Bacterial Spore-Based Hygromorphs: A Novel Active Material with Potential for Architectural Applications
Abstract
1. Introduction
2. Background
2.1. Hygromorphic Materials
2.2. Application of Hygromorphic Mechanisms in Architecture
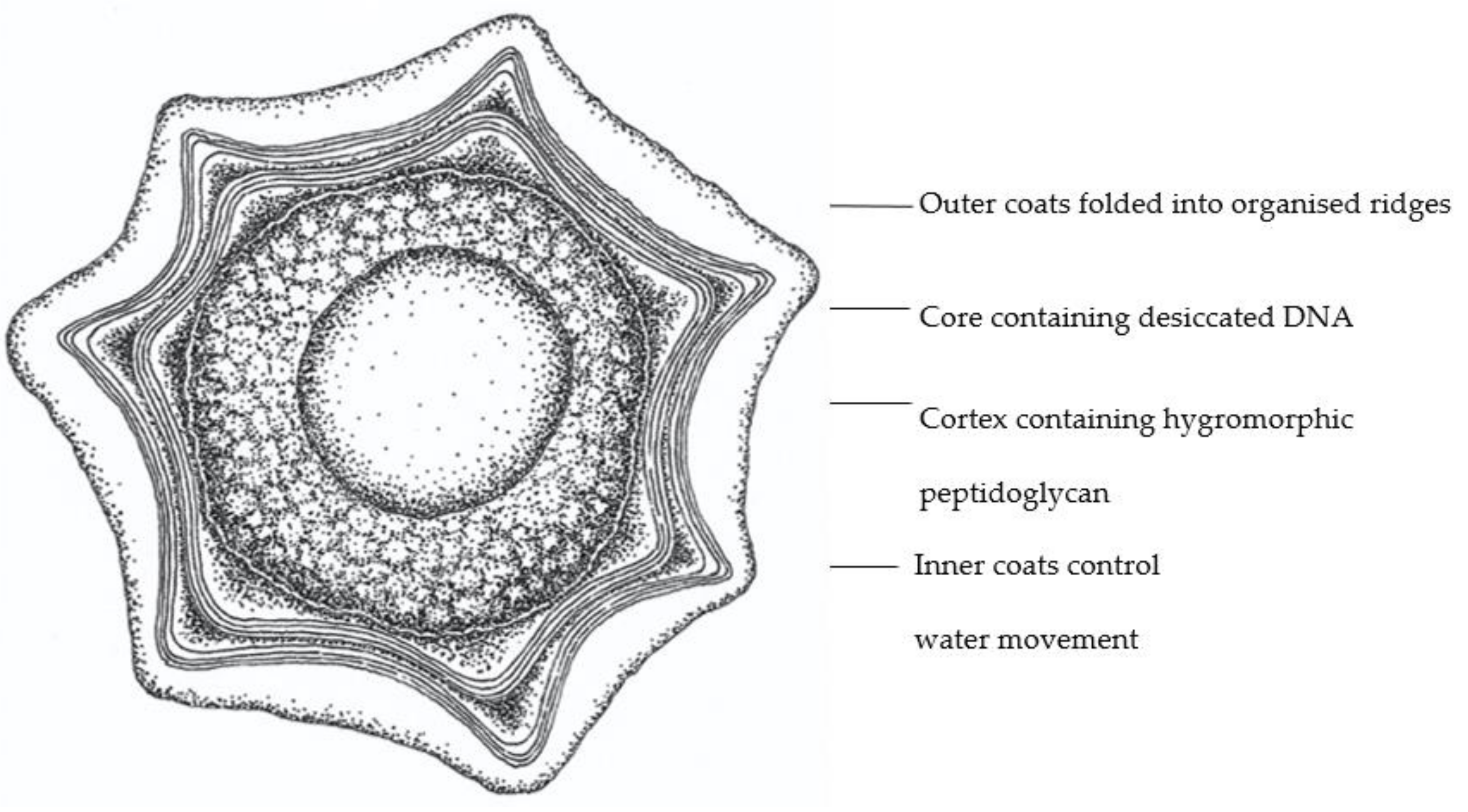
2.3. Hygromorphic Nature of Bacillus subtilis Spores
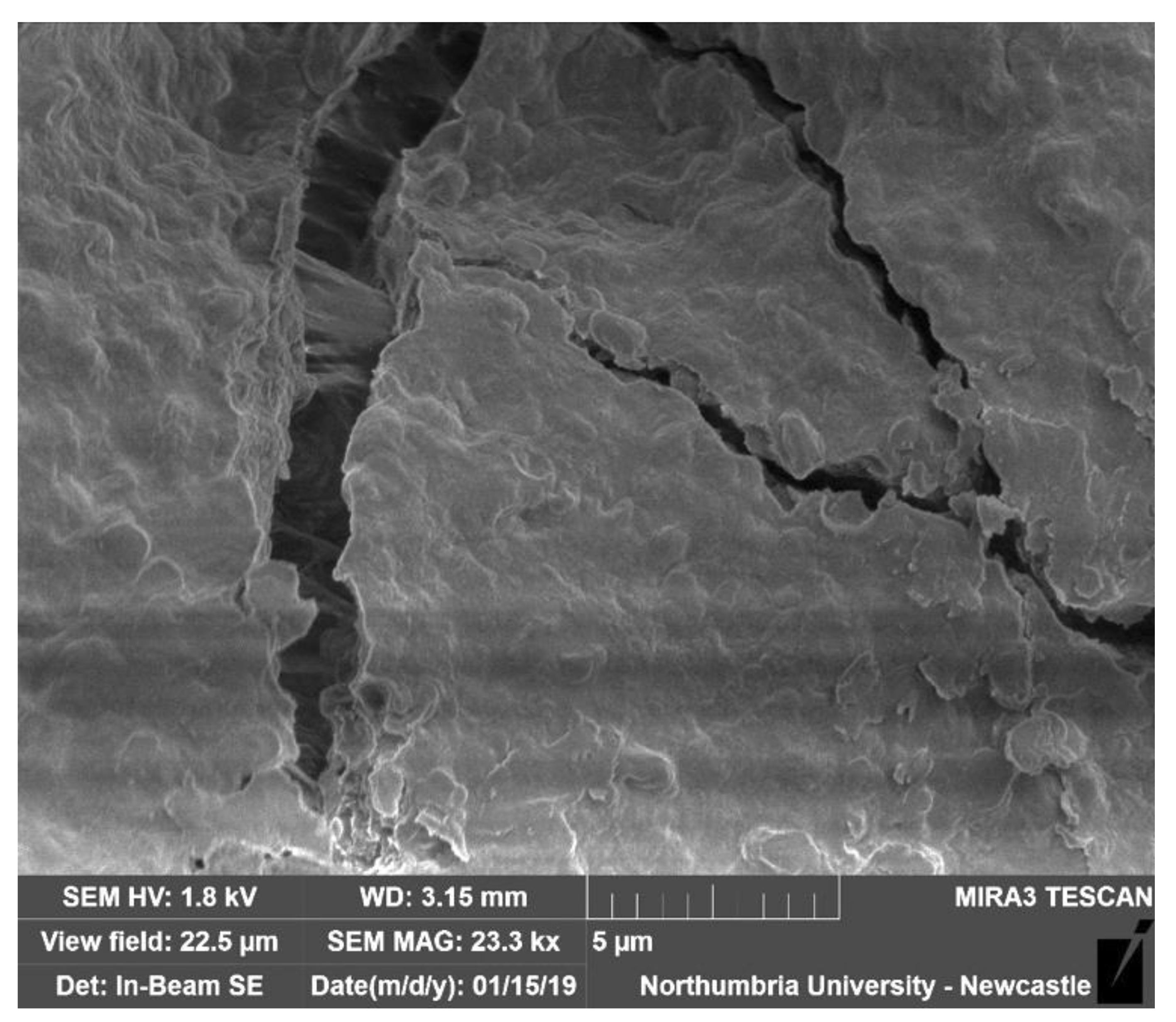
2.4. Spores Implemented as Part of a Bio-Hybrid Material
3. Materials and Methods
3.1. Selection of Spore Type
3.2. Culturing of Bacillus subtilis Spores
3.3. Selection of Inert Substrate Layer from Existing Studies
- Negligible expansion and contraction with changing moisture content;
- Suitable surface for spore dispersion and adhesion;
- Sufficient axial stiffness to ensure that spore expansion induces curvature (as opposed to linear stretching of the substrate);
- Suitable bending stiffness (which is a function of axial stiffness and strip thickness) to give the required balance between deflection and actuation force.
