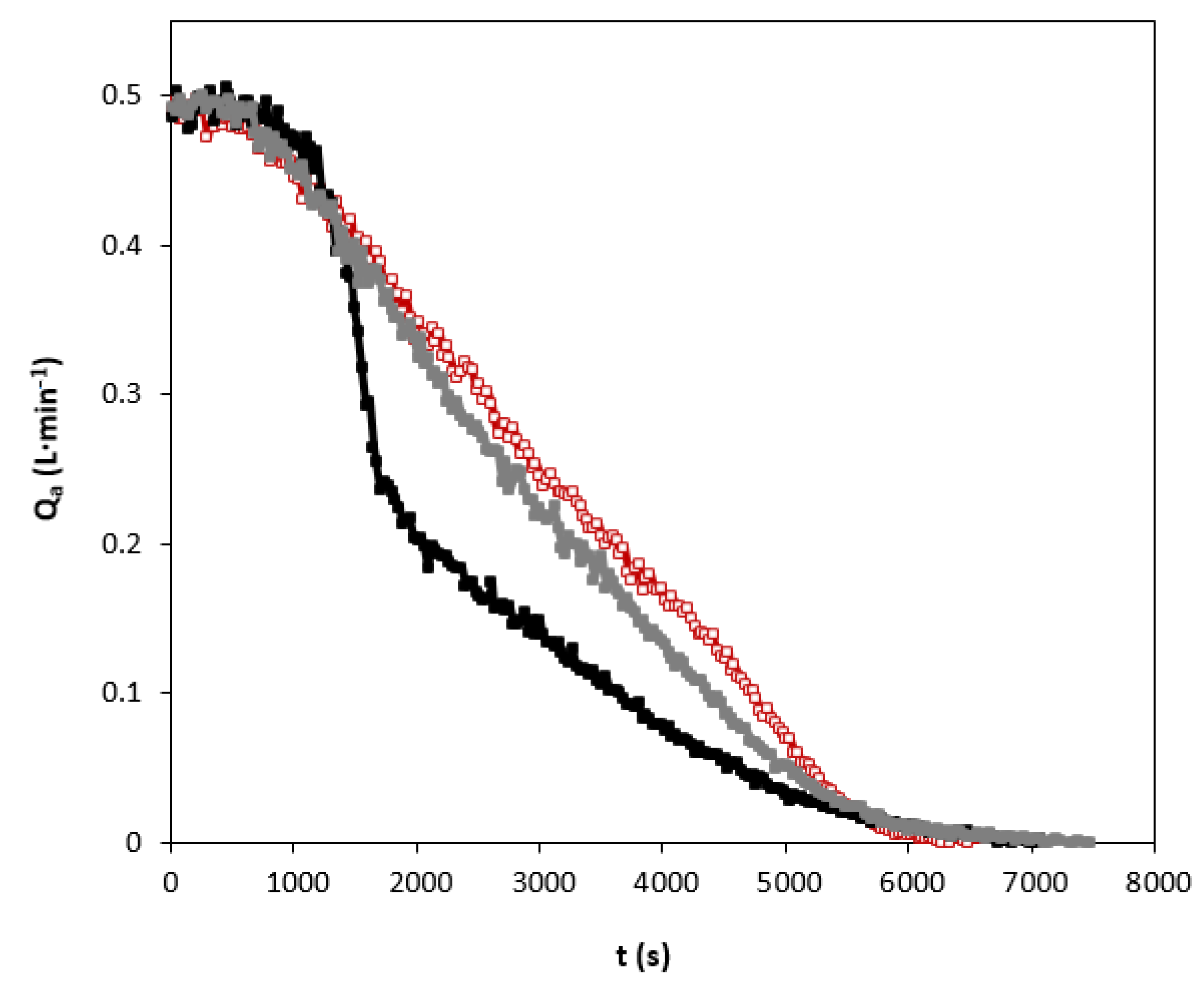
2.6. Solvent Regeneration Studies
3. Results
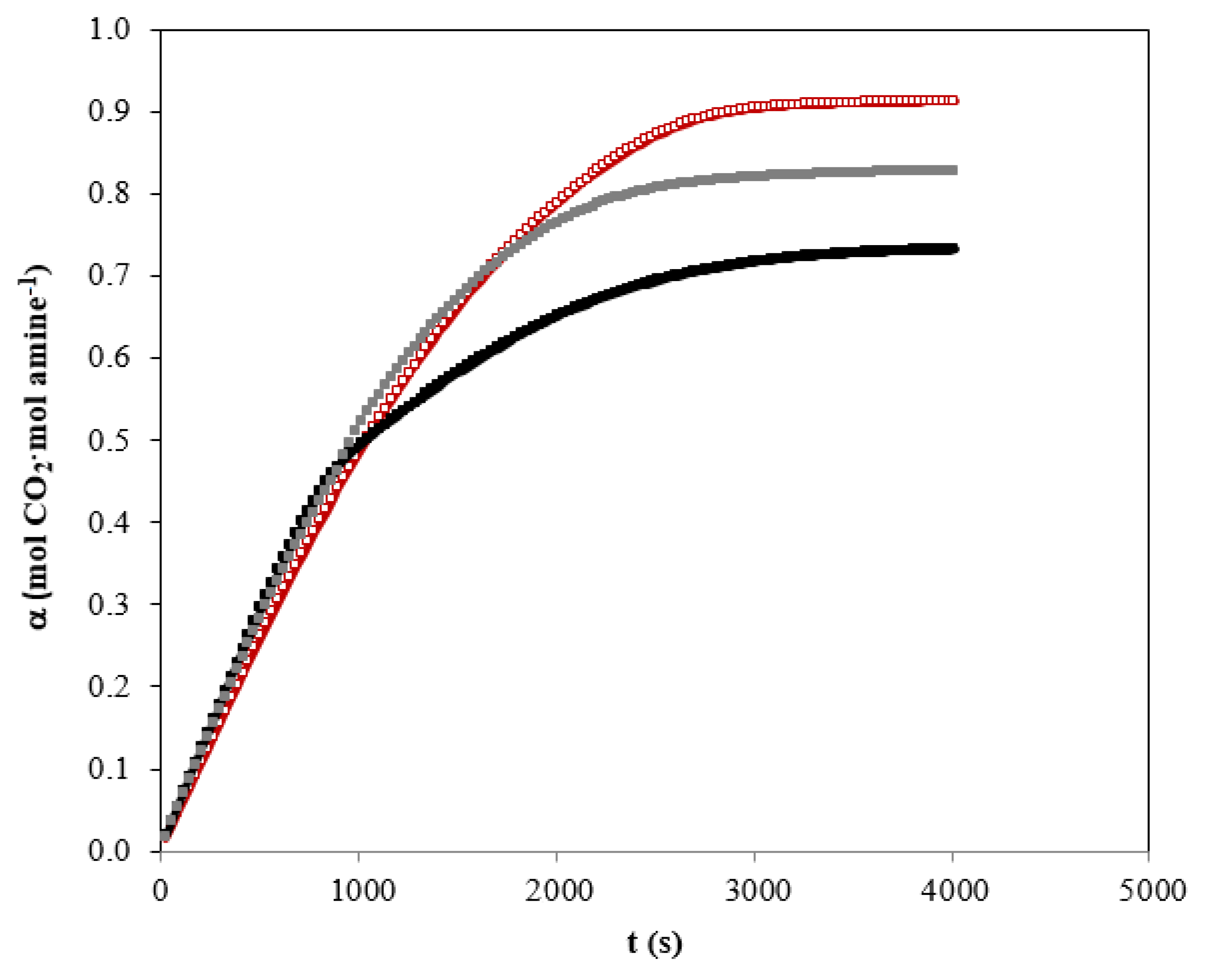
3.1. Absorption Experiments
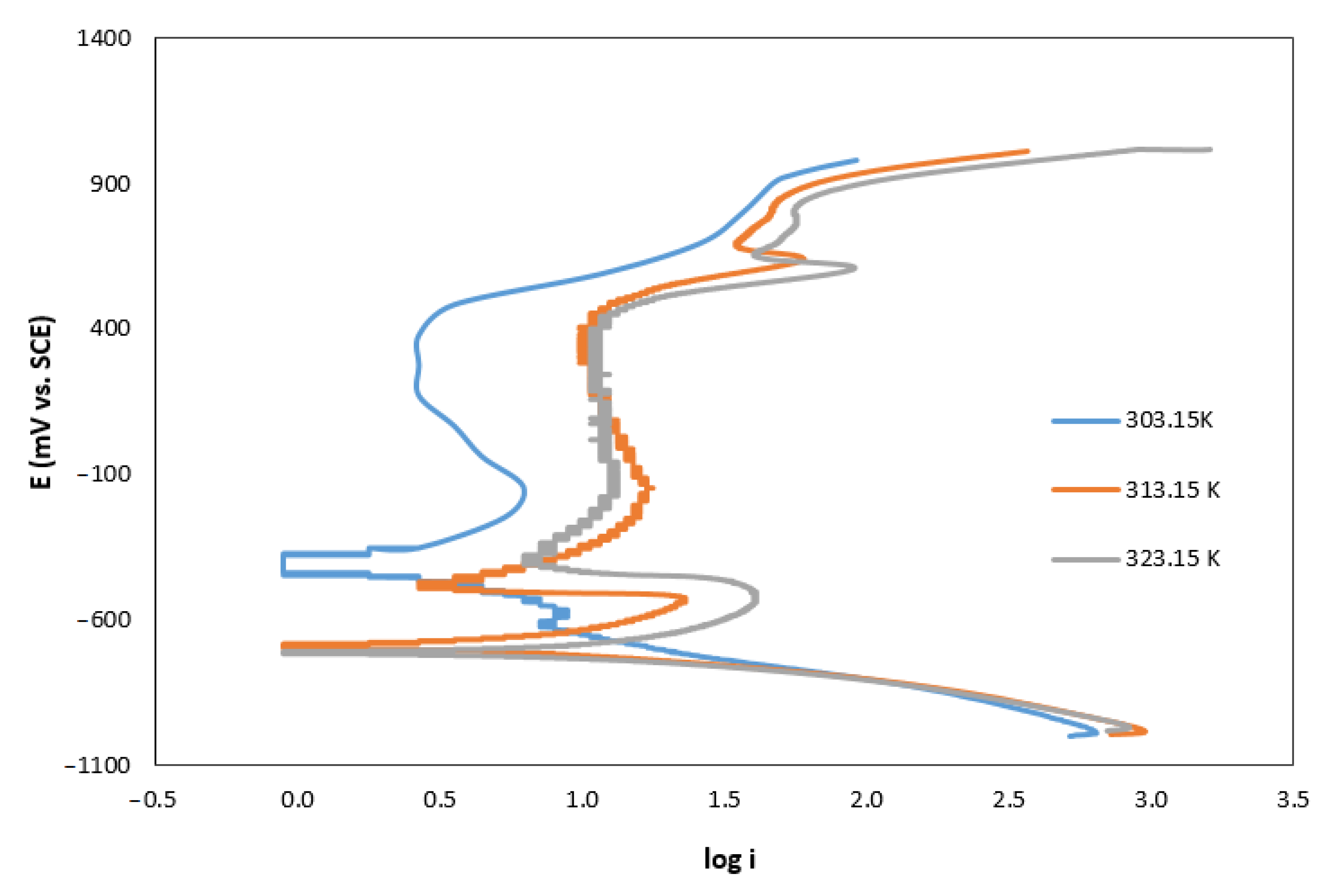
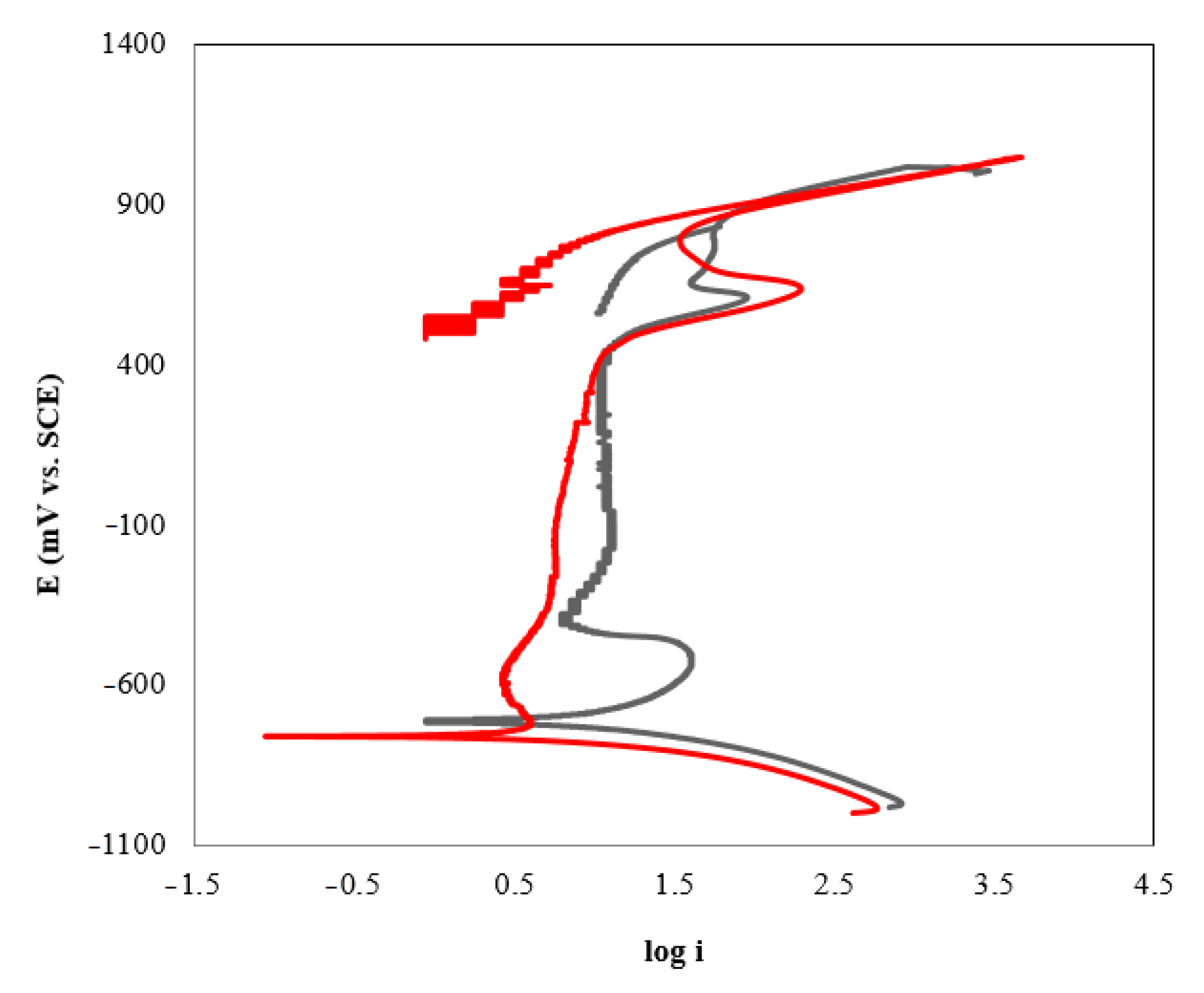
3.2. Corrosion Studies
3.3. Effect of the Absorbent Composition on Corrosion Rate
3.4. Bubble Column Studies
3.5. Solvent Regeneration Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Symbols used | ||
| A | [m2] | Reactor area |
| c | [m·s−1] | Speed of sound |
| CBO | [kmol·m−3] | Concentration |
| CR | [mm·year−1] | Corrosion rate |
| Ecorr | [mV] | Electrochemical potential |
| F | [C ·mol−1] | Faraday constant |
| icorr | [µA·cm−2] | Corrosion density |
| M | [g·mol−1] | Material molecular mass |
| n | Number of electrons | |
| NA | [kmol·m−2·s-1] | Flow density |
| P | [kPa] | Total pressure |
| Q′ | [m3 ·s−1] | Volumetric gas flow rate |
| R | [kPa· m3 ·K−1·kmol−1] | Gas constant |
| S | [cm2] | Surface exposed to the corrosive ambient |
| T | [K] | Temperature |
| t | [s] | Time |
| Greek symbols | ||
| α | [moles of carbon dioxide·moles of amine−1] | CO2 loading |
| η | [moles of carbon dioxide·moles of amine−1] | Carbonation relationship |
| ρ | [g·cm−3] | Density |
| η | [mPa·s] | Viscosity |
| σ | [mN·m−1] | Surface tension |
| βa | [mV·decade−1] | Anodic slope |
| βC | [mV·decade−1] | Cathodic slope |
| K | [-] | Constant of the used capillaries |
| θ | [-] | Correction value |
References
- Paraschiv, S.; Paraschiv, L.S. Trends of carbon dioxide (CO2) emissions from fossil fuels combustion (coal, gas and oil) in the EU member states from 1960 to 2018. Energy Rep. 2020, 6, 237–242. [Google Scholar] [CrossRef]