3.4. Application of Spores to Inert Layer (Latex) to Form a Hygromorphic Bilayer
3.5. Hygromorphic Bilayer Performance Testing
3.6. Preparation of Hygromorphic Actuators for Loading Experiments
4. Results
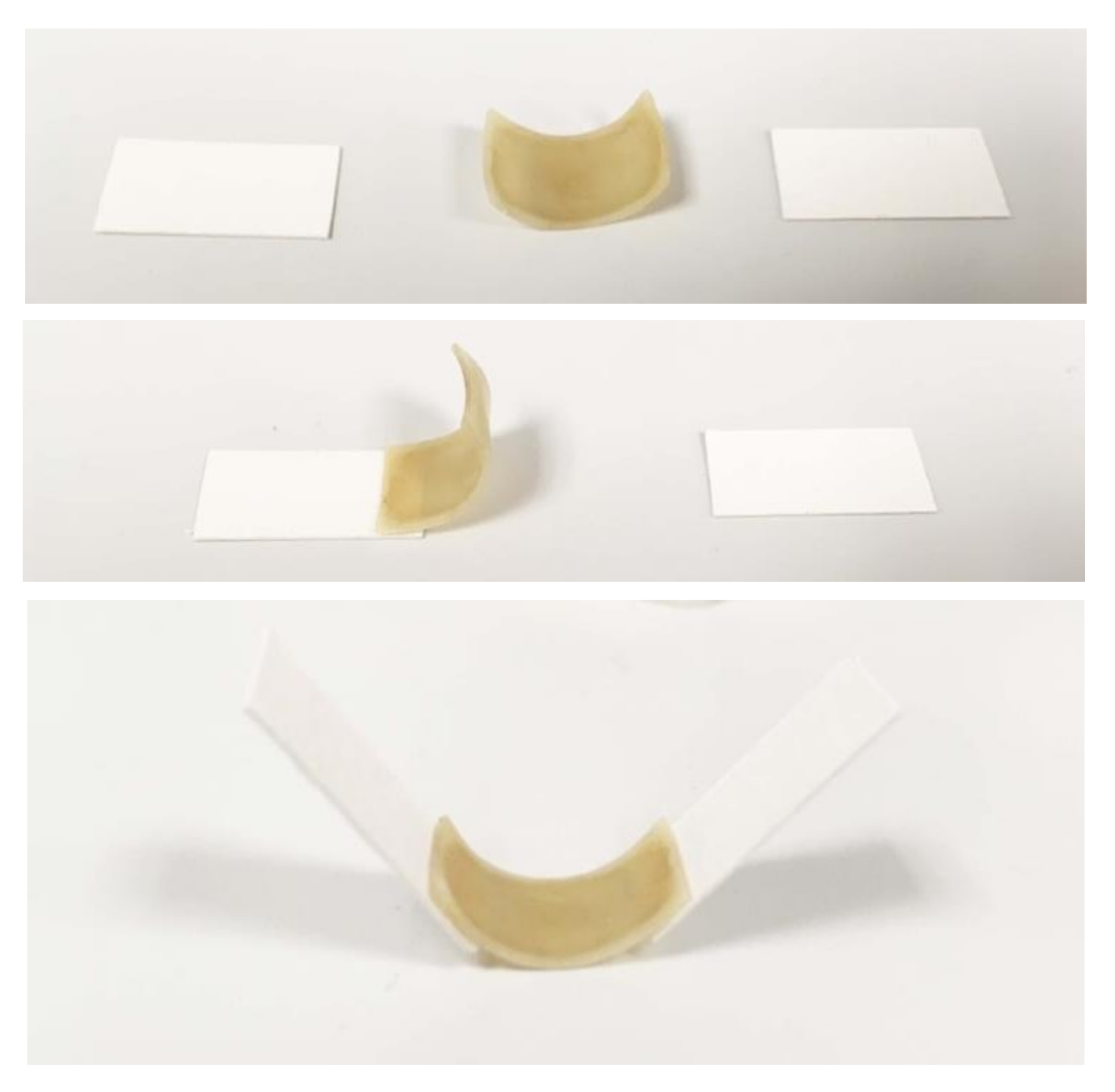
4.1. Actuator Deflection and Programmability Using Multiple Spore Layers
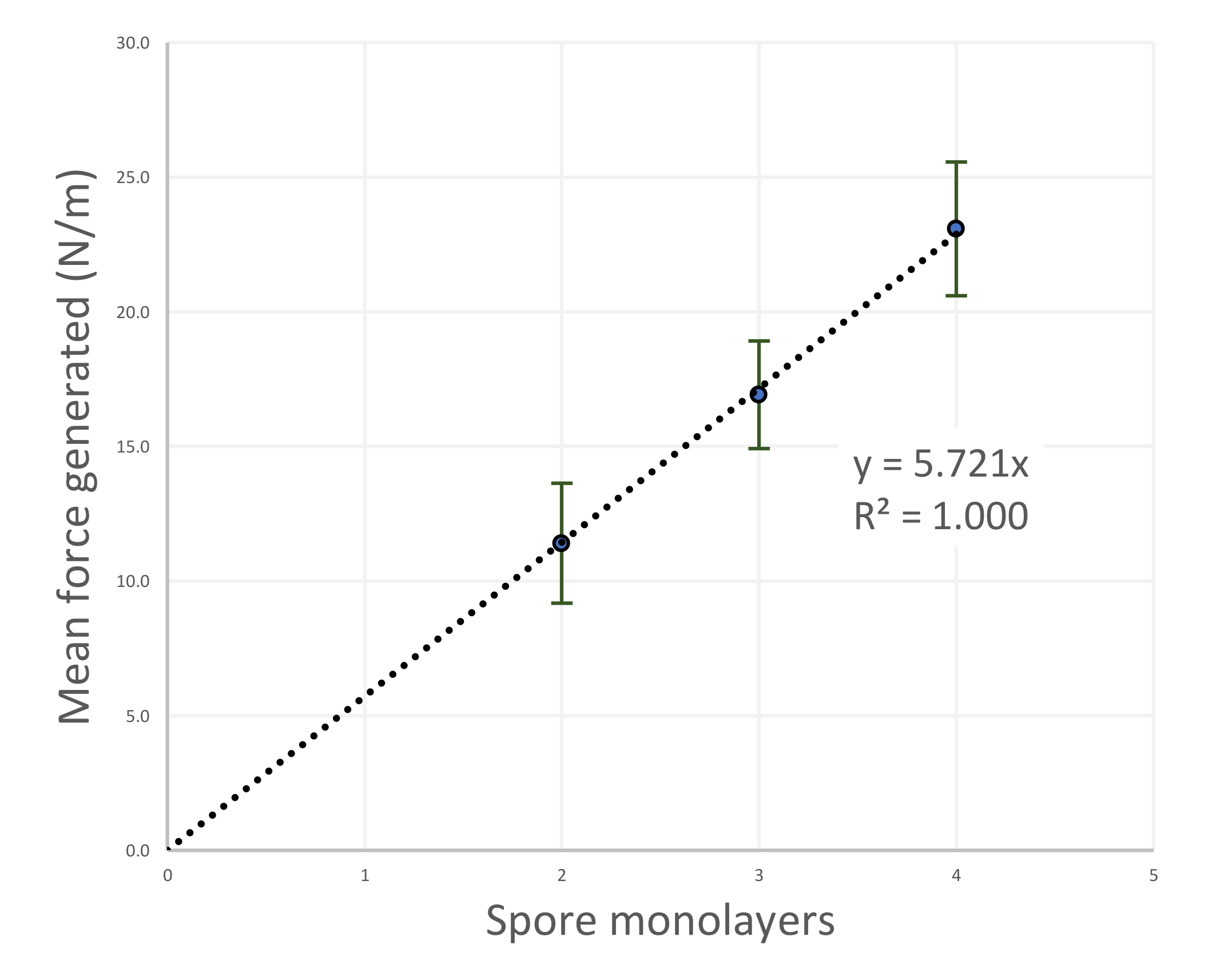
4.2. Forces Generated by the Hygromorphic Actuators
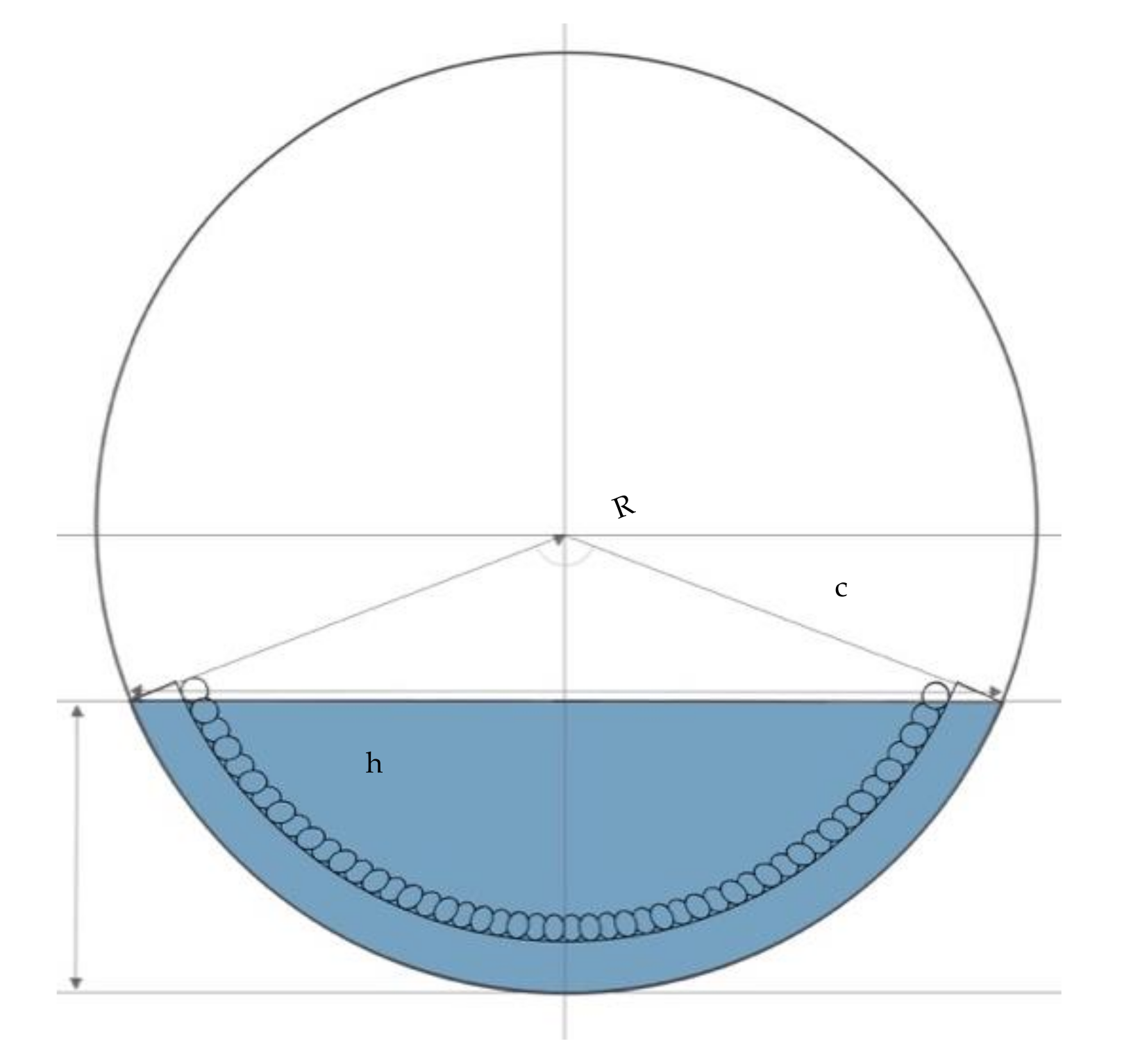
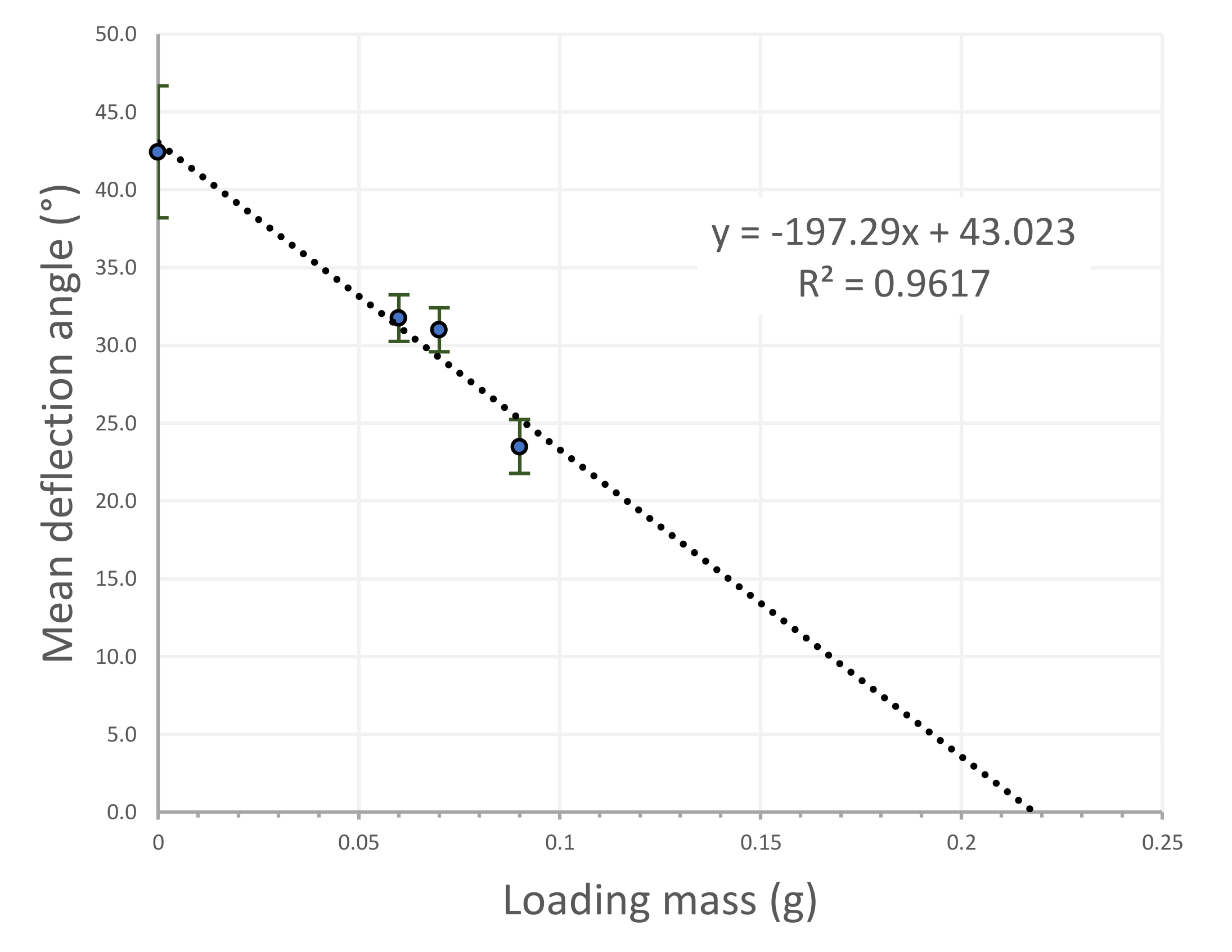
4.3. Capability of Hygromorphic Actuators to Do Work
5. Discussion
5.1. Speed and Angle of Deformation
5.2. Force Output
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Koyaz, M. Adaptability Level of Facade Systems Regarding Facade Adaptability Level of Facade Systems. In Proceedings of the International Conference on Building Envelope Systems and Technologies (ICBEST 2017), Istanbul, Turkey, 15–18 May 2017. [Google Scholar]
- Augustin, N. Motion with Moisture: Creating Passive Dynamic Envelope Systems Using the Hygroscopic Properties of Wood Veneer; University of Waterloo: Waterloo, ON, Canada, 2018. [Google Scholar]
- Banfill, P.F.G.; Peacock, A.D.; Ban¢ll, P.F.G. Energy-efficient new housing–the UK reaches for sustainability. Build. Res. Inf. 2007, 35, 426–436. [Google Scholar] [CrossRef]
- Holstov, A.; Bridgens, B.; Farmer, G. Hygromorphic materials for sustainable responsive architecture. Constr. Build. Mater. 2015, 98, 570–582. [Google Scholar] [CrossRef]
- Reichert, S.; Menges, A.; Correa, D. Meteorosensitive architecture: Biomimetic building skins based on materially embedded and hygroscopically enabled responsiveness. Comput. Des. 2015, 60, 50–69. [Google Scholar] [CrossRef]
- Chen, X.; Mahadevan, L.; Driks, A.; Sahin, O. Bacillus spores as building blocks for stimuli-responsive materials and nanogenerators. Nat. Nanotechnol. 2014, 9, 137–141. [Google Scholar] [CrossRef]
- Hong, H.A.; Duc, L.H.; Cutting, S.M. The use of bacterial spore formers as probiotics: Table 1. FEMS Microbiol. Rev. 2005, 29, 813–835. [Google Scholar] [CrossRef]
- Chen, X.; Goodnight, D.; Gao, Z.; Cavusoglu, A.H.; Sabharwal, N.; DeLay, M.; Driks, A.; Sahin, O. Scaling up nanoscale water-driven energy conversion into evaporation-driven engines and generators. Nat. Commun. 2015, 6, 7346. [Google Scholar] [CrossRef]
- Wang, W.; Yao, L.; Cheng, C.-Y.; Zhang, T.; Atsumi, H.; Wang, L.; Wang, G.; Anilionyte, O.; Steiner, H.; Ou, J.; et al. Harnessing the hygroscopic and biofluorescent behaviors of genetically tractable microbial cells to design biohybrid wearables. Sci. Adv. 2017, 3, e1601984. [Google Scholar] [CrossRef]
- Ramirez-Figueroa, C.; Hernan, L.; Guyet, A.; Dade-Robertson, M. Bacterial Hygromorphs. In Proceedings of the 36th Annual Conference of the Association for Computer Aided Design in Architecture, Cambridge, MA, USA, 2–4 November 2017. [Google Scholar]