- Mardani, A.; Streimikiene, D.; Cavallaro, F.; Loganathan, N.; Khoshnoudi, M. Carbon dioxide (CO2) emissions and economic growth: A systematic review of two decades of research from 1995 to 2017. Sci. Total. Environ. 2019, 649, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Sovacool, B.K.; Griffiths, S.; Kim, J.; Bazilian, M. Climate change and industrial F-gases: A critical and systematic review of developments, sociotechnical systems and policy options for reducing synthetic greenhouse gas emissions. Renew. Sustain. Energy Rev. 2021, 141, 110759. [Google Scholar] [CrossRef]
- Mandald, B.; Bandyopadhyay, S.S. Simultaneous Absorption of CO2 and H2S Into Aqueous Blends ofN-Methyldiethanolamine and Diethanolamine. Environ. Sci. Technol. 2006, 40, 6076–6084. [Google Scholar] [CrossRef] [PubMed]
- Faiz, R.; Al-Marzouqi, M. Mathematical modeling for the simultaneous absorption of CO2 and H2S using MEA in hollow fiber membrane contactors. J. Membr. Sci. 2009, 342, 269–278. [Google Scholar] [CrossRef]
- Soosaiprakasam, I.R.; Veawab, A. Corrosion and polarization behavior of carbon steel in MEA-based CO2 capture process. Int. J. Greenh. Gas Control. 2008, 2, 553–562. [Google Scholar] [CrossRef]
- Kittel, J.; Fleury, E.; Vuillemin, B.; Gonzalez, S.; Ropital, F.; Oltra, R. Corrosion in alkanolamine used for acid gas removal: From natural gas processing to CO2 capture. Mater. Corros. 2010, 63, 223–230. [Google Scholar] [CrossRef]
- Sema, T.; Naami, A.; Fu, K.; Edali, M.; Liu, H.; Shi, H.; Liang, Z.; Idem, R.; Tontiwachwuthikul, P. Comprehensive mass transfer and reaction kinetics studies of CO2 absorption into aqueous solutions of blended MDEA-MEA. Chem. Eng. J. 2012, 209, 501–512. [Google Scholar] [CrossRef]
- DuPart, M.S.; Bacon, T.R.; Edwards, D.J. Understanding corrosion in alkanolamine gas treating plants. 1. Proper mechanism diagnosis optimizes amine operations. Hydrocarb. Process 1993, 72, 75–80. [Google Scholar]
- Kittel, J.; Gonzalez, S. Corrosion in CO2 post-combustion capture with alkanolamines. A review. Oil Gas Sci. Technol. Revue IFP Energies Nouv. 2014, 69, 915–929. [Google Scholar] [CrossRef]
- Schäffer, A.; Brechtel, K.; Scheffknecht, G. Comparative study on differently concentrated aqueous solutions of MEA and TETA for CO2 capture from flue gases. Fuel 2012, 101, 148–153. [Google Scholar] [CrossRef]
- Saha, A.K.; Bandyopadhyay, S.S.; Biswas, A.K. Kinetics of absorption of CO2 into aqueous solutions of 2-amino-2-methyl-1-propanol. Chem. Eng. Sci. 1995, 50, 3587–3598. [Google Scholar] [CrossRef]
- Patil, M.P.; Vaidya, P.D. Characterization of the superior CO2-capturing absorbent blend AMP/PZ/EGMEE/Water. Int. J. Greenh. Gas Control. 2019, 84, 29–35. [Google Scholar] [CrossRef]
- Nwaoha, C.; Tontiwachwuthikul, P.; Benamor, A. CO2 capture from lime kiln using AMP-DA2MP amine solvent blend: A pilot plant study. J. Environ. Chem. Eng. 2018, 6, 7102–7110. [Google Scholar] [CrossRef]
- Shi, H.; Cui, M.; Fu, J.; Dai, W.; Huang, M.; Han, J.; Quan, L.; Tontiwachwuthikul, P.; Liang, Z. Application of “coordinative effect” into tri-solvent MEA+BEA+AMP blends at concentrations of 0.1 + 2 + 2~0.5 + 2 + 2 mol/L with absorption, desorption and mass transfer analyses. Int. J. Greenh. Gas Control. 2021, 107, 103267. [Google Scholar] [CrossRef]
- Barbarossa, V.; Barzagli, F.; Mani, F.; Lai, S.; Stoppioni, P.; Vanga, G. Efficient CO2 capture by non-aqueous 2-amino-2-methyl-1-propanol (AMP) and low temperature solvent regeneration. RSC Adv. 2013, 3, 12349–12355. [Google Scholar] [CrossRef]
- Qian, Z.; Xu, L.-B.; Li, Z.-H.; Li, H.; Guo, K. Selective Absorption of H2S from a Gas Mixture with CO2by AqueousN-Methyldiethanolamine in a Rotating Packed Bed. Ind. Eng. Chem. Res. 2010, 49, 6196–6203. [Google Scholar] [CrossRef]