- Le Duigou, A.; Castro, M. Evaluation of force generation mechanisms in natural, passive hydraulic actuators. Sci. Rep. 2016, 6, srep18105. [Google Scholar] [CrossRef]
- Arends, T.T.; Pel, L.L.; Huinink, H.H. Hygromorphic response dynamics of oak: Towards accelerated material characterization. Mater. Struct. 2017, 50, 181. [Google Scholar] [CrossRef]
- Erb, R.M.; Sander, J.S.; Grisch, R.; Studart, A.R. Self-shaping composites with programmable bioinspired microstructures. Nat. Commun. 2013, 4, 1712. [Google Scholar] [CrossRef]
- Reyssat, E.; Mahadevan, L. Hygromorphs: From pine cones to biomimetic bilayers. J. R. Soc. Interface 2009, 6, 951–957. [Google Scholar] [CrossRef]
- Cavusoglu, A.-H.; Chen, X.; Gentine, P.; Sahin, O. Potential for natural evaporation as a reliable renewable energy resource. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Scott, J. The University Of Leeds Responsive Knit: The evolution of a programmable material system. DRS2018: Catalyst 2018, 4. [Google Scholar] [CrossRef]
- Holstov, A.; Farmer, G.; Bridgens, B. Sustainable Materialisation of Responsive Architecture. Sustain. J. Rec. 2017, 9, 435. [Google Scholar] [CrossRef]
- Van Der Hofstadt, M.; Fabregas, R.; Millan-Solsona, R.; Juarez, A.; Fumagalli, L.; Gomila, G. Internal Hydration Properties of Single Bacterial Endospores Probed by Electrostatic Force Microscopy. ACS Nano 2016, 10, 11327–11336. [Google Scholar] [CrossRef]