- Camacho, F.; Sanchez, S.; Pacheco, R.; Sanchez, A.; La Rubia, M.D. Thermal effects of CO2 absorption in aqueous solutions of 2-amino-2-methyl-1-propanol. AIChE J. 2005, 51, 2769–2777. [Google Scholar] [CrossRef]
- Dubois, L.; Mbasha, P.K.; Thomas, D. CO2 Absorption into Aqueous Solutions of a Polyamine (PZEA), a Sterically Hindered Amine (AMP), and their Blends. Chem. Eng. Technol. 2010, 33, 461–467. [Google Scholar] [CrossRef]
- Bougie, F.; Iliuta, M.C. Sterically Hindered Amine-Based Absorbents for the Removal of CO2 from Gas Streams. J. Chem. Eng. Data 2012, 57, 635–669. [Google Scholar] [CrossRef]
- Veawab, A.; Tontiwachwuthikul, P.; Bhole, S.D. Studies of Corrosion and Corrosion Control in a CO2−2-Amino-2-methyl-1-propanol (AMP) Environment. Ind. Eng. Chem. Res. 1997, 36, 264–269. [Google Scholar] [CrossRef]
- Veawab, A.; Tontiwachwuthikul, P.; Chakma, A. Influence of Process Parameters on Corrosion Behavior in a Sterically Hindered Amine−CO2 System. Ind. Eng. Chem. Res. 1999, 38, 310–315. [Google Scholar] [CrossRef]
- Veawab, A.; Tontiwachwuthikul, P.; Chakma, A. Corrosion Behavior of Carbon Steel in the CO2 Absorption Process Using Aqueous Amine Solutions. Ind. Eng. Chem. Res. 1999, 38, 3917–3924. [Google Scholar] [CrossRef]
- Tanthapanichakoon, W.; Veawab, A.; McGarvey, B. Electrochemical Investigation on the Effect of Heat-stable Salts on Corrosion in CO2 Capture Plants Using Aqueous Solution of MEA. Ind. Eng. Chem. Res. 2006, 45, 2586–2593. [Google Scholar] [CrossRef]
- Palmero, E.M.; La Rubia, M.D.; Sánchez-Bautista, A.; Pacheco, R.; Sanchez, S. Study of corrosion of AISI 420 in the CO2 absorption process using 2-amino-2-methyl-1-propanol aqueous solutions. Corros. Eng. Sci. Technol. 2013, 48, 136–142. [Google Scholar] [CrossRef]
- Camacho, F.; Sánchez, S.; Pacheco, R.; La Rubia, M.D.; Sánchez, A. Kinetics of the reaction of pure CO2 with N -methyldiethanolamine in aqueous solutions. Int. J. Chem. Kinet. 2008, 41, 204–214. [Google Scholar] [CrossRef]
- López, A.B.; Gómez-Díaz, D.; La Rubia, M.D.; Navaza, J.M. Effect of Carbon Dioxide Chemical Absorption on Bubble Diameter and Interfacial Area. Chem. Eng. Technol. 2013, 36, 1968–1974. [Google Scholar] [CrossRef]
- ASTM G1-03 (2017) e1. Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM G5-14e1. Standard Reference Test Method for Making Potentiodynamic Anodic Polarization Measurements; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM G61-86. Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron-, Nickel-, or Cobalt-Based Alloys; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Baltar, A.; Gómez-Díaz, D.; Navaza, J.M.; Rumbo, A. Absorption and regeneration studies of chemical solvents based on dimethylethanolamine and diethylethanolamine for carbon dioxide capture. AIChE J. 2020, 66, 1. [Google Scholar] [CrossRef]
- Astarita, G.; Marrucci, G.; Gioia, F. The influence of carbonation ratio and total amine concentration on carbon dioxide absorption in aqueous monoethanolamine solutions. Chem. Eng. Sci. 1964, 19, 95–103. [Google Scholar] [CrossRef]
- Blauwhoff, P.; Versteeg, G.; Van Swaaij, W. A study on the reaction between CO2 and alkanolamines in aqueous solutions. Chem. Eng. Sci. 1984, 39, 207–225. [Google Scholar] [CrossRef]
- Camacho, F.; Sanchez, S.; Pacheco, R. Absorption of Carbon Dioxide at High Partial Pressures in 1-Amino-2-propanol Aqueous Solution. Considerations of Thermal Effects. Ind. Eng. Chem. Res. 1997, 36, 4358–4364. [Google Scholar] [CrossRef]