- Arora, S.; Kumar, N.; Yadav, A.; Raghu, H.V. Spore: Potential of Invaluable Bacterial Wrap. Int. J. Life-Sci. Sci. Res. 2016, 2, 513–518. [Google Scholar] [CrossRef]
- Henriques, A.O.; Moran, C.P., Jr. Structure, Assembly, and Function of the Spore Surface Layers. Annu. Rev. Microbiol. 2007, 61, 555–588. [Google Scholar] [CrossRef]
- Plomp, M.; Carroll, A.M.; Setlow, P.; Malkin, A.J. Architecture and Assembly of the Bacillus subtilis Spore Coat. PLoS ONE 2014, 9, e108560. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Munakata, N.; Horneck, G.; Melosh, H.J.; Setlow, P. Resistance of Bacillus Endospores to Extreme Terrestrial and Extraterrestrial Environments. Microbiol. Mol. Biol. Rev. 2000, 64, 548–572. [Google Scholar] [CrossRef]
- Driks, A. The dynamic spore. Proc. Natl. Acad. Sci. USA 2003, 100, 3007–3009. [Google Scholar] [CrossRef]
- Zolock, R.A.; Li, G.; Bleckmann, C.; Burggraf, L.; Fuller, D.C. Atomic force microscopy of Bacillus spore surface morphology. Micron 2006, 37, 363–369. [Google Scholar] [CrossRef][Green Version]
- Ghosal, S.; Leighton, T.J.; Wheeler, K.E.; Hutcheon, I.D.; Weber, P.K. Spatially Resolved Characterization of Water and Ion Incorporation in Bacillus Spores. Appl. Environ. Microbiol. 2010, 76, 3275–3282. [Google Scholar] [CrossRef]
- Sahin, O.; Yong, E.H.; Driks, A.; Mahadevan, L. Physical basis for the adaptive flexibility of Bacillus spore coats. J. R. Soc. Interface 2012, 9, 3156–3160. [Google Scholar] [CrossRef]