- Danckwerts, P. The reaction of CO2 with ethanolamines. Chem. Eng. Sci. 1979, 34, 443–446. [Google Scholar] [CrossRef]
- Chakraborty, A.; Astarita, G.; Bischoff, K. CO2 absorption in aqueous solutions of hindered amines. Chem. Eng. Sci. 1986, 41, 997–1003. [Google Scholar] [CrossRef]
- Danckwerts, P.; McNeil, K. The absorption of carbon dioxide into aqueous amine solutions and the effects of catalysis. Insights Into Chem. Eng. 1981, 45, T32–T49. [Google Scholar] [CrossRef]
- Rayer, A.V.; Kadiwala, S.; Narayanaswamy, K.; Henni, A. Volumetric Properties, Viscosities, and Refractive Indices for Aqueous 1-Amino-2-Propanol (Monoisopropanolamine (MIPA)) Solutions from (298.15 to 343.15) K. J. Chem. Eng. Data 2010, 55, 5562–5568. [Google Scholar] [CrossRef]
- Alvarez, E.; Cancela, Á.; Maceiras, R.; Navaza, J.M.; Táboas, R. Surface Tension of Aqueous Binary Mixtures of 1-Amino-2-Propanol and 3-Amino-1-Propanol, and Aqueous Ternary Mixtures of These Amines with Diethanolamine, Triethanolamine, and 2-Amino-2-methyl-1-propanol from (298.15 to 323.15) K. J. Chem. Eng. Data 2003, 48, 32–35. [Google Scholar] [CrossRef]
- DuPart, M.S.; Bacon, T.R.; Edwards, D.J. Understanding corrosion in alkanolamine gas treating plants. 2. Hydrocarb. Process 1993, 72, 89–94. [Google Scholar]
- Davies, D.H.; Burstein, G.T. The Effects of Bicarbonate on the Corrosion and Passivation of Iron. Corrosion 1980, 36, 416–422. [Google Scholar] [CrossRef]
- Fontana, M.G. Corrosion Engineering; McGraw-Hill: New York, NY, USA, 1986. [Google Scholar]
- Tafel, J.; Schmitz, K.; Naremann, K.; Emmert, B. Uber die Polarization bei Kathodischer der Protonuebertragung. Z. Phys. Chem. 1905, 50, 641. [Google Scholar]