- Garg, N. New Technologies in Renewable Power. Международный Научно-Исследовательский Журнал 2014, 2, 988–995. [Google Scholar]
- Joshi, L.T.; Phillips, D.S.; Williams, C.F.; Alyousef, A.; Baillie, L. Contribution of Spores to the Ability of Clostridium difficile to Adhere to Surfaces. Appl. Environ. Microbiol. 2012, 78, 7671–7679. [Google Scholar] [CrossRef]
- Yao, L.; Ou, J.; Wang, G.; Cheng, C.-Y.; Wang, W.; Steiner, H.; Ishii, H. bioPrint: A Liquid Deposition Printing System for Natural Actuators. 3D Print. Addit. Manuf. 2015, 2, 168–179. [Google Scholar] [CrossRef]
- Tehri, N.; Kumar, N.; Raghu, H.V.; Shukla, R.; Vashishth, A. Microbial Bioprospecting for Sustainable Development; Springer: Singapore, 2018; pp. 279–289. [Google Scholar]
- Sunde, E.P.; Setlow, P.; Hederstedt, L.; Halle, B. The physical state of water in bacterial spores. Proc. Natl. Acad. Sci. USA 2009, 106, 19334–19339. [Google Scholar] [CrossRef]
- Westphal, A.J.; Price, P.B.; Leighton, T.J.; Wheeler, K.E. Kinetics of size changes of individual Bacillus thuringiensis spores in response to changes in relative humidity. Proc. Natl. Acad. Sci. USA 2003, 100, 3461–3466. [Google Scholar] [CrossRef]
- Yao, L.; Ou, J.; Cheng, C.-Y.; Steiner, H.; Wang, W.; Wang, G.; Ishii, H. bioLogic. In Proceedings of the 33rd Annual ACM Conference on Human Factors in Computing Systems; ACM: New York, NY, USA, 2015; pp. 1–10. [Google Scholar]
- Zhou, K.X.; Li, N.; Christie, G.; Wilson, D.I. Assessing the Impact of Germination and Sporulation Conditions on the Adhesion ofBacillusSpores to Glass and Stainless Steel by Fluid Dynamic Gauging. J. Food Sci. 2017, 82, 2614–2625. [Google Scholar] [CrossRef]
- Craig, S.; Grinham, J. Breathing walls: The design of porous materials for heat exchange and decentralized ventilation. Energy Build. 2017, 149, 246–259. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birch, E.; Bridgens, B.; Zhang, M.; Dade-Robertson, M. Bacterial Spore-Based Hygromorphs: A Novel Active Material with Potential for Architectural Applications. Sustainability 2021, 13, 4030. https://doi.org/10.3390/su13074030
Birch E, Bridgens B, Zhang M, Dade-Robertson M. Bacterial Spore-Based Hygromorphs: A Novel Active Material with Potential for Architectural Applications. Sustainability. 2021; 13(7):4030. https://doi.org/10.3390/su13074030
Chicago/Turabian StyleBirch, Emily, Ben Bridgens, Meng Zhang, and Martyn Dade-Robertson. 2021. "Bacterial Spore-Based Hygromorphs: A Novel Active Material with Potential for Architectural Applications" Sustainability 13, no. 7: 4030. https://doi.org/10.3390/su13074030
APA StyleBirch, E., Bridgens, B., Zhang, M., & Dade-Robertson, M. (2021). Bacterial Spore-Based Hygromorphs: A Novel Active Material with Potential for Architectural Applications. Sustainability, 13(7), 4030. https://doi.org/10.3390/su13074030