- Lopez, A.; La Rubia, M.D.; Navaza, J.; Pacheco, R.; Gómez-Díaz, D. Characterization of MIPA and DIPA aqueous solutions in relation to absorption, speciation and degradation. J. Ind. Eng. Chem. 2015, 21, 428–435. [Google Scholar] [CrossRef]

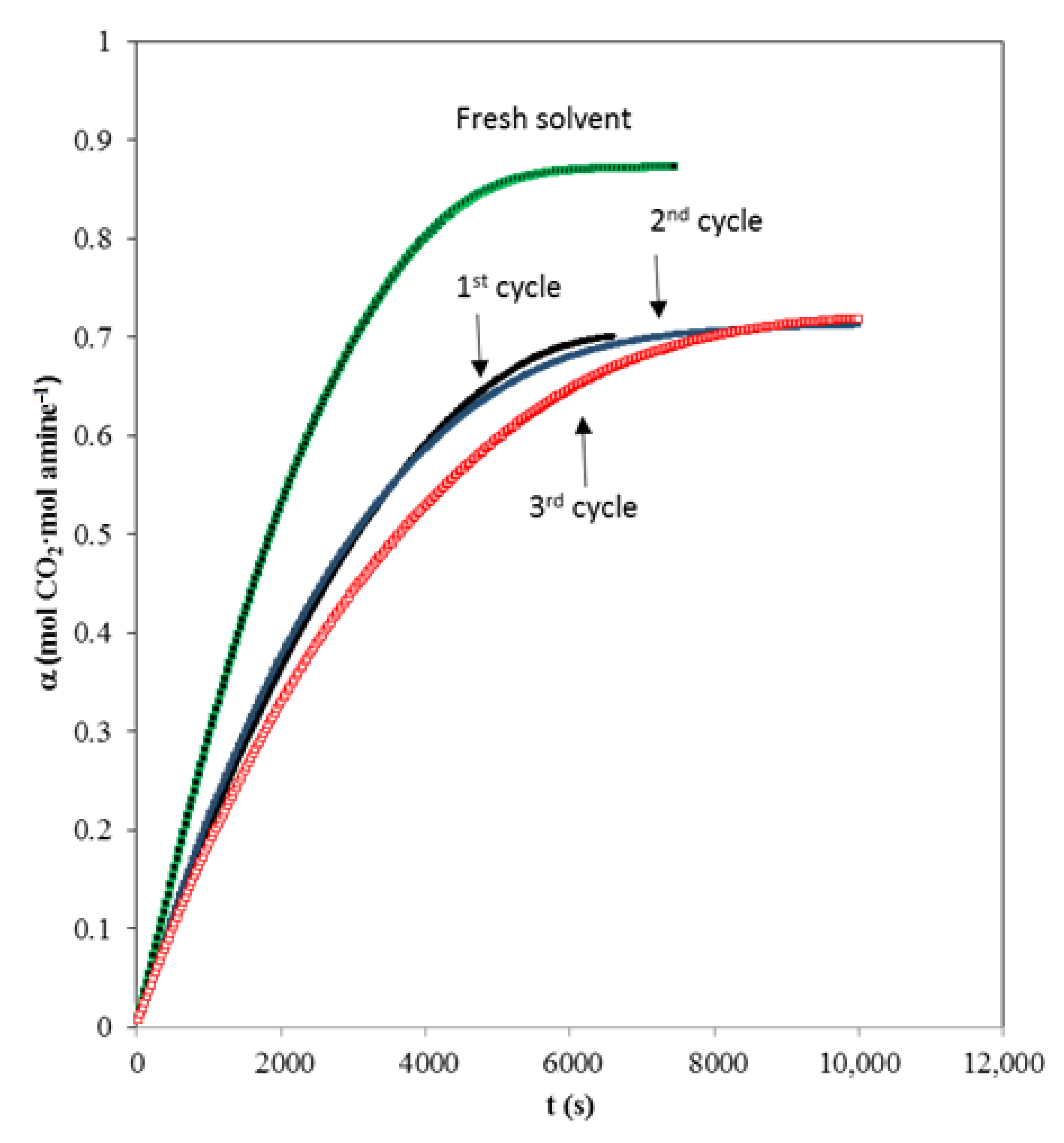
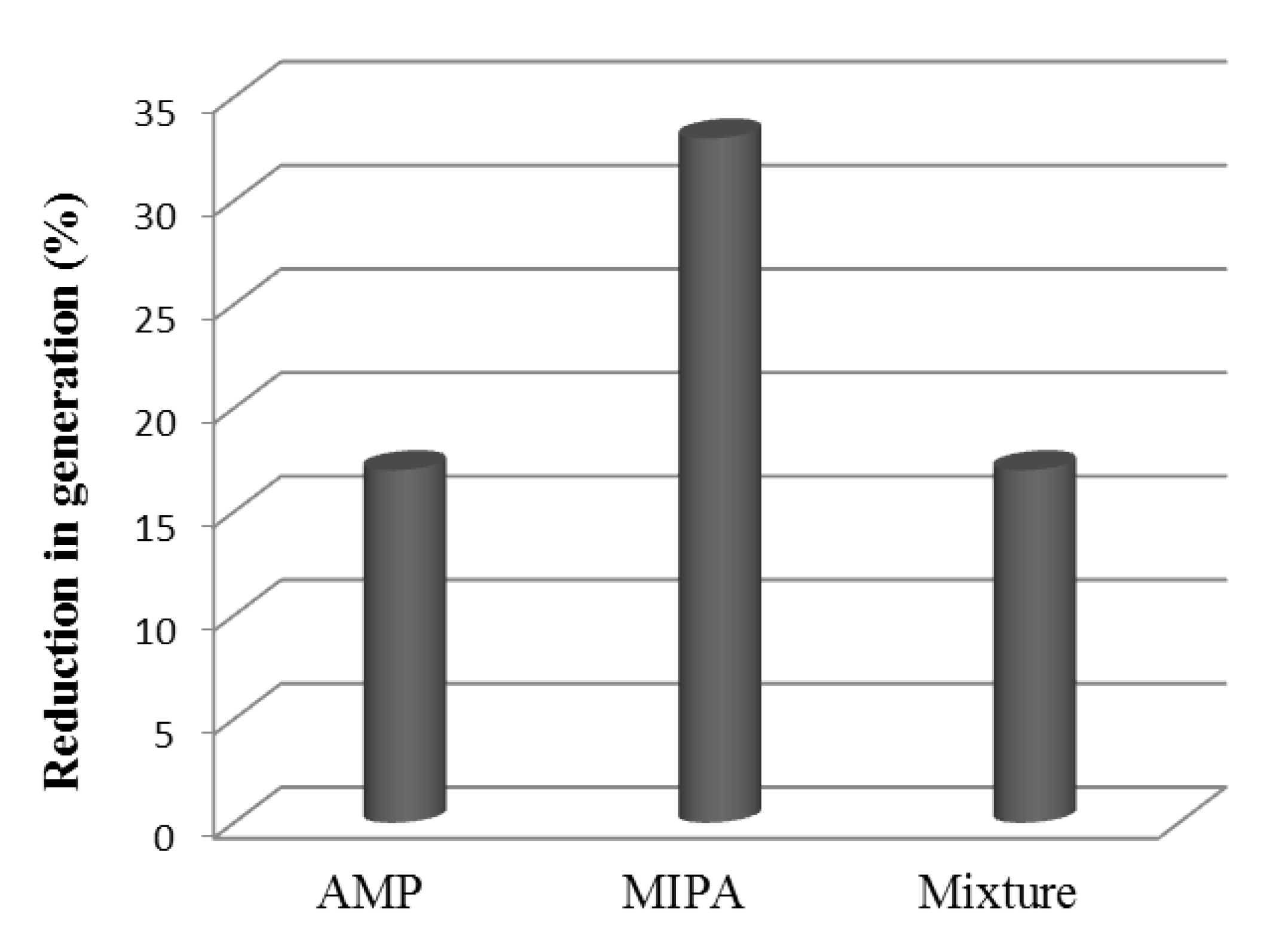
| AMP Concentration (kmol·m−3) | MIPA Concentration (kmol·m−3) | NA·106 (kmol·m−2·s−1) T (K) = 303.15 | NA·106 (kmol·m−2·s−1) T (K) = 313.15 | NA·106 (kmol·m−2·s−1) T (K) = 323.15 |
|---|---|---|---|---|
| 1.0 | 0.00 | 3.876 | 6.859 | 7.36 |
| 0.10 | 5.147 | 7.815 | 8.769 | |
| 0.25 | 5.193 | 7.945 | 8.792 | |
| 0.50 | 4.204 | 5.868 | 6.962 | |
| 0.0 | 0.10 | 1.010 | 2.954 | 4.532 |
| 0.25 | 1.878 | 3.430 | 4.860 | |
| 0.50 | 3.197 | 4.222 | 5.325 |
| AMP Concentration (kmol·m−3) | MIPA Concentration (kmol·m−3) | T (K) = 303.15 | T (K) = 313.15 | T (K) = 323.15 |
|---|---|---|---|---|
| ρ (g·cm−3) | ρ (g·cm−3) | ρ (g·cm−3) | ||
| 1.0 | 0.00 | 0.994111 | 0.98869 | 0.981359 |
| 0.10 | 0.994254 | 0.989225 | 0.98231 | |
| 0.25 | 0.994411 | 0.989863 | 0.982751 | |
| 0.50 | 0.994661 | 0.990185 | 0.983855 | |
| η (mPa·s) | η (mPa·s) | η (mPa·s) | ||
| 1.0 | 0.00 | 1.08935270 | 0.81854676 | 0.62634402 |
| 0.10 | 1.13021224 | 0.85803828 | 0.65123491 | |
| 0.25 | 1.18065910 | 0.89676943 | 0.68355647 | |
| 0.50 | 1.26791521 | 0.96210583 | 0.73541749 | |
| c (m·s−1) | c (m·s−1) | c (m·s−1) | ||
| 1.0 | 0.00 | 1572.66 | 1583.08 | 1588.03 |
| 0.10 | 1576.92 | 1587.00 | 1591.90 | |
| 0.25 | 1583.23 | 1591.57 | 1596.12 | |
| 0.50 | 1594.65 | 1601.33 | 1603.34 | |
| σ (mN·m−1) | σ (mN·m−1) | σ (mN·m−1) | ||
| 1.0 | 0.00 | 52.11 | 43.92 | 40.09 |
| 0.10 | 47.19 | 41.00 | 39.00 | |
| 0.25 | 45.48 | 39.81 | 39.12 | |
| 0.50 | 47.28 | 44.18 | 43.29 |
| AMP Concentration (kmol·m−3) | MIPA Concentration (kmol·m−3) | T (K) | Ecorr (mV) | βa (mV·Decade−1) | βc (mV·Decade−1) | icorr (µA·cm−2) | CR (mm·Year−1) |
|---|---|---|---|---|---|---|---|
| 1.0 | 0.10 | 303 | −391.65 | 150.66 | −161.25 | 2.783 | 0.216 |
| 313 | −691.83 | 171.97 | −72.80 | 4.313 | 0.335 | ||
| 323 | −713.90 | 162.02 | −81.39 | 7.523 | 0.584 | ||
| 0.25 | 303 | −700.70 | 211.44 | −87.97 | 1.185 | 0.092 | |
| 313 | −605.21 | 173.22 | −180.66 | 1.469 | 0.114 | ||
| 323 | −599.02 | 113.98 | −153.02 | 2.963 | 0.230 | ||
| 0.50 | 303 | −447.20 | 151.81 | −155.00 | 0.464 | 0.036 | |
| 313 | −696.27 | 215.03 | −53.64 | 0.609 | 0.047 | ||
| 323 | −737.20 | 64.58 | −43.37 | 2.053 | 0.159 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Bautista, A.; Palmero, E.M.; Moya, A.J.; Gómez-Díaz, D.; La Rubia, M.D. Characterization of Alkanolamine Blends for Carbon Dioxide Absorption. Corrosion and Regeneration Studies. Sustainability 2021, 13, 4011. https://doi.org/10.3390/su13074011
Sánchez-Bautista A, Palmero EM, Moya AJ, Gómez-Díaz D, La Rubia MD. Characterization of Alkanolamine Blends for Carbon Dioxide Absorption. Corrosion and Regeneration Studies. Sustainability. 2021; 13(7):4011. https://doi.org/10.3390/su13074011
Chicago/Turabian StyleSánchez-Bautista, Alfredo, Ester M. Palmero, Alberto J. Moya, Diego Gómez-Díaz, and M. Dolores La Rubia. 2021. "Characterization of Alkanolamine Blends for Carbon Dioxide Absorption. Corrosion and Regeneration Studies" Sustainability 13, no. 7: 4011. https://doi.org/10.3390/su13074011
APA StyleSánchez-Bautista, A., Palmero, E. M., Moya, A. J., Gómez-Díaz, D., & La Rubia, M. D. (2021). Characterization of Alkanolamine Blends for Carbon Dioxide Absorption. Corrosion and Regeneration Studies. Sustainability, 13(7), 4011. https://doi.org/10.3390/su13074011







